Abstract
The recent US Food and Drug Administration licensure of a prophylactic vaccine against oncogenic human papillomavirus (HPV) types 16 and 18, the first of its kind, poses unique challenges in postmarketing vaccine surveillance, especially in measuring vaccine effectiveness against biologic endpoints of HPV infection. Historically, the national system of population-based cancer registries in the US has provided high-quality data on cancer incidence and mortality for the most important biologic endpoints, namely, anogenital cancers and some oral cavity/oropharyngeal cancers. There also has been some data collection on cancer precursors; however, this activity has been inconsistent and of lower priority. Because effectiveness against HPV-associated cancers will not be measurable for several decades, strengthening and possibly expanding the capacity of registries to collect precancer data, which are earlier manifestations of infection, must be considered. Collecting type-specific data on HPV-associated precancers and cancers. While keeping in mind the current limitations of registry operations, they discuss resources that may be needed to implement and sustain these types of activities.
More than 5% of cancers worldwide are attributable to human papillomavirus (HPV) infection, and the greatest attributable fraction is associated with cervical cancer.1 Globally, an estimated 250,000 women die each year of cervical cancer. Prophylactic vaccines against oncogenic types of HPV, which became available to the US market in 2006, hold the promise of becoming a public health triumph in the 21st century. It will be at least 1 to 3 decades after the vaccine becomes available before the population targeted for vaccine (in the United States, this includes girls ages 11–12 years) will be at risk for important endpoints of HPV infection, namely, cervical intraepithelial neoplasia 3 (CIN-3), carcinoma in situ (CIS), and invasive cervical carcinoma (ICC).
The biology of HPV infection and its role as the primary cause of cervical cancer is well established.2–4 Essentially all cervical carcinomas are caused by persistent infection with 1 of 13 to 15 oncogenic HPV types.2–4 Preventing HPV type 16 (HPV-16) and HPV-18 infections could eliminate >70% of all cervical carcinomas, assuming that, as predicted, that there is no type replacement and that other types remain less likely to progress to carcinoma.5,6 Clinical trials evaluating prophylactic HPV vaccines directed specifically against HPV-16 and HPV-18 have reported nearly complete efficacy for preventing type-specific HPV persistence and related cervical, vaginal, and vulvar cancer precursors among adolescent and young women who were naive to the vaccine type and who completed the full 3-dose schedule.7–9 However, the vaccines were not effective in preventing progression to disease among women who already were infected. It has been estimated that vaccination against HPV-16 and HPV-18 would reduce cytologic reports of high-grade squamous intraepithelial lesions by 50% and would have a more modest impact on less severe diagnoses.4 The effectiveness of vaccine in actual use (ie, not in clinical trials) has yet to be determined, and many challenges exist. These effectiveness data are needed to assess the true public health benefit of HPV vaccination.
A key approach to determining the public health benefit and cost-effectiveness of the prophylactic HPV vaccine in the United States is implementation of population-based surveillance activities. In October 2006, the Pan American Health Organization (PAHO) convened experts to discuss the most appropriate and effective strategies for conducting surveillance of the HPV vaccine10 (see Table 1). Surveillance outcomes proposed by the PAHO expert committee included monitoring changes in ICC incidence and mortality and determining trends in the type-specific prevalence and incidence of histologic confirmed CIN-3 lesions. In August 2006, experts convened at the Centers for Disease Control and Prevention (CDC) (Atlanta, Ga) discussed the importance in monitoring the impact of the HPV vaccine in the United States and judged high-grade CIN as the most important cervical cancer precursor endpoint for monitoring purposes. Recommendations from both meetings included outcomes that 1) currently are being collected by US cancer registries and health departments (ICC incidence and mortality), 2) were collected in the past by US cancer registries (CIS; CIN-3), and 3) have the potential to be collected through special projects (HPV type distribution associated with CIN-3, ICC, and noncervical cancers).
TABLE 1.
Results From an Ad Hoc Meeting Conducted by the Pan American Health Organization on Important Biologic Endpoints for Human Papillomavirus Vaccine Surveillance
| Endpoints for HPV Surveillance and Measurement Indicators | |
|---|---|
| Endpoint | Measurement Indicator |
| Genital HPV infections | Changes in prevalence |
| Genital warts (depends on vaccine product being used) | Changes in prevalence |
| Cervical cancer precursors, specifically CIN-3 based on histologic determination | Changes in the overall prevalence |
| Invasive cervical cancer based on histologic determination | Changes in the incidence profiles |
| Invasive cervical cancer mortality | Changes in mortality profiles |
| HPV type-specific changes in histologically defined CIN-3 lesions | Changes in type-specific incidence in CIN-3 precursor lesions |
HPV indicates human papillomavirus; CIN-3, cervical intraepithelial neoplasia grade 3.
See Pan American Health Organization, 2006.10
The US population-based cancer registries include the CDC’s National Program of Cancer Registries (NPCR) and the National Cancer Institute’s (NCI’s) Surveillance, Epidemiology, and End Results (SEER) Program. Together, these registries cover 100% of the US population and serve an essential public health function through the collection and compilation of cancer incidence data that are used for cancer prevention and control efforts and guidance of public health policy.11 The infrastructure to conduct population-based surveillance for the HPV vaccine already is in place through the registries, and they provide a unique opportunity to build upon already existing programs to conduct enhanced HPV-associated precancer and cancer surveillance. These efforts will require a well funded, carefully organized national effort guided by a multidisciplinary team of experts.
In this report, we provide a perspective on the potential role of the US population-based cancer registries in monitoring the effectiveness of prophylactic HPV vaccines beyond their current role of collecting data and monitoring the occurrence of cancers. In addition to discussing the merits of and challenges for using cancer registries for the surveillance of trends in the incidence of HPV-associated disease, we also consider resources that will be needed to implement and sustain these types of surveillance activities within the cancer registry system.
Enhanced Assessment of the Burden of HPV-associated Cancers and Precancers in the United States
Optimally, all measurements used to monitor the impact of the HPV vaccine should be population-based and highly sensitive to early changes in the rates of HPV-associated diseases that are likely to result from vaccine interventions. This commentary is not meant to be a blueprint for future HPV-associated disease surveillance; rather, our objective is to provide an overview of the desired surveillance activities and the critical elements to consider for establishing the system. For brevity and quick reference, this potential surveillance system will be termed Enhanced Assessment of the Burden of HPV-Associated Cancers and Precancers in the United States (E-ABHACUS).
E-ABHACUS recommendations
The overarching goal of E-ABHACUS is to monitor the impact of the HPV vaccine on the incidence of ICC and its precursors (defined herein as CIS, CIN-3, and adenocarcinoma in situ [AIS]) in the United States as the cohort of the vaccinated population ages and vaccination coverage increases. Recommendations for establishing an enhanced surveillance system include:
Systematically monitor age-specific rates of ICC and other invasive HPV-associated carcinomas (currently exists with the cancer registries); other HPV-associated carcinomas include anal, penile, vulvar, vaginal, oropharyngeal, and oral cavity cancers.
Systematically monitor age-specific rates of cervical cancer precursors and precursors for other HPV-associated cancers.
Identify the distribution of HPV types (eg, HPV-16 and HPV-18) associated with HPV-associated carcinoma precursors and invasive carcinoma.
Monitor the incidence of invasive and preinvasive carcinomas along with the prevalence of vaccination; linkages between cancer and immunization registries (which, like many cancer registries, are in state and local health departments) could provide data on the vaccine status of women with diagnoses of these cancers.
Explore and evaluate methods for linking cancer registry data with screening and risk factor data which are already being collected by other surveillance systems.
Although some of the recommendations are existing activities (as noted), ultimately, the identified disparate resources need to be brought together under a single umbrella to maximize the utility of the aggregated information for public health surveillance. Selected scientific and logistical considerations related to addressing these aims are discussed below.
Monitoring rates of ICC and other HPV-associated cancers
Patterns in the occurrence of ICC and other invasive HPV-associated cancers can be monitored through NPCR and SEER. A recent report from the International Agency for Research on Cancer concluded that a significant percentage of several cancers, in addition to cervical cancer and including vulvar, vaginal, anal, penile, and oropharyngeal cancers, are caused by HPV. For these cancers, it is believed that HPV-16 and HPV-18 are responsible for the majority of HPV-positive cases.12 Thus, monitoring postvaccine trends in HPV-associated noncervical carcinomas also appears to be a necessary important surveillance activity. Dillner et al further propose that HPV typing of all HPV-associated cancers will become an essential part of the long-term evaluation and monitoring of HPV vaccination programs13
HPV infection also is related to a subset of head and neck cancers that occur in the oral cavity and oropharynx. Approximately 35% of oropharyngeal cancers and 25% of oral cavity cancers may be related causally to HPV infection in the United States.14 Similar to vulvar carcinomas, >90% of the HPV-associated oropharyngeal cancers are related to HPV-16. The importance of this potential prevention effort is highlighted by data, including those from this supplement, suggesting that the incidence of HPV-associated oropharyngeal/oral cavity cancers is increasing.15,16 These data provide a strong argument for the inclusion of oropharyngeal and oral cavity cancers in enhanced surveillance activities.
Monitoring only invasive carcinoma, however, has limited ability to detect small but meaningful changes in cancer incidence. Monitoring precancers, which present at much higher rates than invasive cancers, provides an opportunity to more accurately detect impact of HPV vaccine and provides a shorter time horizon for measuring impact.
Monitoring rates of cervical cancer precursors and precursors for other HPV-associated cancers
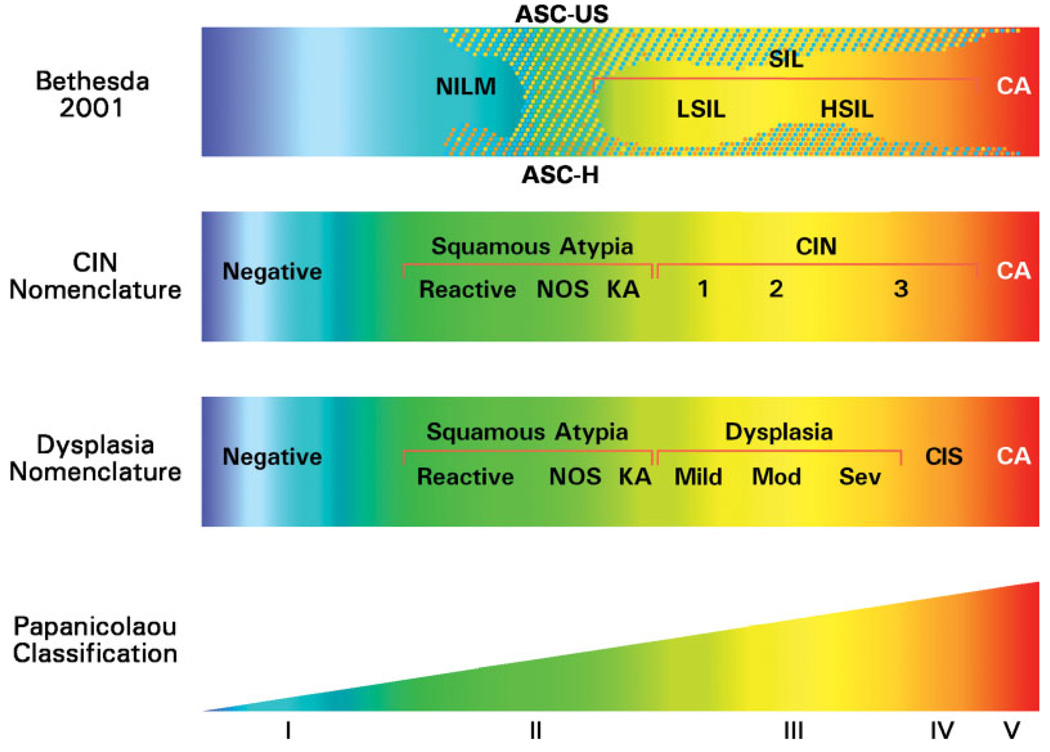
The concept of collecting cervical precancers through US cancer registries is not new. Data on CIS/CIN-3 were collected by the registries starting in 1984 until discontinuation in 1996 based on a joint decision by many national organizations including the NCI and CDC (Table 2).17 Noninvasive cervical lesions display a range of increasing severity. This range historically has been graded by using diverse nomenclatures, including CIN (CIN-1, CIN-2, and CIN-3) and mild to severe dysplasia/CIS. The confusion associated with the use of several contemporaneous histopathologic terminologies (see Fig. 1) created complexities and raised questions regarding the quality of the cervical cancer precursor data.18,19 More recently, US pathologists have widely accepted the ‘intraepithelial neoplasia’ terminology. Specifically, the term CIN-3 largely has superseded the comparable terms of severe dysplasia or CIS.20
TABLE 2.
Historical Rationale for Discontinuing Collection of Cervical Intraepithelial Neoplasia 3 and Cervical Carcinoma In Situ: Conclusions and Recommendations by the American Association for Cancer Research Uniform Data Standards Committee: Subcommittee on Noninvasive Cervix Lesions, 1993*
| Conclusions | |
| 1. | Data on preinvasive cervical neoplasia as currently collected by US registries are no longer comparable historically for monitoring trends over time and cannot be made to be comparable. The problem will get worse as the Bethesda System (TBS) is adopted more widely for histopathology. Collection of only the term carcinoma in situ (CIS) yields undercounts in recent data compared with the past. Collection of both of the terms CIS and cervical intraepithelial neoplasia 3 (CIN-3) yields over counts compared with the past. |
| 2. | In the future, it will not be possible in every case to distinguish moderate dysplasia, severe dysplasia, and CIS on pathology reports. |
| 3. | There is evidence that the relative use of the 3-tier, 4-tier, and TBS systems varies from place to place within the US. Thee comparability of current data across registries is limited by different rates of differences in terminology used on pathology reports. |
| 4. | Data as collected presently can be used to monitor differences in subpopulations within a geographic area as long as biases in diagnostic criteria and case ascertainment are not present. Preinvasive rates help to interpret differences in invasive rates, and in situ/invasive ratios in particular appear to have implications for prevention activities. |
| 5. | Adjacent grades of preinvasive lesions are more difficult to distinguish from one another and are less reproducible than the distinction between invasive and noninvasive lesions. |
| 6. | Treatment is becoming more conservative and more uniform for high-grade squamous intraepithelial lesion (HSIL) and the other equivalent terms. Because colposcopic biopsy and loop electrosurgical excision procedures can be therapeutic as well as diagnostic, histologic diagnoses of preinvasive lesions represent cancer control interventions. Fewer ablative procedures will be done without tissue diagnosis, so this is not expected to be a significant source of missed cases. |
| 7. | Diagnosis and treatment, as also is true to some extent for other cancer sites, are moving out of the hospital setting. It will become more and more difficult and expensive to find cases and collect required demographic data, such as the patient’s race and place of residence. Out of area laboratories likely be a problem and will be an increasingly important source of missed cases. |
| 8. | The Surveillance, Epidemiology, and End Results Program, which sets the de facto reporting standard in this area, likely will drop collection of CIS and CIN-3 in all areas and instead, with special funds, will expand collection to include HSIL and its equivalent terms in selected areas. |
| Recommendations | |
| 1. | The only way to collect histologic data that would be comparable over time in the future is to collect all HSIL and all of its equivalents in the 3- and 4-tier systems. This would increase the number of preinvasive cervix uteri cases in a registry that currently collects CIS and CIN-3 by approximately 2- to 3-fold; however, the new data would not be comparable to data collected earlier. |
| 2. | For research on the natural history of this disease, special studies that include the entire spectrum of low-grade squamous intraepithelial lesion (LSIL) and HSIL will be necessary. |
| 3. | Population-based registries need to assess locally the anticipated uses of the data (are time trends desirable? Are comparisons to data from other areas needed? Will cancer control programs use the data), present and future pathology practices in their areas (Will TBS be used for histopathology?), and available resources (can the registry afford to collect substantially more cases?) and decide whether to: a. Collect CIS, CIN-3, and severe dysplasia, which will not be possible if TBS is used for histopathology; or b. Collect all HSIL and its equivalent terms in the 3- and 4-tier systems, which will substantially added to the caseload; or c. Drop the collection of all data for all but invasive cervical cancers. |
| 4. | The subcommittee strongly recommends that population-based registries discontinue routine collection of data on preinvasive cervical neoplasia unless there is strong local need and interest and sufficient resources are available to collect all HSIL and its equivalent terms. |
2008 Updates: 1) TBS categories have changed from 2-tiered (low grade and high grade) to multiple tiers (atypical squamous cells of undetermined significance [ASC-US]; ASC-US, cannot rule out HSIL; atypical granular cells; LSIL; and HSIL); 2) CIN-3 plays a larger role among the in situ cancers for the 1 registry that has continued collection of this variable (Michigan); 3) TBS is recommended for cytology and the 3-tiered system (CIN-1, CIN-2, and CIN-3) usually is reserved for histology; 4) there still may be pathologists who use the 2-tiered system (low grade and high grade) for histology; and 5) there still may be pathologists who use the dysplasia categories (mild, moderate, severe) for histology.
FIGURE 1.
Comparison of 4 classification systems for squamous cells; Bethesda 2001 (cytology), cervical intraepitheliali neoplasia (CIN) nomenclature (histology), dysplasia nomenclature (cytology and histology), and Papanicolaou classification (cytology; the categories are not shown to scale). ASC-US indicates atypical squamous cells of undetermined significance; NILM, negative for intraepithelial lesion and malignancy; SIL, squamous intraepithelial lesion; LSIL, low-grade SIL; HSIL, high-grade SIL; CA, invasive carcinoma; ASC-H, atypical squamous cells, cannot exclude HSIL; NOS, not otherwise specified; KA, koilocytotic atypia; Mild, mild dysplasia; Mod, moderate dysplasia; Sev, severe dysplasia; CIS, carcinoma in situ. Reproduced with permission from Oxford University Press and the Journal of the National Cancer Institute (Sherman ME, Lorincz AT, Scott DR, et al. Baseline cytology, human papillomavirus testing, and risk for cervical neoplasia: a 10-year cohort analysis. J Natl Cancer Inst. 2003;95:46–5219).
Nosologic concerns aside, current evidence strongly supports the designation of CIN-3 as the most relevant precursor of squamous cell carcinoma. Arguments for the reintroduction of cervical CIN-3/CIS reporting to population-based cancer registries include 1) the acceptably high interobserver reproducibility 21; 2) the reduced likelihood of regression compared with CIN-2 and less severe lesions22; and 3) an HPV type spectrum that more closely matches that of invasive carcinoma rather than milder squamous lesions (≤CIN-2).23
The nomenclature for precursors of invasive adenocarcinoma is simpler than that for squamous cell carcinoma. The only widely accepted precursor of invasive adenocarcinoma is AIS,24 which is a term used consistently by pathologists.
Analogous ‘intraepithelial neoplasia’ terminology has been developed for carcinoma precursors of other epithelial cancer sites: anal intraepithelial neoplasia (AIN), vaginal intraepithelial neoplasia (VaIN), vulvar intraepithelial neoplasia (VIN), and penile intraepithelial neoplasia (PIN). Past challenges to the achievement of consistent systematic coding of cervical cancer precursors have been reduced as a result of these consensus guidelines, and further simplification is possible by adoption of ‘intraepithelial neoplasia’ terminology (eg, CIN-3, VIN-3) in the pathology community.4,20
Challenges to collecting cervical and other HPV-associated precancers exist, but some lessons learned from the articles in this supplement of Cancer offer some insight for future activities. Despite the 1996 decision to stop collecting cervical cancer precursor data, the Michigan Cancer Surveillance Program (MCSP) has continued to collect CIN-3/CIS data, and findings have been published elsewhere in this supplement.25 The MCSP has continued to collect CIN-3 data with minimal additional resources because of the increasing use of electronic case reporting. Expanding outside of Michigan, the CDC is planning a pilot program to look at the feasibility of systematically collecting cervical precancer data (CIN-3, CIS, AIS) through the already existing NPCR infrastructure in selected cancer registries. In the pilot study, central cancer registries will evaluate the feasibility of, the resources required for, and the costs associated with collecting data on cervical precancers. Although such a system leverages the current NPCR infrastructure (data streams from cancer registrars, pathology laboratories, hospitals), additional resources may be needed to collect the relatively large number of precancer cases (compared with the smaller number of invasive cancers). Clearly, evaluating the additional burden and cost of E-ABHACUS and other approaches must be done before large-scale adoption. In fact, large-scale adoption may not even be needed. Findings from the pilot studies for cervical precancers, coupled with information on expected rates of type-specific prevalence in CIN-3 and AIS estimated through selected cancer registries (on a periodic basis), could provide a sentinel populationbased surveillance system for type-specific HPV-associated cervical precancer throughout much of the US population.
After CIN-3 was no longer reportable to cancer registries in 1996, the collection of incidence data of high-grade intraepithelial lesions (often considered synonymous with in situ cancer) of other cancers (AIN-3, VIN-3, VaIN-3, but not PIN-3 after 2000) remained a requirement for SEER and NPCR but not for the Commission on Cancer (led by the American College of Surgeons [ACoS]) (Table 3). Although large percentages of these noncervical in situ lesions are being reported by hospitals and increasingly by nonhospital sources, an accurate assessment of the true burden of these preinvasive lesions will be possible only if the ACoS readopts this reporting requirement. With the effort underway to assemble a multidisciplinary team of experts to monitor the impact of prophylactic HPV vaccines on the rates of cervical carcinoma and its precursors and on the rates of other HPV-associated neoplasms in the United States, key organizations like the CDC-NPCR, NCI-SEER, and ACoS programs, as well as the North American Association of Central Cancer Registries, will need to come to a consensus regarding collection of the new precancerous cases of all HPV-associated epithelial cancers discussed in this supplement. Out of concern regarding the completeness of VIN-3, VaIN-3, and AIN-3 reporting, the NPCR has not required cancer registries to submit these data since 2003, leading to an underestimation of in situ cancers coded as 8077/2 (although cancer registries may collect these data). For the years 1998 through 2003, SEER registries, which continued to require the collection of these data, this code accounts for 46% of in situ anal cancers, 63% of in situ vulvar cancers, and 77% of in situ vaginal cancers. Before any implementation of E-ABHACUS, the quality of these in situ cancer data in SEER and NPCR registries will need to be evaluated carefully.
TABLE 3.
Comparison of Reportable Human Papillomavirus-associated Cancers and Precancers: Commission on Cancer; National Program of Cancer Registries; and Surveillance, Epidemiology, and End Results*
| Reportable Diagnoses on or After Jan. 1, 2005 | COC (Behavior Code of 2 or 3 in ICD-O 3) | SEER (Behavior Code of 2 or 3 in ICD-O 3) | NPCR (Behavior Code of 2 or 3 in ICD-O 3) |
|---|---|---|---|
| Exceptions (not reportable) | • Skin cancers (c44._) with histology 8000–8110 (after Jan. 1, 2003); prior to that date, AJCC stage groups 2–4 in this group were reportable | • Skin cancers (c44._) with histologies 8000–8005, 8010–8046, 8050–8084, and 8090–8110 | • Skin cancers (c44._) with histologies 8000–8005, 8010–8046, 8050–8084, and 8090–8110 |
| • CIS of the cervix and CIN-3 (after Jan. 1, 1996) | • CIS of the cervix and CIN-3 (after Jan. 1, 1996) | • CIS of the cervix and CIN-3 (after Jan. 1, 1996) | |
| • PIN-3 (after Jan. 1 | • PIN-3 (after Jan. 1, 2001) | • PIN-3 (after Jan. 1, 2001) | |
| • VIN-3 (after Jan. 1, 96) | |||
| • VAIN-3 (after Jan. 1, 1996) | |||
| • AIN (after Jan. 1, 1996) |
COC indicates Commission on Cancer; SEER, Surveillance, Epidemiology, and End Results; NPCR, National Program of Cancer Registries; ICD-O 3, International Classification of Diseases for Oncology, 3rd edition; AJCC, American Joint Committee on Cancer; CIS, cervical carcinoma in situ; CIN-3, cervical intraepithelial neoplasia 3; PIN-3, penile intraepithelial neoplasia 3; VIN-3, vulvar intraepithelial neoplasia 3; VAIN-3, vaginal intraepithelial neoplasia 3; AIN, anal intraepithelial neoplasia.
See North American Association of Central Cancer Registries, 2006.18
Population-based assessment of HPV types associated with invasive carcinoma and carcinoma precursors
An essential purpose of E-AHBACUS surveillance systems will be to monitor changes in HPV type distribution in HPV-associated cancers and precancers that could be impacted by vaccination. In 2001, 3 SEER registries (Hawaii, Los Angeles, and Iowa) implemented population-based formalin-fixed, paraffin-embedded tissue block collection within their respective geographic areas, thereby establishing an infrastructure to conduct tissue-based research from a population-based perspective.26 In addition, the CDC is developing a pilot study to systematically monitor HPV types present in tissue specimens from cancers of the cervix, vagina, vulva, anus, penis, and oropharynx/oral cavity obtained with the assistance of 4 participating central cancer registries (Kentucky, Louisiana, Michigan, and Florida) that have a high burden of disease and racial/ethnic diversity. Such carefully annotated population-based tissue banks have the advantage of providing an unbiased sampling frame for the evaluation of HPV DNA in cervical, vaginal, vulvar, penile, anal, and oropharyngeal tissue from several registries in defined communities. By using this approach, it will be possible to monitor changes in HPV type-specific rates for several HPV-associated diseases.
Additional resources will be required to validate that the collected samples are representative of the registry catchment population with regard to key histopathologic, clinical, and patient metrics. Pathologic confirmation of tissue diagnoses, followed by HPV testing distributed across several calendar periods, will permit the estimation of attributable fractions of HPV-16 and HPV-18 for cervical neoplasia and other diseases (precursors of cervical cancer or other HPV-associated cancers) by age, racial/ethnic group, and selected factors over time. Results of an extensive baseline HPV typing effort should lead to the identification of an adequate sampling frame within sentinel registries that would permit selective sampling of tissue for future HPV DNA testing to monitor vaccine impact. The exact design of this approach would require expert multidisciplinary collaborations and detailed knowledge of the screening and treatment practices within the registry’s geographic area.
Relating rates of invasive carcinoma and carcinoma precursors to the vaccinated population
Two approaches for assessing the impact of prophylactic HPV vaccination include 1) linking cancer registry information (registry record, available tissue, and HPV DNA typing) with an individual’s vaccine record and 2) correlating incidence of HPV-associated and/or HPV-typed lesions with vaccination prevalence within defined geographic areas. Relating HPV-associated cervical disease to the vaccination record of individuals could be conducted through existing immunization information systems. Immunization information systems contain confidential and computerized information (http://www.cdc.gov/vaccines/programs/iis/default.htm accessed on July 16, 2008) as do cancer registry data. They collect and consolidate vaccination data from multiple healthcare providers, assess vaccination coverage within a defined geographic area, and have linkages with electronic data sources.27,28 Most data from the immunization information system are initiated through vital records sources at the state or city level and then updated by vaccination providers or billing systems. These records have been implemented widely for the collection of infant immunization data and currently are being expanded to collect vaccination data on all ages with increasing emphasis on adolescent immunization, such as HPV immunization. It is noteworthy that linkages between cancer registries and other information systems are not new; cancer registries routinely link with state and national vital statistics to determine vital status, with hospital discharge records to describe comorbid conditions, and with the Indian Health Services patient database to mitigate the effects of misclassification of American Indian/Alaska Native race.
Although both types of approaches are useful, developing a system for generating population-based summary data is more feasible and perhaps more consistent with the usual goals and functions of cancer surveillance and registration. In addition, the benefits of vaccination at the population level may extend beyond the individual patient to include unvaccinated patient contacts (‘herd immunity’). Thus, as communities debate the various recommendations and protocols for vaccinating adolescents, the possibility exists to define cohorts in which the prevalence of vaccination at early ages is high and is distributed uniformly. An attractive option for surveillance from a public health perspective would be to estimate age-specific vaccination rates by locality (eg, county or zip code) and calendar period and to relate these statistics to the local incidence of (preferably) HPV-typed and pathologically confirmed cancer precursors and carcinoma. This approach would not meet the requirements of evaluating the effectiveness of vaccination from a clinical perspective, but it may allow assessment of vaccination as a public health intervention at the population level.
Interpreting screening information from other surveillance systems
Interpreting cancer registry data along with trends in cervical cancer screening is another recommendation for E-ABHACUS. The paradigm of cervical cancer screening is changing contemporaneously with vaccine introduction, as highlighted by Castle et al29 Cervical cancer screening currently consists of cytologic screening, sometimes in combination with HPV DNA testing and cytology or cytology alone. However, cervical screening with HPV DNA testing followed by reflex cytology30 is being evaluated increasingly as a screening strategy, whereas others are considering the addition of type-specific HPV testing as a part of the screening armamentarium.31 HPV vaccine and HPV DNA testing are most cost-effective in settings in which screening among a vaccinated population starts later and occurs less frequently. 32,33 For these reasons, monitoring the incidence of cervical precancers and cancers needs to be conducted in conjunction with monitoring screening practices. In this supplement, Tiro et al34 have described the different national behavioral surveillance systems that exist to measure screening and vaccination practices among the population as well as providers. It will be important to explore whether linkages of cancer registry data to screening behavior at the individual level can be achieved, although community measures of screening practices also will be informative. Population-based analyses of screening data from various national and state surveillance systems, in conjunction with invasive carcinoma and precursor data from cancer registries, can be used to guide cancer control efforts and intervention research. The interactive State Cancer Profiles website, for example, which was developed by the CDC and NCI, provides cancer registry data and screening information to guide decision-making for cancer control in states and counties.35 In addition, the CDC’s Behavioral Risk Factor Surveillance website includes basic statistical analysis tools to facilitate public health program planning (see http://apps.nccd.cdc.gov/s_broker/htmsql.exe/weat/index.hsql accessed on July 16, 2008).
Considerations and Challenges
The long-term impact of the HPV vaccine on cervical cancer is being measured with pharmaceutical-sponsored efficacy studies of cohorts in several Nordic countries, where the health registry infrastructure allows for active and optimal follow-up.36 Defining vaccine-exposure cohorts and following them prospectively poses major challenges for US registry-based studies. HPV-vaccinated adolescents are likely to be difficult to follow into adulthood, when HPV-16– and HPV-18–associated cervical neoplasia and other outcomes would be assessed. Furthermore, ascertaining individual vaccine histories may require informed consent, could raise serious logistical challenges, and might be subject to recall bias or erroneous self-reporting. In states with high HPV vaccination rates and low in-and-out migration, these concerns may be minimized, but they certainly will not be eliminated.
Of further concern are changes in the treatment of women with HPV-associated lesions less severe than CIN-3; such treatment may reduce the incidence of cervical neoplasia, thus making it difficult to tease out the effects of vaccination versus treatment. This concern is mitigated somewhat by published and widely disseminated treatment guidelines, 37 which endorse the follow-up of women, especially young women, with early HPV-associated lesions. In addition to censoring through treatment, temporal shifts in HPV cofactors, such as smoking, oral contraceptive use, sexual activity, or a shift in cervical cancer screening practices, could affect progression risk among HPV-infected individuals.
An important component of the surveillance plan is the need for adequate statistical power to detect relatively subtle changes in the rates of HPV-associated cancers. To address this concern, the development of an enhanced surveillance system should be implemented as soon as funding and other necessary resources become available to provide a baseline and sufficient lead time for the surveillance process and the inclusion of a large and representative sample. The implementation period must allow ample opportunity to address logistical concerns, identify study population sampling frames, and pilot surveillance methods and the demonstration of feasibility. Vaccination of sexually active women (‘catch-up’) may introduce additional uncertainties about the development of E-ABHACUS, but these effects are likely to be minor compared with the primary effect of preadolescent vaccination. Careful age-period–cohort analyses may permit a partial disentangling of the effects of catch-up versus presexual initiation vaccination efforts.
Logistical Issues
Proposing a specific and comprehensive plan for developing a surveillance system is beyond the scope of this supplement, but possible next steps for implementing such a system include conducting pilot studies in a few selected registries and defining populations that are suitable for baseline determinations of rates of cervical carcinoma precursors and invasive carcinoma. The desired characteristics of these populations are 1) adequate size, 2) racial/ethnic diversity, 3) age distributions that sufficiently represent the target population for screen-detected cervical neoplasia (roughly ages 20–50 years, capturing the rare cancers identified in young groups to the median age of diagnosis), 4) well defined catchments with regard to tissue sources, and 5) potential for tissue access. Given tissue access, representative histologically confirmed CIN-3, AIS, and cervical carcinoma tissue blocks should be collected for HPV DNA typing. These data could be used to provide a prevaccine baseline of the type-specific prevalence of HPV within these tissues by factors of interest, such as age and ethnicity.
Legal Issues
Because all states are required to follow national and state legislation and regulation for cancer reporting, and because the reporting requirements include invasive and in situ cancers, there are no legal impediments (or, otherwise, impediments are minimal) for adopting the collection of CIN-3/CIS, especially given expert consensus that CIN-3 and CIS are the same entity. Collection of CIN-2 data may pose more of a challenge because CIN-2 is less likely to predict the risk of developing invasive cancer. Because HPV vaccine recommendations were released in 2006, legislation has increased in various parts of the United States regarding reporting requirements of HPV-associated conditions, some in conjunction with the cancer registries and some not. The New Mexico Department of Health has designated HPV as a reportable condition in New Mexico; under the auspices of HPV reporting, results from Papanicolaou (Pap) tests, cervical pathology, and HPV test results all are reportable (unpublished results). Since 2006, Florida’s Sexually Transmitted Diseases (STD) Bureau has made some HPV-associated diseases reportable by both practitioners and laboratories (http://www.doh.state.fl.us/Disease_ctrl/std/clinical/STD_Case_Definitions.html accessed on July 16, 2008). Specifically, practitioners who note any HPV-associated laryngeal papillomas or recurrent respiratory papillomatosis, HPV infection in children aged <12 years, or any positive HPV tests should report these findings to Florida’s STD Bureau. In addition, Florida laboratories are required to report any positive HPV DNA tests, HPV type-specific results, and abnormal cervical cytologies and histologies. In January 2008, Connecticut passed legislation that made CIN-2/CIN-3 reportable by pathology laboratories and asked laboratories to submit tissue specimens to the health department for typing. Currently, California and Tennessee are considering making CIN-2/CIN-3 reportable statewide.
Resource Issues
The primary concern of the registries will be the added reporting burden and need for additional resources (both staff and funds). Not only are CIS and CIN-3 much more common than invasive cervical cancer (7-fold in the Michigan data), but the sources of identifying these cases also are diverse. CIS and CIN-3 often are observed and/or treated in physician’s offices, and tissues are sent to free-standing private pathology laboratories. The burden of collecting cancer cases from these nonhospital sources is substantially more labor-intensive and difficult. However, with increased electronic reporting from pathology laboratories, it is potentially feasible to capture cases of these cervical cancer precursors; the underlying assumption is that the registry can identify all pathology laboratories (both in-state and out-of-state) that review and make diagnoses of cancer and precancerous lesions among residents of the catchment area. Additional costs are incurred when cases reported by pathology laboratories are traced back to physician’s offices to obtain other demographics, such as race or address. To determine the cost and staff required by a registry for collecting CIS and CIN-3 data, pilot projects will need to be funded and implemented in selected states to assess the feasibility of the operations and the additional resources required. These pilot projects also should assess the extent to which these additional activities may interfere with routine cancer registry operations. Although the burden would decrease as vaccination coverage increases, resources and relationships that once were used for this activity would have to be revisited. This need to balance public health surveillance efforts (including establishing the prevaccine HPV-associated disease burden and monitoring the impact and effectiveness of the HPV vaccine) with limited resources should be addressed by the national standards organizations.
Conclusions
The development and introduction of prophylactic HPV vaccines creates a public health mandate to monitor their ultimate impact in reducing the HPV-associated disease burden in our nation. The PAHO10 report has acknowledged that implementing the full range of HPV surveillance strategies can be complex and costly. The report suggests that every country should examine its infrastructure and capability to determine the best course of action.
In the United States, the existing public health infrastructure bodes well for the potentially large role that cancer registries may play in evaluating the effectiveness of prophylactic HPV vaccine activities. The HPV vaccine provides a new focus for cancer prevention activities. Clinical trials have demonstrated the tremendous potential of the vaccine for reducing cervical cancer in the United States and worldwide. NPCR and SEER will have major roles in monitoring the vaccine’s impact in the United States; the well developed nationwide cancer registry infrastructure already in place is positioned uniquely to adopt selected recommendations proposed by PAHO.10 NPCR and SEER already collect, for example, information regarding the histology of invasive cervical cancers and other invasive HPV-associated cancers. Expanded roles for monitoring the impact of the HPV vaccine could include the collection of data regarding cervical cancer precursors and precursors of other HPV-associated cancers, the collection of data regarding HPV type, and linkages to vaccine registries. Major resource challenges exist, however, for expanding the current work of registries (eg, the reporting burden of precursors), and feasibility studies are needed to evaluate the resources and costs for registries to implement these new activities. The adoption of E-ABHACUS nationwide offers the opportunity to develop a new paradigm of an integrated partnership approach to cancer surveillance. Accordingly, this initiative should be given careful consideration as a logical avenue for augmenting our existing cancer registry resources for postmarketing HPV vaccine surveillance.
Acknowledgments
This supplement to Cancer was supported by Cooperative Agreement Number U50 DP424071-04 from the Centers for Disease Control and Prevention (CDC).
Marc Goodman has received support from Public Health Service contract N01-PC-67001 from the Department of Health and Human Services, National Institutes of Health. Phyllis Wingo has received support from the Centers for Disease Control and Prevention for her role as a consultant on this project.
The authors thank Mark Sherman, Hannah Weir, Lauri Markowitz, Mark Schiffman, Diane Solomon, Ken Gerlach, and Missy Jamison for reviewing earlier versions of this article.
Footnotes
The findings and conclusions of this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clifford G, Franceschi S, Diaz M, Munoz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24 suppl 3:S26–S34. doi: 10.1016/j.vaccine.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 5.Rousseau MC, Pereira JS, Prado JC, Villa LL, Rohan TE, Franco EL. Cervical coinfection with human papillomavirus (HPV) types as a predictor of acquisition and persistence of HPV infection. J Infect Dis. 2001;184:1508–1517. doi: 10.1086/324579. [DOI] [PubMed] [Google Scholar]
- 6.Liaw KL, Hildesheim A, Burk RD, et al. A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. J Infect Dis. 2001;183:8–15. doi: 10.1086/317638. [DOI] [PubMed] [Google Scholar]
- 7.Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18–27. doi: 10.1097/01.AOG.0000192397.41191.fb. [DOI] [PubMed] [Google Scholar]
- 8.Ault KA. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of 4 randomised clinical trials. Lancet. 2007;369:1861–1868. doi: 10.1016/S0140-6736(07)60852-6. [DOI] [PubMed] [Google Scholar]
- 9.Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 10.Pan American Health Organization (PAHO) Immunization Unit. Ad hoc meeting of experts on surveillance of HPV-associated diseases in the americas. PAHO Immunization Newsletter. 2006 December;:1–4. [Google Scholar]
- 11.US Cancer Statistics Working Group. United States Cancer Statistics 1999–2003 Incidence and Mortality Web-based Report. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2007. [Google Scholar]
- 12.Cogliano V. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Human Papillomavirus [90] Lyon, France: International Agency for Research on Cancer; 2007. [Google Scholar]
- 13.Dillner J, Arbyn M, Dillner L. Translational mini-review series on vaccines: monitoring of human papillomavirus vaccination. Clin Exp Immunol. 2007;148:199–207. doi: 10.1111/j.1365-2249.2007.03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 15.Ryerson AB, Peters ES, Coughlin SS, et al. Burden of potentially HPV-associated cancers of the oropharynx and oral cavity in the United States, 1998–2003. Cancer. 2008;113 suppl 6:000–000. doi: 10.1002/cncr.23745. [DOI] [PubMed] [Google Scholar]
- 16.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110:1429–1435. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 17.American Association of Cancer Registries, Data Standards Committee. Rockville, MD: 1993. Apr 5–6, Subcommittee on Noninvasive Cervix Lesions, Working Group on Preinvasive Cervical Cancer Neoplasia and Population-based Cancer Registries: Final Subcommittee Report. [Google Scholar]
- 18.Havener LA, Hultstrom D, editors. Standards for Cancer Registries Volume II: Data Standards and Data Dictionary, Eleventh Edition, Version 11.1. Springfield, Ill: North American Association of Central Cancer Registries; 2006. [Accessed on July 16, 2008]. Available at: http://www.naaccr.org/filesystem/pdf/Volume%20II%20Version%2011.1.pdf. [Google Scholar]
- 19.Sherman ME, Lorincz AT, Scott DR, et al. Baseline cytology, human papillomavirus testing, and risk for cervical neoplasia: a 10-year cohort analysis. J Natl Cancer Inst. 2003;95:46–52. doi: 10.1093/jnci/95.1.46. [DOI] [PubMed] [Google Scholar]
- 20.Carreon JD, Sherman ME, Guillen D, et al. CIN2 is a much less reproducible and less valid diagnosis than CIN3: results from a histological review of population-based cervical samples. Int J Gynecol Pathol. 2007;26:441–446. doi: 10.1097/pgp.0b013e31805152ab. [DOI] [PubMed] [Google Scholar]
- 21.Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International Biological Study on Cervical Cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 22.Cox JT, Schiffman M, Solomon D. Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol. 2003;188:1406–1412. doi: 10.1067/mob.2003.461. [DOI] [PubMed] [Google Scholar]
- 23.Zuna RE, Allen RA, Moore WE, Mattu R, Dunn ST. Comparison of human papillomavirus genotypes in high-grade squamous intraepithelial lesions and invasive cervical carcinoma: evidence for differences in biologic potential of precursor lesions. Mod Pathol. 2004;17:1314–1322. doi: 10.1038/modpathol.3800223. [DOI] [PubMed] [Google Scholar]
- 24.Wright TC, Kurman RJ, Ferenczy A. Precancerous lesions of the cervix. In: Kurman RJ, editor. Blaustein’s Pathology of the Female Genital Tract. 5th ed. New York, NY: Springer-Verlag; 2001. pp. 253–324. [Google Scholar]
- 25.Copeland G, Datta SD, Spivak G, Garvin AD, Cote ML. Total burden and incidence of in situ and invasive cervix cancer in Michigan, 1985–2003. Cancer. 2008;113 10 suppl:2946–2954. doi: 10.1002/cncr.23747. [DOI] [PubMed] [Google Scholar]
- 26.Goodman MT, Hernandez BY, Hewitt S, et al. Tissues from population-based cancer registries: a novel approach to increasing research potential. Hum Pathol. 2005;36:812–820. doi: 10.1016/j.humpath.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Linkins RW. Immunization registries: progress and challenges in reaching the 2010 national objective. J Public Health Manag Pract. 2001;7:67–74. doi: 10.1097/00124784-200107060-00008. [DOI] [PubMed] [Google Scholar]
- 28.National Vaccine Advisory Committee. Development of Community and State-based Immunization Registries: Report of the National Vaccine Advisory Committee (NVAC) Atlanta, Ga: US Department of Health and Human Services, CDC; 1999. [Google Scholar]
- 29.Castle PE, Solomon D, Saslow D, Schiffman M. Commentary: predicting the effect of successful human papillomavirus vaccination on existing cervical screening programs in the United States. Cancer. 2008;113(10 suppl):3031–3035. doi: 10.1002/cncr.23762. [DOI] [PubMed] [Google Scholar]
- 30.Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 31.Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–1079. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 32.Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol. 2004;103:619–631. doi: 10.1097/01.AOG.0000120143.50098.c7. [DOI] [PubMed] [Google Scholar]
- 33.Goldie SJ, Kohli M, Grima D, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst. 2004;96:604–615. doi: 10.1093/jnci/djh104. [DOI] [PubMed] [Google Scholar]
- 34.Tiro JA, Saraiya M, Jain N, et al. Human papillomavirus and cervical cancer behavioral surveillance in the United States. Cancer. 2008;113(10 suppl):3013–3030. doi: 10.1002/cncr.23760. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention, National Cancer Institute. [Accessed on July 16, 2008];Bethesda, Md: National Cancer Institute; State Cancer Profiles. 2007 Available at http://statecancerprofiles.cancer.gov/
- 36.Lehtinen M, Herrero R, Mayaud P, et al. Chapter 28: studies to assess the long-term efficacy and effectiveness of HPV vaccination in developed and developing countries. Vaccine. 2006;24 suppl 3:S233–S241. doi: 10.1016/j.vaccine.2006.05.109. [DOI] [PubMed] [Google Scholar]
- 37.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 Consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. J Low Genit Tract Dis. 2007;11:223–239. doi: 10.1097/LGT.0b013e318159408b. [DOI] [PubMed] [Google Scholar]