Abstract
Attempts have been made to elevate EAAT2 expression in effort to compensate for loss of function and expression associated with disease or pathology. Increased EAAT2 expression has been noted following treatment with β-lactam antibiotics, and during ischemic preconditioning (IPC). However, both of these conditions induce multiple changes in addition to alterations in EAAT2 expression that could potentially contribute to neuroprotection. Therefore, the aim of this study was to selectively overexpress EAAT2 in astrocytes and characterize the cell type specific contribution of this transporter to neuroprotection. To accomplish this we used a recombinant Adeno-associated virus vector, AAV1-GFAP-EAAT2, designed to selectively drive the overexpression of EAAT2 within astrocytes. Both viral mediated gene delivery and β-lactam antibiotic (penicillin-G) treatment of rat hippocampal slice cultures resulted in a significant increase in both the expression of EAAT2, and dihydrokainate (DHK) sensitive glutamate uptake. Penicillin-G provided significant neuroprotection in rat hippocampal slice cultures under conditions of both moderate and severe oxygen glucose deprivation (OGD). In contrast, the overexpression of EAAT2 in astrocytes provided enhanced neuroprotection only following a moderate OGD insult. These results indicate that functional EAAT2 can be selectively overexpressed in astrocytes, leading to enhanced neuroprotection. However, this cell type specific-increase in EAAT2 expression offers only limited protection compared to treatment with penicillin-G.
INTRODUCTION
Glutamate is the primary excitatory amino acid neurotransmitter in the mammalian central nervous system. When released from presynaptic terminals, glutamate can activate ionotropic receptors such as AMPA and KA to mediate standard fast excitatory signaling, or contribute to the higher order processing required in development, plasticity, learning and memory by activating the NMDA and metabotropic glutamate receptors. Extracellular enzymes do not metabolize free glutamate. Instead, high affinity, Na-dependant excitatory amino acid transport proteins (EAATs), located in the plasma membranes of both neurons and surrounding astrocytes, facilitate cellular uptake (for review see Billups et al., 1998, Bridges et al., 1999; Danbolt., 2001; Seal and Amara, 1999; Takahashi et al., 1997). The regulation of glutamate within the synaptic cleft is critical to limit the over stimulation of excitatory amino acid receptors. Excitatory amino acid transporter 2 (EAAT2) is responsible for up to 90% of all glutamate uptake activity in the brain and is primarily localized on astrocytes (Chen, 2004; Maragakis, 2004).
Given the extensive role of EAAT2 in regulating extracellular glutamate concentrations, alterations in EAAT2 expression and activity can have profound effects on neuroprotection and neuropathology. Multiple neurodegenerative diseases have been associated with reduced EAAT2 expression and function (Boston-Howes, 2006; Cross, 1987; Guo, 2002; Li et al., 1997; Rao, 2001; Rothstein, 1996; Rothstein et al., 1996). In contrast, increased astrocytic EAAT2 expression appears to afford greater neuroprotection under excitotoxic conditions. Rosenberg and Aizenman (1989) demonstrated that cortical neuron cultures were significantly less vulnerable to glutamate when cultured in an astrocyte-rich environment compared to cultures grown with few astrocytes. Recent studies have demonstrated that treatment of cultured neurons with β-lactam antibiotics results in increased expression and activity of EAAT2, which was hypothesized to enhance neuroprotection against hypoxia/ischemia (Rothstein et al., 2005; Lipski et al., 2007). However, an earlier study by Mitani and Tanaka (2003) reported higher extracellular concentrations of glutamate in the brains of wild type mice compared to EAAT2(GLT1) knock out mice following ischemia, suggesting that EAAT2 may actually contribute to neuropathology following ischemia. In addition, Bonde et al (2003) observed a 181% increase in EAAT2(GLT1) expression following the treatment of hippocampal slice cultures with GDNF. This increase in EAAT2 was not associated with greater neuronal survival, but rather with increased neuronal loss following hypoxia/ischemia. The picture is further complicated by the observation that EAAT2(GLT1) expression may shift from astrocytes to neurons following ischemic insult (Danbolt, 2001; Martin et al., 1997, Bonde et al., 2003; Rao et al., 2001a; Fukamachi et al., 2001, Xu et al., 2003), and we have previously shown that over expression of EAAT2 in neurons increases neuronal sensitivity to glutamate mediated excitotoxicity (Selkirk, et al., 2005).
Clearly EAAT2 activity has a profound impact on the regulation of normal glutamatergic neurotransmission, and can profoundly influence neuroprotection or neurodegeneration. However, the inability to selectively study the expression and function of EAAT2 in a cell type specific manner has made it difficult to clearly determine the exact contribution of astrocytic EAAT2 towards neuroprotection or neurodegeneration. Therefore, we used recombinant Adeno-associated viral vectors to transduce rat hippocampal slice cultures and limit the overexpression of human EAAT2 to astrocytes using the GFAP promoter. Under these conditions we demonstrated that functional EAAT2 expression and activity could be selectively increased in astrocytes, leading to a significant increase in neuroprotection following moderate hypoxia/ischemia but not following a more sever insult.
Methods
Virus Preparation
The AAV1-GFAP-hrGRP and AAV1-GFAP-EAAT2 viruses were packaged in HEK293T cells cultures grown in standard growth media (DMEM, 10% heat inactivated FBS, 0.05% penicillin/streptomycin (5000U/ml), 0.1 mM MEM nonessential amino acids, 1 mM MEM sodium pyruvate, and gentamicin (25 mg/ml). Cells were transfected with three plasmids using Polyfect Transfection Reagent (Qiagen, Valencia, CA). The three plasmids used in the transfection were: 1) adeno helper plasmid (pFD6), AAV helper (H21) and the AAV packaging vector containing the glial fibrillary acidic protein (GFAP) promoter followed by either the enhanced green fluorescent protein (eGFP) gene or the human EAAT2 (GLT1a) gene sequence (obtained from J. Rothstein, Johns Hopkins), flanked by AAV2 inverted terminal repeats. Virus was isolated from HEK293T cells through repeated freeze-thaw cycles, incubation for 30 minutes at 37°C with 50U benzonase (Novagen, Madison, WI) and 0.5% sodium deoxycholate, briefly sonicated and further purified by iodixonol density gradient centrifugation as previously reported (Zolotukhin, 1999). The titer (genomic particles/ml (gp/ml)) of final virus isolate was determined by quantitative real time-polymerase chain reaction (RT-PCR) using an ABI Prism 7700 with primer and probe sets specific for the EAAT2 sequence or the WPRE sequence.
Rat hippocampal slice cultures (RHSC)
RHSC were prepared using a modified method of Noraberg (1999). Hippocampal tissue was isolated from 7-day-old Sprague-Dawley rats. Tissue was cut into 400µm slices using a McIlwain tissue chopper and transferred to ice-cold dissection media (Hanks balanced salt solution, 20mM HEPES, 25mM D-glucose, pH 7.3, filter sterilized) and incubated for 30 minutes on ice. Slices presenting clear hippocampal architecture were transferred to dissection media alone or media containing 1 × 1011 gp/ml of AAV1-GFAP-EAAT2, AAV1-GFAP-null or AAV1-GFAP-hrGFP, oxygenated for 30 minutes and placed on inserts in 6-well plates containing 1 ml of primary RHSC media (50% Optimem (Invitrogen, Carlsbad, CA), 25% HBSS, 25% heat inactivated horse serum, 25mM D-Glucose, (+/−) 100µM penicillin G, pH 7.3, filter sterilized). On day three, media was changed to a secondary culture media (Neurobasal-A media, B-27 supplement, 1mM Gluta-max (Invitrogen, Carlsbad, CA), 25mM D-glucose, 2.7 +/− 100µM penicillin G, pH 7.3, filter sterilized). Half of the secondary media was changed every other day. Cultures were maintained at 37°C, 5% CO2 for 10 days.
Oxygen and glucose deprivation (OGD)
OGD studies were performed using a modified method of Bonde, et al. (2003). Propidium iodide (PI) is a polar compound that gains entry into dead and dying neurons and binds to nucleic acid. Binding of PI to DNA results in a red maximum fluorescence emission at 630nm upon excitation at 495nm. At least 6 hours prior to OGD, PI (Molecular Probes, Eugene, OR) was added to the media at a concentration of 2µM (Noraberg, 1999). At this concentration, staining is specific for damaged neurons. OGD was established by transferring inserts to deoxygenated, glucose-free balanced salt solution (BSS) (120 mM NaCl, 5mM KCl, 1.25 mM Na2HPO4, 2mM CaCl2, 25mM NaHCO3, 20mM HEPES, 25mM Sucrose, pH 7.3, filter sterilized). Cultures were placed directly into an oxygen deprivation chamber (37°C, 5% CO2 and 95% N2, Biospheric, PRO-OX 110) in glucose free media for 60 minutes to establish a moderate insult, or incubated under normal oxygen conditions in glucose-free buffer for 15 minutes then placed into the oxygen and glucose free conditions for 60 minutes to establish a severe insult. Non-OGD control slices were transferred to BSS with glucose and incubated in normal O2 for 1 hour. After OGD, inserts were transferred back into wells with 1ml secondary media containing 25 mM D-glucose, B-27 without antioxidants and with 2µM PI then returned to normal oxygen conditions. Fluorescent images were taken of the hippocampal slices prior to OGD and at 18, and 24 hours post-OGD on an Olympus IX71 inverted fluorescent microscope (Melville, NY) attached to an Hamamatsu ORCA-ER digital camera using Image-Pro Plus software package (Media Cybernetics, Bethesda, MD). Four independent experiments were conducted with 8–12 slices/experiment.
Western Blot Analysis
Western blot analysis of rat hippocampal slice cultures were performed to evaluate EAAT2 expression levels in control slices, penicillin G treated slices and cultures transduced with AAV1-GFAP-EAAT2. The RHSC were homogenized in lysis solution (2.5% sodium deoxycholate, 0.1% protease inhibitor cocktail set III (Calbiochem, San Diego, CA), and 0.05% benzonase (EMD Biosciences, San Diego, CA) in PBS). Protein concentrations of lysate samples were determined using the Bio-Rad DC protein assay (Hercules, CA). Aliquots of homogenized RHSC (30µg) were loaded onto NuPAGE 4–12% Bis-Tris gels (Invitrogen, Carlsbad, CA). Proteins were transferred to Immuno-Blot PVDF membrane (Bio-Rad, Hercules, CA) and blocked in Tris buffer containing Tween-20 and 0.5% non-fat milk. Membranes were probed with antibodies to GLT-1 to label EAAT2 (1:500, ABR) and actin (1:1000, Sigma, St. Louis, MO). Proteins were visualized using ECL (Pierce, Rockford, IL) with species-specific HRP conjugated secondary antibodies. Blots were imaged on a Kodak Image Station 440 CF. Dosimetry analysis of regions of interest were used to calculate fold change in EAAT2 protein expression corrected to actin controls. Similar experiments were performed using the anti-EAAC1 (1:100 kindly provided by Dr Jeffery Rothstein) and anti-GLAST (1:100, ABR) to label EAAT3 and EAAT1 respectively.
Immunohistochemistry
Cell type specific expression obtained with the AAV1-GFAP vector was evaluated in RHSC transduced with AAV1-GFAP-GFP. Slice cultures were transduced with AAV1-GFAP-GFP and fixed after 10 days in culture. Slices were fixed with 4% paraformaldehyde for 30 minutes at room temperature, and stored at 4°C in PBS. Slices were cryo-protected by incubation in a sucrose solution at 4°C and cryo-sectioned into 10µM thick sections. Slices were then incubated in blocking buffer (1% normal goat serum, 0.3% Triton-X 100) for 1 hour at room temperature. After initial blocking, neurons were stained with the fluorescent Nissl stain, NeuroTrace Blue (1:400; Invitrogen). Astrocytes were stained with a primary rabbit anti-GFAP antibody (1:500, Chemicon, Temecula, CA) overnight at 4°C, then rinsed in blocking buffer (1% normal goat serum) and incubated for 1 hour in secondary anti-rabbit antibody conjugated to Alexa 546 (1:500, Molecular Probes, Eugene, OR). Fluorescent images were obtained on a Bio-Rad Radiance 2000 MP laser scanning confocal microscope (Molecular Histology Core-University of Montana) and an Olympus IX71 inverted fluorescent microscope attached to a Hamamatsu ORCA-ER camera using Image-Pro plus software.
Glutamate uptake assays
Uptake assays were performed using a modified method of Selkirk (Selkirk, 2005). Briefly, whole slices were homogenized on ice in tissue buffer (50mM Tris, 0.3M sucrose, pH 7.3) and centrifuged at 14,000 × g for 10 minutes at 4°C. The pellet was resuspended in 250µL of either sodium containing Krebs buffer or sodium-free Krebs containing equimolar amount of choline. Hippocampal homogenates were incubated with aspartic acid solution (200nM [3H] D-aspartic acid (PerkinElmer, Boston, MA) and 2.0µM cold D-aspartic acid (Novabiochem, San Diego, CA) in the presence and absence of 500µM dihydrokainic acid (DHK), (Tocris, Ellisville, MO). DHK is an EAAT2 selective uptake inhibitor and allows for assessment of non-EAAT2 mediated aspartic acid uptake. Reactions were incubated at 37°C for 4 minutes and promptly terminated by filtration through Whatman GC/F filter paper (Whatman, Brentford, Middlesex, UK) via a Brandel Cell Harvester (Brandel, Gaithersburg, MD). Filtrate was incubated overnight in scintillation cocktail and counted on a Beckman LS 6500 Scintillation System. Specific EAAT2 mediated uptake of [3H]-D-aspartic acid was calculated as total sodium-dependent uptake (pmol Asp/mg protein/min) less the uptake in the presence of DHK.
Data Analysis
One-way ANOVA was performed to determine statistical significance of western blot dosimetry analysis, functional [3H]D-aspartic acid uptake and level of neuroprotection offered between the control tissue and those treated with either penicillin G or transduced with AAV-GFAP-EAAT2. Statistical analysis was performed using Prism Software (GraphPad Software, Inc., San Diego, CA).
Results
Astrocyte targeted transgene expression following AAV-mediated gene delivery
The GFAP promoter sequence identified by Brenner et. al. (1994) has been used in multiple studies to selectively drive protein expression in astrocytes. Feng et. al. (2004) demonstrated stable, long-term astrocyte specific expression of apolipoprotein E (ApoE) using rAAV containing the GFAP promoter. Guo et. al. (2003) utilized the GFAP promoter sequence to drive EAAT2 expression in a transgenic mouse model and noted a high degree of astrocyte specific transgene expression.
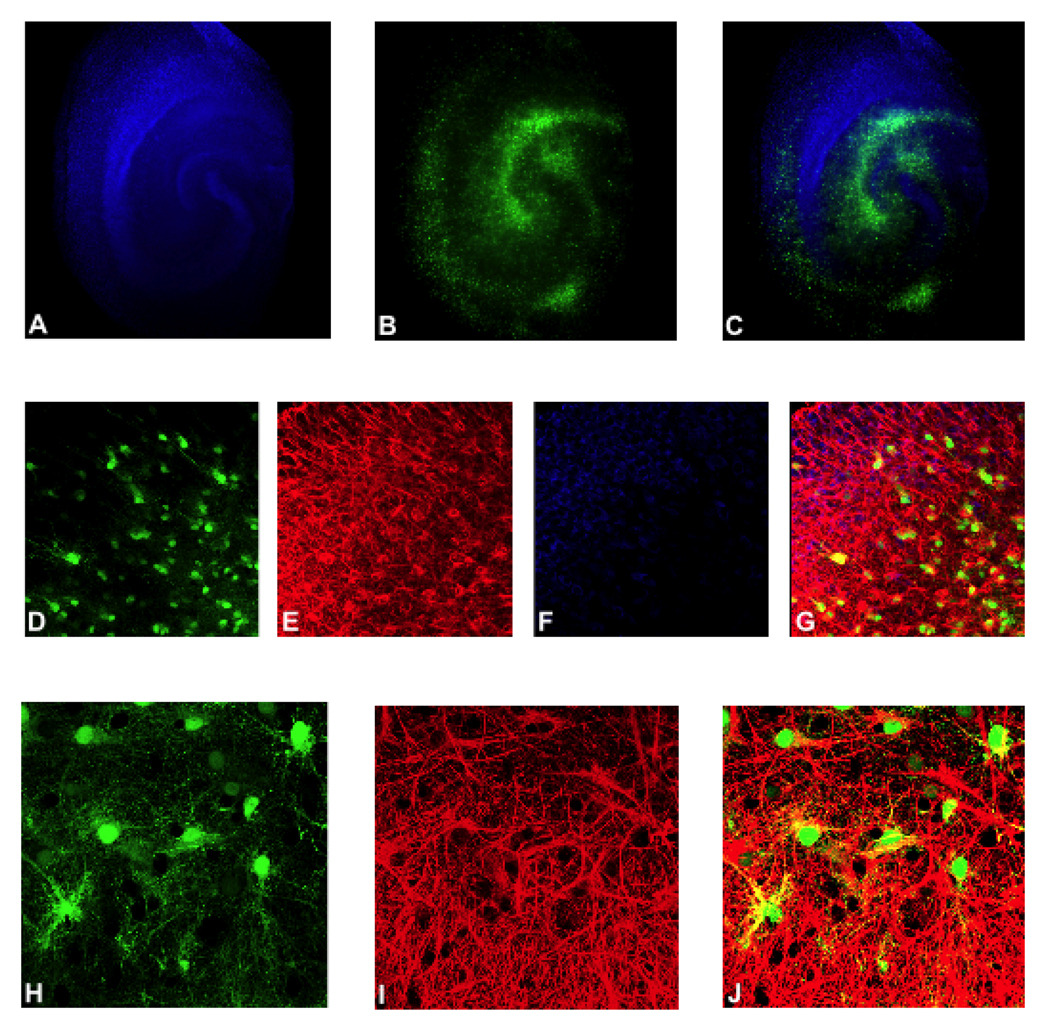
We validated the astrocyte specificity of the AAV1-GFAP viral construct by transducing rat hippocampal slice cultures (RHSC) with AAV1-GFAP-GFP. Extensive GFP expression was observed throughout the slices, with a greater density of GFP positive cells present within the stratum radiatum, stratum oriens, hilus and dentate (Figure 1B & C). Neurons within transduced slices were labeled with NeuroTrace Blue and showed a distinct pattern separate from GFP positive cells (Figure 1A & C). Immunohistochemical analysis was performed on cryosections prepared from RHSCs to confirm astrocyte specificity of transgene expression and to establish the extent to which AAV could penetrate slices and transduce cells within the core of the cultures. Neurons were labeled with NeuroTrace Blue (Figure 1F & G) and astrocytes were labeled with anti-GFAP antibody (Figure 1E & G). Co-labeling of green GFP positive cells with anti-GFAP antibody (Figure 1 G & J) indicated that transgene expression was effectively targeted to astrocytes, with no GFP expression observed in neurons (Figure 1G). Extensive astrocytes-specific GFP expression was seen within all cryosections examined indicating that AAV was capable of efficient astrocytes transduction throughout the entire slice culture.
Figure 1. AAV1-GFAP construct targets transgene delivery and expression to astrocytes.
Representative images of rat hippocampal slice cultures (RHSC) transduced with AAV1-GFAP-GFP are shown. Panels A–C show low magnification images in which GFP expression (green) is observed throughout the slice culture and does not overlap with neurons stained with NeuroTrace (blue). Panels E, G, I and J show slice cultures stained with anti-GFAP antibody. Panels D–G are 40X images and panels H-J are 60X oil immersion images. Panels D,G, H and I show GFP positive astrocytes (green). Panels E, G, I and J show GFAP stained astrocytes and astrocytic processes (red); and panels G (merged image of panels D–F) and J (merged image of panels H–J) show colocalization of GFP and GFAP (yellow).
AAV-mediated delivery of the EAAT2 gene results in increased expression of functional EAAT2 within astrocytes
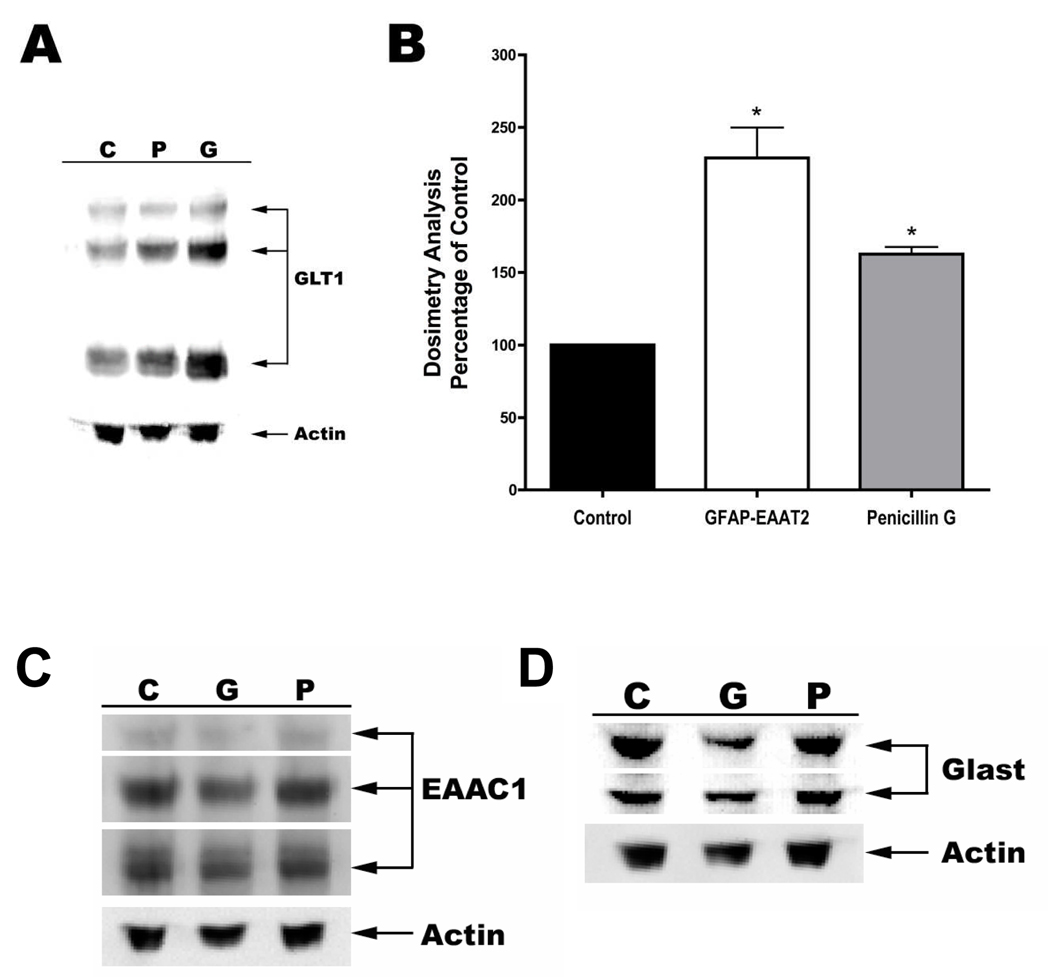
Western blot analysis was performed to determine the level of EAAT2 overexpression that could be achieved within astrocytes of AAV transduced RHSC compared to cultures treated with penicillin-G. Immunoblot analysis indicated that EAAT2 expression was significantly increased to 229% of controls following transduction with AAV1-GFAP-EAAT2 (Figure 2A &B). In comparison, treatment of RHSC with 100µM penicillin-G rendered a somewhat lower, but still statistically significant increase of 163% in EAAT2 expression over that of controls. Total intensity of all three bands characteristic of EAAT2, consisting of a monomer, dimer and multimeric aggregated proteins, were used to calculate the total level of EAAT2 expression normalized against β actin.
Figure 2. Western blot analysis of glutamate transporters in rat hippocampal slice cultures.
Rat hippocampal slice cultures were either untreated (C), or exposed to 100µM penicillin-G (P) or transduced with AAV1-GFAP-EAAT2 (G). Protein extracts were separated by SDS-PAGE, transferred to PVDF membrane and immunoblotted with anti-GLT1 antibody (A), anti-EAAC1(EAAT3) antibody (C) or anti GLAST(EAAT1) antibody (D). All blots were normalized against β-actin. Panel B shows a graphic representation of percent changes in GLT1(EAAT2) observed in the two treatment groups relative to that of controls. (n=6–9 cultures; *=p<0.05)
We further wanted to determine if the overexpression of exogenous EAAT2 caused alterations in the expression of other glutamate transporters. EAAC1(EAAT3) and GLAST(EAAT1) are glutamate transporters also expressed with in the hippocampus. EAAC1 is predominantly expressed in neurons and GLAST is expressed in astrocytes. Western blot analysis indicated no changes in the expression of either of these transporters under any of the conditions examined (Figure 2C & D).
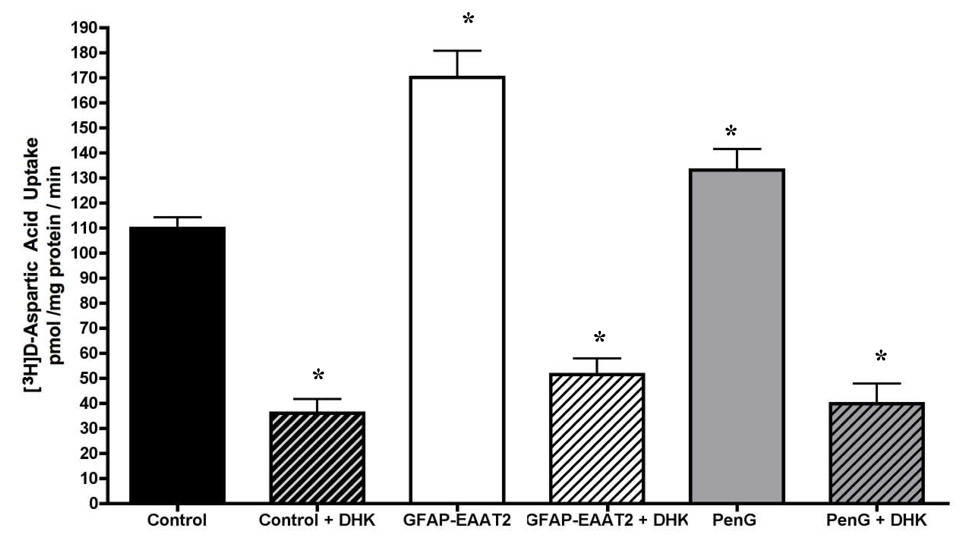
To determine if exogenous EAAT2 transporters were trafficked to the cell surface and contributed to functional uptake activity, we measured [3H]D-aspartate uptake in control, penicillin-G treated and AAV1-GFAP-EAAT2 transduced RHSCs. Crude homogenate preparations from AAV1-GFAP-EAAT2 and penicillin-G treated slices showed a 162% and 128% increase in DHK sensitive glutamate uptake respectively, over that of controls (Figure 3). The disparity observed between the level of EAAT2 expression in western blots to that noted in EAAT2 specific uptake activity could be attributed to the fact that western blot analysis quantifies total EAAT2 levels, which would include both immature and fully processed or functional transporters.
Figure 3. [3H]D-Asp uptake activity.
Total uptake activity within crude preparations of RHSCs were compared between controls, AAV-GFAP-EAAT2 transduced (GFAP-EAAT2) or penicillin G (PenG) treated slices. EAAT2 specific uptake was determined by measuring activity in the presence and absence of the EAAT2 selective inhibitor, DHK.
Selective over expression of EAAT2 in astrocytes provides limited neuroprotection following oxygen-glucose deprivation
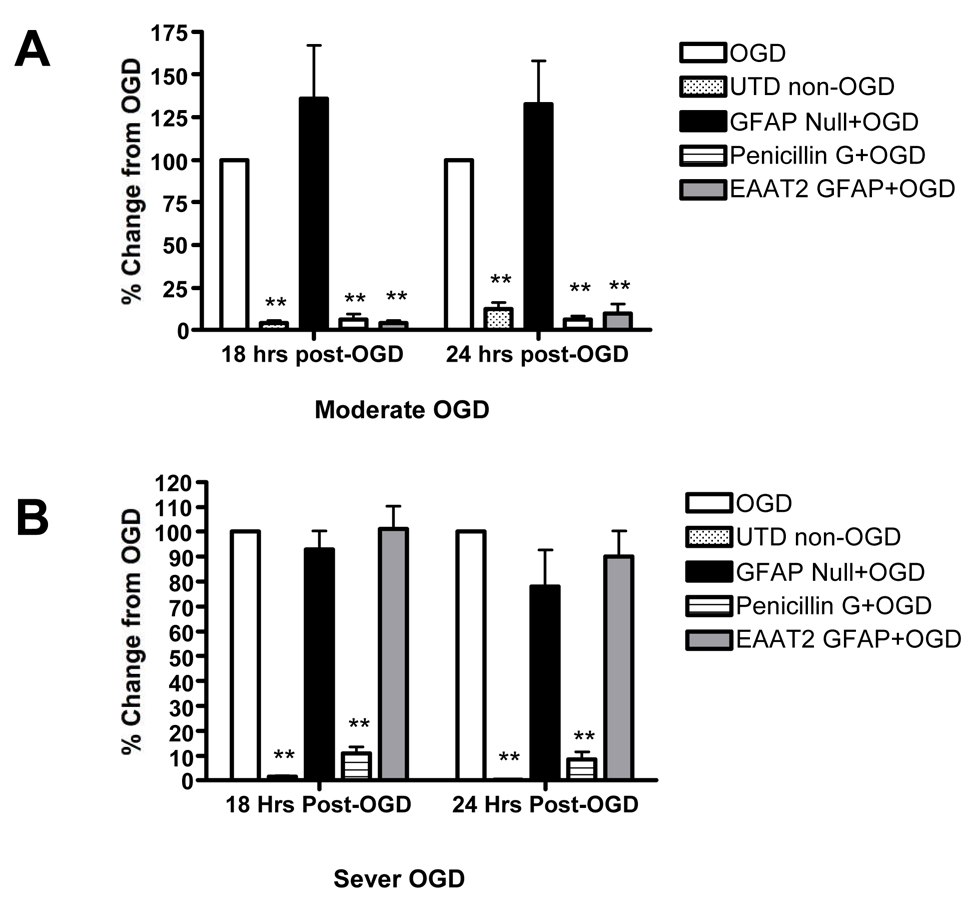
Rothstein et al (2005) suggested that β-lactam antibiotics mediate neuroprotection as a consequence of increased EAAT2 expression and activity. To test this hypothesis, we exposed RHSCs to oxygen-glucose deprivation (OGD) and compared neuronal survival in control slices to that observed in cultures transduced with AAV1-GFAP-EAAT2 or treated with penicillin-G. Neuronal loss following OGD was determined by staining slices with propidium iodide (PI). Significant accumulation of PI staining was not observed until after 12 hours post OGD. Therefore, fluorescent images were collected at 18 and 24 hours post OGD. Treatment with either penicillin G or AAV-GFAP-EAAT2 resulted in equally robust neuroprotection following moderate OGD at both the 18 and 24-hour time points (Figure 4A). We wanted to confirm that the protection observed with AAV-GFAP-EAAT2 was actually due to EAAT2 overexpression in astrocytes rather than a nonspecific consequence of AAV transduction. Therefore, RHSCs were transduced with a control null virus, which contained the GFAP expression cassette minus the EAAT2 sequence. Transduction of slice cultures with the null control virus did not provide any degree of neuroprotection following OGD (Figure 4A).
Figure 4. Over expression of exogenous EAAT2 in astrocytes enhances neuroprotection against oxygen glucose deprivation.
Neuronal damage mediated by OGD was measured by propidium iodide (PI) uptake in control RHSCs exposed to OGD (open white bars); RHSCs not exposed to OGD (stippled bars); RHScs transduced with AAV1-GFAP-null virus (solid black bars); RHSCs transduced with AAV1-GFAP-EAAT2 transduced (gray bars) and RHSCs treated with 100µM penicillin G (horizontal stripped bars). Panel A shows RHSCs exposed to moderate to OGD and imaged for PI fluorescence at 18, and 24 hours post OGD (n=10–13 slices). Panle B shows RHSCs exposed to sever insult and imaged for PI fluorescence at 18, and 24 hours post OGD (n=10–21 slices). (One-way ANOVA analysis, ** = p < 0.01)
We further examined the neuroprotective potential of penicillin G and AAV-GFAP-EAAT2 treatment under the conditions of a more stringent insult by incubating slice cultures in glucose free buffer but under normal oxygen conditions for 15 prior to the 60-minute OGD to more thoroughly deplete glucose reserves. This approach results in approximately three times more neuronal loss following the same 60 minute OGD. Under these conditions, penicillin G provided similarly high levels of neuroprotection as were observed under moderate OGD conditions. In contrast, cultures transduced with AAV-GFAP-EAAT2 showed absolutely no neuroprotection under the more stringent conditions and had neuronal loss similar to control OGD slices and slices treated with the null virus (Figure 4B).
Discussion
Prior attempts to modulate EAAT2 function have been limited to non-specific or indirect approaches. This is due to the fact that only a limited number of inhibitory compounds exhibit transporter isotype specificity. In addition, all currently available inhibitors are unable to target transporters in a cell type specific manner. Likewise, enhancers of EAAT2 function, such as the β-lactam antibiotics, also lack cell type specificity.
In an effort to circumvent this limitation, Guo et. al., (2003) developed a transgenic mouse model using the GFAP promoter to drive EAAT2 expression in astrocytes. Unfortunately, the over expression of EAAT2 during development in these transgenic animals reduced lifespan, litter size and overall growth. Therefore, a true measure of EAAT2 mediated neuroprotection could not be conclusively defined due to the potential compensatory alterations occurring during CNS development. In contrast, the use of AAV to mediate specific changes in EAAT2 expression limits complications due to altering essential glutamate signaling during the developmental processes (Lujan et al., 2005). In contrast, viral mediated gene delivery in post natal animals circumvents results events. Furthermore, results presented here clearly demonstrate that functional EAAT2 protein can be selectively overexpressed in astrocytes through the use of recombinant AAV viral constructs.
Here we have shown that specific overexpression of EAAT2 in astrocytes is neuroprotective under conditions of moderate oxygen glucose deprivation. Prior studies have shown up regulation of EAAT2 expression correlates with neuroprotection but were unable to conclusively state the direct impact of EAAT2 due to the simultaneous activation of other neuroprotective pathways (Rothstein et al., 2005; Ganel et al., 2006; Labrande et al., 2006). In this study we were able to successfully isolate astrocytic EAAT2, and demonstrated its neuroprotective potential. However, our results indicate that increased expression of EAAT2 in astrocytes is not the sole mechanism of β-lactam antibiotic mediated neuroprotection.
Our data suggest that increased astrocyte expression of EAAT2 represents only one of potentially many events that contribute to β-lactam antibiotic mediated neuroprotection. Presumably, transduction of slices with AAV-GFAP-EAAT2 and treatment with penicillin-G invoke similar changes that occur as a result of increased EAAT2 expression and through the heightened removal of glutamate from the synaptic cleft under excitotoxic conditions. Ischemic preconditioning, a plausible mechanism behind neuroprotection noted with β-lactam antibiotic treatment, may be a response to an acute, sub-lethal excitotoxic event. Penicillin-G is an antagonist at the GABA-A receptor (Fujimoto, 1995). Inhibition of this receptor is thought to invoke a dose dependent excitotoxic event through continued glutamate release. Penicillin-G causes a spike in glutamate release and is commonly used to induce seizure at higher doses (Shen and Lai, 2002). Moderate dosing of penicillin-G may elicit the required acute, sub-lethal excitatory event needed to initiate ischemic preconditioning. This initial excitotoxic event could cause numerous changes to protein expression and function (Dirnagl et al., 2003; Stenzel-Poore et al., 2003; Carmel et al., 2004; Romera et al., 2004). Up regulation of GABA-A receptor, hypoxia inducible factor-1 (HIF-1), glucose transporters, and tumor necrosis factor alpha (TNFa), and down regulation of glutamate receptors, transcriptional activators, and vesicular docking proteins are just a few of the proteins changes associated with ischemic preconditioning (Liu et al., 2000; Ruscher et al., 2002; Sommer et al., 2002; Dirnagl et al., 2003; Stenzel-Poore et al., 2003; Carmel et al., 2004; Romera et al., 2004; Dave et al., 2005; Lu et al., 2005). The direct overexpression of EAAT2 in astrocytes through the use of AAV1-GFAP-EAAT2 circumvents the activation of additional pathways associated with ischemic preconditioning.
Although the exact mechanisms mediating neuroprotection in AAV1-GFAP-EAAT2 transduced tissue remain to be defined, a number of possibilities exist. The most obvious mechanism would be that increased EAAT2 membrane expression limits excitotoxicity through the enhanced removal of glutamate and termination of post-synaptic excitatory signaling. Under low glutamate signaling conditions, glutamate receptors have been shown to shift in subunit composition and signaling potential (Molnar and Isaac, 2002). Therefore, increasing the ability of astrocytes to remove glutamate from the synaptic cleft may produce a refined or hypoactive glutamate-signaling environment.
Alternatively, increased supply of glutamate to astrocytes may facilitate an increased production of nutrients and antioxidants to neurons (Benarroch, 2005). Astrocytes possess a cystine-glutamate exchanger that facilitates accumulation of cystine within the astrocyte. Inside the astrocyte, cystine is converted to cysteine and used in the astrocytic production of glutathione precursor for use by neurons. The glutathione precursor, L-cysteinyl-glycine is converted to glutathione in the neuron by interaction with the g-glutamyl transpeptidase coenzyme. Additionally, astrocytes are able to regenerate ascorbic acid to ascorbate for reuse by neurons (Kim et al., 2005). Ascorbic acid recycling is positively coupled to increased glutamate uptake, glucose utilization and glutathione production. Neurons are incapable of producing these potent antioxidants alone. Increased glutamate uptake capacity of astrocytes could enhance the supply of precursors and energy production required to synthesize these potent antioxidants essential for neuronal health.
Increasing glutamate uptake into astrocytes may also create a shift in energy production and utilization that could alter the metabolic coupling between astrocytes and neurons. Glutamate uptake is coupled to a concomitant sodium influx. The Na+/K+ ATPases maintains an intracellular sodium concentration (10–20 mM) (Kimelberg and Goderie, 1993). Increases in intracellular sodium concentrations results in the activation of energy consuming Na+/K+ ATPase and stimulates glucose uptake and glycolytic activity. Accordingly, activation of these pathways results in an increase in astrocytic lactate and pyruvate production and release. Low-level increases in the supply of lactate to neurons up regulates pathways involved in the use of lactate as an alternative energy source and promotes enhanced ability to utilize lactate under successive anaerobic conditions (Dienel and Hertz, 2005). Thus an increase in glutamate uptake could initiate these changes, resulting in increased energy supply to neurons in the form of lactate and to a lesser extent pyruvate. This may promote a more neuroprotective environment with the accumulation of an energy reserve and enhanced lactate utilization capacity to be used during subsequent oxygen glucose deprivation. These three proposed mechanisms of EAAT2 mediated neuroprotection together may promote the overall health of tissue through increased production of anti-oxidants, energy supplies, balanced extracellular environment and induce a general energy depletion tolerance.
Acknowledgements
This publication was supported by grants from NCRR and NINDS (P20 RR15583, P20 RR017670, R21 NS058541-01)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benarroch EE. Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clin Proc. 2005;80:1326–1338. doi: 10.4065/80.10.1326. [DOI] [PubMed] [Google Scholar]
- Billups B, Rossi D, Oshima T, Warr O, Takahashi M, Sarantis M, Szatkowski M, Attwell D. Physiological and pathological operation of glutamate transporters. Prog Brain Res. 1998;116:45–57. doi: 10.1016/s0079-6123(08)60429-x. [DOI] [PubMed] [Google Scholar]
- Bonde C, Sarup A, Schousboe A, Gegelashvili G, Zimmer J, Noraberg J. Neurotoxic and neuroprotective effects of the glutamate transporter inhibitor DL-threo-beta-benzyloxyaspartate (DL-TBOA) during physiological and ischemia-like conditions. Neurochem Int. 2003;43:371–380. doi: 10.1016/s0197-0186(03)00024-x. [DOI] [PubMed] [Google Scholar]
- Boston-Howes W, Gibb SL, Williams EO, Pasinelli P, Brown RH, Jr, Trotti D. Caspase-3 cleaves and inactivates the glutamate transporter EAAT2. J Biol Chem. 2006 doi: 10.1074/jbc.M600653200. [DOI] [PubMed] [Google Scholar]
- Bridges RJ, Kavanaugh MP, Chamberlin AR. A pharmacological review of competitive inhibitors and substrates of high-affinity, sodium-dependent glutamate transport in the central nervous system. Curr Pharm Des. 1999 May 5;(5):363–379. [PubMed] [Google Scholar]
- Carmel JB, Kakinohana O, Mestril R, Young W, Marsala M, Hart RP. Mediators of ischemic preconditioning identified by microarray analysis of rat spinal cord. Exp Neurol. 2004;185:81–96. doi: 10.1016/j.expneurol.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Chen W, Mahadomrongkul V, Berger UV, Bassan M, DeSilva T, Tanaka K, Irwin N, Aoki C, Rosenberg PA. The glutamate transporter GLT1a is expressed in excitatory axon terminals of mature hippocampal neurons. J Neurosci. 2004;24:1136–1148. doi: 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AJ, Slater P, Simpson M, Royston C, Deakin JF, Perry RH, Perry EK. Sodium dependent D-[3H]aspartate binding in cerebral cortex in patients with Alzheimer’s and Parkinson’s diseases. Neurosci Lett. 1987;79:213–217. doi: 10.1016/0304-3940(87)90699-9. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Danbolt NC, Storm-Mathisen J, Kanner BI. An [Na+ + K+]coupled L-glutamate transporter purified from rat brain is located in glial cell processes. Neuroscience. 1992;51:295–310. doi: 10.1016/0306-4522(92)90316-t. [DOI] [PubMed] [Google Scholar]
- Dave KR, Lange-Asschenfeldt C, Raval AP, Prado R, Busto R, Saul I, Perez-Pinzon MA. Ischemic preconditioning ameliorates excitotoxicity by shifting glutamate/gamma-aminobutyric acid release and biosynthesis. J Neurosci Res. 2005;82:665–673. doi: 10.1002/jnr.20674. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Hertz L. Astrocytic contributions to bioenergetics of cerebral ischemia. Glia. 2005 Jun;50(4):362–388. doi: 10.1002/glia.20157. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Munakata M, Akaike N. Dual mechanisms of GABAA response inhibition by beta-lactam antibiotics in the pyramidal neurones of the rat cerebral cortex. Br J Pharmacol. 1995;116:3014–3020. doi: 10.1111/j.1476-5381.1995.tb15957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukamachi S, Furuta A, Ikeda T, Ikenoue T, Kaneoka T, Rothstein JD, Iwaki T. Altered expressions of glutamate transporter subtypes in rat model of neonatal cerebral hypoxia-ischemia. Brain Res Dev Brain Res. 2001 Dec 31;132(2):131–139. doi: 10.1016/s0165-3806(01)00303-0. [DOI] [PubMed] [Google Scholar]
- Ganel R, Ho T, Maragakis NJ, Jackson M, Steiner JP, Rothstein JD. Selective up-regulation of the glial Na+-dependent glutamate transporter GLT1 by a neuroimmunophilin ligand results in neuroprotection. Neurobiol Dis. 2006;21:556–567. doi: 10.1016/j.nbd.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Guo H, Lai L, Butchbach ME, Lin CL. Human glioma cells and undifferentiated primary astrocytes that express aberrant EAAT2 mRNA inhibit normal EAAT2 protein expression and prevent cell death. Mol Cell Neurosci. 2002;21:546–560. doi: 10.1006/mcne.2002.1198. [DOI] [PubMed] [Google Scholar]
- Guo H, Lai L, Butchbach ME, Stockinger MP, Shan X, Bishop GA, Lin CL. Increased expression of the glial glutamate transporter EAAT2 modulates excitotoxicity and delays the onset but not the outcome of ALS in mice. Hum Mol Genet. 2003;12:2519–2532. doi: 10.1093/hmg/ddg267. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Park YG, Baik EJ, Jung SJ, Won R, Nahm TS, Lee BH. Dehydroascorbic acid prevents oxidative cell death through a glutathione pathway in primary astrocytes. J Neurosci Res. 2005;79:670–679. doi: 10.1002/jnr.20384. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK. Effect of ascorbate on Na(+)-independent and Na(+)-dependent uptake of [3H]norepinephrine by rat primary astrocyte cultures from neonatal rat cerebral cortex. Brain Res. 1993;602:41–44. doi: 10.1016/0006-8993(93)90238-i. [DOI] [PubMed] [Google Scholar]
- Labrande C, Velly L, Canolle B, Guillet B, Masmejean F, Nieoullon A, Pisano P. Neuroprotective effects of tacrolimus (FK506) in a model of ischemic cortical cell cultures: role of glutamate uptake and FK506 binding protein 12 kDa. Neuroscience. 2006;137:231–239. doi: 10.1016/j.neuroscience.2005.08.080. [DOI] [PubMed] [Google Scholar]
- Lipski J, Wan CK, Bai JZ, Pi R, Li D, Donnelly D. Neuroprotective potential of ceftriaxone in in vitro models of stroke. Neuroscience. 2007;146:617–629. doi: 10.1016/j.neuroscience.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Li S, Mallory M, Alford M, Tanaka S, Masliah E. Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J Neuropathol Exp Neurol. 1997;56(8):901–911. doi: 10.1097/00005072-199708000-00008. [DOI] [PubMed] [Google Scholar]
- Liu J, Ginis I, Spatz M, Hallenbeck JM. Hypoxic preconditioning protects cultured neurons against hypoxic stress via TNF-alpha and ceramide. Am J Physiol Cell Physiol. 2000;278:C144–C153. doi: 10.1152/ajpcell.2000.278.1.C144. [DOI] [PubMed] [Google Scholar]
- Lu GW, Yu S, Li RH, Cui XY, Gao CY. Hypoxic preconditioning: a novel intrinsic cytoprotective strategy. Mol Neurobiol. 2005;31:255–271. doi: 10.1385/MN:31:1-3:255. [DOI] [PubMed] [Google Scholar]
- Lujan R, Shigemoto R, Lopez-Bendito G. Glutamate and GABA receptor signalling in the developing brain. Neuroscience. 2005;130:567–580. doi: 10.1016/j.neuroscience.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Dykes-Hoberg M, Rothstein JD. Altered expression of the glutamate transporter EAAT2b in neurological disease. Ann Neurol. 2004;55:469–477. doi: 10.1002/ana.20003. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Brambrink AM, Lehmann C, Portera-Cailliau C, Koehler R, Rothstein J, Traystman RJ. Hypoxia-ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Ann Neurol. 1997 Sep;42(3):335–348. doi: 10.1002/ana.410420310. [DOI] [PubMed] [Google Scholar]
- Mitani A, Tanaka K. Functional changes of glial glutamate transporter GLT-1 during ischemia: an in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1. J Neurosci. 2003;23(18):7176–7182. doi: 10.1523/JNEUROSCI.23-18-07176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar E, Isaac JT. Developmental and activity dependent regulation of ionotropic glutamate receptors at synapses. ScientificWorldJournal. 2002;2:27–47. doi: 10.1100/tsw.2002.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VL, Bowen KK, Dempsey RJ. Transient focal cerebral ischemia down-regulates glutamate transporters GLT-1 and EAAC1 expression in rat brain. Neurochem Res. 2001a May;26(5):497–502. doi: 10.1023/a:1010956711295. [DOI] [PubMed] [Google Scholar]
- Rao VL, Dogan A, Todd KG, Bowen KK, Kim BT, Rothstein JD, Dempsey RJ. Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J Neurosci. 2001;21:1876–1883. doi: 10.1523/JNEUROSCI.21-06-01876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera C, Hurtado O, Botella SH, Lizasoain I, Cardenas A, Fernandez-Tome P, Leza JC, Lorenzo P, Moro MA. In vitro ischemic tolerance involves upregulation of glutamate transport partly mediated by the TACE/ADAM17-tumor necrosis factor-alpha pathway. J Neurosci. 2004;24:1350–1357. doi: 10.1523/JNEUROSCI.1596-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PA, Aizenman E. Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex. Neurosci Lett. 1989;103(2):162–168. doi: 10.1016/0304-3940(89)90569-7. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, Priller J, Dirnagl U, Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22:10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Amara SG. Excitatory amino acid transporters: a family in flux. Annu Rev Pharmacol Toxicol. 1999;39:431–456. doi: 10.1146/annurev.pharmtox.39.1.431. [DOI] [PubMed] [Google Scholar]
- Selkirk JV, Stiefel TH, Stone IM, Naeve GS, Foster AC, Poulsen DJ. Over-expression of the human EAAT2 glutamate transporter within neurons of mouse organotypic hippocampal slice cultures leads to increased vulnerability of CA1 pyramidal cells. Eur J Neurosci. 2005;21:2291–2296. doi: 10.1111/j.1460-9568.2005.04059.x. [DOI] [PubMed] [Google Scholar]
- Shen EY, Lai YJ. In vivo microdialysis study of excitatory and inhibitory amino acid levels in the hippocampus following penicillin-induced seizures in mature rats. Acta Paediatr Taiwan. 2002;43:313–318. [PubMed] [Google Scholar]
- Sommer C, Fahrner A, Kiessling M. [3H]muscimol binding to gamma-aminobutyric acid(A) receptors is upregulated in CA1 neurons of the gerbil hippocampus in the ischemia-tolerant state. Stroke. 2002;33:1698–1705. doi: 10.1161/01.str.0000016404.14407.77. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, Meller R, Rosenzweig HL, Tobar E, Shaw TE, Chu X, Simon RP. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362:1028–1037. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Billups B, Rossi D, Sarantis M, Hamann M, Attwell D. The role of glutamate transporters in glutamate homeostasis in the brain. J Exp Biol. 1997;200(Pt 2):401–409. doi: 10.1242/jeb.200.2.401. [DOI] [PubMed] [Google Scholar]
- Xu NJ, Bao L, Fan HP, Bao GB, Pu L, Lu YJ, Wu CF, Zhang X, Pei G. Morphine withdrawal increases glutamate uptake and surface expression of glutamate transporter GLT1 at hippocampal synapses. J Neurosci. 2003 Jun 1;23(11):4775–4784. doi: 10.1523/JNEUROSCI.23-11-04775.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]