Abstract
Helper CD4+ T-cell subsets have improved our understanding of adaptive immunity in humans and in animal models of disease. These include T helper type 1 (Th1), Th2 and the interleukin-17 (IL-17) -producing population ‘Th17’. Th2 cells have been described as orchestrating the immune response in allergic disease based on studies with patient samples and animal models. The cytokine IL-9 has largely been regarded as a Th2 cytokine that makes multifocal contributions to allergic disease. Recent data suggest that under certain conditions relevant to chronic disease (IL-4 and transforming growth factor-β), a distinct population of IL-9-producing ‘Th9’ helper T cells can exist. The contribution of Th9 cells in allergic disease is currently unknown, and this review will propose a model for how these cells may regulate chronic allergic inflammation.
Keywords: airway remodeling, allergy, asthma, IL-9, TGF-beta, Th2
Introduction
Allergic diseases have been increasing in prevalence in the developed world over the past few decades.1 Atopic diseases including atopic dermatitis, food allergy, allergic rhinitis, asthma and anaphylaxis have significant associated morbidity along with large health-care expenditures. Through analysis of samples from patients and animal models of disease, T helper type 2 (Th2) cells and their cytokines, namely interleukin-4 (IL-4), IL-5 and IL-13, have been implicated as orchestrating the allergic immune response. The effector portion of this response includes immunoglobulin E (IgE) production, infiltration of eosinophils and mast cells, and ultimately the propagation of chronic inflammation. Though characterization of T-cell subsets Th1, Th2 and Th17 has allowed for understanding immunity in many disease models, the cytokine profiles of effector T cells can vary within populations, making clear distinctions difficult. Therefore, it seems possible that chronic allergic disease may not be solely a Th2-driven process.
Lessons from human and animal studies demonstrated that IL-9 is implicated in asthma and in the protection against nematode infections. It was originally described as a T-cell and mast cell growth factor. It is a multifunctional cytokine secreted by many cell types including T lymphocytes, eosinophils, mast cells and neutrophils.2–5 Interleukin-9 has largely been described as one of several Th2 cytokines, but this has now become less clear. Recent reports have described a new subset of the T helper population separate from Th2 that produces IL-9 in large quantities and contributes uniquely to immune responses.6,7 This population has been named ‘Th9’. The aim of this review is to combine data from human and animal models of allergic disease, and propose how IL-9 secreted by T cells, specifically Th9 cells, may regulate chronic allergic inflammation. We will begin with a review of T cells as an important source of IL-9, focusing on the novel Th9 subset, then we will translate how IL-9 and possibly Th9 cells may function in allergic disease.
T cells as a major source of IL-9
Initial in vitro studies revealed that IL-9 is preferentially produced by Th2-type lymphocytes, and it was therefore categorized as a member of the Th2 cytokine family.8,9 Subsequent in vivo studies have demonstrated that IL-9 production in response to protein antigen is significantly reduced in the absence of CD4 T cells, implicating T cells as a major source of IL-9 production.10 Consistent with this, IL-9 messenger RNA (mRNA) and protein are highly expressed and can be localized to lymphocytes in the bronchoalveolar lavage (BAL) and CD3+ cells in bronchial tissue from asthmatic patients.11–14 In addition, several reports indicate that IL-9 is expressed during the course of Th2-driven immune responses such as during murine parasitic infections.15,16 The mesenteric lymph nodes from such studies have revealed elevated IL-9 levels along with IL-3, IL-4 and IL-5.17 Hence, IL-9 seems to be produced during some Th2 responses leading to its inclusion in the Th2 subset of cytokines.
In humans, activation of CD4 T cells with phorbol 12-myristate 13-acetate (PMA) or anti-CD3 can induce the expression of IL-918 and IL-9 is further up-regulated by IL-2.19 In addition, signalling through the IL-2 receptor and CD28 can amplify IL-9 secretion by allergen-specific T-cell clones.20 Further analysis has revealed that the synergistic effect of IL-2 for IL-9 production by T cells is dependent on both IL-4 and IL-10.21 Among the different subsets of human CD4 T cells, IL-9 is highly expressed by CD45RO+ CCR8+ memory T cells upon anti-CD3/CD28 stimulation.22 Interestingly, it has also been reported that the transcriptional activator tax protein encoded by human T-cell lymphotropic virus type I (HTLV-I) can induce IL-9 expression by human T cells via a nuclear factor-κB (NF-κB) pathway.23 Taken together, human memory T cells are a possible source of IL-9 and many factors can induce IL-9 expression in T cells, including IL-2 and IL-10.
In mice, IL-9 was first identified as a T helper cell cytokine that promotes T-cell growth and proliferation.2,3 In activated T cells, both IL-1α and IL-1β, were found to enhance the secretion of IL-9.24,25 Similar to human T cells, a cascade of different cytokines can increase IL-9 production by activated mouse T cells. Fifteen years ago, Schmitt et al.26 showed that a combination of transforming growth factor-β (TGF-β) and IL-4 synergistically up-regulate IL-9 production by activated naïve CD4 T cells and interferon-γ (IFN-γ) suppresses this pathway. Interestingly, activation of naïve T cells with only TGF-β has little effect on IL-9 production, indicating the importance of IL-4 in IL-9 production. This study did not evaluate the levels of IL-5 and IL-13 to characterize the T-cell subset, but these authors were using ‘Th9’ conditions at a time when the concept of Th1 and Th2 was being widely accepted.
Although the requirement for IL-4 in enhancing IL-9 production has been suggested in different models,27,28 a significant amount of IL-9 production is present in IL-4-deficient mice upon protein antigen immunization suggesting an IL-4-independent pathway for IL-9 production.10 However, in the later case it is unclear whether IL-9 is produced by CD4+ T cells or innate cells such as CD4+ natural killer T cells or CD4+ lymphoid tissue inducer cells. In contrast to these in vivo results, a reduction in IL-9 production has been found after activating CD4 T cells from IL-4-deficient mice in vitro, or in the presence of neutralizing anti-IL-4 antibody.10 Hence, IL-4 appears to be an important initiator of CD4 T-cell IL-9 synthesis.
To evaluate the functional role of IL-4 in IL-9 synthesis, Schmitt et al. showed that activation of naïve T cells in the presence of TGF-β and anti-IFN-γ significantly enhances IL-9 production. However, compared with the combination of TGF-β and IL-4, the enhancing effect of anti-IFN-γ antibody in the presence TGF-β was attenuated, suggesting that IL-4 is sufficient for IL-9 production in the presence of TGF-β. Consistent with this, defective IL-9 production by signal transducer and activator of transcription 6 (STAT6) -deficient T cells reinforces the importance of IL-4 downstream signalling for IL-9 synthesis.6 Taken together, IL-4 appears to enhance TGF-β-mediated IL-9 production by neutralizing the inhibitory effect of endogenously produced IFN-γ and by directly supporting IL-9 production.26
In addition to IL-4 and TGF-β, IL-10 has also been suggested to promote IL-9 production in murine systems.10 This was shown by the impaired IL-9 production in IL-10-deficient mice that were immunized with keyhole limpet haemocyanin. However, blockade of the IL-10 receptor on T cells has little effect on IL-9 production, indicating that IL-10 may not directly regulate IL-9 synthesis.6 This inconsistency between in vivo and in vitro studies may be explained by an enhanced Th1 response and IFN-γ production in IL-10-deficient mice that may suppress IL-9 production in vivo.26 Unlike the consistent and non-redundant role for IL-2 on T cells for IL-9 synthesis,26 the precise role of IL-10 remains to be determined.
Th9, a new subset of T helper cells
Depending on the signals that innate cells sense from different pathogens and on the subsequent cytokine milieu, naïve CD4+ T cells differentiate into different subsets based on cytokine profile including Th1, Th2, Th17 cells or inducible regulatory T cells (iTreg).29–33 The signalling events downstream of cytokines are essential to polarize T cells into these different fates. In the case of Th1, IL-12 production by antigen-presenting cells acting through the STAT4 can trigger the development of Th1 cells to produce the signature Th1 cytokine IFN-γ, which in turn activates STAT1 and induces expression of the principle Th1 transcription factor T-bet.34–36 Th2 cells express IL-4, IL-5 and IL-13. IL-4 activates STAT6 and induces the Th2 transcription factor GATA3.37,38 When naïve T cells are stimulated in the presence of TGF-β with inflammatory cytokines such as IL-6, IL-21 or IL-23, they differentiate into another subset of T helper cells that produce IL-17 (Th17) and IL-22.39 Interleukins -6, -21 and -23, acting via STAT3 in the presence of TGF-β, induce the key transcription factors RAR-related orphan receptor (ROR)γt and RORα for Th17 development.40 In addition to T helper cells, activation of naïve T cells in the presence of TGF-β and IL-2 results in the generation of iTregs via induction of the transcription factor Foxp3.41 This highlights the differential role of TGF-β in T-cell differentiation, suggesting that the local cytokine milieu is critical to the T-cell response, whether in the presence of IL-6 for Th17 or IL-4 for Th9 differentiation.
Although T-cell subsets differentiated in vitro provide important potential clues to immune responses, adaptive in vivo responses are usually characterized by a heterogeneous population of T helper and regulatory T cells. Even in polarized in vitro conditions, some degree of flexibility exists. Recent studies have shown that Treg and Th17 cells are not stable populations and have the capacity for dedifferentiation.42–44 Therefore, T helper and Treg cells are mixed populations consisting of a committed lineage and an uncommitted subpopulation with developmental plasticity.45,46 Such heterogeneity is also observed in T cells producing IL-9. Although IL-9 production has been generally attributed to Th2 cells, under certain circumstances naturally arising CD4+ CD25+ regulatory T cells (nTreg) in peripheral tissues and iTregs generated in the presence of TGF-β can produce more IL-9 upon activation than Th2 cells.47,48 However, IL-9 production appears to be absent from Foxp3+ nTregs isolated from thymus or Foxp3+ iTregs generated from highly purified naïve T cells.6 The reason for this discrepancy could be that CD4+ CD25+ T cells in the periphery represent a mixture of committed Foxp3+ Treg and recently activated T helper cells that can already produce IL-9.22 Similarly, activation of CD4+ CD25− T cells, which are a mixture of naïve and effector/memory T cells, in the presence of TGF-β gives rise to both Foxp3+ iTregs and Foxp3− effector T cells that subsequently produce IL-9 upon activation.26 Overall, these findings indicate that apart from Th2 cells, another subset of effector T helper cells may exist that produces a significant amount of IL-9.
Recent comparative analysis of different subsets of T helper cells for cytokine production by polymerase chain reaction and intracellular staining revealed that activation of naïve CD4 T cells in the presence of TGF-β and IL-4 significantly enhances IL-9 and IL-10 production but not other Th2 cytokines, indicating that IL-9-producing cells are not Th2 cells.6,7 Interestingly, in the presence of TGF-β and IL-4, IL-9-producing cells are also distinct from Th1, Th17 and Foxp3+ iTreg populations, suggesting that they represent a new subset of T helper cells. Functional analysis of Th9 cells has also confirmed that in contrast to Treg cells, Th9 are neither anergic nor suppressive, as they can vigorously proliferate and along with effector T cells they further enhance T-cell proliferation.7 In addition, Th9 cells do not express any well-defined transcription factors like T-bet, GATA3, RORγt and Foxp3, emphasizing that Th9 cells are different from Th1, Th17 and Foxp3+ iTreg populations.6 In a human T-cell line, it was noted that the promoter region of the IL-9 gene can bind to several transcription factors like activating protein-1 and NF-κB, considered to be universal transcription factors expressed by other T helper cells.49 One report recently showed that GATA1 regulates IL-9 production in mast cells through p38 mitogen-activated protein kinase.50 However, this might be different in the T cell where GATA3 is the only GATA factor known to be expressed. Therefore, the existence of a specific transcription factor that is uniquely expressed by Th9 cells remains to be discovered.
Considering the plasticity in T helper cell responses, it has been shown that TGF-β is a key cytokine at the centre of the differentiation of Th17 cells and iTregs, and reprogrammes already established Th2 cells to lose their ability to produce Th2 cytokines and switch to IL-9 and IL-10 production.6 In contrast to the idea that Th2 cells have an irreversible lineage commitment, fate ‘decisions’ taken by Th2 cells might be more flexible than anticipated. Such flexibility in Th2 cells has only been reported in response to TGF-β, as IL-12 cannot reprogramme Th2 cell to a Th1 phenotype.6 However, how TGF-β inhibits the expression of GATA3 and consequently Th2 cytokines by Th2 cells and instead promotes IL-9 and IL-10 production requires further analysis. In this context, whether TGF-β can reprogramme Th1 cells by modulating T-bet expression also needs to be addressed.
As it is currently unknown whether Th9 cells contribute to the development or maintenance of allergic disease, we will next review human and animal model studies describing the expression and function of IL-9. Should Th9 cells be identified as an important source of IL-9 in these diseases, we may gain some insight from these studies into the effector function of these cells.
IL-9 in human allergic disease
Asthma
Asthma is characterized by chronic inflammation of the airways and bronchial hyper-responsiveness. Genetic predisposition along with environmental exposures appears to be important for the development of clinical allergic disease including asthma. In humans, IL-9 maps to the long arm of chromosome 5 (5q31-35), a region that also contains genes encoding IL-3, IL-4, IL-5, IL-13, CD14 and granulocyte–macrophage colony-stimulating factor.51 Polymorphisms in this region have associations with the development of atopy and bronchial hyper-responsiveness.52,53 Specifically, IL-9 was proposed as a candidate gene for atopy after linkage disequilibrium showed significant association with elevated total IgE levels.54 The homologue of IL-9 in mice is on chromosome 13 and sequence differences between strains of mice have been suggested to account for differences in IL-9 expression and bronchial hyper-responsiveness, a cardinal feature of asthma.55 Additionally, the IL-9 receptor is located in the long arm of the XY pseudoautosomal region and one report has provided evidence of linkage between a polymorphic region containing the IL-9R gene and asthma and bronchial hyper-responsiveness.56 Differences at the IL-9 and IL-9 receptor loci may be partially responsible for the genetic susceptibility to allergic disease including asthma.
Allergic asthma has been characterized by a Th2 inflammatory response to inhaled allergens, orchestrated by CD4 T cells that produce a characteristic cytokine profile responsible for eosinophilic lung inflammation.57 In asthmatics, CD4 cells and Th2 cytokines in BAL specimens are increased after allergen challenge.58,59 Though initial studies evaluated IL-4, IL-5 and IL-13 levels in the BAL, IL-9 levels have also been demonstrated to increase after allergen challenge in asthmatics compared with normal controls.12 Additionally, bronchial biopsies from patients with atopic asthma have shown an elevated number of IL-9 mRNA-positive cells in the airway compared with normal controls and patients with chronic bronchitis and sarcoidosis.11,60 Importantly, two reports have demonstrated that IL-9 can be localized to the lymphocyte population in the BAL, and CD3+ cells in the airway.13,14 However, it remains unclear if the principal cellular sources of IL-9 are Th2 cells that produce IL-4, IL-5 and IL-13 or other T cells that contribute significantly during chronic asthma. These studies have revealed that IL-9 is enhanced during asthmatic inflammation, but the precise role in asthma pathogenesis has relied on murine models for elucidation.
Other allergic diseases
A few studies have evaluated patients with allergic rhinitis and the associations with IL-9 levels. In one report, peripheral blood mononuclear cells (PBMCs) from non-atopic and atopic individuals, including those with asthma and/or allergic rhinitis, were stimulated with allergens in vitro and IL-5, IL-9 and IL-13 levels were measured.61 Interestingly, IL-9 levels were more specifically correlated with allergen-specific IgE levels compared with IL-5 and IL-13, and higher IL-9 levels were present in those with asthma versus allergic rhinitis. A separate study evaluated allergen-specific responses in PBMCs from atopic children, and found a positive correlation between elevated IL-9 production after cat allergen stimulation and presence of asthma, but not allergic rhinitis. These suggest that IL-9 production after in vitro stimulation may be a biomarker of allergen-specific T-cell responses associated with asthma more than upper airway disease. There is limited published data regarding the IL-9 expression in the nasal tissue of patients with allergic rhinitis. One group has evaluated the numbers of IL-9 mRNA-positive cells and used immunohistochemistry to characterize IL-9-producing cells in the nasal mucosa of patients with allergic rhinitis before and after allergen immunotherapy.62 The number of IL-9 mRNA-positive cells correlated with nasal eosinophilia, and about one-third of these cells were T cells. After 2 years of immunotherapy, IL-9 protein level and mRNA-positive cells were significantly reduced.
To our knowledge, there are no published reports regarding the involvement of IL-9 with other human allergic diseases including atopic dermatitis, food allergy and anaphylaxis. It is difficult to conclusively state the role of IL-9 in allergic disease from human studies given that this cytokine may be a bystander of the driving forces behind the pathogenesis and most studies have examined a very limited number of cytokines. However, samples from patients with allergic respiratory disease, principally asthma, have revealed that T cells are a significant source of the IL-9 that is elevated in these diseases compared with normal subjects.
Mouse models of allergic disease
Mouse models of allergic airways disease have been used to evaluate the function of IL-9. Initial genetic studies in B6 mice that have hypo-responsive airways revealed that polymorphisms at the IL-9 locus were associated with reduced levels compared with hyper-responsive DBA/2J (D2) mice55 Subsequently, IL-9 over-expressing transgenic mice were created and found to have increased airway hyper-responsiveness, eosinophilia and IgE levels after antigen challenges.63 Unchallenged transgenic mice in this study appeared to have no evidence of peribronchial inflammation or BAL eosinophilia, suggesting that IL-9 over-expression enhances the asthmatic phenotype only in the presence of antigen-induced inflammatory changes. In contrast, lung selective over-expression of IL-9 using a clara cell (CC10) promoter without the presence of antigen led to profound increases in airway eosinophilia, peribronchial inflammation, mast cell accumulation and hyper-responsiveness.64 In addition, these transgenic mice had epithelial mucus production and subepithelial fibrosis, features of airway remodelling associated with chronic asthma. The dependence upon antigen between systemic and lung-specific IL-9 expression to induce phenotypic changes may be related to differences in strains, IL-9 expression level, or possibly a suppressive role of IL-9 when over-expressed systemically. Regardless, over-expression of IL-9 in the lungs of transgenic mice appears to enhance airway inflammation and remodelling.
It is difficult to draw conclusions about the role of IL-9 from transgenic IL-9 mice because IL-9 is probably highly regulated. The creation of IL-9-deficient mice has allowed for the evaluation of the role of IL-9 in models of allergic disease. Initially, IL-9-deficient mice were tested in a model of granulomatous Th2 lung inflammation that occurs after Schistosoma mansoni egg administration, and is characterized by mastocytosis and mucus metaplasia in addition to lung granuloma formation and eosinophilia.65 The IL-9-deficient mice had a similar amount of eosinophilia, but were significantly impaired in the development of epithelial mucus metaplasia and had reduced numbers of lung mast cells. Using a more conventional acute asthma model with ovalbumin (OVA)/alum immunization followed by six daily aerosolized airway OVA challenges, one group found no difference in airway hyper-responsiveness, BAL cellularity, mucus metaplasia and peribronchial inflammation in IL-9-deficient mice.66 However, another report showed a significant decrease in airway hyper-responsiveness and BAL eosinophilia after administration of an IL-9 blocking antibody was given before a single aerosolized challenge after two immunizations with OVA/alum.67 The conflicting results between the knockout and antibody blocking study may be related to protocol differences, with the former possibly bypassing the requirement for IL-9 (daily challenges for 6 days versus one challenge). In addition, the findings from knockout mice cannot always be applied to physiological disease models because of selective mutations being present from birth.
Studies of IL-9 in mouse models of allergic disease models apart from those involving lung inflammation are limited. However, two reports suggest that IL-9 may have an important role in anaphylaxis. In one report, IL-9 promoted anaphylaxis during both sensitization and effector stages in a passive model of systemic anaphylaxis, IL-9 receptor-deficient mice were not protected from anaphylaxis.68 In a recent report, intestinal anaphylaxis was attenuated in IL-9-deficient mice undergoing oral antigen sensitization and challenge.69 In addition, gut-specific IL-9 transgenic mice had increased mucosal permeability and were predisposed to oral antigen sensitization. Hence, IL-9 appears to be acting during the sensitization and challenge phases of murine anaphylaxis, perhaps through IgE production and mastocytosis.
IL-9 and airway remodelling
Airway remodelling occurs in chronic asthma and is characterized by structural changes mediated by cytokines and growth factors that include epithelial mucus metaplasia, peribronchial fibrosis, increases in airway smooth muscle mass, and angiogenesis.70 Of these features of remodelling, IL-9 has been most associated with epithelial mucus production, which is considered deleterious to symptoms and lung function in asthmatics and is reviewed elsewhere.71 In mouse models of Th2-driven lung inflammation, mucus metaplasia occurs when epithelial cells change their phenotype and rapidly increase their production of mucins. Though data from the IL-9 transgenic mice support a role for IL-9 in induction of mucus metaplasia, this phenotype was initially correlated with the degree of inflammation. However, it is now clear from both in vitro and in vivo studies that IL-9 can directly induce these changes, independent of inflammation.72–75 Initially, IL-9 was shown to directly stimulate bronchial epithelial cells in vitro to increase muc5ac gene expression, the principle mucin in asthmatic airway epithelium.72 Additional studies have reported that direct instillation of recombinant IL-9 into the trachea of mice can induce muc5ac gene expression and histolopathological mucus changes,73,75 without the presence of recruited inflammatory cells in the airway. A few reports have shown that IL-9 induction of mucus metaplasia is dependent on IL-13,76,77 suggesting that there may be direct and indirect effects.
Other features of remodelling including increases in peribronchial subepithelial fibrosis in IL-9 transgenic mice have been reported, but this may be secondary to immune cell recruitment and mediator release.78 For example, when human airway smooth muscle was stimulated with IL-9 in vitro, eotaxin1 and IL-8 are released, suggesting that the interactions between IL-9 and smooth muscle may propagate inflammation through eosinophil and neutrophil recruitment.79,80 As mentioned, IL-10 has been shown to both stimulate IL-9 production and be secreted from Th9 cells.6,10 Interestingly, IL-10 overexpression in the lung has previously been shown to induce STAT6 and IL-13-independent airway inflammation and fibrosis.81
Overall, IL-9 appears to have important roles in airway remodelling, most notably with mucus production in the epithelium. Conceivably, sources of IL-9 during the remodelling process include T cells, eosinophils, mast cells and structural cells. Although CD4 Th2 cells appear critical to the initiation of remodelling changes, we have recently demonstrated that CD4 cells are not required for remodelling, including mucus metaplasia, after the establishment of acute inflammation but remain essential to eosinophilic inflammation even during late antigen challenges. This suggests that IL-9 secreted from a CD4 cell may have a direct role in the early initiation of airway remodelling, but other cell types likely contribute later.82
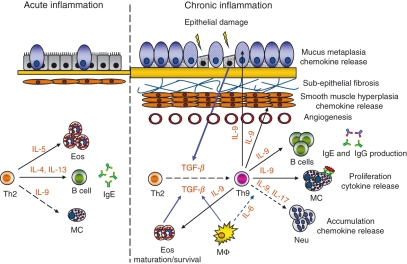
Interestingly, there is significant evidence from human asthmatics and mouse models that TGF-β regulates the airway remodelling process on many levels,83 providing the appropriate milieu to differentiate or possibly ‘reprogramme’ effector CD4 T cells into Th9 cells. The recently published data regarding Th9 subsets suggest a model where Th2 cells that have been demonstrated to play a critical role in acute allergic inflammation have the ability to switch to a Th9 phenotype during chronic inflammation and contribute to the ongoing inflammatory response and perhaps the initiation of airway remodelling (Fig. 1). As mentioned, and reviewed in detail elsewhere,84,85 there are many potential sources of IL-9 in allergic disease, including mast cells and eosinophils, making it difficult to draw conclusions about the presence and/or role of Th9 cells in allergic disease.
Figure 1.
Model of T helper type 2 (Th2) and Th9 responses during acute and chronic lung inflammation. During the acute phase of Th2 driven-lung inflammation, allergen inhalation in predisposed individuals induces activation of Th2 cells to express multiple cytokines. These cytokines are involved in the class-switching of B cells to immunoglobulin E (IgE) synthesis [interleukin-4 (IL-4) and IL-13], activation and recruitment of mast cells (IL-4, IL-9 and IL-13) and the development and maturation of eosinophils (IL-5 and granulocyte–macrophage colony-stimulating factor) and basophils (IL-4) and initiation of mucus metaplasia (IL-13 and other growth factors). During repetitive exposure to allergens or perhaps other stimuli epithelial cells and the underlying mesenchyme participate in the repair process of damaged epithelium through the release of various growth factors and cytokines such as transforming growth factor-β (TGF-β) and vascular endothelial growth factor. In the chronically inflamed airway, eosinophils, macrophages, and to some extent, mast cells produce TGF-β. In the presence of IL-4 and TGF-β, some effector Th2 cells may switch to Th9 cells and become involved in distinct aspects of chronic lung inflammation mediated by IL-9. The IL-9 supports antigen-independent proliferation of T cells, enhances IgE and IgG production by B cells; promotes proliferation/survival and release of proinflammatory cytokines from mast cells and potentiates the maturation and survival of eosinophils. Interleukin-9 can increase IL-8 release from neutrophils and smooth muscle and in combination with IL-17 may increase accumulation of neutrophils in the airways. In epithelial cells, IL-9 induces chemokines that enhance eosinophilia and T-cell infiltration, as well as stimulating mucin production leading to mucus metaplasia. IL-9 can also induce the expression of Th2-associated chemokines in human airway smooth muscle, which in turn increase infiltration of eosinophils and other inflammatory cells. Together, the cellular and structural changes mediated by IL-9 can augment airway remodelling and perpetuate chronic inflammation. MΦ = macrophage, Eos = eosinophil, Neu = neutrophils, MC = mast cells.
Th9 in health and non-allergic disease
Despite the functional role of IL-9 in allergic diseases and resistance against intestinal nematodes, there is little information regarding the significance of IL-9-producing cells in autoimmune disease. There are a few models describing how IL-9-producing cells can be generated in vivo. Using a skin allograft model, Lu et al.47 showed that CD4+ CD25+ T cells, but not CD4+ CD25− cells, are able to secret a significant amount of IL-9, that in turn activates mast cells and induces graft tolerance. In this model, TGF-β either from Tregs or other cells may potentiate IL-9 production by CD4+ CD25+ T cells. In a separate experimental system, direct interaction between effector encephalitogenic T cells and TGF-β+ neuron cells switches the effector T cells into IL-9-producing regulatory T cells that can suppress ongoing experimental autoimmune encephalomyelitis.48 In these two models, TGF-β can ameliorate ongoing tissue damage driven by alloreactive or encephalitogenic T cells probably through deviating uncommitted pathogenic Th0/Th1 cells toward protective IL-9/IL-10-producing cells. However, in a colitis model in which RAG-1-deficient mice were reconstituted with effector Th9 cells alone or in combination with CD45RBhi effector T cells, severe weight loss and moderate colitis were observed.7 Interestingly, 6–8 weeks post transfer, Th9 cells recovered from RAG-1-deficient mice are able to produce both IL-17 and IFN-γ, indicating the flexibility in cytokine production by Th9 cells.7 As a consequence, Th9 cells could contribute to the pathogenesis of many different diseases, in part through a broad repertoire of cytokines that can be produced by these cells.
Concluding remarks
The identification of T-cell subsets and their respective cytokine profiles that are consistently found in patients with different diseases has improved our understanding of these diseases and has led to the creation of relevant animal models. With the recent discovery of a novel T-cell subset, Th9, it seems plausible that these cells may be contributing to allergic disease. Analysis of specimens from allergic patients has revealed that T cells can be a significant source of IL-9 and mouse models have revealed that IL-9 has multiple effects in the development and maintenance of allergic inflammation and airway remodelling. During the allergic response in vivo, it is unknown whether IL-9-secreting T cells are distinct from Th2 cells or whether Th2 cells can be reprogrammed into Th9 cells. This exciting new area in the field of allergy and immunology is now open for further investigation.
Acknowledgments
We thank Dr Michael Croft for his guidance and review of this manuscript.
Acknowledgments
The authors have nothing to disclose.
References
- 1.Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001;344:30–7. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 2.Uyttenhove C, Simpson RJ, Van Snick J. Functional and structural characterization of P40, a mouse glycoprotein with T-cell growth factor activity. Proc Natl Acad Sci USA. 1988;85:6934–8. doi: 10.1073/pnas.85.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Snick J, Goethals A, Renauld JC, Van Roost E, Uyttenhove C, Rubira MR, Moritz RL, Simpson RJ. Cloning and characterization of a cDNA for a new mouse T cell growth factor (P40) J Exp Med. 1989;169:363–8. doi: 10.1084/jem.169.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hultner L, Druez C, Moeller J, Uyttenhove C, Schmitt E, Rude E, Dormer P, Van Snick J. Mast cell growth-enhancing activity (MEA) is structurally related and functionally identical to the novel mouse T cell growth factor P40/TCGFIII (interleukin 9) Eur J Immunol. 1990;20:1413–16. doi: 10.1002/eji.1830200632. [DOI] [PubMed] [Google Scholar]
- 5.Renauld JC, Vink A, Louahed J, Van Snick J. Interleukin-9 is a major anti-apoptotic factor for thymic lymphomas. Blood. 1995;85:1300–5. [PubMed] [Google Scholar]
- 6.Veldhoen M, Uyttenhove C, van Snick J, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–6. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 7.Dardalhon V, Awasthi A, Kwon H, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–55. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dugas B, Renauld JC, Pene J, et al. Interleukin-9 potentiates the interleukin-4-induced immunoglobulin (IgG, IgM and IgE) production by normal human B lymphocytes. Eur J Immunol. 1993;23:1687–92. doi: 10.1002/eji.1830230743. [DOI] [PubMed] [Google Scholar]
- 9.Petit-Frere C, Dugas B, Braquet P, Mencia-Huerta JM. Interleukin-9 potentiates the interleukin-4-induced IgE and IgG1 release from murine B lymphocytes. Immunology. 1993;79:146–51. [PMC free article] [PubMed] [Google Scholar]
- 10.Monteyne P, Renauld JC, Van Broeck J, Dunne DW, Brombacher F, Coutelier JP. IL-4-independent regulation of in vivo IL-9 expression. J Immunol. 1997;159:2616–23. [PubMed] [Google Scholar]
- 11.Shimbara A, Christodoulopoulos P, Soussi-Gounni A, et al. IL-9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. J Allergy Clin Immunol. 2000;105:108–15. doi: 10.1016/s0091-6749(00)90185-4. [DOI] [PubMed] [Google Scholar]
- 12.Erpenbeck VJ, Hohlfeld JM, Discher M, Krentel H, Hagenberg A, Braun A, Krug N. Increased expression of interleukin-9 messenger RNA after segmental allergen challenge in allergic asthmatics. Chest. 2003;123(Suppl. 3):370S. [PubMed] [Google Scholar]
- 13.Erpenbeck VJ, Hohlfeld JM, Volkmann B, Hagenberg A, Geldmacher H, Braun A, Krug N. Segmental allergen challenge in patients with atopic asthma leads to increased IL-9 expression in bronchoalveolar lavage fluid lymphocytes. J Allergy Clin Immunol. 2003;111:1319–27. doi: 10.1067/mai.2003.1485. [DOI] [PubMed] [Google Scholar]
- 14.Tsicopoulos A, Shimbara A, de Nadai P, et al. Involvement of IL-9 in the bronchial phenotype of patients with nasal polyposis. J Allergy Clin Immunol. 2004;113:462–9. doi: 10.1016/j.jaci.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Fallon PG, Smith P, Richardson EJ, et al. Expression of interleukin-9 leads to Th2 cytokine-dominated responses and fatal enteropathy in mice with chronic Schistosoma mansoni infections. Infect Immun. 2000;68:6005–11. doi: 10.1128/iai.68.10.6005-6011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faulkner H, Renauld JC, Van Snick J, Grencis RK. Interleukin-9 enhances resistance to the intestinal nematode Trichuris muris. Infect Immun. 1998;66:3832–40. doi: 10.1128/iai.66.8.3832-3840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svetic A, Madden KB, Zhou XD, Lu P, Katona IM, Finkelman FD, Urban JF, Jr, Gause WC. A primary intestinal helminthic infection rapidly induces a gut-associated elevation of Th2-associated cytokines and IL-3. J Immunol. 1993;1:3434–41. [PubMed] [Google Scholar]
- 18.Renauld JC, Goethals A, Houssiau F, Merz H, Van Roost E, Van Snick J. Human P40/IL-9. Expression in activated CD4+ T cells, genomic organization, and comparison with the mouse gene. J Immunol. 1990;144:4235–41. [PubMed] [Google Scholar]
- 19.Houssiau FA, Renauld JC, Fibbe WE, Van Snick J. IL-2 dependence of IL-9 expression in human T lymphocytes. J Immunol. 1992;148:3147–51. [PubMed] [Google Scholar]
- 20.Kajiyama Y, Umezu-Goto M, Kobayashi N, Takahashi K, Fukuchi Y, Mori A. IL-2-induced IL-9 production by allergen-specific human helper T-cell clones. Int Arch Allergy Immunol. 2007;143(Suppl. 1):71–5. doi: 10.1159/000101409. [DOI] [PubMed] [Google Scholar]
- 21.Houssiau FA, Schandene L, Stevens M, Cambiaso C, Goldman M, van Snick J, Renauld JC. A cascade of cytokines is responsible for IL-9 expression in human T cells. Involvement of IL-2, IL-4, and IL-10. J Immunol. 1995;154:2624–30. [PubMed] [Google Scholar]
- 22.Soler D, Chapman TR, Poisson LR, et al. CCR8 expression identifies CD4 memory T cells enriched for FOXP3+ regulatory and Th2 effector lymphocytes. J Immunol. 2006;177:6940–51. doi: 10.4049/jimmunol.177.10.6940. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Petrus M, Bryant BR, et al. Induction of the IL-9 gene by HTLV-I Tax stimulates the spontaneous proliferation of primary adult T-cell leukemia cells by a paracrine mechanism. Blood. 2008;111:5163–72. doi: 10.1182/blood-2007-09-113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt E, Beuscher HU, Huels C, Monteyne P, van Brandwijk R, van Snick J, Ruede E. IL-1 serves as a secondary signal for IL-9 expression. J Immunol. 1991;147:3848–54. [PubMed] [Google Scholar]
- 25.Helmby H, Grencis RK. Interleukin 1 plays a major role in the development of Th2-mediated immunity. Eur J Immunol. 2004;34:3674–81. doi: 10.1002/eji.200425452. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt E, Germann T, Goedert S, et al. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1994;153:3989–96. [PubMed] [Google Scholar]
- 27.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–8. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 28.Gessner A, Blum H, Rollinghoff M. Differential regulation of IL-9-expression after infection with Leishmania major in susceptible and resistant mice. Immunobiology. 1993;189:419–35. doi: 10.1016/S0171-2985(11)80414-6. [DOI] [PubMed] [Google Scholar]
- 29.Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–56. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 30.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–44. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 31.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 32.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 35.Sinigaglia F, D’Ambrosio D, Panina-Bordignon P, Rogge L. Regulation of the IL-12/IL-12R axis: a critical step in T-helper cell differentiation and effector function. Immunol Rev. 1999;170:65–72. doi: 10.1111/j.1600-065x.1999.tb01329.x. [DOI] [PubMed] [Google Scholar]
- 36.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–57. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 37.Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 2006;16:3–10. doi: 10.1038/sj.cr.7310002. [DOI] [PubMed] [Google Scholar]
- 38.Hebenstreit D, Wirnsberger G, Horejs-Hoeck J, Duschl A. Signaling mechanisms, interaction partners, and target genes of STAT6. Cytokine Growth Factor Rev. 2006;17:173–88. doi: 10.1016/j.cytogfr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z, O’Shea JJ. Th17 cells: a new fate for differentiating helper T cells. Immunol Res. 2008;41:87–102. doi: 10.1007/s12026-007-8014-9. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z, Laurence A, O’Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin Immunol. 2007;19:400–8. doi: 10.1016/j.smim.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang XO, Nurieva R, Martinez GJ, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+ CD25− Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–9. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 44.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 45.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci USA. 2009;106:1903–8. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei G, Wei L, Zhu J, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–67. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu LF, Lind EF, Gondek DC, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med. 2006;12:518–25. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- 49.Zhu YX, Kang LY, Luo W, Li CC, Yang L, Yang YC. Multiple transcription factors are required for activation of human interleukin 9 gene in T cells. J Biol Chem. 1996;271:15815–22. doi: 10.1074/jbc.271.26.15815. [DOI] [PubMed] [Google Scholar]
- 50.Stassen M, Klein M, Becker M, et al. p38 MAP kinase drives the expression of mast cell-derived IL-9 via activation of the transcription factor GATA-1. Mol Immunol. 2007;44:926–33. doi: 10.1016/j.molimm.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 51.van Leeuwen BH, Martinson ME, Webb GC, Young IG. Molecular organization of the cytokine gene cluster, involving the human IL-3, IL-4, IL-5, and GM-CSF genes, on human chromosome 5. Blood. 1989;73:1142–8. [PubMed] [Google Scholar]
- 52.Postma DS, Bleecker ER, Amelung PJ, Holroyd KJ, Xu J, Panhuysen CI, Meyers DA, Levitt RC. Genetic susceptibility to asthma–bronchial hyperresponsiveness coinherited with a major gene for atopy. N Engl J Med. 1995;333:894–900. doi: 10.1056/NEJM199510053331402. [DOI] [PubMed] [Google Scholar]
- 53.Noguchi E, Shibasaki M, Arinami T, et al. Evidence for linkage between asthma/atopy in childhood and chromosome 5q31–q33 in a Japanese population. Am J Respir Crit Care Med. 1997;156:1390–3. doi: 10.1164/ajrccm.156.5.9702084. [DOI] [PubMed] [Google Scholar]
- 54.Doull IJ, Lawrence S, Watson M, Begishvili T, Beasley RW, Lampe F, Holgate T, Morton NE. Allelic association of gene markers on chromosomes 5q and 11q with atopy and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 1996;1:1280–4. doi: 10.1164/ajrccm.153.4.8616554. [DOI] [PubMed] [Google Scholar]
- 55.Nicolaides NC, Holroyd KJ, Ewart SL, et al. Interleukin 9: a candidate gene for asthma. Proc Natl Acad Sci USA. 1997;94:13175–80. doi: 10.1073/pnas.94.24.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holroyd KJ, Martinati LC, Trabetti E, et al. Asthma and bronchial hyperresponsiveness linked to the XY long arm pseudoautosomal region. Genomics. 1998;52:233–5. doi: 10.1006/geno.1998.5445. [DOI] [PubMed] [Google Scholar]
- 57.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 58.Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 59.Huang SK, Xiao HQ, Kleine-Tebbe J, Paciotti G, Marsh DG, Lichtenstein LM, Liu MC. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688–94. [PubMed] [Google Scholar]
- 60.Ying S, Meng Q, Kay AB, Robinson DS. Elevated expression of interleukin-9 mRNA in the bronchial mucosa of atopic asthmatics and allergen-induced cutaneous late-phase reaction: relationships to eosinophils, mast cells and T lymphocytes. Clin Exp Allergy. 2002;32:866–71. doi: 10.1046/j.1365-2222.2002.01376.x. [DOI] [PubMed] [Google Scholar]
- 61.Devos S, Cormont F, Vrtala S, Hooghe-Peters E, Pirson F, Snick J. Allergen-induced interleukin-9 production in vitro: correlation with atopy in human adults and comparison with interleukin-5 and interleukin-13. Clin Exp Allergy. 2006;36:174–82. doi: 10.1111/j.1365-2222.2006.02422.x. [DOI] [PubMed] [Google Scholar]
- 62.Nouri-Aria KT, Pilette C, Jacobson MR, Watanabe H, Durham SR. IL-9 and c-Kit+ mast cells in allergic rhinitis during seasonal allergen exposure: effect of immunotherapy. J Allergy Clin Immunol. 2005;116:73–9. doi: 10.1016/j.jaci.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 63.McLane MP, Haczku A, van de Rijn M, et al. Interleukin-9 promotes allergen-induced eosinophilic inflammation and airway hyperresponsiveness in transgenic mice. Am J Respir Cell Mol Biol. 1998;19:713–20. doi: 10.1165/ajrcmb.19.5.3457. [DOI] [PubMed] [Google Scholar]
- 64.Temann UA, Geba GP, Rankin JA, Flavell RA. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J Exp Med. 1998;188:1307–20. doi: 10.1084/jem.188.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13:573–83. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 66.McMillan SJ, Bishop B, Townsend MJ, McKenzie AN, Lloyd CM. The absence of interleukin 9 does not affect the development of allergen-induced pulmonary inflammation nor airway hyperreactivity. J Exp Med. 2002;195:51–7. doi: 10.1084/jem.20011732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng G, Arima M, Honda K, et al. Anti-interleukin-9 antibody treatment inhibits airway inflammation and hyperreactivity in mouse asthma model. Am J Respir Crit Care Med. 2002;166:409–16. doi: 10.1164/rccm.2105079. [DOI] [PubMed] [Google Scholar]
- 68.Knoops L, Louahed J, Van Snick J, Renauld JC. IL-9 promotes but is not necessary for systemic anaphylaxis. J Immunol. 2005;175:335–41. doi: 10.4049/jimmunol.175.1.335. [DOI] [PubMed] [Google Scholar]
- 69.Forbes EE, Groschwitz K, Abonia JP, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol. 2007;19:676–80. doi: 10.1016/j.coi.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 71.Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med. 2009;15:4–11. doi: 10.1097/MCP.0b013e32831da8d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Longphre M, Li D, Gallup M, et al. Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells. J Clin Invest. 1999;104:1375–82. doi: 10.1172/JCI6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reader JR, Hyde DM, Schelegle ES, Aldrich MC, Stoddard AM, McLane MP, Levitt RC, Tepper JS. Interleukin-9 induces mucous cell metaplasia independent of inflammation. Am J Respir Cell Mol Biol. 2003;28:664–72. doi: 10.1165/rcmb.2002-0207OC. [DOI] [PubMed] [Google Scholar]
- 74.Vermeer PD, Harson R, Einwalter LA, Moninger T, Zabner J. Interleukin-9 induces goblet cell hyperplasia during repair of human airway epithelia. Am J Respir Cell Mol Biol. 2003;28:286–95. doi: 10.1165/rcmb.4887. [DOI] [PubMed] [Google Scholar]
- 75.Louahed J, Toda M, Jen J, Hamid Q, Renauld JC, Levitt RC, Nicolaides NC. Interleukin-9 upregulates mucus expression in the airways. Am J Respir Cell Mol Biol. 2000;22:649–56. doi: 10.1165/ajrcmb.22.6.3927. [DOI] [PubMed] [Google Scholar]
- 76.Steenwinckel V, Louahed J, Orabona C, et al. IL-13 mediates in vivo IL-9 activities on lung epithelial cells but not on hematopoietic cells. J Immunol. 2007;178:3244–51. doi: 10.4049/jimmunol.178.5.3244. [DOI] [PubMed] [Google Scholar]
- 77.Cohn L, Whittaker L, Niu N, Homer RJ. Cytokine regulation of mucus production in a model of allergic asthma. Novartis Found Symp. 2002;20:77–82. [PubMed] [Google Scholar]
- 78.van den Brule S, Heymans J, Havaux X, Renauld JC, Lison D, Huaux F, Denis O. Profibrotic effect of IL-9 overexpression in a model of airway remodeling. Am J Respir Cell Mol Biol. 2007;37:202–9. doi: 10.1165/rcmb.2006-0397OC. [DOI] [PubMed] [Google Scholar]
- 79.Baraldo S, Faffe DS, Moore PE, Whitehead T, McKenna M, Silverman ES, Panettieri RA, Jr, Shore SA. Interleukin-9 influences chemokine release in airway smooth muscle: role of ERK. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1093–102. doi: 10.1152/ajplung.00300.2002. [DOI] [PubMed] [Google Scholar]
- 80.Gounni AS, Hamid Q, Rahman SM, Hoeck J, Yang J, Shan L. IL-9-mediated induction of eotaxin1/CCL11 in human airway smooth muscle cells. J Immunol. 2004;173:2771–9. doi: 10.4049/jimmunol.173.4.2771. [DOI] [PubMed] [Google Scholar]
- 81.Lee CG, Homer RJ, Cohn L, et al. Transgenic overexpression of interleukin (IL)-10 in the lung causes mucus metaplasia, tissue inflammation, and airway remodeling via IL-13-dependent and -independent pathways. J Biol Chem. 2002;277:35466–74. doi: 10.1074/jbc.M206395200. [DOI] [PubMed] [Google Scholar]
- 82.Doherty TA, Soroosh P, Broide DH, Croft M. CD4+ cells are required for chronic eosinophilic lung inflammation but not airway remodeling. Am J Physiol Lung Cell Mol Physiol. 2009;296:L229–35. doi: 10.1152/ajplung.90543.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boxall C, Holgate ST, Davies DE. The contribution of transforming growth factor-beta and epidermal growth factor signalling to airway remodelling in chronic asthma. Eur Respir J. 2006;27:208–29. doi: 10.1183/09031936.06.00130004. [DOI] [PubMed] [Google Scholar]
- 84.Hauber HP, Bergeron C, Hamid Q. IL-9 in allergic inflammation. Int Arch Allergy Immunol. 2004;134:79–87. doi: 10.1159/000078384. [DOI] [PubMed] [Google Scholar]
- 85.Soussi-Gounni A, Kontolemos M, Hamid Q. Role of IL-9 in the pathophysiology of allergic diseases. J Allergy Clin Immunol. 2001;107:575–82. doi: 10.1067/mai.2001.114238. [DOI] [PubMed] [Google Scholar]