Abstract
The ultimate outcome of T cell receptor recognition is determined by the context in which the antigen is encountered. In this fashion both antigen-presenting cells and T cells must integrate multiple environmental cues in the form of pathogen-associated molecular patterns, cytokines and accessory molecule signals. The mammalian target of rapamycin (mTOR) is an evolutionarily conserved serine/threonine kinase that plays a central role in integrating environmental signals critical to regulating metabolism and cell survival. In this paper we review the data demonstrating that mTOR integrates signals from the immune microenvironment and therefore facilitates the generation of the adaptive immune response. Specifically, we review the role of mTOR in promoting dendritic cell activation and maturation, in regulating full T cell activation versus anergy, and influencing the induction of regulatory T cells.
Keywords: anergy, dendritic cells, mammalian target of rapamycin, regulatory T cell, T cell
Environmental cues modulate the immune response
A hallmark of the adaptive T cell response is the ability of T cell receptors (TCR) to specifically recognize a vast array of different antigens. However, while this recognition, termed Signal 1, plays a role in initiating T cell activation, the precise nature of the response is dictated by environmental cues within the inflammatory milieu. That is, in spite of the exquisite specificity of TCR engagement, the outcome of antigen recognition is not determined by the nature of the antigen itself, but rather by the context in which the antigen is encountered.
While TCR engagement heralds recognition, full T cell activation in response to this recognition requires a second signal, Signal 2. Originally proposed by Bretcher and Cohn and modified by Lafferty and Cunningham, the two-signal model provides the foundation for the laws governing T cell activation.1,2 Lafferty and Cunningham initially proposed Signal 2 to be a species-specific accessory signal delivered by the stimulator cell [antigen-presenting cell (APC)] to the T cell.2 Today, the B7-CD28 interaction is recognized as being a critical component of Signal 2. However, in reality T cells receive a multitude of accessory signals from the APC upon TCR engagement. Some of the signals such as CD28, ICOS and 4-1BB deliver costimulatory signals whereas others, like cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1), deliver coinhibitory signals.3,4 In this fashion, Signal 2 represents the net integration of multiple positive and negative accessory molecule signals.
While Signal 2 dictates the outcome of antigen recognition, the regulation of costimulatory molecules on the surface of the APC plays a critical role in determining T cell function. Hence, the ‘decision’ as to whether a particular antigen will induce full activation is determined at the level of the APC. Antigens presented by activated APCs will promote T cell responses while those presented by resting APCs might promote T cell tolerance. Within this framework Medzhitov and Janeway proposed a model whereby immune responses were driven by the presence or absence of ‘infectious non-self’.5 For example, during a viral infection robust T cell responses result from the presentation of viral peptides by activated APCs. The activation of the APC is the result of stimulation by virus-associated pathogen-associated molecular patterns (PAMPs; for example double-stranded RNA activating Toll-like receptor 3). In the absence of PAMPs, the response to virus peptide would be weak or even tolerogenic.6 In an effort to account for non-infectious immune responses (such as to transplanted organs or cancer) Matzinger and Fuchs have proposed the Danger Theory.7,8 In this model, immune activation occurs when antigen is presented in the presence of danger signals, which in turn lead to the activation of APCs. As a consequence, the ability of dendritic cells (DC) to sense and integrate environmental cues is also critical to shaping adaptive immune responses.
When naïve CD4+ helper T cells become activated, the precise effector response is also determined by environmental cues.9 In the past, this was thought to be limited to T helper 1 (Th1) and Th2 phenotypes. Th1 cells, characterized by interferon-γ (IFN-γ), tumour necrosis factor-α, and interleukin-2 (IL-2) production, begin a pattern of immunity well suited for defence against viruses and intracellular bacteria. Th1 effectors are generated when antigen recognition occurs in the setting of IL-12 and IFN-γ. Th2 cells produce IL-4, IL-5, IL-6, IL-10 and IL-13. They promote B cell-mediated immunity, aid in fighting parasitic infections and are associated with the allergic response. Th2 cells are efficiently generated when antigen recognition occurs in the context of increased IL-4 and minimal IFN-γ.9
Recently, however, it has become appreciated that there is a much greater plasticity to T cell responses upon TCR engagement. Th17 cells, characterized by IL-17 production, are associated with defence against extracellular bacteria as well as autoimmunity.10 The generation of this subset of T cells occurs when naïve T cells recognize antigen in the presence of IL-6 and transforming growth factor-β (TGF-β). In addition to IL-17-producing cells, another outcome of antigen recognition is the generation of inducible regulatory cells. For example, when TCR engagement occurs in the presence of TGF-β, a population of Foxp3+ cells that inhibit responses is generated. Likewise, antigen recognition in the presence of IL-10 leads to the generation of IL-10-producing Tr1 cells with suppressive properties.10 While in vitro skewing to these specific subsets (effector and regulatory) is easily achieved by adding exogenous cytokines and blocking antibodies, in vivo, T cells must simultaneously integrate multiple environmental cues to choose a specific effector fate.
mTOR integrates environmental cues
The target of rapamycin (TOR) was originally described as a yeast kinase potently inhibited by rapamycin, a cyclophilin-binding compound produced by Streptomyces hygroscopicus.11 While two TORs exist in yeast, metazoan TOR consists of one TOR protein which can form distinct signalling complexes. Rapamycin interferes with TOR signalling by binding to FKBP12, which can disrupt the interactions between TOR and its binding partners.12
Like its yeast counterpart, the mammalian TOR (also known as FRAP and RAFT1) was shown to integrate numerous environmental cues critical to regulating metabolism and cell survival.13 Mammalian TOR is constitutively expressed and regulated post-translationally. Studies exploring mTOR signalling using rapamycin reveal it to be an integrator of insulin, growth factor and amino acid signalling.14 mTOR phosphorylates downstream substrates to prevent apoptosis and autophagy, reorganize actin, initiate ribosome biogenesis, start translation, and positively influence the G0 to G1 transition of the cell cycle. Incubation of cells with rapamycin mimics a starvation signal leading to cell cycle arrest in G1, the inhibition of translation and ribosome assembly, the suppression of metabolism, and the initiation of autophagy.15
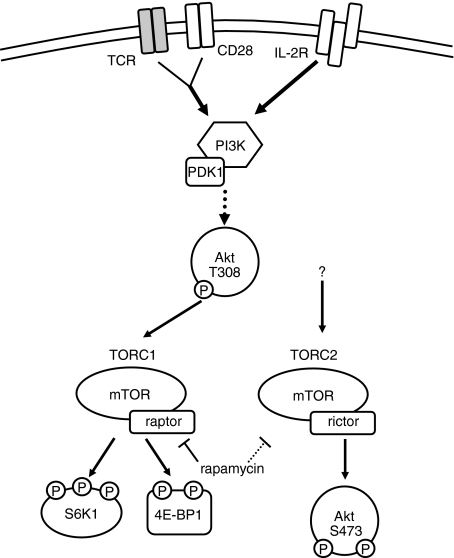
Activation of mTOR proceeds via two distinct signalling complexes: TORC1 and TORC2.16 TORC1 is a rapamycin-sensitive complex consisting of an upstream positive signalling small GTPase called Rheb, the GβL adaptor subunit, mLST8, and PRAS40. In addition to these subunits, a 150 kDa molecular weight scaffolding protein, the regulatory associated protein of TOR (raptor), is responsible for facilitating phosphorylation of substrates by mTOR kinase. TORC1 has been implicated in amino acid and growth factor signalling. The downstream substrates of TORC1 are varied and have yet to be fully catalogued. However, the canonical substrates used in TORC1 studies are the 70 kDa ribosomal S6 kinase (S6K1) and the translational repressor 4E-BP1 (Fig. 1).
Figure 1.
Schematic of mammalian target of rapamycin (mTOR) signalling in T cells. Environmental cues including costimulatory molecule engagement (CD28), growth factors [for example, interleukin-2 (IL-2)], amino acids, and insulin stimulate the activity of the phosphatidylinositol 3-kinase (PI3K) signalling cascade, promoting the partial activation of Akt on threonine 308, upstream of mTOR. The mTOR signalling proceeds via two complexes, TORC1 (characterized by mTOR and raptor) and TORC2 (characterized by mTOR and rictor). While TORC1 is activated in part by the inhibition of TSC2, a biochemical inhibitor of TORC1, the precise upstream events leading to TORC2 activation remain unclear.64 TORC1 phosphorylates (among many substrates) the ribosomal S6K1 and the translational repressor 4E-BP1. TORC2 phosphorylates Akt on serine 473, locking it into a fully activated conformation. Rapamycin interferes with TORC1 signalling by interfering with mTOR–raptor interactions. Rapamycin can also inhibit TORC2 signalling, although the mechanism is not entirely clear.
In 2004 a second signalling complex was reported, initially thought to be insensitive to rapamycin treatment, termed TORC2.17 TORC2 consists of an upstream positive signalling protein called mSin1, the GβL adaptor subunit, mLST8, and protor.16 The 200 kDa molecular weight rapamycin-insensitive companion of TOR (rictor) is the scaffolding protein associated with TORC2 signalling. TORC2 phosphorylates protein kinase B, or Akt, at the specific phosphorylation site at serine 473.18,19 This hydrophobic motif site locks Akt into a fully activated conformation, allowing it to phosphorylate a number of additional proteins.20
Interestingly, Akt is both upstream and downstream of mTOR. Upstream, phosphatidylinositol 3-kinase (PI3K) activation leads to the activation of Akt through the phosphorylation of threonine 308 (Fig. 1). This T308 site partially activates Akt facilitating the activation of mTOR by phosphorylating and inactivating the tuberous sclerosis complex proteins TSC2 and TSC1. TSC2 normally acts a GAP for the GTPase Rheb, hence inactivation of TSC2 promotes TORC1 activation. As previously mentioned, the phosphorylation of S473 of Akt is dependent upon TORC2 activation. These observations must be considered when evaluating the role of Akt in mTOR-dependent functions. Further complicating evaluation of mTOR signalling is the fact that TORC2, which was initially described as a rapamycin-insensitive signalling complex, has been shown to be sensitive to the effects of rapamycin in certain situations.21 The rapamycin sensitivity was first described as a consequence of long-term rapamycin exposure, but it is becoming increasingly evident that treatment with rapamycin can cause TORC2 inhibition (and, consequently, decreased Akt phosphorylation) in certain cell types.21,22 In T lymphocytes, for example, TORC2 can be inhibited by rapamycin over the course of several hours (unpublished findings). Hence, although rapamycin acts by inhibiting the interaction between raptor and mTOR (TORC1 assembly), through mechanisms that are not precisely clear, rapamycin also can inhibit TORC2 and subsequently downstream Akt function.
Much of what we know concerning mTOR has been derived from experiments employing the pharmacological inhibitor rapamycin. Interestingly, rapamycin has been shown to be a potent immunosuppressive agent. Mechanistically, it was initially thought that rapamycin inhibited T cell responses as a direct result of inhibiting T cell proliferation. However, as we will discuss, it has become clear that rapamycin (and other rapalogues) can profoundly affect T cell activation versus anergy, the generation of regulatory T cells (Tregs) and DC activation and function.
mTOR and anergy
When Th1 T cells are stimulated via the TCR in the absence of costimulation the cells not only fail to produce IL-2 and proliferate, but they enter a state of hyporesponsiveness known as T cell anergy. Because TCR engagement without costimulation fails to promote proliferation, it was initially proposed that anergy was the result of TCR engagement in the absence of cell division.23 In a variety of experimental systems several groups have linked cell cycle blockade with anergy induction.24–26 In support of such a model, our laboratory was able to demonstrate that T cells stimulated in the presence of rapamycin (even in the context of costimulation) produce appropriate levels of IL-2 upon initial stimulation, but behave as if they are anergic upon subsequent rechallenge.27 While these effects were initially thought to be caused by the anti-proliferative effects of rapamycin, we and others have disassociated cell cycle progression from anergy.28,29 For example, the novel immunosuppressive compound Sanglifherin A can induce cell cycle arrest in G1 but fails to induce anergy.28 These findings prompted us to propose that it was not the ability of rapamycin to inhibit proliferation that promoted anergy but rather its ability to inhibit mTOR. In support of this hypothesis we have subsequently demonstrated that T cells transfected with a mutant mTOR construct that is resistant to rapamycin fail to become anergized in the presence of rapamycin.30 In addition, anergized Th1 cells display decreased mTOR activation, compared to their activated counterparts.30 In this context, we propose that mTOR senses the environment for accessory signals that are delivered along with TCR engagement. For example, sustained B7/CD28 interactions and subsequent prolonged PI3K activity has been shown to maintain optimal CD4+ T cell activation/division.31 These data also highlight the fact that although PI3K can be activated by TCR engagement alone, enhanced (immunologically relevant) mTOR activation requires costimulation. The role of CD28 in enhancing mTOR activity is in part mediated by IL-2 but there is also an IL-2-independent component.32 Notably, however, the ability of growth factors such as IL-2 to reverse anergy has been shown to be mTOR dependent.33
In the presence of Infectious Non-self or Danger signals, antigen is presented in the context of Signal 2 which in turn is biochemically transmitted in the T cell through mTOR. For this reason, we propose that for Th1 cells, TCR engagement in the context of mTOR activation leads to a full immune response, while TCR engagement in the absence of mTOR activation leads to anergy.
mTOR and the regulatory T cell
In classic experiments, it was determined that day 3 thymectomized mice develop spontaneous autoimmune disease.34 Subsequently it was shown that the prevention of autoimmunity is mediated by thymus-derived Tregs.35 More recently, the generation of inducible Foxp3+ T cells from naïve cells has been demonstrated.36In vitro, this occurs when T cells encounter antigen in the presence of TGF-β and IL-2. In vivo, this can be seen in the context of a tolerogenic environment, such as lymphocytes that infiltrate a tumour.37 In addition to Foxp3, Tregs are characterized by high expression of glucocorticoid-induced tumour necrosis factor receptor (GITR) and CTLA-4. Furthermore, because regulatory cells fail to produce high levels of IL-2 and IFN-γ upon rechallenge with cognate peptide, such cells can be described as being anergic.
When naïve T cells are stimulated in vitro and rested in IL-2, they become activated, but maintain a small population (2–5%) of CD25bright Foxp3+ regulatory T cells, the normal ‘complement’ consisting of the natural Tregs present from the initial naïve culture. When TGF-β is present during the course of stimulation and expansion a significant proportion of the culture becomes Foxp3+. Strikingly, several groups found that treating T cells with rapamycin during stimulation and expansion, in the absence of exogenous TGF-β, also results in a culture containing a relatively high percentage of Treg cells.38–40 That is, when T cells are stimulated in the presence of rapamycin there is enrichment for Foxp3+ regulatory cells. It has been proposed that this is the result of both the de novo generation of Foxp3+ cells from naïve T cells as well as the selective expansion of regulatory cells over effector cells, in both murine and human systems. For example, in one model, systemic antigen administration under the cover of rapamycin treatment promoted de novo induction of Foxp3+ regulatory cells in vivo.41 In another model, mice treated with rapamycin without antigen administration showed an increase in the ratios of Tregs to T effector cells.42 Alternatively, others suggest that rapamycin promotes selective pressure for Treg expansion.43 In these models it is thought that Tregs, through mechanisms such as up-regulation of Pim2 kinase, simply do not require the mTOR machinery and so can out-proliferate effector cells in the setting of mTOR blockade.44
Upstream of mTOR, a signalling cascade from PI3K/Akt leads to the inactivation of TSC2 and subsequently the activation of TORC1. Based on observations that T cells lacking phosphotase and tensin homolog (PTEN), an important negative regulator of PI3K, have decreased expression of Foxp3 and that inhibition of PI3K and Akt leads to increased expression of Foxp3 it has been proposed that the PI3K/Akt/mTOR axis regulates Foxp3 expression.45 Such findings are supported by observations that T cells overexpressing a constitutively active Akt show a decrease in Foxp3-regulated genes.46 In this system rapamycin blocked the ability of the hyperactive Akt to inhibit Foxp3 and so it was concluded that Akt regulates Foxp3 via its ability to activate mTOR. However, it is important to remember that (i) full Akt activation is actually downstream of TORC2, and (ii) rapamycin can inhibit TORC2 signalling. Indeed, by employing T cells that have selective deletion of TORC1 signalling, our laboratory has data supporting a role for TORC2 in regulating Foxp3 expression (manuscript in preparation). Interestingly, the ability of Akt to induce an inhibitory phosphorylation of the forkhead box family members (like Foxo3) in cells other than lymphocytes has been demonstrated.47 In this context, it may be that mTOR signalling via TORC2-induced activation of Akt also plays a role in regulating Foxp3 expression as opposed to Akt regulating Foxp3 expression solely through the activation of TORC1.
mTOR and the dendritic cell
Dendritic cells play a critical role in regulating adaptive immune responses not only by presenting antigens to T cells but also by influencing the outcome of antigen recognition. Like T cells, DC function is also very much influenced by environmental cues and evidence is accumulating that mTOR integrates these signals.
Resting DCs are constantly sampling the environment through macropinocytosis and presenting antigen on their surface.48 When T cells encounter antigen presented by these immature DCs they are suboptimally activated and in some cases T cell tolerance can ensue.49 Using the drug rapamycin, it has been shown that mTOR activity is necessary for the activation/maturation process. Rapamycin has been shown to inhibit maturation of DCs by IL-4, granulocyte–macrophage colony-stimulating factor and IL-1β signalling.50–52 Dendritic cells derived from bone marrow in the presence of rapamycin demonstrate mitigated up-regulation of MHC and costimulatory molecules.53,54 Furthermore, DCs that are matured in the presence of rapamycin are not only poor stimulators of T cells but also promote the induction of T cell tolerance. Rapamycin-treated DC display decreased synthesis and secretion of IL-18, an important potentiator of IFN-γ production by T cells.55 In addition, alloantigen pulsed DCs that are matured in the presence of rapamycin have the ability to induce alloantigen-specific tolerance and promote graft-specific tolerance in mouse models of solid-organ transplantation.54 In part, this tolerance is mediated by the induction of Foxp3+ Tregs. That is, rapamycin-conditioned DC were shown to be poor stimulators of CD4+ effector cells but had the capacity to induce activation and proliferation of CD4+ Foxp3+ regulatory cells.
In addition to promoting DC maturation, mTOR has also been implicated in influencing DC and monocyte-induced cytokine production. Stimulation with the TLR-4 ligand lipopolysaccharide has been shown to lead to the activation of mTOR and the phosphorylation of its downstream substrates.56,57 However, for both human and murine myeloid DC, inhibition of mTOR with rapamycin leads to the enhanced production of IL-12 and the inhibition of IL-10 production. Such observations suggest that TLR-4-induced mTOR activation inhibits pro-inflammatory cytokine production while enhancing the production of the anti-inflammatory cytokine IL-10.56,57 In contrast, mTOR activation appears to promote type I interferon production in plasmacytoid DC.58 It has been shown that TLR-9-induced IFN-α or IFN-β is dependent upon mTOR and the downstream substrate of mTOR, S6K1.
As a consequence, while it is clear that mTOR activation regulates the DC response to environmental cues such as TLR agonists or cytokines, the nature of this regulation is complex. In general, mTOR appears to be required for the generation of activated/mature DCs. Maturation of DC in the absence of mTOR activity can lead to tolerogenic APCs. The TLR-9 activation in plasmacytoid DC activates mTOR and this activation promotes immunity through the production of IFN-α and IFN-β. Alternatively, TLR-4 activation of myeloid DCs leads to the production of IL-10 and the inhibition of IL-12 production, suggesting that under such circumstances mTOR activation negatively regulates a pro-inflammatory response.
A central role for mTOR in directing immune responses
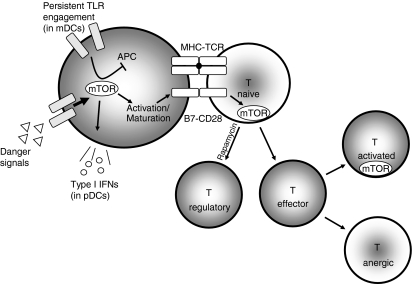
As an integrator of environmental cues in both T cells and APCs, mTOR plays a central role in directing immune responses (Fig. 2). In the presence of danger signals, mTOR facilitates the activation and maturation of DCs, which promotes the presentation of antigen in the context of activating costimulatory molecules. At the same time, continued mTOR activation can enhance IL-10 production and serve as a negative feedback loop limiting aggressive T cell responses. Within the T cell, mTOR activation as a result of costimulatory signals and cytokine production leads to full activation. Interestingly, recently a role for mTOR in T cell trafficking has been demonstrated.59,60 In the absence of such signals, T cell anergy and the generation of Treg cells dominates.
Figure 2.
Environmental signals (Danger) leading to the activation of mammalian target of rapamycin (mTOR) can induce the activation and maturation of immature DCs. This activation leads to the up-regulation of B7 molecules and the production of type I interferons. In mature myeloid dendritic cells (mDCs) and monocyte-derived lineages, persistent Toll-like receptor (TLR) stimulation may stimulate mTOR to induce a negative feedback loop, leading to the inhibition of pro-inflammatory cytokine secretion. Naïve T cells receiving stimulation in the context of mTOR activation differentiate into effector cells, while those receiving T cell receptor stimulation with decreased mTOR activation (or rapamycin treatment) default into regulatory T cells. Furthermore, differentiated effector cells receiving stimulation with full mTOR activation become fully activated, while those receiving suboptimal mTOR activation are rendered anergic.
Given this central role of mTOR in multiple cells critical for the adaptive immune response, it is not surprising that mTOR inhibition is an effective means to suppress the immune system in transplantation.61 However, rapamycin (or other rapalogues) are often used in conjuncion with calcineurin inhibitors such as cyclosporine A(CsA) or FK506. These latter agents block TCR-induced signalling and mitigate the tolerance-inducing properties of mTOR inhibition.62 It has been shown that CsA blocks TCR-induced anergy induction as well as the generation of Tregs.27,63 Alternatively, while mTOR inhibition can ultimately promote Treg cell generation and anergy, it does not ‘acutely’ inhibit cytokine production. As a consequence, optimal ‘tolerance induction therapy’ will involve inhibiting acute inflammation while preserving subsequent TCR signalling under the cover of mTOR inhibition. Thoughtful design of regimens employing rapalogues in conjunction with costimulatory blockade and/or targeted anti-cytokine therapy might prove better to achieving the ultimate goal of long-term tolerance in the absence of long-term immunosuppression.
Acknowledgments
The authors have no conflicts of interest to disclose.
Acknowledgments
We would like to thank members of the Powell laboratory, especially Dr Christopher Gamper and Dr Christian Meyer, as well as Dr Leo Luznik for their critical reading of this manuscript. Work in this laboratory is supported by National Institutes of Health grants R01CA098109 and R01CA14227 and by a Pilot Project Grant from Ajinomoto.
References
- 1.Bretscher P, Cohn M. A theory of self–nonself discrimination. Science. 1970;169:1042–9. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 2.Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975;53:27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 3.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 4.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R, Janeway CA., Jr How does the immune system distinguish self from nonself? Semin Immunol. 2000;12:185–8. doi: 10.1006/smim.2000.0230. [DOI] [PubMed] [Google Scholar]
- 6.Pasare C, Medzhitov R. Toll-like receptors and acquired immunity. Semin Immunol. 2004;16:23–6. doi: 10.1016/j.smim.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs EJ, Matzinger P. Is cancer dangerous to the immune system? Semin Immunol. 1996;8:271–80. doi: 10.1006/smim.1996.0035. [DOI] [PubMed] [Google Scholar]
- 8.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 9.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–69. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–88. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu Rev Immunol. 1996;14:483–510. doi: 10.1146/annurev.immunol.14.1.483. [DOI] [PubMed] [Google Scholar]
- 12.Jozwiak J, Jozwiak S, Oldak M. Molecular activity of sirolimus and its possible application in tuberous sclerosis treatment. Med Res Rev. 2006;26:160–80. doi: 10.1002/med.20049. [DOI] [PubMed] [Google Scholar]
- 13.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–34. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 14.Goberdhan DC, Boyd CA. mTOR: dissecting regulation and mechanism of action to understand human disease. Biochem Soc Trans. 2009;37:213–6. doi: 10.1042/BST0370213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris TE, Lawrence JC., Jr TOR signaling. Sci STKE. 2003;2003 doi: 10.1126/stke.2122003re15. [DOI] [PubMed] [Google Scholar]
- 16.Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17:666–81. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 17.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 18.Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–16. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 19.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 20.Franke TF. Intracellular signaling by Akt: bound to be specific. Sci Signaling. 2008;1 doi: 10.1126/scisignal.124pe29. [DOI] [PubMed] [Google Scholar]
- 21.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 22.Zeng Z, Sarbassov dos D, Samudio IJ, et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509–12. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins MK. The role of cell division in the induction of clonal anergy. Immunol Today. 1992;13:69–73. doi: 10.1016/0167-5699(92)90137-V. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert KM, Weigle WO. Th1 cell anergy and blockade in G1a phase of the cell cycle. J Immunol. 1993;151:1245–54. [PubMed] [Google Scholar]
- 25.Vanasek TL, Khoruts A, Zell T, Mueller DL. Antagonistic roles for CTLA-4 and the mammalian target of rapamycin in the regulation of clonal anergy: enhanced cell cycle progression promotes recall antigen responsiveness. J Immunol. 2001;167:5636–44. doi: 10.4049/jimmunol.167.10.5636. [DOI] [PubMed] [Google Scholar]
- 26.Wells AD, Walsh MC, Sankaran D, Turka LA. T cell effector function and anergy avoidance are quantitatively linked to cell division. J Immunol. 2000;165:2432–43. doi: 10.4049/jimmunol.165.5.2432. [DOI] [PubMed] [Google Scholar]
- 27.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–84. [PubMed] [Google Scholar]
- 28.Allen A, Zheng Y, Gardner L, Safford M, Horton MR, Powell JD. The novel cyclophilin binding compound, sanglifehrin A, disassociates G1 cell cycle arrest from tolerance induction. J Immunol. 2004;172:4797–803. doi: 10.4049/jimmunol.172.8.4797. [DOI] [PubMed] [Google Scholar]
- 29.Colombetti S, Benigni F, Basso V, Mondino A. Clonal anergy is maintained independently of T cell proliferation. J Immunol. 2002;169:6178–86. doi: 10.4049/jimmunol.169.11.6178. [DOI] [PubMed] [Google Scholar]
- 30.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–70. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 31.Colombetti S, Basso V, Mueller DL, Mondino A. Prolonged TCR/CD28 engagement drives IL-2-independent T cell clonal expansion through signaling mediated by the mammalian target of rapamycin. J Immunol. 2006;176:2730–8. doi: 10.4049/jimmunol.176.5.2730. [DOI] [PubMed] [Google Scholar]
- 32.Appleman LJ, Berezovskaya A, Grass I, Boussiotis VA. CD28 costimulation mediates T cell expansion via IL-2-independent and IL-2-dependent regulation of cell cycle progression. J Immunol. 2000;164:144–51. doi: 10.4049/jimmunol.164.1.144. [DOI] [PubMed] [Google Scholar]
- 33.Powell JD, Bruniquel D, Schwartz RH. TCR engagement in the absence of cell cycle progression leads to T cell anergy independent of p27(Kip1) Eur J Immunol. 2001;31:3737–46. doi: 10.1002/1521-4141(200112)31:12<3737::aid-immu3737>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 34.Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–5. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- 35.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–8. [PubMed] [Google Scholar]
- 36.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25– naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–47. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 39.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–8. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 40.Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, Ayyoub M. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J Immunol. 2006;177:944–9. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]
- 41.Kang J, Huddleston SJ, Fraser JM, Khoruts A. De novo induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J Leukoc Biol. 2008;83:1230–9. doi: 10.1189/jlb.1207851. [DOI] [PubMed] [Google Scholar]
- 42.Qu Y, Zhang B, Zhao L, Liu G, Ma H, Rao E, Zeng C, Zhao Y. The effect of immunosuppressive drug rapamycin on regulatory CD4+CD25+Foxp3+ T cells in mice. Transpl Immunol. 2007;17:153–61. doi: 10.1016/j.trim.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178:320–9. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 44.Basu S, Golovina T, Mikheeva T, June CH, Riley JL. Cutting edge: Foxp3-mediated induction of pim 2 allows human T regulatory cells to preferentially expand in rapamycin. J Immunol. 2008;180:5794–8. doi: 10.4049/jimmunol.180.9.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sauer S, Bruno L, Hertweck A, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–74. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guertin DA, Stevens DM, Thoreen CC, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–71. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Reis e Sousa C. Activation of dendritic cells: translating innate into adaptive immunity. Curr Opin Immunol. 2004;16:21–5. doi: 10.1016/j.coi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–8. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, Thomson AW. Rapamycin inhibits IL-4-induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–63. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 51.Turnquist HR, Sumpter TL, Tsung A, et al. IL-1beta-driven ST2L expression promotes maturation resistance in rapamycin-conditioned dendritic cells. J Immunol. 2008;181:62–72. doi: 10.4049/jimmunol.181.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woltman AM, van der Kooij SW, Coffer PJ, Offringa R, Daha MR, van Kooten C. Rapamycin specifically interferes with GM-CSF signaling in human dendritic cells, leading to apoptosis via increased p27KIP1 expression. Blood. 2003;101:1439–45. doi: 10.1182/blood-2002-06-1688. [DOI] [PubMed] [Google Scholar]
- 53.Taner T, Hackstein H, Wang Z, Morelli AE, Thomson AW. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce ag-specific T cell regulation and prolong graft survival. Am J Transplant. 2005;5:228–36. doi: 10.1046/j.1600-6143.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- 54.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–31. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 55.Ko H, Hambly BD, Eris JM, Levidiotis V, Wyburn K, Wu H, Chadban SJ, Yin JL. Dendritic cell derived IL-18 production is inhibited by rapamycin and sanglifehrin A, but not cyclosporine A. Transpl Immunol. 2008;20:99–105. doi: 10.1016/j.trim.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Weichhart T, Costantino G, Poglitsch M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–77. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 57.Ohtani M, Nagai S, Kondo S, et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–43. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, Pulendran B. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–64. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reichardt W, Durr C, von Elverfeldt D, et al. Impact of mammalian target of rapamycin inhibition on lymphoid homing and tolerogenic function of nanoparticle-labeled dendritic cells following allogeneic hematopoietic cell transplantation. J Immunol. 2008;181:4770–9. doi: 10.4049/jimmunol.181.7.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sinclair LV, Finlay D, Feijoo C, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–21. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ponticelli C. Can mTOR inhibitors reduce the risk of late kidney allograft failure? Transpl Int. 2008;21:2–10. doi: 10.1111/j.1432-2277.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 62.Powell JD, Zheng Y. Dissecting the mechanism of T cell anergy with immunophilin ligands. Curr Opin Investig Drugs. 2006;7:1002–7. [PubMed] [Google Scholar]
- 63.Kopf H, de la Rosa GM, Howard OM, Chen X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int Immunopharmacol. 2007;7:1819–24. doi: 10.1016/j.intimp.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]