Abstract
It has been well established that CD45 is a key receptor-type protein tyrosine phosphatase (PTPase) regulating Src-family protein tyrosine kinase (Src-PTK) in T and B lymphocytes. However, precisely how CD45 exerts its effect in these lymphocytes remains controversial. We recently reported that Jacalin, an α-O-glycoside of the disaccharide Thomsen–Friedenreich antigen-specific lectin from jackfruit seeds, caused marked T-cell activation in response to T-cell receptor ligation and CD28 costimulation by binding to CD45. On extending the reported research, we found that CD45 and isoforms are major Jacalin receptors on B lymphocytes, and that the glycosylation of CD45 is involved in the interaction of Jacalin with the PTPase. In contrast to Jacalin-stimulated T-cell activation, we found that Jacalin induced human B-lymphocyte apoptosis, resulting in calcium mobilization and calpain activation, suggesting that the calcium–calpain pathway may mediate the Jacalin-induced apoptosis. Importantly, the apoptosis was effectively blocked by a specific CD45 PTPase inhibitor, indicating that Jacalin induces human B-lymphocyte apoptosis through CD45 triggering. Furthermore, we found that Jacalin significantly increased the C-terminal inhibitory tyrosine (Tyr507) phosphorylation of Src-PTK Lyn, one of the major substrates of CD45 PTPase, and this effect was also observed on incubation of B lymphocytes with the specific CD45 PTPase inhibitor, suggesting that Jacalin stimulation results in increasing C-terminal tyrosine phosphorylation of the kinase through inhibition of CD45 tyrosine phosphatase activity in human B lymphocytes. Therefore, the down-modulation of Lyn kinase may play a role in the regulation of B-lymphocyte viability.
Keywords: apoptosis, B cells, CD45, lectin, protein tyrosine kinase
Introduction
Glycosylation of cell surface proteins controls critical lymphocyte processes, including lymphocyte homing, thymocyte selection, the amplitude of an immune response, lymphocyte activation and cell death.1 The roles of glycosylation in these functions are specific, i.e. different functions require specific sugars on specific glycoprotein acceptors.2 Regulated glycosylation of specific acceptor substrates can affect immune functions by creating or masking ligands for endogenous and extraneous lectins.2 Plant lectins have long been used as surrogates for authentic lymphocyte activational or inhibitory stimuli. These carbohydrate-binding proteins activate or inhibit lymphocytes by cross-linking many lymphocyte cell surface glycoproteins, including some that contribute to the immunological synapse.3 Although a number of observations have suggested that lectin–glycan interactions contribute to immune system functions, nonetheless, it is still difficult to attach any physiological significance to or to propose any molecular basis for plant lectin-dependent activation events.
Jacalin is highly specific for the α-O-glycoside of the disaccharide Thomsen–Friedenreich antigen (Galβ1-3GalNAc, T-antigen, and its sialylated form), and is a tetrameric two-chain lectin (molecular mass, 66 000) comprising a heavy α chain of 133 amino acid residues and a light β chain of 20 amino acid residues.4 Jacalin was first described as a general T-cell mitogen, however, little is known about the molecular basis.5 Recently, we isolated CD45, a type I receptor protein tyrosine-phosphatase (RPTPase), and identified it as one of the major Jacalin targets in both the CD4+ and CD8+ T-cell subsets through affinity chromatography and mass spectrometry.6 Consequently, we found that the lectin induced significant T-cell activation in response to T-cell receptor (TCR) ligation and CD28 costimulation in a CD45-positive Jurkat T-cell line (JE6.1) and primary T cells, and that the effect was blocked completely by a specific CD45 protein tyrosine phosphatase (PTPase) inhibitor. However, Jacalin-induced T-cell activation did not occur in a CD45-negative Jurkat T-cell line (J45.01). Furthermore, we also observed that glycosylation-dependent interaction of Jacalin with CD45 on T cells elevates TCR-mediated signalling, thereby up-regulating the T-cell activation threshold and T helper type 1 (Th1)/Th2 cytokine secretion.6
CD45 is highly glycosylated and has been estimated to comprise up to 10% of the surface areas of nucleated haematopoietic cells and their precursors.7 The molecule undergoes complex alternative splicing in the extracellular domain, and different patterns of CD45 splicing are associated with distinct functions. In particular, CD45 has been shown to play an essential role in signal transduction via the B-cell antigen receptor.8,9 Therefore, we inferred that Jacalin may modulate the viability of B lymphocytes through CD45 signal initiation. In the present study, we identified CD45 and isoforms as Jacalin major receptors on human B lymphocytes and suggested that the glycosylation of CD45 is involved in the interaction of Jacalin with the PTPase. Consequently, the binding of Jacalin to CD45 induces human B-lymphocyte apoptosis (programmed cell death) in a dose-dependent manner, and this effect is significantly blocked by a specific CD45 PTPase inhibitor. Furthermore, in an attempt to better elucidate the mechanism of Jacalin-induced B-lymphocyte apoptosis through triggering of CD45, which has been known to selectively associate with Lyn, one of the Src family protein tyrosine kinases (PTKs), we here provide original data regarding the CD45-mediated signal transduction through control of the tyrosine phosphorylation of Lyn in human B-lymphocyte apoptosis induced by Jacalin.
Materials and methods
Reagents and antibodies
Jacalin, fluorescein isothiocyanate (FITC) -conjugated Jacalin and agarose-conjugated Jacalin were purchased from USBiological (Swampscott, MA). A PTPase CD45 inhibitor, N-(9,10-dioxo-9,10-dihydro-phenanthren-2-yl)-2,2-dimethyl-propionamide, was obtained from Calbiochem (La Jolla, CA). Polyclonal antibodies against human nuclear poly (ADP-ribose) polymerase (PARP), Lyn and phospho-Lyn (Tyr507) were purchased from Cell Signaling Technology (Beverly, MA). Anti-human phospho-Lyn (Tyr396) and anti-human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibodies (mAbs) were purchased from Epitomics (Burlingame, CA) and Abcam (Cambridgeshire, UK), respectively. Phycoerythrin (PE) -conjugated anti-human pan-CD45 mAb was obtained from Beckman Coulter (Fullerton, CA). The FITC-conjugated anti-human immunoglobulin D (IgD) mAb and anti-human CD45RA, CD45RB and CD45RO mAbs were obtained from BD PharMingen (San Diego, CA). FITC-conjugated anti-human pan-CD45 mAb was purchased from Immunotech (Marseille, France) and Chemicon (Temecula, CA). Alexa546-conjugated anti-mouse IgG secondary antibody and an intracellular Ca2+ indicator, fluo-3-acetoxymethylester (Fluo-3-AM), were obtained from Molecular Probes (Eugene, OR). The calpain inhibitor Z-LLY-FMK and caspase inhibitor negative control Z-FA-FMK were purchased from Calbiochem (San Diego, CA). A protease inhibitor cocktail and several kinds of monosaccharides were obtained from Nacalai Tesque (Kyoto, Japan). All chemicals for gel electrophoresis and Western blotting were purchased from ATTO (Tokyo, Japan), Bio-Rad (Hercules, CA), Pierce (Rockford, IL) or Zymed (South San Francisco, CA).
Cell line, cell culture and cell stimulations
The human B-lymphoma cell line, Raji, was obtained from the American Type Culture Collection (ATCC; Rockville, MD). The Raji B cells were cultured at 37° under 5% CO2 in RPMI-1640 containing 10% fetal calf serum, 2 mm glutamine and 50 μg/ml kanamycin. For B-cell stimulation, cells were diluted to 1 × 106 cells/ml and then incubated in the medium alone or with the specified additions for the times indicated.
Preparation of membrane fractions and affinity chromatography
Raji B cells were resuspended in ice-cold Dounce buffer [10 mm Tris–HCl (pH 7·6), 0·5 mm MgCl2 and protease inhibitor cocktail], and then homogenized with a Dounce homogenizer. Tonicity restoration buffer [10 mm Tris–HCl (pH 7·6), 0·5 mm MgCl2, 0·6 m NaCl and protease inhibitor cocktail] was added to the homogenized cells to give 150 mm NaCl (final concentration), and then the homogenate was centrifuged at 500 g for 5 min at 4° to remove cell debris and nuclei. The supernatant was centrifuged at 150 000 g for 45 min at 4°. The resulting total membrane pellet was solubilized with lysis buffer [150 mm NaCl, 20 mm Tris–HCl (pH 7·6), 1 mm ethylenediaminetetraacetic acid, 1% Nonidet P-40 (NP-40), and protease inhibitor cocktail] for 60 min on a rotary shaker at 4°, and then centrifuged at 10 000 g for 20 min at 4°. The supernatant was saved as the Raji B-cell membrane proteins and was applied to a Jacalin-agarose affinity column. After washing the column with TBS buffer (pH 7·6) containing 20 mm CaCl2 and 0·1% NP-40, the proteins bound to the column were eluted with TBS buffer (pH 7·6) containing 100 mm Me-α-Gal and 0·1% NP-40. The eluted proteins were resolved on a 5–20% Tris–HCl gradient gel (ATTO), followed by Western blot and lectin blot detection using anti-human pan-CD45 antibody and Jacalin, respectively.
Separation and culture of human primary B lymphocytes
Peripheral blood lymphocytes were isolated from healthy donors by Ficoll–Paque Plus (Amersham Biosciences, Piscataway, NJ) density gradient centrifugation at 1500 gfor 25 min. CD19+ B lymphocytes were purified by positive selection with anti-human CD19 antibody-coated magnetic beads according to the manufacturer’s instructions (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Flow cytometry of the resulting cell population indicated that more than 95% of the remaining cells expressed CD19. The separated cells were incubated in a similar medium to that for Raji B cells. For stimulation, cells were diluted to 1 × 106 cells/ml and then incubated in the medium alone or with the specified additions for the times indicated.
Flow cytometry
Cells were washed once in phosphate-buffered saline (PBS) and pelleted, and then viable cells were resuspended in fluorescence-activated cell sorting (FACS) buffer (PBS containing 2% fetal calf serum). To assess human CD19, pan-CD45, CD45 isoforms and Jacalin receptor surface expression on B lymphocytes, 20 μl of FITC/PE-conjugated antibodies specific to the respective CDs or FITC-conjugated Jacalin were added to 1·0 × 106 cells in a volume of 200 μl for 1 hr, followed by three washes in FACS buffer. All incubations were conducted on ice to prevent receptor internalization. After 1 hr on ice, the cells were washed once with FACS buffer, pelleted by centrifugation, and finally suspended in 500 μl FACS buffer before analysis with a FACScan (Becton Dickinson, San Jose, CA) flow cytometer equipped with the lysis II software program. The double receptor expression on the surface of primary CD19+ B cells or Raji B cells was determined by two-colour flow cytometry using anti-human pan-CD45-PE antibodies and Jacalin-FITC.
Confocal laser scan microscopy
This experiment was carried out on two-well chamber glass slides. Human primary CD19+ B cells and Raji B cells were incubated overnight at 37° and monitored under a confocal microscope (FV1000; Olympus, Tokyo, Japan). For double immunofluorescence, the B cells were stained with FITC-conjugated Jacalin and anti-human CD45 mAb in the presence or absence of 100 mm Me-α-Gal, a strong inhibitor of Jacalin–glycan interactions, followed by Alexa546-conjugated anti-mouse IgG1 polyclonal antobidy.
Western blotting and lectin blotting
One per cent NP-40 lysis buffer cell extracts were boiled with the sample buffer. Samples were resolved on a 4–20% gradient sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) gel (ATTO), and then transferred to nitrocellulose membranes, followed by immunoblot or lectin-blot detection. For visualization, a SuperSignal West Pico Chemiluminescent kit (Pierce) was used with horseradish peroxidase-conjugated second antibody (Zymed) or horseradish peroxidase-conjugated Jacalin (US Biological, Swampscott, MA).
Apoptosis assay
Apoptosis was analysed by means of a nuclear PARP cleavage assay. Primary B lymphocytes and Raji B cells were treated with Jacalin at the indicated concentrations in the presence or absence of 1 μm specific CD45 PTPase inhibitor, calpain inhibitor Z-LLY-FMK and caspase inhibitor negative control Z-FA-FMK, respectively, for 3 days. The cells were harvested and subjected to analysis of PARP cleavage by Western blotting as reported previously.10 For preparation of a positive apoptosis control, primary B or Raji B cells were exposed to ultraviolet (UV) irradiation at 254 nm for 15 min, and then cultured in RPMI-1640 containing 10% fetal calf serum for 3·5 hr at 37° in 5% CO2. The UV-exposed cells served as a positive control.
Calcium mobilization
Live Raji B cells (1·0 × 106) in culture medium were labelled with 10 μm Fluo-3-AM, an intracellular Ca2+ indicator (Molecular Probes), at 37° for 30 min. The cells were washed and resuspended in culture medium. Intracellular Ca2+ signals were recoded for 45 seconds by a flow cytometer without any stimuli. After 45 seconds, stimuli were added and Ca2+ fluxes were measured for a total of 5 min. Intracellular Ca2+ signals were recoded on a FACSCalibur.
Tyrosine phosphorylation of Src-family PTKs
For tyrosine phosphorylation of Lyn, one of the Src-family PTKs, Raji B cells were serum-starved overnight and then stimulated with or without 50 μg/ml Jacalin in the presence or absence of 1 μm specific CD45 PTPase inhibitor for the indicated stimulation periods. Cells in the medium alone were used as a negative control. Whole cell lysates were analysed by Western blotting with specific antibodies for human Lyn, phospho-Lyn (Tyr507) (Cell Signaling Technology), phospho-Lyn (Tyr396) (Epitomics) or GAPDH (Abcam), followed with the horseradish peroxidase-conjugated relative second antibody (Zymed).
Results
Jacalin receptors are expressed on both human peripheral B cells and Raji B cells
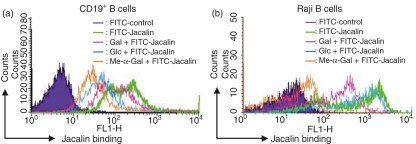
To obtain an insight into the expression profile of Jacalin receptors on B lymphocytes, human peripheral CD19+ B cells (Fig. 1a) and the human Raji B-cell line (Fig. 1b) were stained with FITC-conjugated Jacalin and then analysed by flow cytometry. As shown in Fig. 1, both peripheral CD19+ B cells and Raji B cells exhibited high levels of Jacalin receptor expression. Based on these binding data, we investigated whether Jacalin binding to the surface of B cells is dependent upon carbohydrate interactions. We examined the ability of several free monosaccharides to competitively block the interaction of Jacalin with B cells. The addition of 50 mm methyl-α-galactose (Me-α-Gal) significantly abrogated Jacalin binding to peripheral CD19+ B cells as well as to Raji B cells. In contrast, glucose (Glc) and galactose (Gal), at a concentration of 50 mm, exhibited substantially less inhibition of the binding of Jacalin to B cells (Fig. 1). This competitive inhibition by free monosaccharides is consistent with the carbohydrate recognition specificity of Jacalin previously reported.4 The data suggest that the glycans expressed on B cells may mediate the biological function of Jacalin induction through interaction between the glycans and Jacalin.
Figure 1.
Expression of Jacalin receptors on the surfaces of human primary CD19+ B cells and Raji B cells. CD19+ B cells from healthy human peripheral blood lymphocytes were separated with a QuadroMACS separation unit. The separated CD19+ B cells (a) and Raji B cells (b) were stained with fluorescein isothiocyanate-labelled Jacalin, and then analysed by flow cytometry, respectively. The cells were prepared and labelled as described in the Materials and methods. As a negative control, the autofluorescence of the cells was measured (purple area). As shown in each histogram, the sugar specificity of the binding of Jacalin to primary CD19+ B and Raji B cells was analysed in the presence of 50 mm Gal (pink), Glc (blue), or Me-α-Gal (orange), respectively.
Carbohydrates mediate the colocalization of Jacalin receptors with CD45 on both human peripheral B cells and Raji B cells
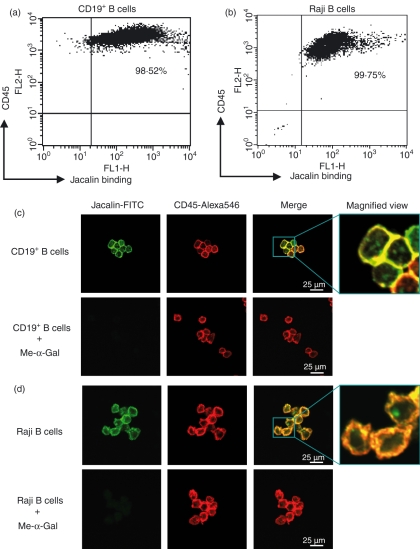
To investigate the expression and localization of Jacalin receptors and CD45 on B lymphocytes in detail, both human peripheral CD19+ B cells and human Raji B cells were analysed by dual-colour flow cytometry. As shown in Fig. 2a, there was a very strong correlation between CD45 and Jacalin staining on both peripheral CD19+ B cells (Fig. 2a) and Raji B cells (Fig. 2b). Next, we investigated whether CD45 can be directly stained by Jacalin and whether carbohydrates could mediate the colocalization of Jacalin receptors with CD45 on the B-cell surface. When we examined B lymphocytes by confocal microscopy, we observed that CD45 was substantially colocalized with FITC-conjugated Jacalin (green) and anti-human pan-CD45 mAb followed by Alexa546-conjugated second antibody (red) in both primary B (Fig. 2c) and Raji B (Fig. 2d) cell membranes in the absence of Me-α-Gal; however, the colocalization was completely inhibited by 100 mm Me-α-Gal. Collectively, the results of flow cytometry and confocal microscopy suggest that CD45 may be a major Jacalin target expressed in the B-cell membrane, and that the carbohydrates on CD45 mediate the colocalization of Jacalin receptors with CD45.
Figure 2.
Glycosylation-dependent colocalization of Jacalin receptors with CD45 on human primary CD19+ B cells and Raji B cells. (a, b) Expression of Jacalin receptors and CD45 on human CD19+ B and Raji B cells. The separated human CD19+ B cells (a) and Raji B cells (b) were analysed by two-colour flow cytometry using fluorescein isothiocyanate (FITC) -labelled Jacalin and phycoerythrin (PE) -labelled anti-human pan-CD45. The proportions of costained primary CD19+ B cells and Raji B cells are indicated in the upper-right portions of the histograms. The results are representative of three independent experiments. (c, d) Costained primary CD19+ B cells and Raji B cells with Jacalin-FITC and pan-CD45-Alexa546. Human primary CD19+ B cells (c) and Raji B cells (d) were costained with FITC-conjugated Jacalin and anti-human pan-CD45 monoclonal antibodies, followed by Alexa546-conjugated second antibody, in the absence (upper panels) and presence (lower panels) of 100 mm Me-α-Gal, respectively, and then monitored by confocal microscopy. The yellow fluorescence shows the combined fluorescence of FITC (green) and Alexa546 (red). The magnified views of colocalization are shown in right panels.
Glycosylation-dependent interaction of Jacalin with CD45 and its isoforms on Raji B cells
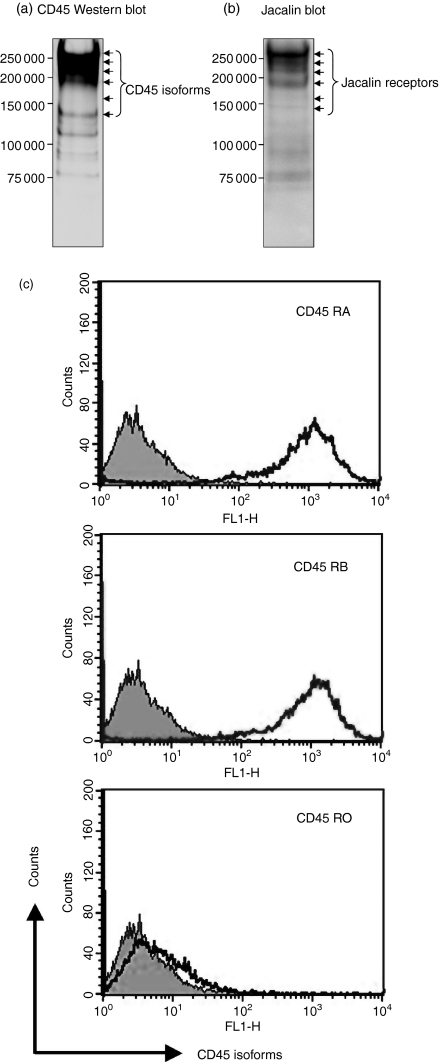
Next, to determine whether CD45 and its isoforms are the Jacalin receptors expressed on B lymphocytes, we isolated the Jacalin receptors from a Raji B-cell membrane fraction as described in the Materials and methods. The Raji membrane proteins were applied to an affinity column of Jacalin–agarose and the bound proteins were eluted with 100 mm Me-α-Gal. The eluted proteins were separated by SDS–PAGE, and then identified by Western blotting with anti-human pan-CD45 mAb and by lectin blotting with Jacalin, respectively. As shown in Fig. 3(a,b), the results of CD45 Western blotting corresponded to those of Jacalin blotting, as the major receptor bands appeared in the molecular mass range of 150 000–250 000, particularly higher molecular mass forms, under reducing conditions. Furthermore, the proportion and density of CD45 isoform bands detected by CD45 Western blotting and Jacalin blotting were quite different, suggesting that Jacalin may bind to CD45 isoforms with different affinities. CD45 is known to be expressed as multiple isoforms, which are generated through complex alternative splicing at exons 4 (A), 5 (B), and 6 (C), in the extracellular domain of the molecule.9 According to the molecular mass of Jacalin major receptors, the isoform containing all exons (CD45RABC) appears to be the predominant form on B cells, which is consistent with previous findings.9 Importantly, the proportion and expression of CD45 and its isoforms as Jacalin receptors on Raji B cells are significantly different from those of the lectin receptors on Jurkat T cells.6 In addition, to further examine the expression of CD45 isoforms on Raji B cells, we performed flow cytometry using specific antibodies against CD45RA, CD45RB and CD45RO, respectively. As shown in Fig. 3(c), CD45RA and CD45RB, but not CD45RO, were present at high levels on Raji B cells. Taken together, these data indicated that CD45 and its isoforms are Jacalin major receptors on B lymphocytes, and that the glycans expressed on CD45 are involved in the interaction of Jacalin with CD45 and its isoforms. Future studies will analyse the differences of composition and glycosylation of CD45 isoforms and quantify the binding affinities between Jacalin and CD45 isoforms.
Figure 3.
Purification and detection of CD45 and its isoforms as Jacalin receptors on Raji B cells. (a, b) Purification and detection of Jacalin receptors by affinity chromatography, and Western and lectin blotting. Jacalin receptors were purified from human Raji B-cell membrane proteins on a Jacalin-agarose affinity column and fractionated on a 5–20% reducing gradient sodium dodecyl sulphate–polyacrylamide gel electrophoresis gel, and then transferred to nitrocellulose membranes, followed by pan-CD45 Western blot (a) and Jacalin lectin blot (b) detection. The arrows indicate the purified Jacalin receptors on Raji B cells. The molecular weight markers are shown on the left. (c) Expression of CD45 isoforms on Raji B cells revealed on flow cytometry analysis. Raji B cells were stained with fluorescein isothiocyanate-conjugated anti-human CD45RA, CD45RB and CD45RO monoclonal antibodies, respectively, and then analysed by flow cytometry. As a negative control, the autofluorescence of the cells was measured (grey area).
Jacalin induces apoptosis in human peripheral B lymphocytes
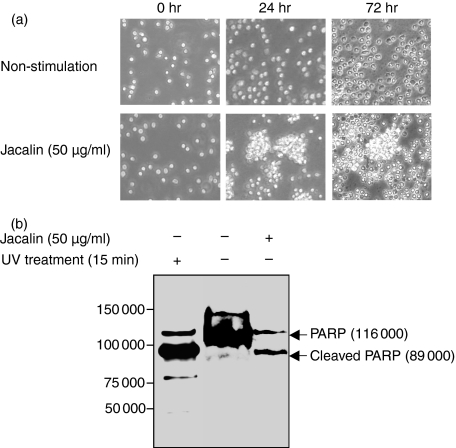
Signalling through the B-cell receptor (BCR) is required for peripheral B-lymphocyte maturation, activation, maintenance and silencing.8 CD45 is the major PTPase on the surface of B lymphocytes, and functions as both a positive and a negative regulator of BCR signalling.8 In mature B cells, the antigen receptor normally consists of two isotypes, membrane IgD and IgM. With our recent finding that Jacalin induces T-lymphocyte activation and Th1/Th2 cytokine secretion through binding to CD45,6 we hypothesized that CD45 might also play a promotive role in the modulation of the B-lymphocyte differentiation or maturation initiated by Jacalin stimulation in a similar way to in T lymphocytes. To determine whether the Jacalin-CD45 interaction affected the alteration in B-cell morphology, we first observed that, after Jacalin treatment, primary CD19+ B cells underwent morphological changes characteristic of apoptosis, such as membrane blebbing and cell shrinkage, whereas the B cells did not show a change in morphology with no Jacalin treatment (Fig. 4a). This suggests that Jacalin may induce apoptosis selectively in B lymphocytes. To verify this possibility, we next examined the Jacalin-induced B-lymphocyte apoptosis characterized by the proteolytic cleavage of PARP, which is one of the substrates of activated caspase-3.10 As shown in Fig. 4(b), Western blot analysis apparently indicated that Jacalin treatment resulted in an 89 000 molecular weight apoptosis-related cleavage fragment in primary B lymphocytes. UV treatment was used as a positive control for apoptotic cells. Collectively, these results clearly demonstrated that Jacalin induces apoptosis in primary B lymphocytes.
Figure 4.
Effects of Jacalin-stimulated human primary B lymphocytes. (a) Differential morphological changes of Jacalin-stimulated human primary B lymphocytes. The separated human CD19+ B cells were stimulated without (upper panels) or with (lower panels) Jacalin at 50 μg/ml for 24 and 72 hr, respectively. Photographs were taken after 24 and 72 hr stimulation using an Olympus microscope. (b) Determination of Jacalin-induced human primary B-lymphocyte apoptosis. The separated human CD19+ B cells were incubated without or with Jacalin at 50 μg/ml for 72 hr, followed by assessment of apoptosis. Poly (ADP-ribose) polymerase (PARP) cleavage was analysed by Western blot for apoptosis detection. The arrows indicate 116 000 PARP (upper bands) and 89 000 apoptosis-related cleavage fragment (lower bands). As a positive apoptosis control, primary B cells were exposed to ultraviolet irradiation at 254 nm for 15 min.
Jacalin induces Raji B-cell apoptosis in a dose-dependent manner
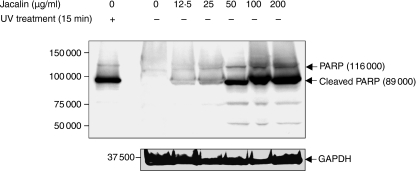
To further examine Jacalin-induced apoptosis in detail, human Raji B cells were treated with Jacalin at the indicated concentrations and then analysed using a PARP cleavage assay. It is interesting that, as shown in Fig. 5, the level of the 89 000 molecular weight apoptosis-related cleavage fragment increased with the increase in the Jacalin concentration, suggesting that Jacalin induced apoptosis in a dose-dependent manner in Raji B cells. UV treatment was used as a positive control for apoptotic cells, and GAPDH expression was examined as a control for protein loading.
Figure 5.
Dose-dependent Raji B-cell apoptosis induced by Jacalin. Raji B cells were treated with Jacalin at various concentrations, as indicated above, for 72 hr, followed by assessment of apoptosis. Poly (ADP-ribose) polymerase (PARP) cleavage was analysed by Western blotting for apoptosis detection. The arrows indicate 116 000 PARP (upper bands) and the 89 000 apoptosis-related cleavage fragment (lower bands). As a positive apoptosis control, Raji B cells were exposed to ultraviolet irradiation at 254 nm for 15 min. GAPDH is shown as a control for protein loading (bottom). The results are representative of three independent experiments.
Jacalin induces human B-lymphocyte apoptosis through CD45 triggering
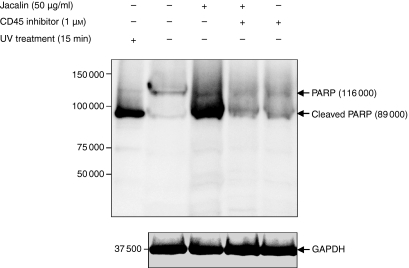
Next, to determine the role of CD45 signalling in B-lymphocyte apoptosis triggered by Jacalin induction, we incubated Raji B cells with a specific PTPase CD45 inhibitor, N-(9,10-dioxo-9,10-dihydro-phenanthren-2-yl)-2,2-dimethyl-propionamide,6,11 at 1 μm during 50 μg/ml lectin stimulation. As shown in Fig. 6, the Jacalin-induced apoptosis was effectively blocked in the presence of the specific PTPase CD45 inhibitor compared with in its absence. These results indicate that Jacalin induces B-lymphocyte apoptosis through CD45 triggering, suggesting that the level of signalling achieved on initiation of CD45 signalling is intimately associated with the induction of B-lymphocyte apoptosis.
Figure 6.
CD45-mediated Jacalin-induced Raji B-cell apoptosis. Raji B cells were treated with Jacalin at 50 μg/ml in the absence or presence of the PTPase CD45 inhibitor for 72 hr, followed by assessment of apoptosis. PARP cleavage was analysed by Western blotting for apoptosis detection. The arrows indicate 116 000 PARP (upper bands) and the 89 000 apoptosis-related cleavage fragment (lower bands). As a positive apoptosis control, Raji B cells were exposed to ultraviolet irradiation at 254 nm for 15 min. GAPDH is shown as a control for protein loading (bottom). The results are representative of three independent experiments.
Calcium–calpain pathway may mediate Jacalin-induced human B-lymphocyte apoptosis
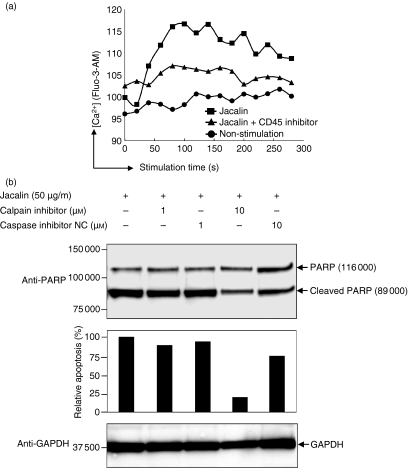
It has been reported that calcium mobilization is impaired in CD45-deficient B cells and CD45 expression is required for calcium mobilization through BCR triggering.12,13 Calcium signalling is originated with intracellular and extracellular calcium flux. To examine whether calcium influx is required for Jacalin-induced B-cell apoptosis, we performed a calcium mobilization assay in Raji B cells. The Raji B cells incubated with 10 μm Fluo-3-AM, an intracellular Ca2+ indicator, were stimulated by Jacalin in the presence or absence of CD45 inhibitor, and then the intracellular calcium signals were recoded on a FACSCalibur. As shown in Fig. 7(a), Jacalin stimulation increased intracellular calcium concentration in Raji B cells and the calcium influx was partially suppressed by CD45 inhibitor, suggesting that Jacalin–CD45 interaction results in calcium influx and this calcium influx may be required for Jacalin-induced B-cell apoptosis.
Figure 7.
Calcium–calpain pathway may mediate Jacalin-induced Raji B-cell apoptosis. (a) Jacalin-induced calcium mobilization. Raji B cells were loaded with 10 μm Fluo-3-AM following treatment without or with Jacalin labelled with Jacalin treatment (50 μg/ml) in the presence or absence of 1 μm CD45 inhibitor for the indicated time above, and then intracellular Ca2+ concentrations were recoded on a FACSCalibur. (b) Calpain activation is involved in Jacalin-induced Raji B-cell apoptosis. Raji B cells were incubated with a calpain inhibitor or a caspase inhibitor negative control at 1 μm and 10 μm, respectively, in the presence of 50 μg/ml Jacalin for 72 hr, followed by assessment of apoptosis. Poly (ADP-ribose) polymerase (PARP) cleavage was analysed by Western blotting for apoptosis detection (upper panel). The arrows indicate 116 000 PARP and the 89 000 apoptosis-related cleavage fragment as indicated above. The relative percentage of the apoptosis was determined by comparing the decreases in the 89 000 apoptosis-related cleavage fragment in the absence and presence of calpain inhibitor or caspase inhibitor negative control (middle panel). The 89 000 apoptosis-related cleavage bands were scanned by laser densitometry. GAPDH is shown as a control for protein loading (lower panel). The results are representative of three independent experiments.
Since calpain, a calcium-dependent protease, and calcium influx are required for downstream proteases of the calcium–calpain apoptosis pathway, we next examined whether calpain activation is involved in Jacalin-induced apoptosis in Raji B cells. We incubated Raji B cells with a calpain inhibitor or a caspase inhibitor negative control at 1 μm and 10 μm, respectively, during stimulation with 50 μg/ml Jacalin. The results in Fig. 7(b) show that Jacalin-induced B-cell apoptosis was significantly suppressed by the calpain inhibitor at 10 μm compared with by the caspase inhibitor negative control. Taken together, these data indicate that Jacalin induces B-lymphocyte apoptosis following Jacalin–CD45 interaction, resulting in calcium mobilization and calpain activation, which suggests that the calcium–calpain pathway may mediate Jacalin-induced B-lymphocyte apoptosis. It would be an important and critical next step to demonstrate the molecular mechanism underlying Jacalin-induced B-cell death through CD45 triggering.
Jacalin increases the C-terminal tyrosine phosphorylation of Lyn kinase through inhibition of CD45 tyrosine phosphatase activity in human B lymphocytes
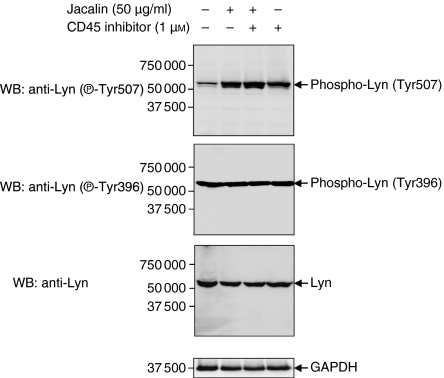
Stimulation of B lymphocytes by a stimulator, antigen or anti-BCR antibodies is associated with a rapid increase in intracellular protein tyrosine phosphorylation.8 As the BCR complex does not exhibit any intrinsic kinase activity, the induction of protein tyrosine phosphorylation must involve the participation of cytoplasmic tyrosine kinases. Accordingly, there is accumulating evidence that Lyn and Syk are responsible for the initiation of BCR-induced signalling.14 Lyn, one of the major Src-PTKs, plays both stimulatory and inhibitory roles in B lymphocytes.8 The regulation of Lyn kinase by phosphorylation is complex in that there are two identified tyrosine phosphorylation sites: C-terminal phosphorylation (Tyr507) is inhibitory and autophosphorylation (Tyr396) is stimulatory.8 Recent studies have shown that Lyn is one of the major substrates of CD45 tyrosine phosphatase in B lymphocytes,14 and that CD45 inhibits Lyn kinase activity through dephosphorylation of the C-terminal tyrosine (Tyr507) of Lyn, and negatively regulates BCR-initiated effector phenomena such as growth arrest and apoptosis.8 To further investigate CD45-regulated B-cell apoptosis after Jacalin stimulation and to correlate BCR signalling with Jacalin induction, we analysed the CD45-mediated tyrosine phosphorylation of Lyn kinase upon Jacalin treatment in Raji B cells in the absence and presence of the specific PTPase CD45 inhibitor. As shown in Fig. 8, Jacalin significantly increased the C-terminal negative regulatory residue (Tyr507) phosphorylation of Lyn after 3 hr stimulation compared with the non-Jacalin stimulation control, and the same levels of the positive regulatory residue (Tyr396) phosphorylation and equal amounts of total 56 000 molecular weight Lyn kinase were observed in the cell lysates obtained for the experiment groups. The effect of Jacalin-increased Lyn-Tyr507 phosphorylation was also observed on incubation of Raji B cells with the specific CD45 PTPase inhibitor both with and without Jacalin stimulation (Fig. 8), suggesting that Jacalin-induced CD45 ligation results in increasing C-terminal negative regulatory tyrosine (Tyr507) phosphorylation of Lyn kinase through inhibition of CD45 tyrosine phosphatase activity in B lymphocytes, and that the positive regulatory residue (Tyr396) is autophosphorylated before Jacalin stimulation. Therefore, the down-modulation of Lyn kinase, as an initiating step for Jacalin–CD45 interaction-induced signal transduction, may contribute to the regulation of B-lymphocyte viability.
Figure 8.
Enhancement of C-terminal negative regulatory residue (Tyr507) phosphorylation of Lyn kinase by Jacalin through inhibition of CD45 protein tyrosine phosphatase (PTPase) activity in Raji B cells. Raji B cells were treated with Jacalin at 50 μg/ml in the absence or presence of the PTPase CD45 inhibitor (1 μm) for 3 hr, followed by assessment of tyrosine phosphorylation of Lyn kinase. The levels of Lyn phosphorylated at tyrosine 507 and 396 and total 56 000 Lyn were determined by Western blotting with specific antibodies against phospho-Lyn (Tyr507), phospho-Lyn (Tyr396) and total Lyn, respectively. The arrows indicate phospho-Lyn (Tyr507) (upper panel), phospho-Lyn (Tyr396) (middle panel) and total 56 kDa Lyn (lower panel). GAPDH expression was examined as a loading control (bottom panel). The results are representative of three independent experiments.
Discussion
Protein tyrosine phosphorylation is a key mechanism for nearly every aspect of cell regulation, ranging from cell survival and proliferation to apoptotic cell death in multicellular eukaryotes. Tyrosine phosphorylation itself is regulated through the concerted actions of PTKs and PTPs. CD45 is the prototypic member of the RPTPase family and plays essential roles in immune functions. Although the requirement of invariant cytoplasmic domains with intrinsic PTPase activity has been documented, no data are available concerning the function of the CD45 extracellular domain in lymphocyte signal transduction. Recently, we showed that CD45 and its isoforms are major Jacalin receptors expressed on CD4+ and CD8+ T lymphocytes, and that Jacalin induced significant interleukin-2 (IL-2) production by both a CD45-positive Jurkat T-cell line (JE6.1) and primary T lymphocytes, but not by a CD45-negative Jurkat T-cell line (J45.01).6 Moreover, we also observed that Jacalin caused a striking increase in IL-2 secretion in response to TCR ligation and CD28 costimulation, and contributed to Th1/Th2 cytokine secretion, both actions that were completely blocked by a specific CD45 PTPase inhibitor, suggesting that Jacalin induces T-cell activation through CD45 triggering.6 Since CD45 is a heavily glycosylated haemopoietic cell-specific two-domain tyrosine phosphatase essential for efficient T and B cells, we therefore hypothesized that Jacalin may also interact with CD45 expressed on B lymphocytes and modulate the viability of B lymphocytes through the Jacalin–CD45 interaction.
In this study, we have identified, by affinity chromatography, and Western and lectin blot experiments, that CD45 and its isoforms are major Jacalin targets on the membranes of B lymphocytes (Fig. 3). Importantly, the proportions and expression of CD45 and its isoforms as Jacalin receptors on B cells are significantly different from those of the lectin receptors on T cells as determined in our previous report.6 CD45 exists as several isoforms because of alternative splicing at three consecutive exons (4, 5 and 6, designated as A, B and C) in the extracellular domain. The difficulty of working with cells expressing many isoforms has led to efforts to detect convincing differences in the functions of individual CD45 isoforms, and the altered combinations of isoforms might influence the CD45 function by affecting segregation into lipid rafts, dimerization or interactions with other molecules.15 Moreover, the putative O-glycosylation sites are clustered in the domains encoded by exons 4–6, and these domains contain more sialylated O-linked sugar chains with the core 2 structure.9 Since Jacalin predominantly binds to O-linked glycoproteins containing carbohydrate moieties such as GalNAc, Galβ1-3GalNAc, Neu5Acα2-3GalNAc, Galβ1-3(Galβ1-4GlcNAcβ1-6)GalNAc, and GlcNAcβ1-3GalNAc,4,14,16 we hypothesize that Jacalin may bind to these Jacalin epitopes, which are present on the CD45 extracellular domains encoded by exons 4–6.9 It was previously reported that the ligation of CD45 on human T or B cells by certain mAbs induces cell death.17,18 However, until now the putative physiological inducer of CD45 ligation remained unknown. In the present work, our observations evidently indicate that Jacalin induces B-lymphocyte apoptosis in a carbohydrate-dependent manner through the CD45–Jacalin interaction.
Apoptosis is an important regulatory process in the immune system, and is essential for the balanced cellular homeostasis needed to maintain immune responsiveness, and to avoid the aberrations of immunodeficiency, autoimmunity and cancer. In addition, the induction of apoptosis is usually an active process, accompanied by distinct morphological and biochemical events, although these vary in different cell types and also depend on the apoptosis-inducing stimulus.19 Several groups have shown that galectins, a family of highly conserved β-galactoside-binding lectins that are expressed in a wide variety of mammalian tissues, induce the death of various cell types including T and B lymphocytes, eosinophils, keratinocytes, adipocytes and tumour cells.20–26 However, the death pathways induced by galectins in these cells are still poorly understood. Cell death in many cell types is a complex process, involving multiple death signals and several parallel death pathways.27–29 The complexity of cell death pathways reflects a requirement for tight control of the death process, while redundancy is ensured by the overlapping of cell death inducers and effectors. In this study, we found that Jacalin induces human B-lymphocyte apoptosis following Jacalin–CD45 interaction, resulting in calcium mobilization and calpain activation, suggesting that the calcium–calpain pathway may mediate Jacalin-induced human B-lymphocyte apoptosis. Although it is clear that CD45 phosphatase plays a key role in the life and death of lymphocytes, the great conundrum regarding CD45 is what the extracellular domain does. Equally intriguing is what role the alternative splicing plays in regulation of phosphatase activity. Recent human and animal data have revealed extensive polymorphism in the extracellular domain and some CD45 isoforms affect alternative splicing with different glycosylation, which changes during lymphocyte differentiation, activation and cell death.9,30,31 Cell-specific glycosylation of CD45 could provide a mechanism for influencing various immunological pathways, including TCR and BCR signalling. Here our results suggest that Jacalin binds to CD45 isoforms with different affinities through lectin–glycan interactions and induces B-lymphocyte apoptosis, which may provide an opportunity to investigate the effects of changes in the CD45 extracellular domain, and to determine the importance of CD45 polymorphisms in immune functions and diseases. Given the potential for glycosylation changes to affect the binding of lectin ligands to CD45, dimerization and other protein–protein interactions, we need to understand the glycobiology of CD45 much better.
The cytoplasmic region of CD45, like those of many other RPTPases, contains two homologous PTPase domains, active domain 1 (D1) and catalytically impaired domain 2 (D2). To date, all the functions attributed to CD45 are inherently coupled to its phosphatase activity. For instance, the regulation of lymphocyte antigen receptor signalling is mediated through the dephosphorylation, and hence activation, of Src-PTKs by CD45.8,32,33 Stimulation of the TCR results in the activation of a series of PTKs and culminates in a variety of distal events that include transcriptional activation of the IL-2 gene.34 Costimulation through T-cell surface CD28 increases the stability of phosphorylated proteins,35 thereby amplifying TCR signalling and facilitating T-cell activation. It is clearly established that CD45 is required for TCR signalling and this appears to be caused by the up-regulation of kinase activity in p56Lck (also probably p59Fyn,36 and CD45 may be required to act dynamically during the very early stages of TCR signalling.37 To date, the best described bioaction for CD45 is as an activator of the Src-PTKs Lck and Lyn in TCR and BCR complexes, respectively. Our recent findings demonstrated that Jacalin causes a marked increase in IL-2 secretion in response to TCR ligation and CD28 costimulation, and the ability of the lectin to act as a positive inducer of the TCR signalling threshold to modulate CD45 PTPase, which results in activation of the mitogen-activated protein kinase cascade leading to up-regulation of the T-cell response.6 On the other hand, signal transduction via the BCR complex is regulated through changes in the tyrosine phosphorylation of several proteins. The equilibrium between tyrosine phosphorylation and dephosphorylation is regulated by the combined actions of PTKs and PTPs. In particular, CD45 has been shown to play an essential role in signal transduction via the BCR and to selectively associate with Lyn kinase as its major PTPase substrate in B lymphocytes.14 Whether Lyn promotes or inhibits immune cell activation depends on the stimulus and the developmental state, meaning that the consequences of Lyn activity are context-dependent.8 Moreover, the cascades of signalling induced by CD45 ligation have not been fully elucidated. The experiments reported here indicate that Jacalin stimulation results in increasing C-terminal negative regulatory residue (Tyr507) phosphorylation of Lyn kinase through inhibition of CD45 tyrosine phosphatase activity in B lymphocytes (Fig. 8), suggesting that the intracellular signalling events associated with B-cell viability may be triggered by the CD45–Jacalin interaction. Our results are in agreement with previous findings of Katagiri et al. and Yanagi et al.;38,39 who reported that Lyn can be activated through dephosphorylation of the C-terminal regulatory tyrosine by CD45 in B cells. Understanding the molecular mechanisms involved in the immunoregulatory functions of Jacalin might help to delineate novel therapeutic targets based on lectin–glycan interactions for autoimmune diseases and chronic inflammatory disorders. Accumulating evidence indicates that Lyn is the predominantly expressed Src-SFK in B cells, and that it plays both positive and negative regulatory roles in BCR-induced signal transduction.8,14 The positive functions of Lyn after BCR ligation are probably context-dependent, such that Lyn can be substituted by other SFKs depending on the nature of the stimulation.14 The irreplaceable function of Lyn in B cells is to set the threshold of negative feedback control of signalling after BCR ligation, and this is achieved through multiple mechanisms that may act synergistically and independently.8,14 In this context, the data presented here constitute evidence of the involvement of a signal transduction pathway, initiated through inhibition of the tyrosine kinase activity of Lyn, in Jacalin-stimulated B lymphocytes.
Intriguingly, understanding of the pathway of Jacalin-induced B-lymphocyte apoptosis is critical for the development of new approaches for regulating cell viability. Our findings may well contribute not only to our knowledge of B-cell biology but also to the development of pharmaceutical reagents for better management of human diseases that may involve dysregulated Lyn-related pathways as contributing or causative factors. Further elucidation of the Jacalin-induced B-lymphocyte apoptosis mechanism will facilitate identification of target cells susceptible to this type of death, and the design of agents to therapeutically manipulate the death pathway.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research B (18370057) and C (18590471), Core-to-Core Program-Strategic Research Networks (17005) from the Japan Society for the Promotion of Science, and Ritsumeikan Research Proposal Grant from Ritsumeikan University (Shiga, Japan). The authors would like to thank the Shiga Prefecture Red Cross Blood Center (Shiga, Japan) for kindly supplying the human peripheral blood mononuclear cells from healthy donors and Ms Tomoko Tominaga for secretarial assistance.
Glossary
Abbreviations:
- Galβ1-3GalNAc
galactose β1-3 N-acetylgalactosamine
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- Me-α-Gal
methyl-α-galactose
- PARP
poly (ADP-ribose) polymerase
- PTK
protein tyrosine kinase
- PTPase
protein tyrosine phosphatase
- RPTP
receptor-type protein tyrosine phosphatase
References
- 1.Lowe JB. Glycosylation, immunity, and autoimmunity. Cell. 2001;104:809–12. doi: 10.1016/s0092-8674(01)00277-x. [DOI] [PubMed] [Google Scholar]
- 2.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2002;291:2370–6. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 3.Dustin LM, Chan AC. Signaling takes shape in the immune system. Cell. 2000;103:283–94. doi: 10.1016/s0092-8674(00)00120-3. [DOI] [PubMed] [Google Scholar]
- 4.Kabir S. Jacalin: a jackfruit (Artocarpus heterophyllus) seed-derived lectin of versatile applications in immunobiological research. J Immunol Methods. 1998;212:193–211. doi: 10.1016/s0022-1759(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 5.Gattass CR, Ghobrial I, Bunn-Moreno MM. Specific inhibition of OKT8 binding to peripheral blood mononuclear cells by jacalin. Immunol Lett. 1988;17:133–8. doi: 10.1016/0165-2478(88)90081-8. [DOI] [PubMed] [Google Scholar]
- 6.Baba M, Ma BY, Nonaka M, et al. Glycosylation-dependent interaction of CD45 with Jacalin induces T lymphocyte activation and Th1/Th2 cytokine secretion. J Leukoc Biol. 2007;81:1002–11. doi: 10.1189/jlb.1106660. [DOI] [PubMed] [Google Scholar]
- 7.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling in immune cells. Annu Rev Immunol. 2003;21:107–37. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity. 2005;22:9–18. doi: 10.1016/j.immuni.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Furukawa K, Funakoshi Y, Autero M, Horejsi V, Kobata A, Gahmberg CG. Structural study of the O-linked sugar chains of human leukocyte tyrosine phosphatase CD45. Eur J Biochem. 1998;251:288–94. doi: 10.1046/j.1432-1327.1998.2510288.x. [DOI] [PubMed] [Google Scholar]
- 10.Ma BY, Mikolajczak SA, Danesh A, Hosiawa KA, Takaori-Kondo A, Uchiyama T, Kelvin DJ, Ochi A. The expression and the regulatory role of OX40 and 4-1BB heterodimer in activated human T cells. Blood. 2005;106:2002–10. doi: 10.1182/blood-2004-04-1622. [DOI] [PubMed] [Google Scholar]
- 11.Van Vliet SJ, Gringhuis SI, Geijtenbeek TBH, van Kooyk Y. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat Immunol. 2006;7:1200–8. doi: 10.1038/ni1390. [DOI] [PubMed] [Google Scholar]
- 12.Qin S, Chock PB. Tyrosine phosphatase CD45 regulates hydrogen peroxide-induced calcium mobilization in B cells. Antioxid Redox Signal. 2001;4:481–90. doi: 10.1089/15230860260196281. [DOI] [PubMed] [Google Scholar]
- 13.Pao LI, Cambier JC. Syk, but not Lyn, recruitment to B cell antigen receptor and activation following stimulation of CD45– B cells. J Immunol. 1997;158:2663–9. [PubMed] [Google Scholar]
- 14.Shrivastava P, Katagiri T, Ogimoto M, Mizuno K, Yakura H. Dynamic regulation of Src-family kinases by CD45 in B cells. Blood. 2004;103:1425–32. doi: 10.1182/blood-2003-03-0716. [DOI] [PubMed] [Google Scholar]
- 15.Penninger JM, Irie-Sasaki J, Sasaki T, Oliveira-dos-Santos AJ. CD45: new jobs for an old acquaintance. Nat Immunol. 2001;2:389–96. doi: 10.1038/87687. [DOI] [PubMed] [Google Scholar]
- 16.Tachibana K, Nakamura S, Wang H, et al. Elucidation of binding specificity of Jacalin toward O-glycosylated peptides: quantitative analysis by frontal affinity chromatography. Glycobiology. 2006;16:46–53. doi: 10.1093/glycob/cwj038. [DOI] [PubMed] [Google Scholar]
- 17.Klauss SJ, Sidorenko SP. CD45 ligation induces programmed cell death in T and B lymphocytes. J Immunol. 1996;156:2743–53. [PubMed] [Google Scholar]
- 18.Lesage S, Steff AM, Philippoussis F, Page M, Trop S, Mateo V, Hugo P. CD4+CD8+ thymocytes are preferentially induced to die following CD45 cross-linking, through a novel apoptotic pathway. J Immunol. 1997;159:4762–71. [PubMed] [Google Scholar]
- 19.Opferman JT, Korsmeyer SJ. Apoptosis in the development and maintenance of the immune system. Nat Immunol. 2003;4:410–5. doi: 10.1038/ni0503-410. [DOI] [PubMed] [Google Scholar]
- 20.Rabinovich GA, Baum LG, Tinari N, Paganelli R, Natoli C, Liu FT, Iacobelli S. Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response. Trends Immunol. 2002;23:313–20. doi: 10.1016/s1471-4906(02)02232-9. [DOI] [PubMed] [Google Scholar]
- 21.Perillo NL, Pace K, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–9. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 22.Perillo NL, Uittenbogaart CH, Nguyen JT, Baum LG. Galectin-1, an endogenous lectin produced by thymic epithelial cells, induces apoptosis of human thymocytes. J Exp Med. 1997;185:1851–8. doi: 10.1084/jem.185.10.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA. 1996;93:6737–42. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fouillit M, Joubert-Caron R, Poirier F, et al. Regulation of CD45-induced signaling by galectin-1 in Burkitt lymphoma B cells. Glycobiology. 2000;10:413–9. doi: 10.1093/glycob/10.4.413. [DOI] [PubMed] [Google Scholar]
- 25.Pace KE, Lee C, Stewart PL, Baum LG. Restricted receptor segregation into membrane microdomains occurs on human T cells during apoptosis induced by galectin-1. J Immunol. 1999;163:3801–11. [PubMed] [Google Scholar]
- 26.Kashio Y, Nakamura K, Abedin MJ, Seki M, Nishi N, Yoshida N, Nakamura T, Hirashima M. Galectin-9 induces apoptosis through the calcium–calpain–caspase-1 pathway. J Immunol. 2003;170:3631–6. doi: 10.4049/jimmunol.170.7.3631. [DOI] [PubMed] [Google Scholar]
- 27.Hunot S, Flavell RA. Death of a monopoly. Science. 2001;292:865–6. doi: 10.1126/science.1060885. [DOI] [PubMed] [Google Scholar]
- 28.Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589–98. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 29.Jaattela M, Tschopp J. Caspase-independent cell death in T lymphocytes. Nat Immunol. 2000;4:416–23. doi: 10.1038/ni0503-416. [DOI] [PubMed] [Google Scholar]
- 30.Tchilian EZ, Beverley PCL. Altered CD45 expression and disease. Trends Immunol. 2006;27:146–53. doi: 10.1016/j.it.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Amano M, Galvan M, He J, Baum LG. The ST6Gal I sialyltransferase selectively modifies CD45 and negatively regulates galectin-1 induced CD45 clustering, phosphatase modulation and T cell death. J Biol Chem. 2003;278:7469–75. doi: 10.1074/jbc.M209595200. [DOI] [PubMed] [Google Scholar]
- 32.Thomas ML, Brown EJ. Positive and negative regulation of Src-family membrane kinases by CD45. Immunol Today. 1999;20:406–11. doi: 10.1016/s0167-5699(99)01506-6. [DOI] [PubMed] [Google Scholar]
- 33.Ashwell JD, D’Oro U. CD45 and Src-family kinases: and now for something completely different. Immunol Today. 1999;20:412–6. doi: 10.1016/s0167-5699(99)01505-4. [DOI] [PubMed] [Google Scholar]
- 34.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–74. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 35.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–2. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 36.Tamma SML, Chung KW, Patel T, Balan SP, Pahwa S. P38 MAPK plays a role in IL-4 synthesis in jacalin plus CD28-stimulated CD4+ T cells II. J Leukoc Biol. 2006;79:1339–47. doi: 10.1189/jlb.0905513. [DOI] [PubMed] [Google Scholar]
- 37.Stone JD, Conroy LA, Byth KF, Hederer RA, Howlett S, Takemoto Y, Holmes N, Alexander DR. Aberrant TCR-mediated signaling in CD45-null thymocyte involves dysfunctional regulation of Lck, Fyn, TCR-zeta and ZAP-70. J Immunol. 1997;158:5773–82. [PubMed] [Google Scholar]
- 38.Katagiri T, Ogimoto M, Hasegawa K, et al. CD45 negatively regulates lyn activity by dephosphorylating both positive and negative regulatory tyrosine residues in immature B cells. J Immunol. 1999;163:1321–6. [PubMed] [Google Scholar]
- 39.Yanagi S, Sugawara H, Kurosaki M, Sabe H, Yamamura H, Kurosaki T. CD45 modulates phosphorylation of both autophosphorylation and negative regulatory tyrosines in B cells. J Biol Chem. 1996;271:30487–92. doi: 10.1074/jbc.271.48.30487. [DOI] [PubMed] [Google Scholar]