Abstract
Glucocorticoid-induced tumour necrosis factor receptor-related receptor (GITR) costimulates functions of both effector and regulatory T cells. The administration of agonistic anti-GITR monoclonal antibodies efficiently enhances various T-cell-mediated immune responses; however, it is unknown to what extent the ligand of GITR (GITRL) contributes to T-cell responses. We investigated the involvement of endogenously expressed GITRL on dendritic cells and ectopically expressed GITRL on tumours in T-cell-mediated immunity. Expression of GITRL on dendritic cells in secondary lymphoid organs was limited, and treatment with anti-GITRL monoclonal antibodies did not substantially affect T-cell-mediated immunity to alloantigens, a specific protein antigen (ovalbumin), or tumour antigens. The introduction of GITRL promoted anti-tumour immunity in four tumour models. Tumour-associated GITRL greatly augmented the effector function of CD8+ T cells and enhanced the contribution of CD8+ T cells. These events reduced the crucial contribution of CD25+ CD4+ regulatory T cells, which were found to inhibit immunity against tumours lacking GITRL. Peritumoral injection of GITRL tumour vaccine efficiently inhibited the growth of established tumours. Our results suggest that the ectopic expression of GITRL in tumour cells enhances anti-tumour immunity at peripheral tumour sites. Consequently, the combined use of a GITRL tumour vaccine with methods aimed at enhancing the activation of host antigen-presenting cells in secondary lymphoid tissues may be a promising strategy for tumour immunotherapy.
Keywords: cancer vaccines, CD8/cytotoxic T cell, costimulation, dendritic cells
Introduction
Glucocorticoid-induced tumour necrosis factor receptor (TNFR) -related receptor (GITR, TNFR18) is a type I transmembrane protein and a member of the TNFR superfamily.1 GITR is expressed at high levels, predominantly, on CD25+ CD4+ regulatory T (Treg) cells, but it is also constitutively expressed at low levels on conventional CD25− CD4+ and CD8+ T cells and is rapidly upregulated after activation.2 The cytoplasmic domain of GITR shares strong homology with a TNFR superfamily subgroup that lacks the death domain, which includes CD27, CD134 (OX40) and CD137 (4-1BB).1 The ligand for GITR (GITRL) is a type II transmembrane protein belonging to the TNF superfamily and expression of its messenger RNA has been detected in the heart, thymus, lung and spleen.3,4 GITRL messenger RNA and cell surface protein expression has also been observed in B cells, macrophages and dendritic cells (DC).4,5
In vitro studies using an agonistic anti-GITR monoclonal antibody (mAb; DTA-1)2,6,7 or GITRL transfectants and soluble GITRL5,8,9 have shown that the GITR–GITRL pathway induces positive costimulatory signals leading to the activation of CD4+ and CD8+ effector T cells as well as Treg cells, despite their opposing effector functions. The administration of DTA-1 or soluble GITRL immunoglobulin has been shown to enhance anti-tumour immunity by augmenting CD4+ and/or CD8+ T-cell activation.10–14 Collectively, these studies have demonstrated the T-cell costimulatory functions of GITR in vitro and in vivo; however, they have not addressed the direct contribution of endogenous GITRL expressed on antigen-presenting cells. Additionally, the recent reports in studies using inflammatory disease models that include shock and injury suggest that GITR participates in various aspects of the inflammatory responses mediated by macrophages, neutrophils and tissue parenchymal cells, and greatly contributes to innate immune responses.15,16
The purpose of this study was to investigate the contribution of endogenously expressed GITRL on DC as potent antigen-presenting cells and ectopically transduced GITRL on tumours in T-cell-mediated immunity. We investigated GITRL expression on DC in the lymph nodes before and after antigen priming and the functional involvement of GITRL using anti-GITRL mAb. We also compared the contribution of CD4+ or CD8+ effector T cells with that of Treg cells to immunity against GITRL− and GITRL+ tumours. Finally, we examined the therapeutic efficacy of a GITRL-transfected tumour cell vaccine.
Materials and methods
Mice
Female 6-week-old BALB/c, C57BL/6 (B6), B6xBALB/cF1 (CBF1), DBA/2, C3H/HeN (C3H), BALB/c nu/nu, and BALB/c-Rag1tm1Mom (BALB/c Rag1−/−) mice were purchased from Japan SLC (Hamamatsu, Japan) and the Jackson Laboratory (Bar Harbor, ME). Ovalbumin (OVA) -specific T-cell receptor transgenic OT-I mice17 were kindly provided by W. R. Heath (The Walter and Eliza Hall Institute of Medical Research, Melbourne, Vic., Australia). All mice were maintained under specific pathogen-free conditions, respectively. All procedures were reviewed and approved by the Animal Care and Use Committee of the Tokyo Medical and Dental University and the Evanston Northwestern Healthcare Institutional Animal Care and Use Committee.
Generation of GITRL transfectants
The full-length mouse GITRL in a pMKITneo expression vector was generated as previously described.9 The SCCVII (squamous cell carcinoma),18 P815 (mastocytoma),19 Colon26 (colorectal adenocarcinoma)20 and B16 (melanoma)19 cell lines were transfected with the GITRL/pMKITneo plasmid using electroporation or Lipofectamine 2000 (Invitrogen, Carlsbad, CA). After drug selection, transfectants expressing high levels of GITRL were sorted by flow cytometry, as previously described.21
Monoclonal antibodies and flow cytometry
The mAb against CD3 [145-2C11, hamster immunoglobulin G (IgG)], CD4 (GK1.5, rat IgG2b), CD8 (53-6.72, rat IgG2a), CD45R (RA3-6B2, rat IgG2a), CD11b (M1/70, rat IgG2b), CD25 (PC61, rat IgG1; 7D4, rat IgM), CD49b (HMα2, hamster IgG), CD11c (N418, hamster IgG), I-A/I-E (M5/114, rat IgG2b), I-Ad (AMS-32.1, IgG2b), I-Ak (11-5.2, mouse IgG2b), H-2Db (KH95, mouse IgG2b), H-2Dd (34-2-12, mouse IgG2a), H-2Kd (SF1.1.1, mouse IgG2a), H-2Kk (36-7-5, mouse IgG2a) CD54 (YN1/1.7.4, rat IgG2b), CD80 (16-10A1, hamster IgG), interferon-γ (IFN-γ; XMG1.2, rat IgG1), PDCA-1 (eBio927/BST2, rat IgG2b)22 and Foxp3 (FJK-16s, rat IgG2a) were used. All fluorescein isothiocyanate-conjugated, phycoerythrin-conjugated, peridinin–chlorophyll-protein complex–carbocyanin5.5-conjugated, allophycocyanin (APC)-conjugated and biotinylated mAb, and isotype control immunoglobulins were obtained from eBioscience (San Diego, CA) or BD-Pharmingen (San Diego, CA). A hybridoma producing mAb against CD25 (PC61.5) was provided by H. R. MacDonald (Ludwig Institute for Cancer Research, Lausanne, Switzerland); the mAb was purified from ascites using a protein G column. An anti-GITRL mAb (MIH44, rat IgG2b,κ)9 was purified from ascites using the caprylic acid and ammonium sulphate precipitation method and the biotinylation was performed according to the protocol of our laboratory. For biotinylated mAb, APC–streptavidin was used. Culture supernatant from the 2.4G2 hybridoma (anti-CD16/CD32 mAb) was used to block non-specific binding. Multicolour staining for intracellular cytokine and cell surface antigens was performed, as previously described.23 Foxp3 staining was performed according to the manufacturer’s protocol (eBioscience). The stained cells were then analysed using the BD FACSCalibur system and cellquest software (BD Biosciences, San Jose, CA).
Tumour inoculation and evaluation of tumour growth
The SCCVII (C3H-originated, 3 × 105), P815 (DBA/2-originated, 1 × 105), Colon26 (BALB/c-originated, 5 × 105) and B16 (B6-originated, 1 × 105) parental cells and each transfectant were injected subcutaneously (s.c.) into the shaved right flank of syngeneic mice and tumour volumes were evaluated.24 To deplete CD4+ or CD8+ T cells, 0·5 mg of either anti-CD4 (GK1.5) or anti-CD8 (53.6.72) mAb24 was administered intraperitoneally (i.p.) on days −4, −1, 3 and 7. This protocol of anti-CD4 and anti-CD8 mAb treatments resulted in > 98% and > 95% depletion of CD4+ and CD8+ T cells, respectively, as assessed by flow cytometric analysis on peripheral blood lymphocytes. To deplete Treg cells, 0·5 mg anti-CD25 mAb (PC61.5) was administered i.p. on days −4 and −1. This anti-CD25 mAb treatment reduced the percentage of Foxp3+ cells within peripheral blood CD4+ T cells from 6–7% to < 2%. In experiments examining the effects of anti-GITRL mAb, 200 μg anti-GITRL mAb or control rat IgG (MP Biomedicals, Solon, OH) was injected i.p. three times a week after tumour inoculation.
In the experiments investigating therapeutic effects, parental SCCVII (3 × 105) cells were injected s.c. into the right flank on day 0, and then mitomycin C (MC)-treated parental SCCVII or GITRL–SCCVII (3 × 105) cells as tumour vaccine were injected s.c. into the peritumoral sites at days 3, 6, 9 and 15.
Carboxyfluorescein diacetate succinimidyl ester (CFSE) labelling and transfer of allogeneic splenocytes
Whole splenocytes from BALB/c mice were labelled with CFSE (Molecular Probes, Eugene, OR) as previously described,2 and CFSE-labelled cells (1 × 108 cells) were transferred into CBF1 recipient mice. On day 2, splenocytes were stained with anti-H-2b and anti-CD4 mAb and cell division was analysed by flow cytometry.
Cardiac allograft
Heterotopic cardiac allograft transplantation was performed as previously described25,26 and graft survival was evaluated by daily palpation. The mice received i.p. injections of control rat IgG or anti-GITRL mAb (200 μg/mouse) every other day after transplantation. The day of rejection was defined as the day of cessation of palpable graft beating, and visual examination after laparotomy.
Skin graft and T-cell reconstitution
The CD25− CD4+ T cells were isolated from BALB/c splenocytes by negative selection, using the mouse CD25+ CD4+ T-cell isolation kit according to the manufacturer’s protocol (Miltenyi Biotec, Tokyo, Japan). The purity of CD25− CD4+ cells (> 97%), was confirmed by flow cytometry. BALB/c Rag1−/− mice received transplants of full-thickness B6 skin grafts. The grafts were covered with sterile dressings for 7 days. Four weeks after skin transplantation, recipient mice were reconstituted with an intravenous injection of purified CD25− CD4+ T cells (3 × 105 cells). These mice received i.p. injections of control rat IgG or anti-GITRL mAb (100 μg/mouse) for every third day after T-cell injection. Skin graft rejection was defined as the loss of > 80% of the surface of the implanted tissue.
Preparation of splenocytes and lymph node cells for analyses of DC
Single cell suspensions of splenocytes and lymph node (LN) cells from naive and tumour-bearing mice were isolated using type I collagenase at 37° for 30 min as previously described27 and used for flow cytometric analyses.
OVA-specific CD8+ T-cell responses in OT-I mice
OT-I mice17 received intravenous injections of OVA (100 μg/mouse; Sigma, St Louis, MO) and were treated with either control rat IgG or anti-GITRL mAb (200 μg/mouse) at days 0 and 2. The LN cells were analysed after 3 days. OT-I CD8+ T cells were isolated by negative selection using a magnetic antibody cell sorting system (Miltenyi Biotec) with biotinylated anti-CD4, anti-CD45R, anti-CD11b and anti-CD49b mAb and were used as effector cells. Cytotoxicity against E.G7 was measured by 6-hr JAM test as previously described.23 E.G7, an EL-4 cell line expressing OVA,28 was provided by M. J. Bevan (University of Washington, Seattle, WA). [3H]Thymidine-labelled E.G7 cells were used as target cells (5 × 103/well) and the incorporated radioactivity was measured.
Immunization with SCCVII tumours
C3H mice were given 2 × 106 MC-treated parental or GITRL-transfected SCCVII tumour cells i.p. every week for four weeks with a final boost 3 days before the assay. Single-cell suspensions of peritoneal exudate cells (PEC) and LN cells (submandibular, cervical, axillary and inguinal) were prepared. CD8+ T cells were isolated as described above. Cytotoxicity against parental and GITRL-transfected SCCVII tumours was assessed by a flow cytometric PKH-26 assay as described previously.29 Effector CD8+ T cells and PHK-26-labelled targets were cocultured for 6 hr at the indicated effector : target ratios. The cells were then stained with propidium iodide and analysed by flow cytometry. The percentage of cytotoxicity in the PKH-26+ gated cell population was calculated by subtracting the non-specific propidium iodide-positive target cells, measured with appropriate controls without effector cells.
Results
GITRL transfection reduces tumorigenicity
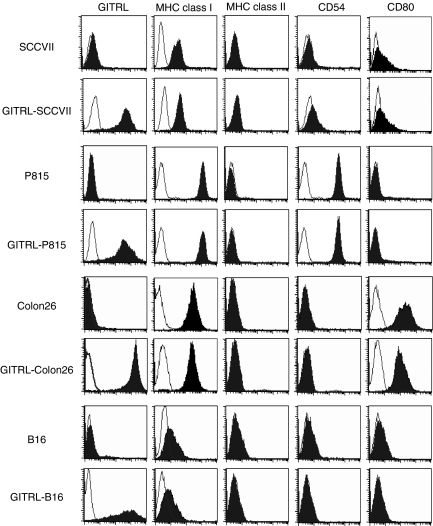
We recently reported that the transfection of GITRL into P815 cells enhanced both the effector and regulatory functions of conventional CD4+ T cells and Treg cells in an in vitro anti-CD3-induced costimulation assay.9 To investigate the effects of GITRL transduction on tumour immunity, we obtained four groups of GITRL-transfected tumour cells that stably expressed GITRL at high levels (Fig. 1). The level of major histocompatibility complex class I and class II, CD54 and CD80 expression in the GITRL transfectants was comparable to that in the parental tumours.
Figure 1.
Expression of cell surface antigens on parental and glucocorticoid-induced tumour necrosis factor receptor-related receptor ligand (GITRL)-transfected tumours. Parental and GITRL-transfected tumour cell lines were stained with biotinylated anti-GITRL, followed by phycoerythrin-streptavidin, fluorescein isothiocyanate-conjugated or phycoerythrin-conjugated anti-major histocompatibility complex (MHC) class I, anti-MHC class II, anti-CD54, or anti-CD86 monoclonal antibody, or with the appropriate fluorochrome-conjugated control immunoglobulin. Data are displayed as histograms (4-decade logarithm scales) with the control histograms nearest the ordinate (open histograms).
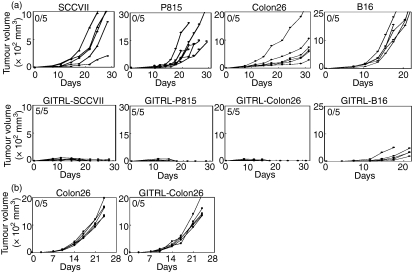
To examine tumorigenicity, GITRL-SCCVII, GITRL-P815, GITRL-Colon26 and GITRL-B16 cells and their respective parental tumours were injected s.c. into syngeneic C3H, DBA/2, BALB/c and B6 mice, respectively. All of the parental tumours grew progressively and some of the mice died before the final observation period (day 20 or 30; Fig. 2a). In contrast, the GITRL-transfected SCCVII, P815 and Colon26 tumours completely regressed after transient growth. Rechallenge of respective parental tumour cells into the GITRL-transfected tumour-rejected mice completely eliminated the growth of tumours (data not shown), suggesting the induction of tumour-specific immunity. The growth rate of the GITRL-B16 tumours was markedly reduced; however, the tumours were not completely eradicated.
Figure 2.
Glucocorticoid-induced tumour necrosis factor receptor-related receptor ligand (GITRL)-transfected tumours lose tumorigenicity. (a) Parental GITRL− and GITRL-transfected tumours were subcutaneously injected into syngeneic mice and tumour volume was monitored in each mouse. The values in the upper left corners are the ratio of tumour rejection at day 30. Similar results were obtained from three different GITRL-transfected clones of each tumour. (b) Parental Colon26 and GITRL-Colon26 tumour cells were subcutaneously injected into BALB/c nude mice and tumour volume was monitored.
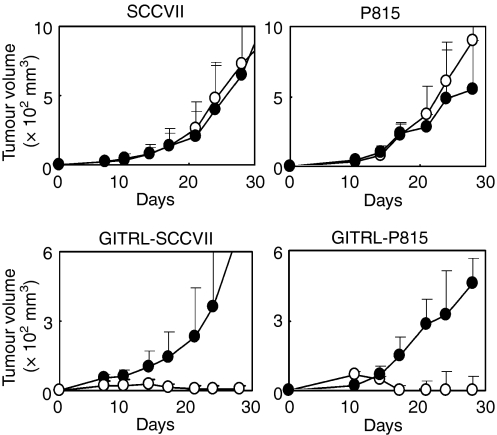
To determine the mechanism of GITRL-induced anti-tumour immunity, we examined the effects of a blocking anti-GITRL mAb on tumour growth in SCCVII and P815 tumours. The treatment of parental tumour-inoculated mice with the mAb did not overtly alter tumour growth (Fig. 3). In contrast, the treatment of GITRL-SCCVII-inoculated mice and GITRL-P815-inoculated mice with the same mAb reversed the effects of tumour reduction and permitted new tumour growth. These results suggest that the enhanced immunity of GITRL-transfected tumours is dependent on the induction of GITRL in tumour cells and that endogenous GITRL is not clearly involved in tumour immunity.
Figure 3.
Effects of anti-glucocorticoid-induced tumour necrosis factor receptor-related receptor ligand (GITRL) monoclonal antibody (mAb) treatment on tumour growth. Tumours were inoculated as described in the Materials and methods. Tumour-inoculated mice received an intraperitoneal injection of control rat immunoglobulin (IgG; open circles) or anti-GITRL mAb (closed circles; 200 μg/mouse) three times a week. The mean tumour volume ± SD was determined in each group of five to eight mice, and the data are representative of two independent experiments.
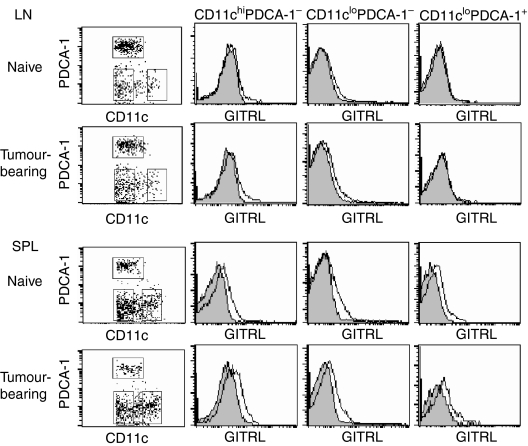
GITRL is weakly expressed on LN DC
Previous reports have shown the cell-surface expression of GITRL in immature and mature bone marrow-derived DC and splenic B220+ CD11c+ plasmacytoid DC (pDC);3–5,30 however, its expression was unstable and diminished after activation.3,4 To verify GITRL expression on the DC in secondary lymphoid tissues, we used flow cytometry to analyse GITRL expression in three subpopulations of LN and splenic DC from naive and tumour-bearing mice. GITRL expression was analysed in CD11chi PDCA-1− DC, CD11clo PDCA-1− DC, and CD11clo PDCA-1+ pDC (Fig. 4). GITRL expression was not clearly detected on the CD11clo PDCA-1+ pDC in naive and tumour-bearing LN cells, although it was detected on splenic CD11clo PDCA-1+ pDC. CD11chi PDCA-1− and CD11clo PDCA-1− DC in both LN cells and splenocytes from naive and tumour-bearing mice expressed GITRL at a low but significant level. No clear differences were observed between the naive and tumour-bearing mice, although the level of expression in each group varied individually. Similar results were obtained using the mAb YGL386 (data not shown).4
Figure 4.
Expression of glucocorticoid-induced tumour necrosis factor receptor-related receptor ligand (GITRL) on lymph node (LN) and splenic dendritic cells (DC). The LN cells and splenocytes from naive BALB/c mice and Colon26 tumour-bearing BALB/c mice at 30 days after tumour inoculation were stained with allophycocyanin-conjugated anti-PDCA-1 monoclonal antibody (mAb), fluorescein isothiocyanate-conjugated CD11c mAb, and biotinylated anti-GITRL mAb, followed by phycoerythrin-streptavidin or the appropriate fluorochrome-conjugated control immunoglobulin. Stained cells were analysed by flow cytometry. An electronic gate was first placed on CD11c+ lymphocytes and the expression of CD11c and PDCA-1 was displayed as dotted plots. The data for GITRL expressions on CD11chi PDCA-1−, CD11clo PDCA-1−, and CD11clo PDCA-1+ cells are presented as histograms (4-decade logarithm scales), in addition to control staining histograms (shaded histograms). Data are representative of three individuals.
Minor involvement of GITRL expressed on DC in antigen-specific T-cell responses
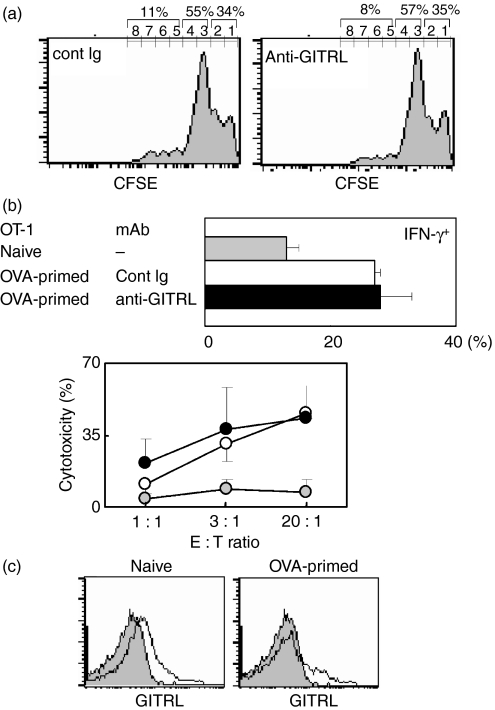
To confirm the minor involvement of endogenous GITRL expressed on DC, we tested the effects of anti-GITRL mAb on the response of T cells to alloantigens known to induce a T-cell response via DC. In transfer experiments, treatment with anti-GITRL mAb did not clearly affect donor cell division (Fig. 5a). Consistent with this, no obvious improvement in cardiac allograft survival was observed after treatment with anti-GITRL mAb in either strain combination (BALB/c into B6 or BALB/c into C3H; Table 1). To rule out the effects of the anti-GITRL mAb on Treg-mediated function, we performed an allogeneic skin graft using BALB/c Rag1−/− mice that had been reconstituted by the transfer of CD25− CD4+ T cells and examined the effects of anti-GITRL mAb treatment (Table 2). Anti-GITRL mAb treatment did not prolong graft survival. The minor involvement of GITRL on DC in CD8+ T-cell responses was also confirmed by experiments using OT-1 mice. In OVA-primed mice, the total number of LN CD8+ T cells was increased twofold compared with the number in unprimed mice. The percentage of IFN-γ+ cells among the CD8+ T cells and the level of cytotoxicity against OVA-expressing E.G7 cells by CD8+ T cells were not clearly affected by anti-GITRL mAb treatment (Fig. 5b). Again, a low level of GITRL expression was seen on CD11chi LN DC from naïve and OVA-primed mice (Fig. 5c). Our results suggest that endogenously expressed GITRL on DC is not overtly involved in antigen-specific T-cell responses.
Figure 5.
Treatment with anti-glucocorticoid-induced tumour necrosis factor receptor-related receptor ligand (GITRL) monoclonal antibody (mAb) does not affect antigen-specific T-cell responses. (a) Carboxyfluorescein diacetate succinimidyl ester (CFSE)-labelled donor BALB/c (H-2d) splenocytes were injected intravenously into CBF1 (H-2d/b) recipient mice, and recipient mice were then treated with control immunoglobulin G (IgG) or anti-GITRL mAb (400 μg/mouse). On day 2, recipient splenocytes were stained with biotinylated anti-H-2b, followed by allophycocyanin-streptavidin, or the appropriate control immunoglobulin, and analysed by flow cytometry. An electronic gate was placed on the H-2b− cells; the peaks of CFSE fluorescence are presented as histograms. The values shown are total percentages from the indicated generations. Similar results were obtained from three independent experiments. Cell division of CD4+ T cells assessed by H-2b− CD4+ T cells also showed no difference between control and anti-GITRL mAb-treated mice (data not shown). (b, c) Lymph node (LN) cells from naive OT-I mice (shaded circles/column) and ovalbumin (OVA) -primed mice treated with control rat IgG (open circles/column) or anti-GITRL mAb (closed circles/column) were analysed. In (b) cells were stained with fluorescein isothiocyanate-conjugated anti-CD3, allophycocyanin-conjugated anti-CD8, and phycoerythrin-conjugated anti-interferon-γ (IFN-γ) mAb or the appropriate control immunoglobulin, and the percentages of IFN-γ+ cells within CD8+ CD3+ lymphocytes were analysed (upper panel). Cytotoxicity of primed OT-1 CD8+ T cells against OVA-expressing E.G7 was measured (lower panel). The values are the mean ± SD from each group of three mice. (c) LN cells were stained with fluorescein isothiocyanate-conjugated anti-CD11c mAb and biotinylated anti-GITRL mAb, followed by phycoerythrin-streptavidin or the appropriate control immunoglobulin. An electronic gate was placed on the CD11chi lymphocytes and the expression of GITRL is presented as histograms (open histograms) with control immunoglobulin-stained cells (shaded histograms).
Table 1.
Effects of treatment with anti-GITRL monoclonal antibody on cardiac allograft
| Donor | Recipient | Antibody treatment | Graft survival days |
|---|---|---|---|
| C57BL/6 | C57BL/6 | cont. Ig | 69, >100, >100, >100, >100 |
| BALB/c | C57BL/6 | cont. Ig | 7, 8, 11, 11, 13 |
| BALB/c | C57BL/6 | anti-GITRL | 9, 10, 12, 12 |
| BALB/c | C3H | cont. Ig | 5, 6, 6, 6, 7, 8 |
| BALB/c | C3H | anti-GITRL | 6, 6, 6, 6, 6 |
cont. Ig, control immunoglobulin; GITRL, glucocorticoid-induced tumour necrosis factor receptor-related receptor ligand.
Table 2.
Effects of treatment with anti-GITRL monoclonal antibody on allogeneic skin graft
| Donor skin | Recipient | Transferred cells | Antibody treatment | Graft survival days |
|---|---|---|---|---|
| BALB/c | BALB/c | (−) | (−) | >100, >100, >100, >100 |
| C57BL/6 | BALB/c | (−) | (−) | 5, 6, 7, 7, 8, 8, 8, 8 |
| C57BL/6 | BALB/c Rag1−/− | CD25− CD4+ T | Cont. Ig | 9, 9, 11, 11, 11, 13, 13 |
| C57BL/6 | BALB/c Rag1−/− | CD25− CD4+ T | anti-GITRL | 9, 10, 10, 12, 12, 14, 14 |
cont. Ig, control immunoglobulin; GITRL, glucocorticoid-induced tumour necrosis factor receptor-related receptor ligand.
Requirements of CD4+ or CD8+ effector T cells and Treg cells for tumour-eradicating immunity
To examine the requirements for GITRL-induced anti-tumour immunity, parental Colon26 and GITRL-Colon26 were inoculated into syngeneic nude mice and the tumour growth was evaluated. As shown in Fig. 2(b), immunoincompetent nude mice lacking T cells permitted the growth of GITRL-Colon26 tumours and no difference in growth of either tumour was observed. These results suggest that T cells are required for rejection of GITRL+ tumour and innate immunity is not obviously involved in the anti-tumour immunity enhanced by GITRL.
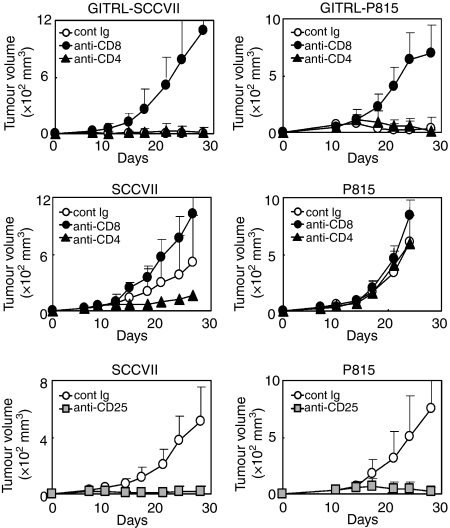
Next, to see the contribution of CD4+ and CD8+ T cells, we depleted either CD4+ or CD8+ T cells and examined the effects on tumour growth. The depletion of CD8+ T cells dramatically promoted tumour growth in GITRL-SCCVII and GITRL-P815, in opposition to the effects of GITRL transduction, whereas the depletion of CD4+ T cells had no clear effect (Fig. 6, upper panels). These results suggest that CD8+ T cells alone are essential for GITRL+ tumour-eradicating immunity.
Figure 6.
Effects of CD4+, CD8+ or regulatory T cell depletion on tumour growth. Mice were pretreated with control reagent, anti-CD4 or anti-CD8 monoclonal antibody (mAb) and then glucocorticoid-induced tumour necrosis factor receptor-related receptor ligand (GITRL)-SCCVII and GITRL-P815 (upper panels) or SCCVII and P815 (middle panels) tumours were inoculated. Mice were pretreated with anti-CD25 mAb and then SCCVII and P815 (bottom panels) tumours were inoculated. Values are expressed as the mean tumour volume ± SD in each group of five to eight mice, and data are representative of two independent experiments.
In parental GITRL− tumours, the depletion of CD8+ T cells promoted both SCCVII and P815 tumour growth, whereas the depletion of CD4+ T cells inhibited the growth of SCCVII, but not P815, tumours (Fig. 6, middle panels). These results indicate the consistent involvement of CD8+ T cells in anti-tumour immunity. However, the contribution of CD4+ T cells was dependent on the tumour type. In SCCVII tumours, regulatory CD4+ T-cell function appeared to dominate effector CD4+ T-cell function. The actual contribution of CD4+ Treg cells was confirmed using Treg-depleted mice. The depletion of CD25+ Treg cells completely eradicated all inoculated tumours, regardless of the tumour type, which suggests the crucial involvement of Treg cells in the regulation of tumour immunity (Fig. 6, bottom panels). Our results suggest that Treg cells are critically involved in the induction of tolerance against tumours lacking GITRL, whereas in tumours expressing GITRL at high levels, the effector function of CD8+ T cells overpowers the regulatory function of Treg cells.
Enhancement of CD8+ T-cell function by tumour-associated GITRL
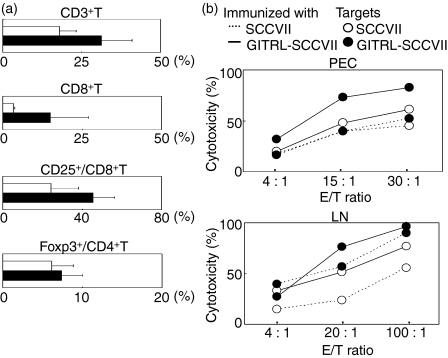
To investigate whether tumour-associated GITRL induces T-cell activation at the induction or effector phase, we immunized C3H mice with parental or GITRL+ SCCVII tumour cells and analysed the states of the T cells in PEC and LN cells. Intraperitoneal immunization with GITRL+ tumours enhanced the percentages of CD3+ T cells and CD8+ T cells compared with mice immunized with parental tumour cells (Fig. 7a). The activation state of the CD8+ T cells as assessed by CD25 expression was also enhanced. In contrast, the percentages of CD4+ T cells (data not shown) and Foxp3+ Treg cells within the CD4+ T cells (Fig. 7a) were not clearly affected. Immunization with GITRL+ tumours enhanced the cytotoxicity of both PEC-CD8+ and LN-CD8+ T cells against parental and GITRL+ SCCVII tumours (Fig. 7b). In addition, when the same T cells were used, CD8+ T-cell-mediated cytotoxicity against GITRL+ tumours was consistently higher than that against GITRL− tumours, although PEC-CD8+ T cells from the parental tumour-immunized mice did not show any clear differences. These results indicate that tumour-associated GITRL augments the expansion and function of cytotoxic T lymphocytes (CTL) at the induction phase and enhances their function at the effector phase.
Figure 7.
Immunization with tumour-associated glucocorticoid-induced tumour necrosis factor receptor-related receptor ligand (GITRL) enhances the expansion and activation of CD8+ T cells. (a) Peritoneal exudate cells (PEC) from mice immunized with SCCVII (open column) or GITRL-SCCVII (closed column) tumour cells were stained with either peridinin chlorophyll protein–carbocyanin 5.5 (PerCP-Cy5.5)-anti-CD3, allophycocyanin (APC)-anti-CD8 and phycoerythrin-anti-CD25 monoclonal antibodies (mAb) or fluorescein isothiocyanate-anti-CD4, PerCP-Cy5.5-anti-CD3, and APC-anti-Foxp3 mAb or with the appropriate fluorochrome-conjugated control immunoglobulin. The stained cells were then analysed by flow cytometry. An electronic gate was placed on the CD3+ CD8+ lymphocytes and the percentages of CD25+ cells were analysed. An electronic gate was also placed on the CD4+ CD3+ lymphocytes, and the percentages of Foxp3+ cells were determined. (b) Isolated CD8+ PEC and lymph node cells from mice immunized with SCCVII (dotted lines) or GITRL-SCCVII (solid lines) tumour cells were used as effector cells. PKH-26-labelled SCCVII (open circles) and GITRL-SCCVII (closed circles) cells were used as target cells. Cytotoxicity at each effector to target (E : T) ratio is shown. The data are representative of two independent experiments.
Inoculation of GITRL tumour vaccine into the peritumoral sites shows therapeutic efficacy
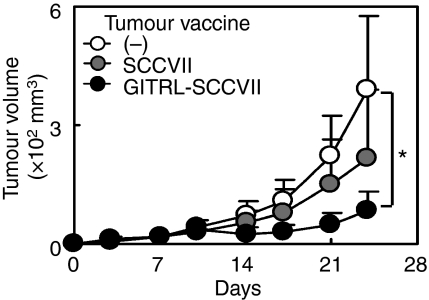
To see the therapeutic effects of GITRL-transfected tumour vaccine, either MC-treated SCCVII or GITRL-SCCVII tumour cells were inoculated into peritumoral sites at days 3, 6, 9 and 15 after the injection of parental SCCVII tumour cells. GITRL tumour vaccine efficiently inhibited the growth of already established tumours, although GITRL− parental tumour vaccine also showed some inhibitory effects (Fig. 8). Inoculation of GITRL tumour vaccine into the peritoneal cavity or into the more distant sites from the tumours did not clearly show the therapeutic efficacy over the effects of parental tumour cell vaccine (data not shown).
Figure 8.
Inoculation of glucocorticoid-induced tumour necrosis factor receptor-related receptor ligand (GITRL) tumour vaccine into the peritumoral sites shows therapeutic efficacy. SCCVII cells (3 × 105) were inoculated into the right flank on day 0. Mice were divided into three groups and for two groups, either mitomycin C-treated SCCVII or GITRL-SCCVII tumour cells (3 × 105) were injected subcutaneously into the peritumoral sites at days 3, 6, 9 and 15 and the tumour growth was evaluated. Values are expressed as the mean tumour volume ± SD in each group of five to eight mice. *Statistically different at P < 0·05.
Discussion
We demonstrated that GITRL was weakly expressed on DC in secondary lymphoid tissues and the administration of a blocking anti-GITRL mAb did not substantially affect the antigen-specific T-cell responses mediated by DC. However, tumour-associated GITRL efficiently enhanced tumour-eradicating immunity by augmenting the production and effector function of CTL and the GITRL-transfected tumour vaccine showed therapeutic efficacy.
We extensively analysed the cell surface expression of GITRL in splenic and LN DC using several mAb (MIH44, MIH459 and YGL3864) with optimal control staining and suitable blocking reagents for non-specific binding. GITRL expression on splenic and LN DC was limited and was not clearly enhanced in tumour-bearing or antigen-primed mice. Previous reports demonstrated that GITRL expression on DC is limited in immature bone marrow-derived DC or freshly isolated splenic DC, and that continuous activation downregulates their expression of GITRL.4,5 In humans, GITRL is preferentially expressed on activated pDC but not on monocyte-derived immature or mature myeloid DC.31 Elevated cell surface GITRL expression was also detected in mouse splenic CD11clo B220+ pDC.5,30 However, substantial GITRL expression on CD11clo PDCA-1+ pDC in LN cells from naive or tumour-bearing mice was not detected. The CD11clo B220+ pDC population contained at least two distinct fractions: CD11clo B220+ PDCA-1+ Ly-6C+ pDC and CD11clo B220+ PDCA-1− Ly-6C− CD49b+ (NK1.1+) natural killer (NK) -like IFN-producing killer DC (IKDC).22,32 We observed weak, but significant, GITRL expression on CD11clo PDCA-1− DC, which may contain NK-like IKDC.
Our in vivo blocking experiments revealed that the endogenous expression of GITRL on DC is not functionally involved in T-cell-mediated immune responses to alloantigens, an exogenous protein antigen (OVA), or tumour antigens (Tables 1 and 2, Figs 3 and 5). A previous report5 has shown the inhibitory effects of anti-GITRL mAb (clone 5F1) on the proliferation of LN cells or splenocytes stimulated with anti-CD3 mAb in vitro; however, these results have not directly indicated the contribution of GITRL expressed on DC because they cannot negate the effects of anti-GITRL mAb to macrophages and other cells. There is currently no clear evidence of the involvement of the GITRL expressed on antigen-presenting DC in T-cell interactions in vivo. Since MIH44 efficiently inhibited GITRL-mediated T-cell responses in vitro9 and in vivo (Fig. 3), it is unlikely that the anti-GITRL mAb used in this study (MIH44) lacked blocking ability. In fact, we observed marked effects of MIH44 treatment on hapten-induced contact hypersensitivity and corneal allografts; in these cases, the interactions between endogenously expressed GITRL on Langerhans cells and GITR on epidermal keratinocytes, and the interactions between endogenously expressed GITRL on corneal epithelium and GITR on tissue-infiltrating Treg cells seem to be involved in the local immune responses, respectively (manuscripts in preparation). Consequently, our results suggest that the GITRL expressed on DC is not substantially involved in the interactions between antigen-presenting DC and T cells in secondary lymphoid organs. The expression and functional involvement of GITRL on non-immune cells has been reported in mouse sympathetic neurons33 and human retinal pigment epithelium.34 It is possible that the physiological impact of the GITR–GITRL pathway may be in its interactions with tissue cells and infiltrating effector or regulatory T cells at local sites of inflammation. Additional studies are needed to explore this possibility.
Here, we demonstrated that the transduction of GITRL into tumours rendered them immunogenic and reduced tumorigenicity (Fig. 2a). Our depletion experiments revealed that CD8+ T cells contribute to the eradication of GITRL+ tumours, independent of CD4+ T cells (Fig. 6). Based on the results of our immunization studies, tumour-associated GITRL augments CTL function at both the induction and effector phases (Fig. 7). Additionally, GITRL tumour vaccine using SCCVII showed therapeutic efficacy when the tumour vaccine was injected only into the peritumoral sites (Fig. 8). These results suggest that tumour-associated GITRL may interact with the GITR expressed on tumour-infiltrating CD8+ T cells, thereby providing costimulatory signals for local CTL activation. It has been shown that the activation of GITR−/− T cells through CD28 costimulation is particularly impaired in CD8+ T cells and GITR lowers the threshold of CD28 costimulation in CD8+ T cells.35 In the studies using systemic administration of agonistic anti-GITR mAb or GITRL immunoglobulin, the contribution of CD4+ and CD8+ T cells to the enhanced anti-tumour immunity was variable and this was dependent on treatment schedules, methods for injection and tumour cells used.10–14 Unlike systemic administration, tumour-associated GITRL seems to enhance preferential activation of CD8+ T cells especially at the local tumour sites. Consistent with our observations, the therapeutic efficacy of GITRL introduction into tumours has been reported.36,37 The injection of recombinant GITRL adenovirus constructs into already established B16 melanoma cells transfected with coxsackie adenovirus receptor (B16-F0 CAR) inhibited the tumour growth.36 In a CMS5 sarcoma cell line expressing a CTL epitope, plasmid immunization with a mixture of a CTL epitope and GITRL enhanced the CTL epitope-specific CD8+ T cells independently of CD4+ T cells, and the activated CD8+ T cells acquired Treg cell resistance.37 Similarly, our studies also showed that functionally enhanced CTL became resistant to Treg-mediated suppression (Fig. 6). Fortunately, the unwanted expansion of Foxp3+ Treg cells observed in some tumour experiments following the systemic administration of anti-GITR mAb12,13 was not seen in the GITRL-transfected tumours. The acceptance of GITRL+ Colon26 tumours and the comparable growth rates between parental and GITRL-transfected tumours in nude mice (Fig. 2b) suggest that innate immunity mediated by NK cells or macrophages is not directly involved in the GITRL-induced anti-tumour immunity, although a regulatory role of tumour-associated GITRL against NK cell activation has been shown in humans.38
In conclusion, tumour-associated GITRL greatly augmented the effector function of CD8+ T cells and enhanced the contribution of CD8+ T cells while reducing the crucial contribution of Treg cells, which is involved in immunity against tumours lacking GITRL. Our results suggest the minor involvement of GITRL in T-cell–DC interactions in secondary lymphoid organs and the efficacy of ectopically expressed GITRL on tumours in enhancing anti-tumour immunity at the peripheral sites. Recent reports have suggested several differences between humans and mice in the expression of and the structural and biochemical properties of GITRL.38,39 Therefore, the results obtained using murine tumour models may not apply directly to humans; however, our results indicate the possibility of tumour immunotherapy using a GITRL-transfected tumour vaccine. Using both a GITRL tumour vaccine and methods aimed at enhancing the activation of host APCs may be a promising strategy for enhancing tumour immunity.
Acknowledgments
We thank Drs K. Maruyama, T. Kitamura, M. Sho, K. Takeda, H. R. MacDonald, M. J. Bevan, W. R. Heath and H. Udono for kindly providing vectors, cells and transgenic mice. We also thank Ms A. Yoshino for expert assistance with the flow cytometry. This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Disclosures
The authors declare no conflict of interest.
References
- 1.Nocentini G, Giunchi L, Ronchetti S, Krausz LT, Bartoli A, Moraca R, Migliorati G, Riccardi C. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:6216–21. doi: 10.1073/pnas.94.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanamaru F, Youngnak P, Hashiguchi M, Nishioka T, Takahashi T, Sakaguchi S, Ishikawa I, Azuma M. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J Immunol. 2004;172:7306–14. doi: 10.4049/jimmunol.172.12.7306. [DOI] [PubMed] [Google Scholar]
- 3.Yu KY, Kim HS, Song SY, Min SS, Jeong JJ, Youn BS. Identification of a ligand for glucocorticoid-induced tumor necrosis factor receptor constitutively expressed in dendritic cells. Biochem Biophys Res Commun. 2003;310:433–8. doi: 10.1016/j.bbrc.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Tone M, Tone Y, Adams E, Yates SF, Frewin MR, Cobbold SP, Waldmann H. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci USA. 2003;100:15059–64. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–20. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 6.Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, Ayroldi E, Riccardi C. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–22. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 7.Esparza EM, Arch RH. Glucocorticoid-induced TNF receptor, a costimulatory receptor on naive and activated T cells, uses TNF receptor-associated factor 2 in a novel fashion as an inhibitor of NF-kappa B activation. J Immunol. 2005;174:7875–82. doi: 10.4049/jimmunol.174.12.7875. [DOI] [PubMed] [Google Scholar]
- 8.Ji HB, Liao G, Faubion WA, Abadia-Molina AC, Cozzo C, Laroux FS, Caton A, Terhorst C. Cutting edge: the natural ligand for glucocorticoid-induced TNF receptor-related protein abrogates regulatory T cell suppression. J Immunol. 2004;172:5823–7. doi: 10.4049/jimmunol.172.10.5823. [DOI] [PubMed] [Google Scholar]
- 9.Igarashi H, Cao Y, Piao J, Kamimura Y, Hashiguchi M, Amagasa T, Azuma M. GITR ligand-costimulation activates effector and regulatory functions of CD4+ T cells. Biochem Biophys Res Commun. 2008;369:1134–8. doi: 10.1016/j.bbrc.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Ko K, Yamazaki S, Nakamura K, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202:885–91. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen AD, Diab A, Perales MA, et al. Agonist anti-GITR antibody enhances vaccine-induced CD8+ T-cell responses and tumor immunity. Cancer Res. 2006;66:4904–12. doi: 10.1158/0008-5472.CAN-05-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez-Montagut T, Chow A, Hirschhorn-Cymerman D, et al. Glucocorticoid-induced TNF receptor family related gene activation overcomes tolerance/ignorance to melanoma differentiation antigens and enhances antitumor immunity. J Immunol. 2006;176:6434–42. doi: 10.4049/jimmunol.176.11.6434. [DOI] [PubMed] [Google Scholar]
- 13.Zhou P, L’Italien L, Hodges D, Schebye XM. Pivotal roles of CD4+ effector T cells in mediating agonistic anti-GITR mAb-induced-immune activation and tumor immunity in CT26 tumors. J Immunol. 2007;179:7365–75. doi: 10.4049/jimmunol.179.11.7365. [DOI] [PubMed] [Google Scholar]
- 14.Hu P, Arias RS, Sadun RE, et al. Construction and preclinical characterization of Fc-mGITRL for the immunotherapy of cancer. Clin Cancer Res. 2008;14:579–88. doi: 10.1158/1078-0432.CCR-07-0940. [DOI] [PubMed] [Google Scholar]
- 15.Nocentini G, Cuzzocrea S, Bianchini R, Mazzon E, Riccardi C. Modulation of acute and chronic inflammation of the lung by GITR and its ligand. Ann N Y Acad Sci. 2007;1107:380–91. doi: 10.1196/annals.1381.040. [DOI] [PubMed] [Google Scholar]
- 16.Krausz LT, Bianchini R, Ronchetti S, Fettucciari K, Nocentini G, Riccardi C. GITR–GITRL system, a novel player in shock and inflammation. Sci World J. 2007;7:533–66. doi: 10.1100/tsw.2007.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed SU, Okamoto M, Oshikawa T, et al. Anti-tumor effect of an intratumoral administration of dendritic cells in combination with TS-1, an oral fluoropyrimidine anti-cancer drug, and OK-432, a streptococcal immunopotentiator: involvement of toll-like receptor 4. J Immunother. 2004;27:432–41. doi: 10.1097/00002371-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Kayagaki N, Yamaguchi N, Nakayama M, et al. Expression and function of TNF-related apoptosis-inducing ligand on murine activated NK cells. J Immunol. 1999;163:1906–13. [PubMed] [Google Scholar]
- 20.Nakagawa R, Motoki K, Ueno H, Iijima R, Nakamura H, Kobayashi E, Shimosaka A, Koezuka Y. Treatment of hepatic metastasis of the colon26 adenocarcinoma with an alpha-galactosylceramide, KRN7000. Cancer Res. 1998;58:1202–7. [PubMed] [Google Scholar]
- 21.Otsuki N, Kamimura Y, Hashiguchi M, Azuma M. Expression and function of the B and T lymphocyte attenuator (BTLA/CD272) on human T cells. Biochem Biophys Res Commun. 2006;344:1121–7. doi: 10.1016/j.bbrc.2006.03.242. [DOI] [PubMed] [Google Scholar]
- 22.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–5. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 23.Oikawa T, Kamimura Y, Akiba H, et al. Preferential involvement of Tim-3 in the regulation of hepatic CD8+ T cells in murine acute graft-versus-host disease. J Immunol. 2006;177:4281–7. doi: 10.4049/jimmunol.177.7.4281. [DOI] [PubMed] [Google Scholar]
- 24.Mogi S, Sakurai J, Kohsaka T, Enomoto S, Yagita H, Okumura K, Azuma M. Tumour rejection by gene transfer of 4-1BB ligand into a CD80+ murine squamous cell carcinoma and the requirements of co-stimulatory molecules on tumour and host cells. Immunology. 2000;101:541–7. doi: 10.1046/j.1365-2567.2000.t01-1-00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ono K, Lindsey ES. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969;57:225–9. [PubMed] [Google Scholar]
- 26.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–50. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Kawashima N, Suda H, Nakano Y, Takano Y, Azuma M. The existence of CD11c+ sentinel and F4/80+ interstitial dendritic cells in dental pulp and their dynamics and functional properties. Int Immunol. 2006;18:1375–84. doi: 10.1093/intimm/dxl070. [DOI] [PubMed] [Google Scholar]
- 28.Brossart P, Goldrath AW, Butz EA, Martin S, Bevan MJ. Virus-mediated delivery of antigenic epitopes into dendritic cells as a means to induce CTL. J Immunol. 1997;158:3270–6. [PubMed] [Google Scholar]
- 29.Hatam L, Schuval S, Bonagura VR. Flow cytometric analysis of natural killer cell function as a clinical assay. Cytometry. 1994;16:59–68. doi: 10.1002/cyto.990160109. [DOI] [PubMed] [Google Scholar]
- 30.Grohmann U, Volpi C, Fallarino F, et al. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007;13:579–86. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 31.Hanabuchi S, Watanabe N, Wang YH, Ito T, Shaw J, Cao W, Qin FX, Liu YJ. Human plasmacytoid predendritic cells activate NK cells through glucocorticoid-induced tumor necrosis factor receptor-ligand (GITRL) Blood. 2006;107:3617–23. doi: 10.1182/blood-2005-08-3419. [DOI] [PubMed] [Google Scholar]
- 32.Vosshenrich CA, Lesjean-Pottier S, Hasan M, Richard-Le Goff O, Corcuff E, Mandelboim O, Di Santo JP. CD11clo B220+ interferon-producing killer dendritic cells are activated natural killer cells. J Exp Med. 2007;204:2569–78. doi: 10.1084/jem.20071451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Keeffe GW, Gutierrez H, Pandolfi PP, Riccardi C, Davies AM. NGF-promoted axon growth and target innervation requires GITRL–GITR signaling. Nat Neurosci. 2008;11:135–42. doi: 10.1038/nn2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahesh SP, Li Z, Liu B, Fariss RN, Nussenblatt RB. Expression of GITR ligand abrogates immunosuppressive function of ocular tissue and differentially modulates inflammatory cytokines and chemokines. Eur J Immunol. 2006;36:2128–38. doi: 10.1002/eji.200635893. [DOI] [PubMed] [Google Scholar]
- 35.Ronchetti S, Nocentini G, Bianchini R, Krausz LT, Migliorati G, Riccardi C. Glucocorticoid-induced TNFR-related protein lowers the threshold of CD28 costimulation in CD8+ T cells. J Immunol. 2007;179:5916–26. doi: 10.4049/jimmunol.179.9.5916. [DOI] [PubMed] [Google Scholar]
- 36.Calmels B, Paul S, Futin N, Ledoux C, Stoeckel F, Acres B. Bypassing tumor-associated immune suppression with recombinant adenovirus constructs expressing membrane bound or secreted GITR-L. Cancer Gene Ther. 2005;12:198–205. doi: 10.1038/sj.cgt.7700781. [DOI] [PubMed] [Google Scholar]
- 37.Nishikawa H, Kato T, Hirayama M, et al. Regulatory T cell-resistant CD8+ T cells induced by glucocorticoid-induced tumor necrosis factor receptor signaling. Cancer Res. 2008;68:5948–54. doi: 10.1158/0008-5472.CAN-07-5839. [DOI] [PubMed] [Google Scholar]
- 38.Baltz KM, Krusch M, Baessler T, Schmiedel BJ, Bringmann A, Brossart P, Salih HR. Neutralization of tumor-derived soluble glucocorticoid-induced tnfr-related protein (GITR) ligand increases NK cell anti-tumor reactivity. Blood. 2008;112:3735–43. doi: 10.1182/blood-2008-03-143016. [DOI] [PubMed] [Google Scholar]
- 39.Chattopadhyay K, Ramagopal UA, Brenowitz M, Nathenson SG, Almo SC. Evolution of GITRL immune function: murine GITRL exhibits unique structural and biochemical properties within the TNF superfamily. Proc Natl Acad Sci USA. 2008;105:635–40. doi: 10.1073/pnas.0710529105. [DOI] [PMC free article] [PubMed] [Google Scholar]