Abstract
We sought to determine whether Litomosoides sigmodontis, a filarial infection of rodents, protects against type 1 diabetes in non-obese diabetic (NOD) mice. Six-week-old NOD mice were sham-infected or infected with either L3 larvae, adult male worms, or adult female worms. Whereas 82% of uninfected NOD mice developed diabetes by 25 weeks of age, no L. sigmodontis-infected mice developed disease. Although all mice had evidence of ongoing islet cell inflammation by histology, L. sigmodontis-infected mice had greater numbers of total islets and non-infiltrated islets than control mice. Protection against diabetes was associated with a T helper type 2 (Th2) shift, as interleukin-4 (IL-4) and IL-5 release from α-CD3/α-CD28-stimulated splenocytes was greater in L. sigmodontis-infected mice than in uninfected mice. Increased circulating levels of insulin-specific immunoglobulin G1, showed that this Th2 shift occurs in response to one of the main autoantigens in diabetes. Multicolour flow cytometry studies demonstrated that protection against diabetes in L. sigmodontis-infected NOD mice was associated with significantly increased numbers of splenic CD4+ CD25+ FoxP3+ regulatory T cells. Interestingly, injection of crude worm antigen into NOD mice also resulted in protection against type 1 diabetes, though to a lesser degree than infection with live L. sigmodontis worms. In conclusion, these studies demonstrate that filarial worms can protect against the onset of type 1 diabetes in NOD mice. This protection is associated with a Th2 shift, as demonstrated by cytokine and antibody production, and with an increase in CD4+ CD25+ FoxP3+ regulatory T cells.
Keywords: autoimmune, diabetes, helminth, immunomodulation, Litomosoides sigmodontis
Introduction
Type 1 diabetes (insulin-dependent diabetes mellitus) is a chronic autoimmune disease characterized by the loss of insulin-producing β-islet cells.1 While the immunological mechanisms responsible for this disease are not completely understood, self-reactive CD4+ and CD8+ T cells probably play a significant role in the damage that occurs to insulin-producing β-islet cells.1–3
The prevalence of type 1 diabetes and other autoimmune diseases has increased dramatically over the past few decades.4–6 An analysis of 37 epidemiological studies conducted from 1960 to 1996 reports that there has been an increase in the incidence of type 1 diabetes worldwide of 3% per year over that time period, and that by the year 2010 the incidence of type 1 diabetes may be as high as 50/100 000 persons in some developed countries.7 While genetic factors may play a role in susceptibility to type 1 diabetes, it is probable that the dramatic worldwide increase in type 1 diabetes prevalence is the result of environmental factors.
One environmental change that may be responsible for the recent increase in autoimmune diseases is the loss of chronic parasitic infections in developed countries. Multiple studies have found that individuals infected with chronic parasitic worm infections have lower rates of autoimmune diseases than others living in the same environment8–11 and a recent epidemiological study associated falling infection rates of humans with pinworms or Strongyloides stercoralis with increased prevalence of type 1 diabetes.6
Experimentally, a number of helminth parasites, including the trematode Schistosoma mansoni and the nematodes Heligmosomoides polygyrus and Trichinella spiralis, prevent the onset or suppress the severity of type 1 diabetes in non-obese diabetic (NOD) mice,12–14 experimental autoimmune encephalitis15 and experimental colitis.16–18 A clinical trial demonstrated that oral administration of porcine whipworm eggs improves symptoms in patients with inflammatory bowel disease.19,20 To date, however, the exact mechanisms by which helminths protect against autoimmune diseases remain unknown.
In this study, we investigated whether Litomosoides sigmodontis, a tissue-invasive filarial nematode, prevents the onset of type 1 diabetes in NOD mice. As a broad range of different helminth parasites have been found to have beneficial effects on autoimmune diseases, we hypothesized that filarial infection would be protective against type 1 diabetes. Furthermore, as filarial infections induce both a T helper type 2 (Th2)-skewed immune response and, over time, a strong immunoregulatory response, we hypothesized that helminth-mediated protection against autoimmunity would be associated with an autoantigen-specific Th2 shift and with increases in regulatory T-cell numbers.
Materials and methods
Mice and parasites
Female BALB/c mice (NCI Mouse Repository, Frederick, MD) and female NOD mice (Jackson Laboratory, Bar Harbor, ME) were maintained at the Uniformed Services University (USUHS) animal facility with free access to food and water. All experiments were performed under protocols approved by the USUHS Institutional Animal Care and Use Committee.
Infective-stage L3 larvae from L. sigmodontis were isolated by saline lavage from the pleural cavity of 4-day infected jirds (Meriones unguiculatus, obtained from TRS Laboratory Inc, Athens, GA). Adult worms were obtained by physical extraction from the pleural cavity and peritoneum of killed female BALB/c mice that had been infected for 6 weeks.
Infection of mice
Six-week-old female NOD mice were infected by subcutaneous injection of L3 larvae or by intraperitoneal surgical implantation of adult worms. For subcutaneous inoculation, 40 infective-stage L3 larvae in 100–150 μl RPMI-1640 medium (Mediatech, Herndon, VA), or RPMI-1640 medium alone for control mice, were injected between the shoulder blades using a 21-gauge needle. For surgical implantation of adult worms, mice were anaesthetized with a mixture of 46 mg/kg body weight ketamine (Ketaject; Phoenix Pharmaceutical, Inc., St Joseph, MO), 0·225 mg/kg acetopromazine (PromAce Injectable; Fort Dodge Animal Health, Fort Dodge, IO) and 5 mg/kg xylazine (TranquiVed; Vedco, Inc., St Joseph, MO). Then, a small 1–1·5 cm vertical incision penetrating the skin, subcutaneous tissues and peritoneal membrane was made just lateral to the midline in the ventral abdominal wall near the umbilicus. Five adult female or five adult male L. sigmodontis worms in 0·5–1 ml RPMI-1640 medium were then injected into the abdominal cavity through the bore of a flexible, plastic Pasteur pipette (Fisher Scientific, Pittsburgh, PA). Control groups were sham-implanted by injection of 0·5–1 ml RPMI-1640 medium. The incision site was closed with one absorbable deep suture (5–0) to close the peritoneal membrane and two superficial absorbable sutures (5–0) to close the subcutaneous tissue and skin.
Assessment of diabetes
Glucose levels of NOD mice were determined using a standard blood glucose meter (Accu-Check Advantage; Roche Diagnostics GmbH, Mannheim, Germany) from blood taken by orbital bleeds every 2 weeks. Mice with glucose levels > 230 mg/dl were considered diabetic. Immunological studies were performed 4 weeks after the uninfected controls developed diabetes (range: 17–24 weeks of age).
Preparation of L. sigmodontis adult worm antigen (LsAg)
Frozen adult L. sigmodontis worms were lyophilized overnight, resuspended in phosphate-buffered saline (PBS) and stirred overnight at 4°. After centrifugation (750 g for 10 min at 4°) the supernatant was collected. The pellet was stirred again overnight, centrifuged and the supernatant was combined with the first supernatant. After a final centrifugation at 12 000 g for 30 min at 4°, supernatant was collected, passed through a 0·22-μm filter (Milex – GV; Millipore Corporation, Bedford, MA) and the protein content was measured with the BCA Protein Assay kit (Pierce, Rockford, IL).
Injection of LsAg in NOD mice
Beginning at 6 weeks of age, NOD mice were injected intraperitoneally with 100 μg LsAg in PBS (Mediatech), or PBS alone for controls, once a week. At the age of 24 weeks mice were killed and immunological studies were performed. Because the control mice died before this time-point, we compared the immunoglobulin, cytokine and histology data with the control group of the implantation experiments.
Assessment of pancreas inflammation
Four weeks after the uninfected NOD mice developed diabetes, mice were killed using CO2 and pancreases were isolated immediately and fixed in 10% formalin (Protocol; Fisher Scientific Company, Kalamazoo, MI). Sections stained with haematoxylin & eosin were assessed for inflammation by a pathologist (J.T.S.) blinded to the intervention group. Numbers of islets of four longitudinal sections of each pancreas were assessed. The severity of insulitis was scored as non-infiltrated (healthy islet), periinsulitis (lymphocytes at the periphery of the islets) or intrainsulitis (lymphocyte infiltration into the interior of the islets lower or greater than 50%).
Assessment of cellular proliferation
Single-cell suspensions of spleen cells were obtained by mechanically forcing splenocytes through a 70μm cell filter (BD Biosciences, San Jose, CA). Red blood cell lysis was then performed (ACK Lysing Buffer; Invitrogen Inc., Carlsbad, CA), followed by one washing step. Cells were plated in triplicate at a concentration of 2 × 106 cells/ml in a volume of 100 μl immunoglobulin E (IgE) medium (Iscove’s modified Dulbecco’s medium; Mediatech) including 10% fetal calf serum (Valley Biomedical, Winchester, VA), 1%l-glutamine (Mediatech), 1% insulin-transferrin-selenium medium (Invitrogen Inc.) and 80 μg/ml gentamicin (Invitrogen Inc.). Cells were stimulated with 20 μg/ml LsAg or insulin (recombinant human insulin; Cell Sciences, Canton, MA) or 5 μg/ml plate-coated α-CD3 (eBioscience, San Diego, CA) and 2 μg/ml α-CD28 (eBioscience) and cultured at 37° in 5% CO2. After 2 days, bromodeoxyuridine (BrdU) was added and cells were cultured for an additional 16 hr. Cell proliferation was assessed using a BrdU chemiluminescent assay per the manufacturer’s instructions (Roche Diagnostics GmbH).
Flow cytometric detection of regulatory T cells and intracellular cytokine production by T cells
Spleen cells were isolated and stimulated as described above, at a total of 1 × 107 cells in 5 ml IgE media. After 2 hr of incubation, BD GolgiStop was added (BD Biosciences) and cells were incubated for an additional 4 hr. Collected cells were incubated in Fixation/Permeabilization Solution (eBioscience) overnight and finally cryopreserved in PBS/10% dimethyl sulphoxide (Sigma, St Louis, MO). For analysis cells were washed once with PBS/1% bovine serum albumin (BSA; Sigma), followed by a blocking step with PBS/1% BSA.
Cells were stained for four-colour flow cytometry with rat anti-mouse CD4 conjugated with peridinin chlorophyll protein (PerCP; BD Biosciences), rat anti-mouse FoxP3 with fluorescein isothiocyanate (FITC; eBioscience), rat anti-mouse CD25 with allophycocyanin (APC)-Alexa Fluor 750 (eBioscience) and rat anti-mouse interleukin-10 (IL-10) with phycoerythrin (PE; eBioscience) or rat anti-mouse CD4 with PerCP (BD Biosciences), rat anti-mouse CD8a with PE (eBioscience), rat anti-mouse IL-4 with APC (BD Biosciences) and rat anti-mouse interferon-γ (IFN-γ) with FITC (eBioscience).
Flow cytometry was performed using a BD LSRII system and subsequently analysed with facsdiva 6.0 software (BD Biosciences). All flow cytometry antibodies were individually titrated and, before each experiment, compensation was conducted with BD CompBeads (BD Biosciences) bound to the flow cytometry antibodies used in that experiment. During analysis, cut-offs for cytokine and CD25-positivity were set using the fluorescence minus one approach.
Measurement of cytokines and antibodies by enzyme-linked immunosorbent assays
Cytokine enzyme-linked immunosorbent assays (ELISA) were performed from spleen cells that were stimulated as described above, at a concentration of 2 × 106 cells in 1 ml IgE media. Culture supernatants were collected after 72 hr and kept at −20° until assays were performed. Interferon-γ, IL-4, IL-5 and IL-10 were quantified according to the manufacturer’s instructions (OptEIA™ Set Mouse; BD Biosciences). Spontaneous cytokine production of unstimulated cells was subtracted.
Insulin-specific IgG1 as well as total IgE, were analysed 4 weeks after the control groups developed diabetes. All samples were analysed as duplicates at the same time on the same plate to allow accurate comparison between groups by optical density (OD). Mice were killed by terminal bleeding and plasma was isolated using heparinized microtubes (BD Microtainer, Franklin Lakes, NJ). Plates (Corning Inc., Corning, NY) were coated with 20 μg/ml insulin (recombinant human insulin; Cell Sciences) or 10 μg/ml purified rat anti-mouse IgE (BD Biosciences) in PBS. Detection occurred using a biotinylated anti-mouse IgE or IgG1 detection antibody (BD Biosciences) followed by addition of alkaline phosphatase-conjugated streptavidin (Jackson ImmunoResearch Laboratories). 4-Nitrophenyl phosphate disodium salt hexahydrate (Sigma) was used as substrate and absorbance was measured at 405 nm (Victor3V; Perkin Elmer, Waltham, MA). Purified mouse IgE was used as standard for total IgE (BD Biosciences).
Statistical analysis
Statistical analyses were performed with graphpad prism software (GraphPad Software, San Diego, CA). Differences between multiple groups were tested for significance using the Kruskal–Wallis test, followed by Dunn’s post-hoc multiple comparisons. Comparisons between two groups were performed by Mann–Whitney U-test. P-values < 0·05 were considered significant. Data are shown as median. Glucose levels and percentages of diabetic mice are shown as means ± SEM.
Results
Litomosoides sigmodontis infection prevents the development of diabetes in NOD mice
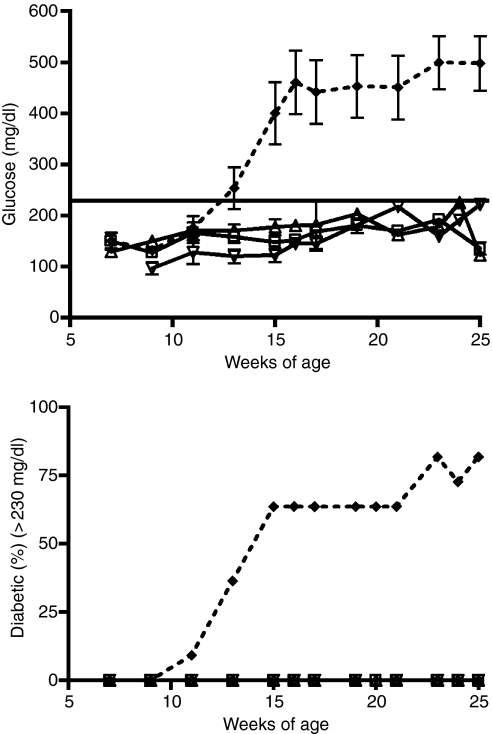
To determine whether infections with L. sigmodontis filarial worms prevent the onset of diabetes in NOD mice, 6-week-old female NOD mice were infected with either 40 L3-stage larvae (n= 3), five adult female worms (n= 7), or five adult male worms (n= 5) and glucose levels were monitored over time. As seen in Fig. 1, infection of NOD mice with either L3 larvae, adult female, or adult male worms prevented the onset of diabetes (glucose levels > 230 mg/dl) in all mice tested until the end of the experiment at 25 weeks of age (Fig. 1). In contrast, 63% of the sham-treated controls developed diabetes at 15 weeks of age (mean 400 mg/dl); rising up to 82% at 25 weeks of age (mean 500 mg/dl).
Figure 1.
Glucose levels (mg/dl) and percentages of diabetic mice (glucose > 230 mg/dl) over time. Mice were infected with either 40 L3 larvae (n= 3, triangles) or implanted intraperitoneally with five adult female worms (n= 7, squares) or five adult male worms (n= 5, upside down triangles). The dashed line shows sham-treated controls (n= 11). Means ± SEM are shown.
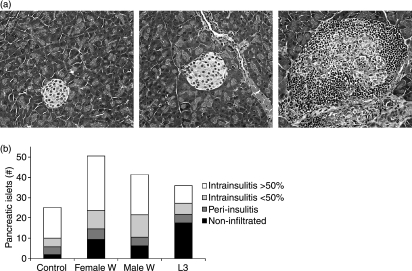
Histological examinations were performed 4 weeks after the control mice developed diabetes (17–24 weeks of age) to investigate whether worm infections decreased the destruction of pancreatic islets. For each pancreas, the total number of islets present in four different slides was counted and the degree of lymphocytic infiltration into each islet was classified as non-infiltrated, peri-insulitis, or intrainsulitis of less than or greater than 50% (Fig. 2a).
Figure 2.
(a) Representative examples of the classification of islets as non-infiltrated (left panel), peri-insulitis (middle panel) and intrainsulitis > 50% (right panel). (b) Mean total numbers of pancreatic islets counted from four slides. Pancreatic islets were classified as non-infiltrated, peri-insulitis, and intrainsulitis with less than or greater than 50% infiltrated lymphocytes. Mice were infected with either 40 L3 larvae (n= 6), implanted intraperitoneally with five adult female worms (n= 7) or five adult male worms (n= 5), or were sham-treated (n= 7). Combined data from two independent experiments are shown.
Histological examinations showed that mice infected with female adult worms (mean 51 islets ± 36), male adult worms (mean 41 ± 13) and L3 larvae (mean 36 ± 19) had, on average, more total pancreatic islets than sham-treated mice (mean 25 ± 13) (Fig. 2b). Litomosoides sigmodontis infection did not prevent the development of peri- or intrainsulitis in the animals, but the numbers of non-infiltrated islets were greater in both female worm-infected and L3-infected mice than in sham-treated mice. Interestingly, five out of six L3-infected mice had healthy islets, compared to only four out of seven mice in the female adult worm-infected or sham-treated groups and just one out of five mice in the male-implanted group (data not shown).
Necropsy studies revealed that, as expected, adult worms surgically implanted into the peritoneal cavity of NOD mice migrated within the peritoneal cavity, but did not leave that anatomic space. While mice implanted with adult worms did not have viable worms remaining in the peritoneal cavity at the end of the study, anatomic studies of mice infected with L3 larvae demonstrated that these infectious-stage larval worms had successfully migrated to the pleural cavity (the usual habitat of adult L. sigmodontis worms) and developed into adult worms. At the study end-point one to three viable worms remained present in the pleural cavity of all L3-infected NOD mice (data not shown). Infection with neither L3-stage larvae nor adult L. sigmodontis worms resulted in circulating microfilariae (L1-stage larvae) at any time-point, suggesting that NOD mice are not permissive for L. sigmodontis reproduction.
Litomosoides sigmodontis infection of NOD mice is associated with a Th2 cytokine shift
To examine if L. sigmodontis-infected NOD mice develop a Th2 immune shift, we isolated spleen cells from infected or non-infected NOD mice 17–24 weeks old and cultured them in the presence of α-CD3/α-CD28 or without stimulant. Flow analysis and ELISA were performed to assess total numbers of Th1- and Th2-cytokine-producing CD4+ T cells and cytokine concentrations.
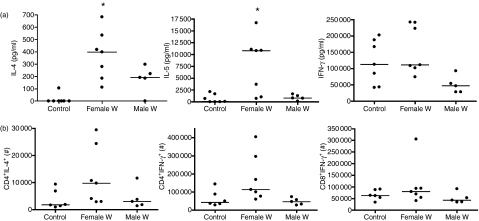
Implantation of female worms in NOD mice resulted in a Th2 immune shift which was characterized by significantly greater IL-4 and IL-5 release by splenocytes in response to α-CD3/α-CD28 stimulation compared to sham-treated controls (Fig. 3a, IL-4: female worms median 398 pg/ml, control 0 pg/ml, P < 0·01; IL-5: female worms 10 815 pg/ml, control 67 pg/ml, P < 0·05). Concentrations of IL-4 and IL-5 in the culture supernatants of α-CD3/α-CD28-stimulated splenocytes of NOD mice infected with male worms were also greater compared to the cytokine levels released from control groups (Fig. 3a, male worms median: IL-4 192 pg/ml, IL-5 799 pg/ml), although the differences did not reach statistical significance.
Figure 3.
(a) In vitro interleukin-4 (IL-4), IL-5 and interferon-γ (IFN-γ) production from α-CD3/α-CD28-stimulated spleen cell culture supernatants and (b) total numbers of α-CD3/α-CD28-stimulated splenic CD4+ IL-4+, CD4+ IFN-γ+ and CD8+ IFN-γ+ T cells from sham-treated controls (n= 11) or mice that were intraperitoneally implanted with five female (n= 7) or male (n= 5) adult worms. Statistical significances between groups were analysed by the Kruskal–Wallis test, followed by Dunn’s post-hoc multiple comparisons. Significant differences are shown between infected mice and controls (*P < 0·05). Combined data from two independent experiments are shown.
Flow analysis from spleen cells revealed that mice infected with female worms showed a tendency to increased numbers of CD4+ IL-4+ spleen cells after stimulation with α-CD3/α-CD28 in comparison to sham-treated controls or mice with male worms but this difference did not reach statistical significance (Fig. 3b).
In contrast to the findings with Th2 cytokines, infection of NOD mice with female or male worms did not change the concentrations of IFN-γ released in spleen cell culture supernatants (Fig. 3a) or the numbers of CD4+ IFN-γ+ or CD8+ IFN-γ+ spleen cells in response to α-CD3/α-CD28 (Fig. 3b) compared to the sham-treated controls.
With regards to the autoantigen-specific immune response, we examined the cytokine response of spleen cells from infected NOD mice and controls to insulin. In all mice studied, insulin stimulation did not result in elevated IL-4, IL-5 or IFN-γ cytokine concentrations in spleen cell culture supernatants or in increased numbers of IL-4-positive or IFN-γ-positive CD4+ or CD8+ spleen cells compared to unstimulated cells. Consequently, no differences in cytokine production between infected or non-infected mice were observed in response to insulin (data not shown).
General and insulin-specific Th2 shift in antibody production in infected NOD mice
To investigate if the observed Th2 shift in cytokine production in L. sigmodontis-infected NOD mice was also present at the antibody level, especially with regard to a diabetes-associated autoantigen, we analysed total IgE and insulin-specific IgG1 antibody concentrations in the plasma from 17- to 24-week-old L. sigmodontis-infected NOD mice and controls.
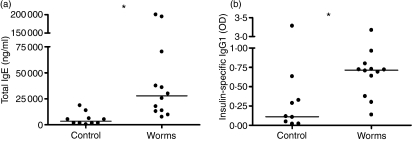
As seen in Fig. 4(a), infection of NOD mice with L. sigmodontis worms resulted in a significant increase in total circulating IgE compared to controls (median 27 791 versus 3536 ng/ml, P < 0·001), indicating a generalized Th2 shift in antibody production. Total IgE levels were highest in mice infected with adult female worms (P < 0·01), and intermediate in mice infected with male adult worms (P < 0·05) compared to uninfected controls (data not shown).
Figure 4.
(a) Concentrations of total immunoglobulin E (IgE; ng/ml) and (b) levels of insulin-specific IgG1 in the plasma of mice that were implanted intraperitoneally with five female or male adult worms (n= 12) or sham-treated controls (n= 11). Significant differences between groups were analysed by the Mann–Whitney U-test (*P < 0·05). Plasma samples were obtained from two independent experiments and were analysed the same day.
Interestingly, insulin-specific IgG1 levels were also significantly increased in infected NOD mice compared to uninfected mice (median OD of 0·71 versus 0·11, P < 0·01, Fig. 4b). As IgG1 antibodies are observed in settings of Th2-skewed immune responses, this finding is consistent with an autoantigen-specific Th2 shift.
Infection with female worms increased the numbers of splenic CD4+ CD25+ FoxP3+ regulatory T cells
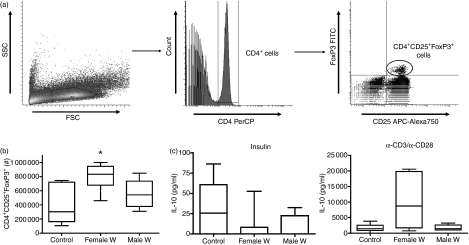
As regulatory T cells are known to induce immunological tolerance, we examined if the protection against diabetes by implantation of L. sigmodontis worms is associated with increased CD4+ CD25+ FoxP3+ regulatory T-cell numbers by measuring total numbers of splenic CD4+ CD25+ FoxP3+ regulatory T cells with multicolour flow cytometry (Fig. 5a).
Figure 5.
(a) Gating strategy for flow cytometric identification of regulatory T cells. Lymphocytes were gated by forward-scatter (FSC) and side-scatter (SSC) characteristics (left panel). CD4 peridinin chlorophyll protein (PerCP)-positive lymphocytes were gated (middle panel) and analysed for CD25 allophycocyanin (APC) -Alexa750 positivity and FoxP3 fluorescein isothiocyanate (FITC) positivity (right panel). (b) Total numbers of spontaneous splenic CD4+ CD25+ FoxP3+ regulatory T cells and (c) interleukin-10 (IL-10) production of insulin (middle panel) or α-CD3/α-CD28 (right panel)-stimulated spleen cell cultures from sham-treated controls (n= 11) or mice that were intraperitoneally implanted with five female (n= 7) or male (n= 5) adult worms. Significant differences between groups were analysed by the Kruskal–Wallis test, followed by Dunn’s post-hoc multiple comparisons. Significant differences are shown between infected mice and controls (*P < 0·05). Combined data from two independent experiments are shown.
Mice that were infected with female worms had significantly increased numbers of splenic CD4+ CD25+ FoxP3+ regulatory T cells compared to uninfected controls (median 835 000 versus 301 076, P < 0·05, Fig. 5b), whereas infection with male worms did not significantly increase regulatory T-cell numbers (median 541 620) compared to controls (Fig. 5b). When analysis was performed with respect to the percentages of CD4+ T cells that were also CD25+ FoxP3+, regulatory T cells were found to be greater in both mice infected with male worms (median 11·5% of CD4+ T cells) and those infected with female worms (median 9·4%) when compared to uninfected controls (median 7·5%), though only the difference between male worm-infected mice and uninfected controls was significant (P < 0·05, data not shown).
Spleen cell cultures from infected mice and controls were performed to investigate if L. sigmodontis infection of NOD mice is associated with increased splenocyte production of IL-10 in response to insulin or α-CD3/α-CD28 or with changes in the proliferative responses of spleen cells to these stimuli.
No differences were found in IL-10 concentrations in spleen cell cultures of mice infected with either adult male or adult female worms compared to uninfected controls (Fig. 5c). While there was a trend for splenocytes of mice infected with adult female worms to produce more IL-10 in response to α-CD3/α-CD28 than splenocytes of uninfected mice, this difference was not statistically significant. More CD4+ FoxP3− cells released IL-10 than CD4+ FoxP3+ cells, suggesting that inducible T-regulatory cells were the predominant source of IL-10 in these mice (data not shown). Also, there were no significant changes between infected or sham-treated controls in spleen cell proliferation in response to insulin or α-CD3/α-CD28 stimulation (data not shown).
Crude homogenate of L. sigmodontis antigens protects against diabetes in NOD mice
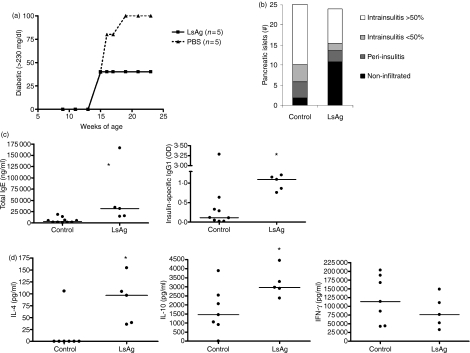
To test if infections with living worms are required to prevent the onset of diabetes, we administered a crude homogenate of adult L. sigmodontis worm antigens (LsAg) or PBS weekly to NOD mice. At the age of 15 weeks, two of five mice developed diabetes, irrespective of injections with either LsAg or PBS (Fig. 6a). While treatment with LsAg prevented any additional cases of diabetes through the end of the experiment at 24 weeks of age, all of the control mice developed diabetes by week 19 (Fig. 6a). Histological examinations revealed that LsAg-treated mice had greater numbers of non-infiltrated islets than control mice, though the average numbers of total pancreatic islets remaining in LsAg-treated mice were not greater than controls (Fig. 6b). Repetition of this experiment to 21 weeks demonstrated similar results, with mean glucose levels of 402 mg/dl in control mice and 213 mg/dl in LsAg-treated mice.
Figure 6.
Comparison of non-obese diabetic (NOD) mice that were injected weekly with parasite antigen (LsAg, n= 5) and controls. (a) Percentages of diabetic mice (glucose > 230 mg/dl) over time and (b) number of pancreatic islets counted from four slides. Pancreatic islets were classified into non-infiltrated, peri-insulitis and intrainsulitis with less than or greater than 50% infiltrating lymphocytes. (c) Concentrations of total immunoglobulin E (IgE; left panel) and levels of insulin-specific IgG1 (right panel) in the sera of mice. (d) Concentrations of interleukin-4 (IL-4), IL-10 and interferon-γ (IFN-γ) in spleen cell culture supernatants in response to α-CD3/α-CD28. Significant differences were analysed by Mann–Whitney U-test (*P < 0·05).
To compare the results from LsAg-injected mice with our previous data from worm-infected mice and controls, immunological studies that evaluated splenic Th1 and Th2 cytokine production, peripheral antibody levels, splenocyte IL-10 production and numbers of splenic CD4+ CD25+ FoxP3+ regulatory T cells were conducted.
These studies revealed a Th2 shift in cytokine levels as well as antibody levels in mice that were treated with LsAg. The antibody concentrations of total IgE and insulin-specific IgG1 were significantly greater in LsAg-injected NOD mice compared to controls (total IgE: median 31 811 versus 3536 ng/ml, P = 0·003; insulin-specific IgG1: median OD of 1·09 versus 0·11, P = 0·0127, Fig. 6c). Furthermore, concentrations of IL-4 (Fig. 6d, median 97 pg/ml) and IL-5 (data not shown, median 2570 pg/ml) obtained from α-CD3/α-CD28 stimulated spleen cell cultures from LsAg-injected mice were significantly higher than from the control groups (median IL-4: 0 pg/ml, IL-5: 67 pg/ml), whereas IFN-γ concentrations were similar among the different groups (Fig. 6d).
Compared to mice infected with live worms, Th2 cytokine production was lower in LsAg-treated mice (see Fig. 3b: median IL-4: female worms 398 pg/ml, male worms 192 pg/ml; median IL-5: female worms 10 815 pg/ml and male worms 799 pg/ml). However, the insulin-specific-IgG1 levels were highest in the LsAg-treated group when plasma from all the groups of mice were run concurrently on the same ELISA plates.
In contrast to infections with live adult female worms, LsAg injections increased the frequencies, but not the total numbers, of splenic CD4+ CD25+ FoxP3+ regulatory T cells (data not shown). Administration of LsAg also increased significantly the IL-10 concentration in α-CD3/α-CD28 stimulated spleen cell culture supernatants compared to controls (Fig. 6d).
Discussion
The results of this study demonstrate that filarial worms prevent the onset of type 1 diabetes in NOD mice. The immunological studies conducted show for the first time that helminth-mediated protection against autoimmunity is associated with increases in numbers of natural T-regulatory cells and that helminth infections can induce a Th2 shift in autoantigen-specific antibody production. As has been shown with other helminths,21–23 live infection is not required for protection against autoimmune disease as treatment with a crude homogenate of L. sigmodontis antigens also protected against diabetes.
Consistent with our findings, several previous animal studies with diverse helminth parasites, including S. mansoni,12,14–16T. spiralis13,18 and H. polygyrus,13,17 have shown that helminth infections exert a beneficial effect on the outcome of autoimmune diseases. Indeed, animal models of arthritis,22 type I diabetes,12–14,21,24 Graves hyperthyroidism,25 experimental autoimmune encephalitis15,26 and colitis16–18 have all been inhibited or ameliorated by infections with helminth parasites or parasite-derived products. In this study, we found that infection with filarial helminths also protects against autoimmunity. The broad range of helminth pathogens that can be immunoprotective and the large number of autoimmune models that can be improved by helminths suggest that a common mechanism of protection is in effect. This study demonstrates that different life cycle stages and worm genders of the filaria L. sigmodontis all have the ability to prevent diabetes onset in NOD mice, suggesting that the mechanisms by which helminths protect against autoimmunity are specific to neither worm stage nor gender.
The results of the histopathological studies conducted on infected mice suggest that autoimmune protection induced by L. sigmodontis is dependent on the continuous presence of the worms. While all worm groups in our study exerted protective effects against type 1 diabetes in NOD mice, pancreases of mice with L3 infections had the greatest percentages of normal, non-infiltrated islets. Unlike the implantation experiments with adult worms, in which no adult worms were found by weeks 17–24, all mice initially infected with L3-stage larval worms still had a few worms present at study end-point. Furthermore, immunological studies performed on two mice infected with L3 larvae showed the highest numbers of regulatory T cells and the strongest Th2 shift compared to the other groups. These findings suggest that the protective effects mediated by worm infections are dependent on the continuous presence of the worms. Also, while no infected mice developed overt clinical diabetes, the presence of at least some infiltrated islets in virtually all infected mice at the study end-point suggest that infection with L. sigmodontis inhibits rather than completely prevents disease progression.
As in our study, protection against the onset of diabetes by helminths or their antigens in other studies has been uniformly associated with a general Th2 shift.12–14,21,24 However, none of these studies were able to show an autoantigen-specific shift in the immune response towards Th2. Although we were also not able to detect autoantigen-specific cytokine responses, we were able to demonstrate a general increase in IL-4 and IL-5 production from spleen cells in helminth-infected NOD mice. That this generalized Th2 shift was accompanied by an increase in circulating levels of insulin-specific IgG1 antibodies suggests that helminth infection also induces a Th2 shift with respect to the autoantigens involved in diabetes.
This Th2 shift may play a role in helminth-mediated autoimmune protection. Since IL-4 suppresses differentiation of naïve T cells into Th1 cells, a Th2 shift might protect against diabetes by reducing the Th1-driven β-islet cell destruction by autoreactive T cells in diabetes. Indeed, there are several lines of evidence that IL-4 can protect against Th1-driven autoimmune diseases. Administration of IL-4-expressing cells improves proteoglycan-induced arthritis in BALB/c mice27 and local delivery of IL-4 by retrovirus-transduced lymphocytes or plasmid DNA vaccination against GAD65 has been shown to improve the courses of experimental autoimmune encephalomyelitis28 and type 1 diabetes in NOD mice,29 respectively. Future experiments will assess if helminth infections protect against the onset of diabetes in IL-4-deficient NOD mice.
Another possible mechanism by which helminths may protect against autoimmune diseases may be through the induction of regulatory T cells. While regulatory T cells can have several effects on multiple cell types, their main role appears to be suppression of antigen-reactive T cells. In the case of type 1 diabetes, regulatory T cells may be able to suppress the destruction of β-islet cells by self-reactive CD4+ or CD8+ T cells. Before this study, however, frequencies of CD4+ CD25+ FoxP3+ regulatory T cells had not been evaluated in models of helminth protection against autoimmunity. In this study, we demonstrated by multicolour fluorescence-activated cell sorting analysis that helminth-mediated protection against the development of type 1 diabetes was associated with an increase in splenic CD4+ CD25+ FoxP3+ regulatory T cells. As they are FoxP3+, these regulatory T cells are most consistent with ‘natural’ regulatory T cells, which characteristically exert their suppressive effects in an IL-10-independent but contact-dependent manner. While development of diabetes in NOD mice is not associated with changes in the numbers of natural regulatory T cells,30,31 there is mounting evidence that development of type 1 diabetes is associated with a qualitative defect in regulatory T-cell function. The suppressive capability of natural regulatory T cells of NOD mice decreases with age32 and T-regulatory cells expanded from 4-week-old NOD mice are more protective against diabetes than those expanded from older NOD mice.33 Since a single administration of islet-specific regulatory T cells can block diabetes development in 13-week-old prediabetic NOD mice,34 we hypothesize that the increase in CD4+ CD25+ FoxP3+ regulatory T cells observed in L. sigmodontis-infected NOD mice may be a mechanism by which helminths protect against autoimmune disease. Determining whether this hypothesis is correct, and assessing whether helminth infection affects regulatory T-cell functionality, will be the focus of future studies.
The administration of a crude homogenate of L. sigmodontis worm antigens showed that a protective effect against the onset of diabetes in NOD mice can be achieved without the need for living worm infections. Similar to infections with living worms, LsAg injections induced a Th2 immune response. However, this Th2 shift was not as strong as after infection with live L. sigmodontis worms, and was associated with a greater increase in splenic IL-10 production than was seen in NOD mice infected with live worms. Also, while LsAg increased frequencies of splenic CD4+ CD25+ FoxP3+ regulatory T cells, it did not increase the total numbers of these natural regulatory T cells. Similar to the LsAg administration in our experiment, studies with S. mansoni egg or worm antigens and infection with H. polygyrus were associated with increased IL-10 levels in NOD mice.13,24 This inconsistency between infections with live L. sigmodontis worms and injections with the crude antigen preparation may be the result of experimental variability or may be the result of actual differences in the mechanisms by which these interventions protect against type 1 diabetes. Furthermore, as the crude homogenate may contain substances that reduce or inhibit the protective components within the homogenate, isolation of the protective components within LsAg in the future may improve the protective effect of parasite antigen.
In summary, we have shown that infection of NOD mice with filarial worms prevents the onset of diabetes. This protection is specific to neither worm stage nor gender and is associated with a generalized Th2 shift in cytokine production, a Th2 shift in insulin-specific antibody production, and with increased numbers of CD4+ CD25+ FoxP3+ regulatory T cells. Future studies will attempt to determine exactly which mechanisms are most responsible for helminth-mediated protection against autoimmunity, with particular focus on the role of helminth-induced natural regulatory T cells. Additionally, as administration of a homogenate of crude L. sigmodontis worm antigens was also protective, future identification of the exact molecules within LsAg responsible for protection against type 1 diabetes may result in the discovery of novel therapeutic compounds for autoimmune diseases.
Acknowledgments
We thank David Larson, Ellen Mueller and Marina N. Torrero for technical assistance on this project. Additionally, we thank Karen Wolcott and Kateryna Lund at the USUHS Biomedical Instrumentation Center for their valuable assistance with flow cytometry. This work was supported by grant number R073MX from the Uniformed Services University of the Health Sciences.
References
- 1.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–7. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 2.Kay TW, Chaplin HL, Parker JL, Stephens LA, Thomas HE. CD4+ and CD8+ T lymphocytes: clarification of their pathogenic roles in diabetes in the NOD mouse. Res Immunol. 1997;148:320–7. doi: 10.1016/s0923-2494(97)87241-0. [DOI] [PubMed] [Google Scholar]
- 3.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268:1185–8. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 4.Pugliatti M, Sotgiu S, Rosati G. The worldwide prevalence of multiple sclerosis. Clin Neurol Neurosurg. 2002;104:182–91. doi: 10.1016/s0303-8467(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 5.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 6.Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 2002;51:3353–61. doi: 10.2337/diabetes.51.12.3353. [DOI] [PubMed] [Google Scholar]
- 7.Onkamo P, Vaananen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of type I diabetes – the analysis of the data on published incidence trends. Diabetologia. 1999;42:1395–403. doi: 10.1007/s001250051309. [DOI] [PubMed] [Google Scholar]
- 8.Elliott DE, Summers RW, Weinstock JV. Helminths as governors of immune-mediated inflammation. Int J Parasitol. 2007;37:457–64. doi: 10.1016/j.ijpara.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Zaccone P, Fehervari Z, Phillips JM, Dunne DW, Cooke A. Parasitic worms and inflammatory diseases. Parasite Immunol. 2006;28:515–23. doi: 10.1111/j.1365-3024.2006.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61:97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- 11.Fleming JO, Cook TD. Multiple sclerosis and the hygiene hypothesis. Neurology. 2006;67:2085–6. doi: 10.1212/01.wnl.0000247663.40297.2d. [DOI] [PubMed] [Google Scholar]
- 12.Cooke A, Tonks P, Jones FM, O’Shea H, Hutchings P, Fulford AJ, Dunne DW. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21:169–76. doi: 10.1046/j.1365-3024.1999.00213.x. [DOI] [PubMed] [Google Scholar]
- 13.Saunders KA, Raine T, Cooke A, Lawrence CE. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect Immun. 2007;75:397–407. doi: 10.1128/IAI.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Wakil HS, Aboushousha TS, El Haddad O, Gamil NB, Mansour T, El-Said H. Effect of Schistosoma mansoni egg deposition on multiple low doses streptozotocin induced insulin dependent diabetes. J Egypt Soc Parasitol. 2002;32:987–1002. [PubMed] [Google Scholar]
- 15.La Flamme AC, Ruddenklau K, Backstrom BT. Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infect Immun. 2003;71:4996–5004. doi: 10.1128/IAI.71.9.4996-5004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF, Jr, Weinstock JV. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G385–91. doi: 10.1152/ajpgi.00049.2002. [DOI] [PubMed] [Google Scholar]
- 17.Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Jr, Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur J Immunol. 2004;34:2690–8. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- 18.Khan WI, Blennerhasset PA, Varghese AK, Chowdhury SK, Omsted P, Deng Y, Collins SM. Intestinal nematode infection ameliorates experimental colitis in mice. Infect Immun. 2002;70:5931–7. doi: 10.1128/IAI.70.11.5931-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Summers RW, Elliott DE, Urban JF, Jr, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn’s disease. Gut. 2005;54:87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Summers RW, Elliott DE, Urban JF, Jr, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–32. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Imai S, Tezuka H, Fujita K. A factor of inducing IgE from a filarial parasite prevents insulin-dependent diabetes mellitus in nonobese diabetic mice. Biochem Biophys Res Commun. 2001;286:1051–8. doi: 10.1006/bbrc.2001.5471. [DOI] [PubMed] [Google Scholar]
- 22.McInnes IB, Leung BP, Harnett M, Gracie JA, Liew FY, Harnett W. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol. 2003;171:2127–33. doi: 10.4049/jimmunol.171.4.2127. [DOI] [PubMed] [Google Scholar]
- 23.Zaccone P, Burton OT, Cooke A. Interplay of parasite-driven immune responses and autoimmunity. Trends Parasitol. 2008;24:35–42. doi: 10.1016/j.pt.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Zaccone P, Fehervari Z, Jones FM, Sidobre S, Kronenberg M, Dunne DW, Cooke A. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol. 2003;33:1439–49. doi: 10.1002/eji.200323910. [DOI] [PubMed] [Google Scholar]
- 25.Nagayama Y, Watanabe K, Niwa M, McLachlan SM, Rapoport B. Schistosoma mansoni and alpha-galactosylceramide: prophylactic effect of Th1 immune suppression in a mouse model of Graves’ hyperthyroidism. J Immunol. 2004;173:2167–73. doi: 10.4049/jimmunol.173.3.2167. [DOI] [PubMed] [Google Scholar]
- 26.Sewell D, Qing Z, Reinke E, Elliot D, Weinstock J, Sandor M, Fabry Z. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int Immunol. 2003;15:59–69. doi: 10.1093/intimm/dxg012. [DOI] [PubMed] [Google Scholar]
- 27.Finnegan A, Mikecz K, Tao P, Glant TT. Proteoglycan (aggrecan)-induced arthritis in BALB/c mice is a Th1-type disease regulated by Th2 cytokines. J Immunol. 1999;163:5383–90. [PubMed] [Google Scholar]
- 28.Shaw MK, Lorens JB, Dhawan A, et al. Local delivery of interleukin 4 by retrovirus-transduced T lymphocytes ameliorates experimental autoimmune encephalomyelitis. J Exp Med. 1997;185:1711–4. doi: 10.1084/jem.185.9.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tisch R, Wang B, Weaver DJ, Liu B, Bui T, Arthos J, Serreze DV. Antigen-specific mediated suppression of beta cell autoimmunity by plasmid DNA vaccination. J Immunol. 2001;166:2122–32. doi: 10.4049/jimmunol.166.3.2122. [DOI] [PubMed] [Google Scholar]
- 30.Gregori S, Giarratana N, Smiroldo S, Adorini L. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J Immunol. 2003;171:4040–7. doi: 10.4049/jimmunol.171.8.4040. [DOI] [PubMed] [Google Scholar]
- 31.Mellanby RJ, Thomas D, Phillips JM, Cooke A. Diabetes in non-obese diabetic mice is not associated with quantitative changes in CD4+ CD25+ Foxp3+ regulatory T cells. Immunology. 2007;121:15–28. doi: 10.1111/j.1365-2567.2007.02546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tritt M, Sgouroudis E, d’Hennezel E, Albanese A, Piccirillo CA. Functional waning of naturally occurring CD4+ regulatory T-cells contributes to the onset of autoimmune diabetes. Diabetes. 2008;57:113–23. doi: 10.2337/db06-1700. [DOI] [PubMed] [Google Scholar]
- 33.Gregg RK, Jain R, Schoenleber SJ, Divekar R, Bell JJ, Lee HH, Yu P, Zaghouani H. A sudden decline in active membrane-bound TGF-beta impairs both T regulatory cell function and protection against autoimmune diabetes. J Immunol. 2004;173:7308–16. doi: 10.4049/jimmunol.173.12.7308. [DOI] [PubMed] [Google Scholar]
- 34.Tarbell KV, Petit L, Zuo X, et al. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med. 2007;204:191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]