Abstract
Matrix metalloproteinases (MMP) can degrade all components of pulmonary extracellular matrix. Mycobacterium tuberculosis induces production of a number of these enzymes by human macrophages, and these are implicated in the pathogenesis of pulmonary cavitation in tuberculosis. The active metabolite of vitamin D, 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3], has previously been reported to inhibit secretion of MMP-9 in human monocytes (MN), but its influence on the secretion and gene expression of MMP and tissue inhibitors of MMP (TIMP) in M. tuberculosis-infected cells has not previously been investigated. We therefore determined the effects of 1α,25(OH)2D3 on expression, secretion and activity of a number of MMP and TIMP in M. tuberculosis-infected human leucocytes; we also investigated the effect of 1α,25(OH)2D3 on the secretion of interleukin-10 (IL-10) and prostaglandin E2 (PGE2), both transcriptional regulators of MMP expression. We found that M. tuberculosis induced expression of MMP-1, MMP-7 and MMP-10 in MN and MMP-1 and MMP-10 in peripheral blood mononuclear cells (PBMC). 1α,25(OH)2D3 significantly attenuated M. tuberculosis-induced increases in expression of MMP-7 and MMP-10, and suppressed secretion of MMP-7 by M. tuberculosis-infected PBMC. MMP-9 gene expression, secretion and activity were significantly inhibited by 1α,25(OH)2D3 irrespective of infection. In contrast, the effects of 1α,25(OH)2D3 on the expression of TIMP-1, TIMP-2 and TIMP-3 and secretion of TIMP-1 and TIMP-2 were small and variable. 1α,25(OH)2D3 also induced secretion of IL-10 and PGE2 from M. tuberculosis-infected PBMC. These findings represent a novel immunomodulatory role for 1α,25(OH)2D3 in M. tuberculosis infection.
Keywords: interleukin-10, matrix metalloproteinases, mycobacteria, TB, tissue inhibitors of matrix metalloproteinases, vitamin D
Introduction
Tuberculosis (TB) is the world’s leading bacterial cause of death.1 The ability of Mycobacterium tuberculosis to induce degradation of pulmonary extracellular matrix contributes to its success as a pathogen: induction of cavitation allows bacilli to replicate in an immunologically privileged site, promoting persistence and transmission, while penetration of the alveolar basement membrane allows extra-pulmonary dissemination of infection. Matrix metalloproteinases (MMP), a family of zinc- and calcium-dependent endopeptidases, are capable of degrading all components of pulmonary extracellular matrix. Generally, MMP are not expressed in healthy non-calcified tissues, but they are upregulated in activated cells where their primary role is to facilitate tissue remodelling and repair. They also regulate the innate immune response by controlling cytokine and chemokine processing, apoptosis and antimicrobial peptide activation (for review see ref. 2) Excess MMP activity in response to M. tuberculosis infection may therefore lead to excessive tissue degradation and, ultimately, pulmonary cavitation.
There are 24 known mammalian MMP which possess broad and overlapping specificities. They are expressed by a wide variety of cells, including lymphocytes, resting MN and activated macrophages. MMP-1 (interstitial collagenase) and MMP-9 (92 000 molecular weight gelatinase B) are the major secreted MMP of human MN and alveolar macrophages under basal conditions and on stimulation with lipopolysaccharide, phorbol 12-myristate 13-acetate or concanavalin A.3Mycobacterium tuberculosis induces the expression of MMP-1, MMP-7 and MMP-10 in human macrophages4 and increased expression of MMP-1, MMP-7 and MMP-9 has also been demonstrated in cells isolated from the lungs of TB patients, with MMP-1 and MMP-7 colocalizing to macrophages around the central area of necrosis in tuberculous granulomata.4,5 Furthermore, circulating concentrations of MMP-9 have also been shown to correlate with the severity of pulmonary TB.6
The catalytic activity of MMP is primarily transcriptionally regulated, with a more delicate control achieved via pro-enzyme proteolytic activation and enzyme inhibition. Tissue inhibitors of matrix metalloproteinases (TIMP) are the major inhibitors of MMP activity; they function by binding the MMP catalytic site with 1 : 1 stoichiometry.7,8 The four TIMP that have been identified share broad specificities and show constitutive expression in a variety of cells. Expression of TIMP-2 and TIMP-3 has been shown to decrease during M. tuberculosis infection of human macrophages, while TIMP-1 expression is suppressed by M. tuberculosis in human pulmonary epithelial cells.4,9
The regulation of MMP and TIMP expression is complex and not yet fully understood. The anti-inflammatory cytokine interleukin-10 (IL-10) has previously been reported to inhibit mononuclear phagocyte MMP-1, MMP-7 and MMP-9 secretion and to induce TIMP-1 expression.10,11 The eicosanoid inflammatory mediator prostaglandin E2 (PGE2) also regulates MMP expression and secretion: in mononuclear phagocytes, secretion of MMP-1 and MMP-7 is PGE2-dependent,4,11,12 while in cocultures of MN and fibroblasts, PGE2 suppresses the secretion and activation of MMP-1.13
The active metabolite of vitamin D, 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3], has previously been reported to inhibit both basal and staphylococcus-stimulated production of MMP-9, but not MMP-1, in human MN and alveolar macrophages.14 1α,25(OH)2D3 also upregulates the IL-10 receptor,15 induces IL-10 synthesis16,17 and modulates gene expression of inducible prostaglandin H synthase-2 (PGHS-2, a rate-limiting enzyme in PGE2 synthesis) and type 1 15-hydroxyprostaglandin dehydrogenase (15-PGDH, the key enzyme in PGE2 catabolism) in vitro.18,19 Specific affinity receptors for 1α,25(OH)2D3 are present on a number of leucocytes, including resting MN, macrophages and T and B lymphocytes.20,21 Furthermore, it has recently been shown that synthesis of 1α,25(OH)2D3 may occur in tuberculous granulomata as a consequence of M. tuberculosis-induced upregulation of CYP27B1, the enzyme that converts 25-hydroxyvitamin D3 to 1α,25(OH)2D3.22 Reports that interactions between T cells and mononuclear phagocytes enhance MMP expression in both cell types11,22–25 provide a rationale for investigating MMP regulation in peripheral blood mononuclear cells (PBMC) as well as MN. We therefore investigated whether 1α,25(OH)2D3 modulates expression, secretion or activity of MMP-1, MMP-7, MMP-9 and MMP-10 in M. tuberculosis-infected PBMC and MN, and whether the changes observed were associated with changes in the expression or secretion of TIMP-1, TIMP-2, TIMP-3, IL-10, PGHS-2, 15-PGDH and PGE2.
Materials and methods
Culture of M. tuberculosis
Mycobacterium tuberculosis H37Rv was grown to mid-log phase in Middlebrook 7H9 broth supplemented with 10% albumin dextrose catalase (Difco, Detroit, MI, USA) and 0·04% Tween 80 (Sigma, St Louis, MO, USA). An equal volume of 30% glycerol was added, and aliquots of the resulting suspension of bacilli were placed into vials. Colony forming units (CFU) per millilitre for each batch of aliquots was determined by plating serial dilutions onto 7H11 agar before freezing at −80°. Vials were defrosted immediately before tissue culture experiments, and knowledge of CFU/ml determined at the time of freezing was used to calculate the volume of inoculum necessary to achieve the desired multiplicity of infection (MOI). For each inoculum the CFU/ml was also determined by plating onto 7H11 agar at the time of infection to confirm that MOI was correct.
Culture of PBMC and MN with 1α,25(OH)2D3 and M. tuberculosis
The PBMC were isolated from buffy coats of healthy blood donors over Ficoll, and MN preparations were obtained by adherence as previously described.26,27 For initial experiments to determine the influence of 1α,25(OH)2D3 and M. tuberculosis on messenger RNA (mRNA) levels, cells from 10 donors were plated in six-well plates at 5 × 106 PBMC/MN in 2 ml RPMI 1640/10% fetal calf serum and incubated with either 10−6 m 1α,25(OH)2D3 dissolved in ethanol (0·1% final concentration) or 0·1% ethanol alone for 72 hr. Cells were then infected with M. tuberculosis H37Rv. For MN, the MOI bacillus : mononuclear phagocyte was 1 : 1; non-phagocytosed bacilli were washed off 4 hr postinfection. For PBMC, MOI was normalized to the average MN count (10% PBMC), giving MOI bacillus : PBMC 0·1 : 1. Infected cells were cultured in the continued presence or absence of 1α,25(OH)2D3 for a further 96 hr. Supernatants were harvested immediately before infection (for determination of constitutive cytokine and MMP secretion), at 24 hr postinfection (for determination of PGE2 concentration) and at 96 hr postinfection (for determination of IL-10 concentration). Supernatants from M. tuberculosis-infected cell culture were sterilized with polysulphone filters (Whatman, Brentford, UK). For subsequent experiments to determine the influence of 1α,25(OH)2D3 and M. tuberculosis on supernatant concentration and activity of MMP, PBMC from six donors were plated in 48-well plates at 2·5 × 106 PBMC in 1 ml RPMI-1640/10% fetal calf serum and incubated with either 0·1% ethanol, 10−6 m 1α,25(OH)2D3 dissolved in ethanol (0·1% final concentration), M. tuberculosis, or both M. tuberculosis and 1α,25(OH)2D3. The MOI bacillus : PBMC was 0·1 : 1. Supernatants were harvested after 72 hr incubation, and sterilized with polysulphone filters (Whatman).
RNA extraction and quantitative reverse transcription–polymerase chain reaction
RNA samples were extracted immediately following isolation of PBMC/MN (0 hr), immediately before infection (72 hr) and at 6 hr and 24 hr postinfection (78 hr and 96 hr post-seeding respectively). At each time-point, supernatants were aspirated and the cell monolayer was immediately lysed and shredded using the RNeasy extraction kit (Qiagen, Valencia, CA). RNA was reverse transcribed using the Quantitect reverse transcription kit (Qiagen) that includes a DNase digest step. Complementary DNA was used in quantitative polymerase chain reaction for MMP-1, MMP-7, MMP-9, MMP-10, TIMP-1, TIMP-2, TIMP-3, IL-10, PGHS-2, 15-PGDH and β-actin on the ABI Prism 7000 platform. Primers and probes were obtained as predeveloped assay reagents (Applied Biosystems, Foster City, CA) with the exception of β-actin, for which primer and probe sequences were as follows: forward primer: CCT GGCACCCAGCACAAT; reverse primer: GCCGATCCACACGGAGTACT; probe: 5′-VIC-ATCAAGATCATTGCTCCTCCTGAGCGC-BQ.
Each reaction was multiplexed by, and normalized to, the β-actin content. Fold induction over unseeded cell samples (0 hr) was calculated by the ΔΔCT method as described elsewhere (User Bulletin #2, available for download from http://www.appliedbiosystems.com).
Enzyme-linked immunosorbent assay
Supernatant concentrations of MMP-1, MMP-7, MMP-9, MMP-10, TIMP-1, TIMP-2, IL-10 and PGE2 were determined by enzyme-linked immunosorbent assay using kits purchased from the following manufacturers: R&D Systems, Abingdon, UK: IL-10 (sensitivity, 27 pg/ml), total MMP-7 (0·2 ng/ml), total MMP-9 (9·7 ng/ml), total TIMP-1 (1·2 ng/ml) and PGE2 (58 pg/ml); Amersham Biosciences, Little Chalfont, UK: total MMP-1 (2·4 ng/ml); and RayBiotech, Norcross, GA: total MMP-10 (1·5 pg/ml), and TIMP-2 (10 pg/ml).
Enzyme activity assays
Supernatant activities of MMP-1 and MMP-9 were determined with colorimetric assays using modified pro-urokinase (Amersham Biosciences).28 Supernatant activity of MMP-7 was measured with a fluorimetric assay using 5-FAM labelled FRET peptide substrates (Anaspec, San Jose, CA). No kits for MMP-10 secretion were available with sufficient sensitivity.
Statistical analysis
RNA fold induction values were normalized by log10 transformation before statistical analysis. Gaussian distribution was confirmed using the D’Agostino and Pearson test. Statistical significance was tested using repeated measures analysis of variance with Bonferroni post hoc correction applied to tested comparisons (significance level, P < 0·05).
Results
1α,25(OH)2D3 inhibits constitutive expression, but not activity, of MMP
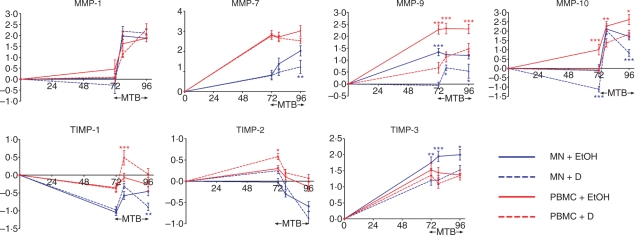
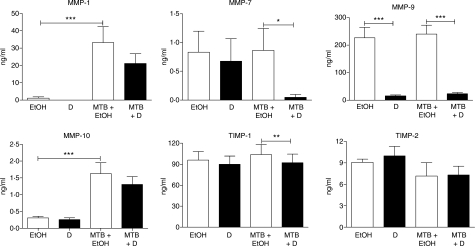
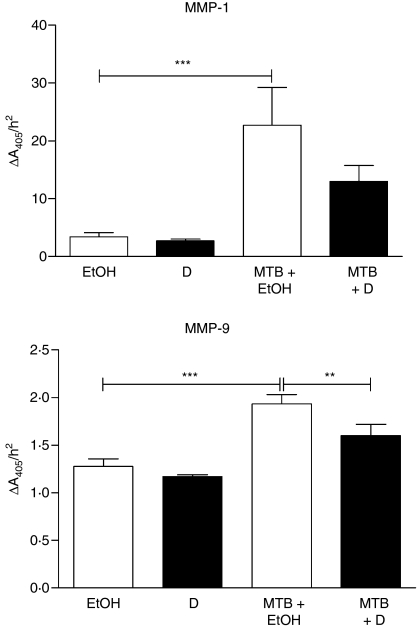
The mRNA levels of four MMP previously reported to be regulated by M. tuberculosis infection were analysed in human PBMC and MN cultured for 72 hr in the presence or absence of 1α,25(OH)2D3 (Fig. 1). In the absence of 1α,25(OH)2D3 an increase in gene expression of MMP-7 (664-fold in PBMC and sevenfold in MN, P < 0·01), MMP-9 (191-fold in PBMC and 22-fold in MN, P < 0·001) and MMP-10 (10-fold in PBMC, P < 0·01) was observed; no change in expression of MMP-1 was seen. Treatment with 1α,25(OH)2D3 inhibited constitutive expression of MMP-9 (40-fold in PBMC and 25-fold in MN, P < 0·001) and MMP-10 (12-fold in PBMC and 10-fold in MN, P < 0·001) but did not influence constitutive expression of MMP-1 or MMP-7. Concentrations of MMP were also determined in supernatants harvested after 72 hr of culture (Fig. 2): 1α,25(OH)2D3 suppressed the secretion of MMP-9 in PBMC (14-fold suppression, P < 0·001) but did not influence the secretion of MMP-7 or MMP-10. MMP-1 was undetectable in 10 of 10 1α,25(OH)2D3-treated samples versus 8 of 10 untreated samples. Activity of MMP-1, MMP-7 and MMP-9 in these supernatants was also assayed (Fig. 3): 1α,25(OH)2D3 did not influence the activity of MMP-1 or MMP-9, and MMP-7 activity was undetectable in all samples.
Figure 1.
Influence of 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3; D] and Mycobacterium tuberculosis (MTB) on gene expression of matrix metalloproteinases (MMP) and tissue inhibitors of matrix metalloproteinases (TIMP) in peripheral blood mononuclear cells (PBMC) and monocytes (MN). 1α,25(OH)2D3 suppressed expression of MMP-9 and MMP-10 in uninfected cells, while M. tuberculosis increased expression of MMP-1, MMP-7 and MMP-10 in untreated cells. When 1α,25(OH)2D3-treated cells were infected with M. tuberculosis, preinfection decreases in expression of MMP-9 and MMP-10 were maintained during infection; 1α,25(OH)2D3 also suppressed MN expression of MMP-7 in an infection-specific manner. In contrast, the effects of 1α,25(OH)2D3 and M. tuberculosis on TIMP gene expression were small and variable. Ten donors; error bars, SE; x-axis, hours postseeding; y-axis, mean log10 fold induction; SE; *P < 0·05; **P < 0·01; ***P < 0·001; EtOH = 0·1% ethanol vehicle.
Figure 2.
Influence of 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3; D] and Mycobacterium tuberculosis (MTB) on secretion of matrix metalloproteinases (MMP) and tissue inhibitors of matrix metalloproteinases (TIMP) by peripheral blood mononuclear cells (PBMC). 1α,25(OH)2D3 suppressed secretion of MMP-9 in uninfected cells, while M. tuberculosis stimulated secretion of MMP-1 and MMP-10 in untreated cells. Treatment with 1α,25(OH)2D3 induced biologically significant suppression of secretion of MMP-7 and MMP-9 in M. tuberculosis-infected cells. Six donors; error bars, SE; *P < 0·05; **P < 0·01; ***P < 0·001; EtOH = 0·1% ethanol vehicle.
Figure 3.
Influence of 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3; D] and Mycobacterium tuberculosis (MTB) on matrix metalloproteinase (MMP) activity of peripheral blood mononuclear cell supernatants. 1α,25(OH)2D3 did not influence activity of MMP-1 or MMP-9 in uninfected cells. Mycobacterium tuberculosis significantly induced activity of MMP-1 and MMP-9, and this effect was significantly attenuated by treatment with 1α,25(OH)2D3 for MMP-9. Six donors; error bars, SE; **P < 0·01; ***P < 0·001; EtOH = 0·1% ethanol vehicle.
Three regulators of MMP activity, TIMP-1, TIMP-2 and TIMP-3, were also analysed to determine the effect of 1α,25(OH)2D3 on their constitutive expression. In vitro culture had a variable effect on TIMP expression: TIMP-1 expression decreased 11-fold in MN (P < 0·001) while TIMP-2 expression increased twofold in PBMC (P < 0·05) and TIMP-3 expression increased 34-fold in PBMC and 56-fold in MN (P < 0·001) (Fig. 1). 1α,25(OH)2D3 induced a twofold increase in TIMP-2 expression in PBMC (P < 0·05) and a fourfold decrease in TIMP-3 expression in MN (P < 0·01) (Fig. 1) but did not influence expression of TIMP-1 or secretion of TIMP-1 or TIMP-2 (Fig. 2).
Mycobacterium tuberculosis induces MMP expression, secretion and activity
Cells cultured for 72 hr with and without 1α,25(OH)2D3 were infected with M. tuberculosis H37Rv, and MMP and TIMP mRNA levels were determined at 6 and 24 hr postinfection. The end-point observations are summarized in Table 1. Mycobacterium tuberculosis upregulated gene expression of MMP-1 (26-fold at 24 hr in PBMC, P < 0·05; 103-fold at 6 hr and 57-fold at 24 hr in MN, P < 0·001), MMP-7 (16-fold at 24 hr in MN, P < 0·001) and MMP-10 (18-fold at 6 hr and 41-fold at 24 hr in PBMC, P < 0·001; 170-fold at 6 hr and 68-fold at 24 hr in MN, P < 0·001), but did not influence gene expression of MMP-9 in either PBMC or MN. Mycobacterium tuberculosis induced secretion of MMP-1 (30-fold increase, P < 0·001) and MMP-10 (fivefold increase, P < 0·001) from PBMC at 72 hr postinfection but did not influence the secretion of MMP-7 or MMP-9 (Fig. 2). It also induced the activity of MMP-1 (sevenfold increase, P < 0·001) and MMP-9 (1·5-fold increase, P < 0·001) (Fig. 3). In contrast, the influence of M. tuberculosis infection on TIMP expression was small and variable: M. tuberculosis induced TIMP-1 gene expression fourfold in MN at 24 hr postinfection (P < 0·001), and suppressed TIMP-2 expression threefold in PBMC and fourfold in MN at 24 hr postinfection (P < 0·001) but did not influence the expression of TIMP-3 or the secretion of TIMP-1 or TIMP-2 (Figs 1, 2).
Table 1.
Summary of significant (P < 0·05) changes in gene expression following 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] treatment and Mycobacterium tuberculosis infection of human peripheral blood mononuclear cells (PBMC) and monocytes (MN)
| 1α,25(OH)2D3 versus 0·1% ethanol |
M. tuberculosis versus uninfected |
M. tuberculosis + 1α,25(OH)2D3 versus M. tuberculosis + 0·1% ethanol |
||||
|---|---|---|---|---|---|---|
| Gene | PBMC | MN | PBMC | MN | PBMC | MN |
| MMP-1 | – | – | ↑↑ | ↑↑ | – | – |
| MMP-7 | – | – | – | ↑↑ | – | ↓ |
| MMP-9 | ↓↓ | ↓↓ | – | – | ↓↓ | ↓ |
| MMP-10 | ↓↓ | ↓↓ | ↑↑ | ↑↑ | ↓ | ↓ |
| TIMP-1 | – | – | – | ↑ | ↑ | ↓ |
| TIMP-2 | ↑ | – | ↓ | ↓ | – | – |
| TIMP-3 | – | ↓ | – | – | – | ↓ |
| IL-10 | – | – | – | ↑↑ | ↑ | ↑ |
| 15-PGDH | ↑ | – | ↑ | ↑ | – | – |
| PGHS-2 | ↓ | – | ↑↑ | ↑↑ | – | ↑↑ |
↑, < 10-fold increase; ↑↑, ≥ 10-fold increase; ↓, < 10-fold decrease; ↓↓, ≥ 10-fold decrease; –, no significant effect.
IL-10, interleukin-10; MMP, matrix metalloproteinase; 15-PGDH, 15-hydroxyprostaglandin dehydrogenase; PGHS-2, prostaglandin H synthase-2; TIMP, tissue inhibitor of matrix metalloproteinase.
1α,25(OH)2D3 inhibits M. tuberculosis-induced increases in MMP expression, secretion and activity
When 1α,25(OH)2D3-treated cells were infected with M. tuberculosis, the pretreatment decreases in mRNA level induced by 1α,25(OH)2D3 were maintained during infection for both MMP-9 and MMP-10 (for MMP-9, 16-fold decrease at 6 hr postinfection and sevenfold decrease at 24 hr postinfection in PBMC (P < 0·001) and fourfold decrease at 6 hr postinfection and fivefold decrease at 24 hr postinfection in MN (P < 0·05); for MMP-10, eightfold decrease at 6 hr postinfection (P < 0·01) and sixfold decrease at 24 hr postinfection (P < 0·05) in PBMC and eightfold decrease at 24 hr postinfection in MN, P < 0·001) (Fig. 1). Additionally, 1α,25(OH)2D3 exerted an infection-specific effect on MMP-7, downregulating gene expression in MN sixfold at 24 hr postinfection (P < 0·01). Preincubation with 1α,25(OH)2D3 also inhibited secretion of MMP-7 (17-fold, P < 0·05) and MMP-9 (10-fold, P < 0·001) from M. tuberculosis-infected PBMC (Fig. 2). A trend towards decreased secretion of MMP-1 and MMP-10 was also observed, but this did not attain statistical significance after correction for multiple analyses. A moderate decrease in activity of MMP-9 (1·2-fold, P < 0·01) was also observed (Fig. 3). The effects of preincubation with 1α,25(OH)2D3 on TIMP expression and secretion in M. tuberculosis-infected cells were small and variable: 1α,25(OH)2D3 induced TIMP-1 gene expression in M. tuberculosis-infected PBMC at 6 hr postinfection (fourfold increase, P < 0·001), but suppressed TIMP-1 gene expression in MN at 24 hr postinfection (threefold decrease, P < 0·01). 1α,25(OH)2D3 also suppressed expression of TIMP-3 (sixfold decrease in MN at 6 hr postinfection, P < 0·001, Fig. 1) but did not induce biologically significant changes in secretion of TIMP-1 or TIMP-2 by M. tuberculosis-infected PBMC (Fig. 2).
1α,25(OH)2D3 induces IL-10 secretion in M. tuberculosis-infected PBMC
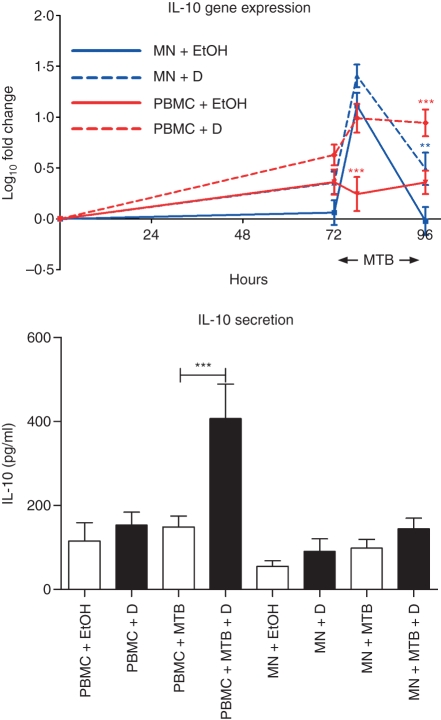
The regulatory cytokine IL-10 has previously been reported to modulate the expression of MMP and TIMP10 and it is also 1α,25(OH)2D3-inducible.16,17 We therefore proceeded to determine the effects of 1α,25(OH)2D3 and M. tuberculosis on IL-10 expression and secretion (Fig. 4). 1α,25(OH)2D3 had no significant effect on constitutive expression of IL-10 in PBMC or MN. Mycobacterium tuberculosis upregulated IL-10 mRNA levels in MN at 6 hr postinfection (11-fold increase, P < 0·001), but not at 24 hr postinfection and not in PBMC at either time-point. 1α,25(OH)2D3 augmented the IL-10 response to M. tuberculosis in PBMC at 6 hr postinfection, causing a sixfold increase in gene expression that was maintained at 24 hr postinfection (P < 0·001). This was reflected by a threefold increase in IL-10 secretion by M. tuberculosis-infected PBMC (P < 0·001, Fig. 4). 1α,25(OH)2D3 induced a more modest increase in IL-10 expression in M. tuberculosis-infected MN (threefold increase at 24 hr post-infection, P < 0·01) but did not induce a statistically significant increase in IL-10 secretion by M. tuberculosis-infected MN at 72 hr postinfection (Fig. 4).
Figure 4.
Influence of 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3; D] and Mycobacterium tuberculosis (MTB) on interleukin-10 (IL-10) gene expression and secretion by peripheral blood mononuclear cells (PBMC) and monocytes (MN). 1α,25(OH)2D3 had no effect on constitutive IL-10 gene expression in PBMC or MN. Mycobacterium tuberculosis induced IL-10 gene expression in MN at 6 hr postinfection. 1α,25(OH)2D3 induced IL-10 gene expression in M. tuberculosis-infected PBMC and MN, and induced IL-10 secretion from PBMC in the presence, but not in the absence, of M. tuberculosis. Ten donors; error bars, SE; **P < 0·01; ***P < 0·001; EtOH = 0·1% ethanol vehicle.
1α,25(OH)2D3 upregulates PGE2 secretion in MN, both in the presence and absence of M. tuberculosis infection
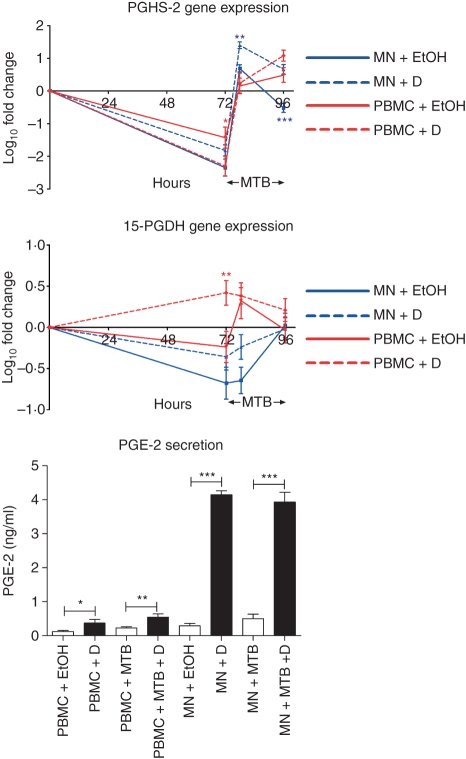
As macrophage MMP expression is largely PGE2-dependent12,29 and because 1α,25(OH)2D3 has been shown to modulate mRNA levels of rate-limiting enzymes in PGE2 synthesis and catabolism (PGHS-2 and 15-PGDH, respectively)18,19 we investigated whether expression of these genes and secretion of PGE2 were modulated by 1α,25(OH)2D3 and M. tuberculosis (Fig. 5). In the absence of 1α,25(OH)2D3 a significant decrease in expression of 15-PGDH (fivefold in MN, P < 0·01) and PGHS-2 (27-fold in PBMC and 223-fold in MN, P < 0·001) was observed. In PBMC, 1α,25(OH)2D3 attenuated this constitutive decrease in 15-PGDH expression (fivefold increase, P < 0·01), but induced a further decrease in PGHS-2 expression (sevenfold decrease, P < 0·05); 1α,25(OH)2D3 did not influence the expression of either gene in MN. Mycobacterium tuberculosis increased mRNA levels of PGHS-2 in both PBMC (39-fold at 6 hr postinfection and 83-fold at 24 hr postinfection, P < 0·001) and MN (1097-fold at 6 hr postinfection and 67-fold at 24 hr postinfection, P < 0·001) and also induced expression of 15-PGDH in PBMC at 6 hr postinfection (fourfold, P < 0·05) and in MN at 24 hr postinfection (fivefold, P < 0·01). 1α,25(OH)2D3 further increased PGHS-2 mRNA levels in M. tuberculosis-infected MN at both 6 hr and 24 hr postinfection (fivefold and 15-fold increase respectively, P < 0·01) but did not influence expression of PGHS-2 in M. tuberculosis-infected PBMC or expression of 15-PGDH in M. tuberculosis-infected PBMC or MN. Mycobacterium tuberculosis did not influence the secretion of PGE2 from PBMC or MN, but 1α,25(OH)2D3 stimulated the secretion of PGE2 from all cells both in the absence and in the presence of M. tuberculosis (threefold in uninfected PBMC, twofold in M. tuberculosis-infected PBMC, 14-fold in uninfected MN and eightfold in M. tuberculosis-infected MN, P < 0·05; Fig. 5).
Figure 5.
Influence of 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3; D] and Mycobacterium tuberculosis (MTB) on gene expression of prostaglandin H synthase-2 (PGHS-2) and 15-hydroxyprostaglandin dehydrogenase (15-PGDH) and secretion of prostaglandin E2 (PGE2) by peripheral blood mononuclear cells (PBMC) and monocytes (MN). 1α,25(OH)2D3 induced constitutive expression of 15-PGDH and suppressed constitutive expression of PGHS-2 in PBMC. M. tuberculosis induced gene expression of PGHS-2 (in both PBMC and MN) and 15-PGDH (in MN). Treatment with 1α,25(OH)2D3 further upregulated PGHS-2 gene expression in M. tuberculosis-infected MN but had no effect on 15-PGDH expression in M. tuberculosis-infected cells. 1α,25(OH)2D3 induced secretion of PGE2 in M. tuberculosis-uninfected and M. tuberculosis-infected PBMC and MN. Mean of 10 donors; error bars, SE. *P < 0·05; **P < 0·01; ***P < 0·001; EtOH = 0·1% ethanol vehicle.
Discussion
The MMP family of enzymes has been a therapeutic target for over 20 years, but dose-limiting side-effects of candidate agents have prevented any from entering clinical trials.30 We have previously shown that the administration of large bolus doses of vitamin D is safe, and suppresses circulating concentrations of MMP-931 as well as modulating antimycobacterial immune responses.32 We and others have also previously demonstrated that 1α,25(OH)2D3 induces the antimicrobial peptide cathelicidin LL-37, which possesses antituberculous activity.22,33,34 This report describes a complementary mechanism whereby vitamin D could exert a beneficial effect on host response to M. tuberculosis by inhibiting the expression, secretion and activity of a number of MMP induced by M. tuberculosis, consequently limiting the degradation of extracellular matrix and decreasing pulmonary cavitation to preserve lung function and reduce infectiousness.
Mononuclear phagocytes are the site of M. tuberculosis replication and containment, and the principal source of MMP. It has previously been shown that 1α,25(OH)2D3 regulates the secretion of MMP-9, but not MMP-1, by human MN and alveolar macrophages.14 Our experiments confirm this previous observation in MN and show for the first time that 1α,25(OH)2D3 also regulates MMP-7 and MMP-10 expression in MN under basal conditions and/or during M. tuberculosis infection. As previous studies have suggested that an interaction between T cells and MN/macrophages enhances MMP expression by both cell populations11,22–25 we also investigated the effects of 1α,25(OH)2D3 on MMP regulation in a mixed population of adherent and non-adherent PBMC. Our observation that each MMP analysed had higher constitutive expression in PBMC than in MN led us to investigate the influence of 1α,25(OH)2D3 and M. tuberculosis on MMP secretion and activity in PBMC. We found that the only significant effect of 1α,25(OH)2D3 on basal MMP secretion by PBMC was a decrease in MMP-9, consistent with Lacraz et al.14 More significantly, we found that there was a greater effect of 1α,25(OH)2D3 when cells were infected with M. tuberculosis (Table 1).
Consistent with previous observations in macrophages4 we found that M. tuberculosis induced expression of MMP-1 and MMP-10 (in both PBMC and MN) and MMP-7 (only in MN), but did not influence the expression of MMP-9. The cell-type specific effect on expression of MMP-7 may reflect the fact that MMP-7 is predominantly expressed in mononuclear phagocytes35,36 or that interferon-γ produced from T lymphocytes in response to M. tuberculosis infection may be responsible for suppressing MMP-7 expression in PBMC.11 Significantly, 1α,25(OH)2D3 inhibited the expression of MMP-7, MMP-9 and MMP-10 in M. tuberculosis-infected PBMC, and this was reflected by a decrease in secretion of MMP-7 and MMP-9 and a decrease in MMP-9 activity. In contrast, the influence of 1α,25(OH)2D3 on the expression and secretion of TIMP was small. Taken together, these results suggest that 1α,25(OH)2D3 primarily influences MMP secretion and activity in M. tuberculosis-infected leucocytes at the transcriptional level.
We therefore investigated the effect of 1α,25(OH)2D3 on the secretion of known regulators of MMP gene expression, and found that 1α,25(OH)2D3 induced secretion of both PGE2 (in MN and PBMC, irrespective of infection) and IL-10 (only in M. tuberculosis-infected PBMC). It has previously been reported that 1α,25(OH)2D3 augments the development of IL-10-secreting T helper type 2 cells in mice,17 and this may explain our observation that induction of IL-10 by 1α,25(OH)2D3 was PBMC-specific. Both IL-10 and PGE2 have been reported to suppress the expression and secretion of MMP10,11,13 and it is possible that the effects of 1α,25(OH)2D3 on MMP expression that we report here are IL-10-mediated or PGE2-mediated. Experiments to determine the effect of blocking the actions of IL-10 and PGE2 on MMP expression in 1α,25(OH)2D3-treated cells are required to investigate this possibility.
Although excess MMP activity is implicated in the pathogenesis of TB, suppression of MMP activity also has the potential to impair host responses by inhibiting the recruitment of cells required to contain infection.37 This dichotomy is illustrated in a study reporting that MMP-9 knockout mice infected with M. tuberculosis exhibit impaired macrophage recruitment and granuloma development, while administration of a broad-range MMP inhibitor to wild-type mice exposed to M. tuberculosis infection reduces haematogenous spread of infection.38 In humans, administration of vitamin D to patients with pulmonary TB has been reported to reduce both cavitation39 and time to sputum smear conversion40 suggesting that vitamin D supplementation may afford a net clinical benefit associated with enhanced resolution of pulmonary cavitation. Clinical studies of the effect of vitamin D supplementation on MMP regulation in TB patients are required, and are a current focus of our work.
Acknowledgments
We thank Professor Jonathon Friedland, Dr Paul Elkington and Dr Justin Green of Imperial College, London, and Professor Robert D. Cohen, of Barts and The London School of Medicine and Dentistry for helpful reviews of the manuscript. This work was supported by the British Lung Foundation (TB05/11), the Wellcome Trust (064261, 072070) and Northwick Park Hospital Tropical Research Fund.
Competing interests
The authors have no competing interests to disclose.
References
- 1.Maartens G, Wilkinson RJ. Tuberculosis. Lancet. 2007;370:2030–43. doi: 10.1016/S0140-6736(07)61262-8. [DOI] [PubMed] [Google Scholar]
- 2.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–29. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro SD, Campbell EJ, Kobayashi DK, Welgus HG. Immune modulation of metalloproteinase production in human macrophages. Selective pretranslational suppression of interstitial collagenase and stromelysin biosynthesis by interferon-gamma. J Clin Invest. 1990;86:1204–10. doi: 10.1172/JCI114826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elkington PT, Nuttall RK, Boyle JJ, O’Kane CM, Horncastle DE, Edwards DR, Friedland JS. Mycobacterium tuberculosis, but not vaccine BCG, specifically upregulates matrix metalloproteinase-1. Am J Respir Crit Care Med. 2005;172:1596–604. doi: 10.1164/rccm.200505-753OC. [DOI] [PubMed] [Google Scholar]
- 5.Chang JC, Wysocki A, Tchou-Wong KM, Moskowitz N, Zhang Y, Rom WN. Effect of Mycobacterium tuberculosis and its components on macrophages and the release of matrix metalloproteinases. Thorax. 1996;51:306–11. doi: 10.1136/thx.51.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hrabec E, Strek M, Zieba M, Kwiatkowska S, Hrabec Z. Circulation level of matrix metalloproteinase-9 is correlated with disease severity in tuberculosis patients. Int J Tuberc Lung Dis. 2002;6:713–9. [PubMed] [Google Scholar]
- 7.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;2:267–83. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 8.Baragi VM, Fliszar CJ, Conroy MC, Ye QZ, Shipley JM, Welgus HG. Contribution of the C-terminal domain of metalloproteinases to binding by tissue inhibitor of metalloproteinases. C-terminal truncated stromelysin and matrilysin exhibit equally compromised binding affinities as compared to full-length stromelysin. J Biol Chem. 1994;269:12692–7. [PubMed] [Google Scholar]
- 9.Elkington PT, Emerson JE, Lopez-Pascua LD, O’Kane CM, Horncastle DE, Boyle JJ, Friedland JS. Mycobacterium tuberculosis up-regulates matrix metalloproteinase-1 secretion from human airway epithelial cells via a p38 MAPK switch. J Immunol. 2005;175:5333–40. doi: 10.4049/jimmunol.175.8.5333. [DOI] [PubMed] [Google Scholar]
- 10.Lacraz S, Nicod LP, Chicheportiche R, Welgus HG, Dayer JM. IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J Clin Invest. 1995;96:2304–10. doi: 10.1172/JCI118286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busiek DF, Baragi V, Nehring LC, Parks WC, Welgus HG. Matrilysin expression by human mononuclear phagocytes and its regulation by cytokines and hormones. J Immunol. 1995;154:6484–91. [PubMed] [Google Scholar]
- 12.Zhang Y, McCluskey K, Fujii K, Wahl LM. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF, and IL-1 beta through prostaglandin-dependent and -independent mechanisms. J Immunol. 1998;161:3071–6. [PubMed] [Google Scholar]
- 13.Zhu Y, Liu X, Skold CM, Wang H, Kohyama T, Wen FQ, Ertl RF, Rennard SI. Collaborative interactions between neutrophil elastase and metalloproteinases in extracellular matrix degradation in three-dimensional collagen gels. Respir Res. 2001;2:300–5. doi: 10.1186/rr73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacraz S, Dayer JM, Nicod L, Welgus HG. 1,25-dihydroxyvitamin D3 dissociates production of interstitial collagenase and 92-kDa gelatinase in human mononuclear phagocytes. J Biol Chem. 1994;269:6485–90. [PubMed] [Google Scholar]
- 15.Michel G, Gailis A, Jarzebska-Deussen B, Muschen A, Mirmohammadsadegh A, Ruzicka T. 1,25-(OH)2-vitamin D3 and calcipotriol induce IL-10 receptor gene expression in human epidermal cells. Inflamm Res. 1997;46:32–4. doi: 10.1007/s000110050042. [DOI] [PubMed] [Google Scholar]
- 16.Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1alpha,25-Dihydroxyvitamin D3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 18.Pichaud F, Roux S, Frendo JL, Delage-Mourroux R, Maclouf J, de Vernejoul MC, Moukhtar MS, Jullienne A. 1,25-dihydroxyvitamin D3 induces NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase in human neonatal monocytes. Blood. 1997;89:2105–12. [PubMed] [Google Scholar]
- 19.Moreno J, Krishnan AV, Swami S, Nonn L, Peehl DM, Feldman D. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005;65:7917–25. doi: 10.1158/0008-5472.CAN-05-1435. [DOI] [PubMed] [Google Scholar]
- 20.Tsoukas CD, Provvedini DM, Manolagas SC. 1,25-Dihydroxyvitamin D3: a novel immunoregulatory hormone. Science. 1984;224:1438–40. doi: 10.1126/science.6427926. [DOI] [PubMed] [Google Scholar]
- 21.Cohen MS, Mesler DE, Snipes RG, Gray TK. 1,25-Dihydroxyvitamin D3 activates secretion of hydrogen peroxide by human monocytes. J Immunol. 1986;136:1049–53. [PubMed] [Google Scholar]
- 22.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 23.Malik N, Greenfield BW, Wahl AF, Kiener PA. Activation of human monocytes through CD40 induces matrix metalloproteinases. J Immunol. 1996;156:3952–60. [PubMed] [Google Scholar]
- 24.Oviedo-Orta E, Bermudez-Fajardo A, Karanam S, Benbow U, Newby AC. Comparison of MMP-2 and MMP-9 secretion from T helper 0, 1 and 2 lymphocytes alone and in coculture with macrophages. Immunology. 2008;124:42–50. doi: 10.1111/j.1365-2567.2007.02728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacraz S, Nicod L, Galve-de Rochemonteix B, Baumberger C, Dayer JM, Welgus HG. Suppression of metalloproteinase biosynthesis in human alveolar macrophages by interleukin-4. J Clin Invest. 1992;90:382–8. doi: 10.1172/JCI115872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkinson RJ, DesJardin LE, Islam N, et al. An increase in expression of a Mycobacterium tuberculosis mycolyl transferase gene (fbpB) occurs early after infection of human monocytes. Mol Microbiol. 2001;39:813–21. doi: 10.1046/j.1365-2958.2001.02280.x. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson KA, Stewart GR, Newton SM, et al. Infection biology of a novel alpha-crystallin of Mycobacterium tuberculosis: Acr2. J Immunol. 2005;174:4237–43. doi: 10.4049/jimmunol.174.7.4237. [DOI] [PubMed] [Google Scholar]
- 28.Verheijen JH, Nieuwenbroek NM, Beekman B, Hanemaaijer R, Verspaget HW, Ronday HK, Bakker AH. Modified proenzymes as artificial substrates for proteolytic enzymes: colorimetric assay of bacterial collagenase and matrix metalloproteinase activity using modified pro-urokinase. Biochem J. 1997;3:603–9. doi: 10.1042/bj3230603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapiro SD, Kobayashi DK, Pentland AP, Welgus HG. Induction of macrophage metalloproteinases by extracellular matrix. Evidence for enzyme- and substrate-specific responses involving prostaglandin-dependent mechanisms. J Biol Chem. 1993;268:8170–5. [PubMed] [Google Scholar]
- 30.Fingleton B. Matrix metalloproteinases as valid clinical targets. Curr Pharm Des. 2007;13:333–46. doi: 10.2174/138161207779313551. [DOI] [PubMed] [Google Scholar]
- 31.Timms PM, Mannan N, Hitman GA, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95:787–96. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- 32.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–13. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 33.Martineau AR, Wilkinson KA, Newton SM, et al. IFN-γ- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178:7190–8. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 34.Martineau AR, Newton SM, Wilkinson KA, et al. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest. 2007;117:1988–94. doi: 10.1172/JCI31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Busiek DF, Ross FP, McDonnell S, Murphy G, Matrisian LM, Welgus HG. The matrix metalloprotease matrilysin (PUMP) is expressed in developing human mononuclear phagocytes. J Biol Chem. 1992;267:9087–92. [PubMed] [Google Scholar]
- 36.De Waal-Malefyt R, Abrams J, Bennett B, Figdor CG, De Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elkington PT, O’Kane CM, Friedland JS. The paradox of matrix metalloproteinases in infectious disease. Clin Exp Immunol. 2005;142:12–20. doi: 10.1111/j.1365-2249.2005.02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor JL, Hattle JM, Dreitz SA, et al. Role for matrix metalloproteinase 9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infect Immun. 2006;74:6135–44. doi: 10.1128/IAI.02048-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol. 2007;103:793–8. doi: 10.1016/j.jsbmb.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 40.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38:3–5. [PubMed] [Google Scholar]