Abstract
Chemokines and chemokine receptors are likely to play important roles in the pathogenesis of Epstein–Barr virus (EBV) -associated disease. The primary EBV infection occurs in the oropharynx where the virus infects mainly tonsillar B cells. We have previously shown that CXCR4 expression on tonsillar B cells is modulated by EBV. Here, CXCR5 and CCR7 expression, which is important for migration into lymphoid tissue, was followed for 14 days after EBV infection of tonsillar B cells. Early after infection (2 days) there were only minor changes in CXCR5 and CCR7 expression. However, at day 7 the expression of CXCR5, as well as of CCR7, was decreased and by day 14 these molecules were no longer present at the cell surface. Furthermore, EBV infection affects the chemotactic response to CXCL13 and CCL21 (the ligands for CXCR5 and CCR7, respectively) with a reduction of ligand-induced migration at day 2. Using gene expression profiling, we identified an additional set of chemokines and chemokine receptors that were changed upon EBV infection in comparison with non-infected tonsillar B cells. In particular, messenger RNA expression for CCR9 and the complement receptor C5AR1 was increased. Both receptors mediate homing to mucosal tissue. The alterations of the expression of these molecules may lead to retention of EBV-infected tonsillar B cells in the interfollicular region of the tonsil.
Keywords: chemokine receptors, chemokines, Epstein–Barr virus, migration, tonsillar B cells
Introduction
Epstein–Barr virus (EBV), a ubiquitous B-lymphotropic human γ-herpesvirus, infects all human populations with a great majority of adults having antibodies to the virus.1 Primary infection by EBV is usually asymptomatic in young children but may cause infectious mononucleosis in adolescents and young adults. The virus is associated with a number of malignancies like Burkitt’s lymphoma, nasopharyngeal cancer and B-cell lymphomas arising in immunocompromised patients.2 Primary infection occurs by the oral route and involves viral replication in permissive cells of the mucosal epithelium associated with pharyngeal lymphoid tissues.3 At the same time, the virus initiates infection of the B-cell pool in tonsils where immunohistochemical staining has shown EBV-positive B cells in the extrafollicular areas of the tonsil.4 Following primary infection, EBV establishes latency in the memory B-cell pool that preferentially populates the pharyngeal lymphoid tissue.5,6 In latent infection, distinct EBV gene expression patterns have been described (for review see ref. 7). In vivo, latency III is found in infected B cells that are driven to proliferate by the virus. In latency III, only found in B immunoblasts, all EBV-encoded transcripts and proteins are expressed including nine virally encoded proteins. These are designated as the growth transformation-associated proteins; six are nuclear proteins (EBNA1–6), whereas three are membrane bound, (LMP1, 2A and 2B). Two non-translated small non-polyadenylated RNAs (EBERs) are also expressed. Latency II, characterized by expression of EBERs, EBNA1 and LMP1, -2A and -2B is found in non-immunoblastic malignancies like Hodgkin’s disease and nasopharyngeal cancer. Latency I is found in cells from Burkitt’s lymphoma patients ex vivo and these cells only express EBNA1 and EBERs. In healthy seropositive individuals the infected cells mainly have a resting phenotype expressing no EBV-encoded proteins or occasionally expressing LMP2A or EBNA1 transcripts, i.e. latency 0–I. BamHI-A rightward transcripts are also expressed in all EBV latency programmes, including EBV-infected B cells in healthy carriers.8
Recent studies have shown that migration and tissue microenvironmental localization of various lymphocyte subclasses are finely regulated through the expression of chemokine receptors, depending on the differentiation and activation stage.9–12 It is conceivable that chemokines and their receptors play important roles in the migration and tissue localization of EBV-infected B cells in diseases such as infectious mononucleosis and opportunistic B-cell lymphomas. Most herpes viruses encode homologues of chemokines and chemokine receptors13 and EBV encodes a distinct G-coupled receptor with unknown function as well as a homologue for interleukin-10.14 In addition, increasing attention has been given to the potential role of viruses in interference with chemokine receptor expression, binding and signalling.15,16 Expression of chemokine receptors and chemokines in EBV-immortalized cell lines has been studied in comparison with peripheral blood B cells and Burkitt’s lymphoma cell lines.17–19 The EBV-immortalized human B cells express CCR6 and CCR10 at high levels and CXCR4 and CXCR5 at low levels17 and it was further shown that EBNA2, as well as LMP1, downregulates CXCR4. Expression of EBNA4 by infected B cells has also been shown to repress CXCR4 expression in cell lines.20 The expression and upregulation of the chemokine receptor CCR7 has also been associated with EBNA2 expression in different human B-cell lines.21,22
We have previously described the downregulation of CXCR4 and the subsequent impairment of ligand (CXCL12)-induced migration of tonsillar B cells infected with EBV in vitro.23 Here we continued our study with EBV-infected tonsillar B cells focusing on the expression of CXCR5 and CCR7, two receptors that are important for secondary lymphoid tissue homing, after EBV infection in vitro. Using gene expression profiling of chemokine receptors and chemokines comparing EBV-infected B cells with uninfected cells from the same donor, we also define a number of genes that are targets for EBV in tonsillar B cells.
Materials and methods
B-cell preparation
Tonsil B cells were prepared from human tonsils. Tonsils obtained from routine tonsillectomy after informed consent of the patient (Karolinska University Hospital, Stockholm, Sweden) were cut into fragments and dispersed into cell suspensions. T cells were removed by E rosetting followed by separation on Lymphoprep (Axis-Shield PoC AS, Oslo, Norway). The remaining cells were suspended in RPMI-1640 supplemented with 10% fetal calf serum (FCS; Gibco, Paisley, UK), glutamine (2 mm), penicillin and streptomycin and the cultures contained > 90% CD19-positive cells. Peripheral blood mononuclear cells were isolated using the Lymphoprep (as described above), and washed once with isotonic saline followed by a wash with Versene [phosphate-buffered saline (PBS), pH 7·4 with 0·5 mm ethylenediaminetetraacetic acid] and finally resuspended in PBS. For B-cell separation, the peripheral blood mononuclear cells were incubated rotating with Dynabeads Pan-B, four beads per target cell, at 4° for 30 min. Thereafter, the cells were washed five to seven times until non-rosetted cells were below 1%. After an overnight incubation, the beads that were shed were removed using a magnet.
Cell culture
All cultures were carried out in HEPES-buffered RPMI-1640 supplemented with 10% FCS, glutamine (2 mm), penicillin and streptomycin. For EBV infection, B cells were incubated with B95-8, a sub-strain of EBV. After 1 hr the virus was washed away and medium containing 10% FCS was added at a concentration of 1 ml/106 cells. Cells were harvested after approximately 48 hr, 7 and 14 days for further analysis. ER/EB2-5 cells were cultured in HEPES-buffered RPMI-1640 supplemented with 10% FCS, glutamine (2 mm), β-oestradiol (1 μl/ml) (Sigma Chemicals, Stockholm, Sweden), penicillin and streptomycin. For oestradiol starvation, ER/EB2-5 cells were washed with PBS three times and then kept in medium without oestradiol [HEPES-buffered RPMI-1640 supplemented with 10% FCS, glutamine (2 mm), penicillin and streptomycin] for 3 days.
Flow cytometry
One million cells were incubated with saturating amounts of monoclonal antibodies for 30 min, at + 4°. The monoclonal antibodies used were: anti-CCR7 (FAB197F), anti-CXCR5 (FAB190P), isotype-matched controls, mouse immunoglobulin G2A (IgG2A; IC003F) and mouse 2B (IC0041P) (R&D Systems, Minneapolis, MN); R-phycoerythrin-conjugated mouse anti-human CD19 and R-phycoerythrin-conjugated mouse IgG1 (DAKO A/S, Glostrup, Denmark). After washing, the cells were fixed in 1% formaldehyde in PBS and analysed in a FACScan using cell quest software (Becton Dickinson, Stockholm, Sweden).
Transmigration
A Transwell culture system was performed in duplicates on primary tonsillar B cells using 5-μm-diameter pore filters (Transwell, 24-well plate; Costar, Cambridge, MA): 2·5 × 105 cells were resuspended in 100 μl RPMI-1640 medium supplemented with 5% FCS, 2 mm glutamine, penicillin and streptomycin and thereafter loaded into the upper chamber of the Transwell filter. To examine the migration of the cells towards CXCL13 and CCL21, 600 μl medium containing 1 μg/ml of the respective recombinant chemokine (R&D Systems) was added to the lower well and the plate was incubated at 37° for 4 hr. Thereafter, the migrated cells in the lower wells were collected, fixed with 200 μl 1% formaldehyde in PBS and counted in a FACScan flow cytometer (Becton Dickinson). The results of three independent experiments are included.
Western blot
The cells were lysed in lysis buffer (60% glycerol, 50 mm Tris–HCl, pH 6·5; 2% sodium dodecyl sulphate, 0·1% bromophenol blue and 0·02% 2-mercaptoethanol), and then denatured by boiling for 10 min. Aliquots of total cell lysates corresponding to 2·5 × 105 cells were loaded in each well. The samples were electrophoresed on 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis gels and transferred to polyvinylidene fluoride membranes at 80 V for 2 hr. After blocking the membranes for 1 hr with 7·5% non-fat dried milk in PBS–Tween-20, they were incubated with the primary antibodies overnight at 4°. The blots were then incubated with horseradish peroxidase-conjugated donkey anti-rabbit immunoglobulin antibody (Amersham, Arlington Heights, IL) or horseradish peroxidase-conjugated goat anti-mouse immunoglobulin antibody (DAKO A/S) and detected with ECL Plus detection reagent (Amersham). Primary antibodies were as follows: mouse anti-EBNA2 (DAKO A/S), mouse anti-LMP-1 (1-4 DAKO A/S) and mouse anti-β actin (AC-15; Sigma Chemicals).
Complementary DNA expression array
Cultured tonsillar B cells (n = 2 donors) were collected after 48 hr and 7 days and RNA was extracted using the RNeasy Mini Kit (Qiagen, Stockholm, Sweden). Quality and concentration of total RNA were evaluated by spectrophotometry (Bio-Rad, Stockholm, Sweden). Approximately 10–20 μg RNA was purified from each sample and complementary DNA (cDNA) expression microarray analysis was performed using the GEArray Q series Human Chemokines and Receptors Gene Array (SuperArray, Frederick, MD) measuring 96 genes encoding for the small inducible cytokine subfamily A (Cys–Cys) (n = 23), subfamily B (Cys–X–Cys) (n = 14), other subfamily members (n = 5), chemokine receptor family (n = 28), chemokine-like factor superfamily (n = 7) and other related genes (n = 19). About 1.5 μg total RNA was reverse-transcribed using an Ampolabelling (LPR) Kit (SuperArray) together with biotin-16-dUTP (Enzo Life Science, Famingdale, NY). Amplification of cDNA was performed for 30 cycles. The biotinylated cDNA probes were denatured and added to the hybridization solution. GEArray Q Series membranes were prehybridized at 60° for 2 hr and thereafter hybridized overnight with the cDNA probes. Membranes were then washed, blocked and incubated with alkaline phosphatase-conjugated streptavidin. The labelled biotin on the membrane was detected by chemoluminescence using a GEArray Chemoluminescence Detection Kit (D-01) (SuperArray).
Analysis of cDNA microarray
The luminescence intensities of hybridized cDNA probes were analysed by GEarrayexpressionanalysis Suite software (SuperArray). Local background was subtracted for each point and signal intensity was normalized against the housekeeping gene Ribosomal protein L13a. For the analysis of the overall changes in gene expression between EBV-infected samples and uninfected controls, the significantly dysregulated genes were selected according to a boundary (cut-off) level of 2·5-fold expression difference, as calculated by the microarray software (SABiosciences, Frederick, MD).
Results
Altered expression of chemokine receptors that influence migration within lymphoid tissue
Primary EBV infection occurs in the oropharynx and infectious mononucleosis is characterized by hyperplasia of lymphoid tissues including tonsils. Therefore, we studied the expression of CXCR5 and CCR7, receptors that are important for migration within lymphoid tissue, in tonsillar B cells upon EBV infection in vitro.
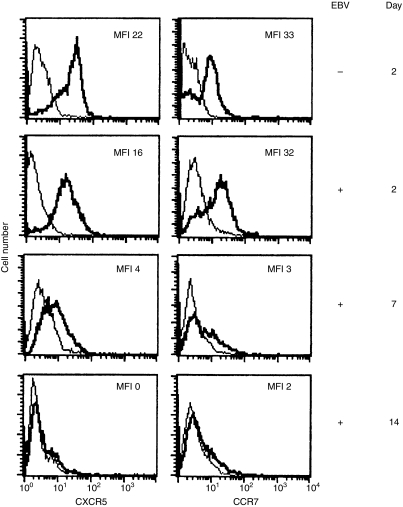
After 2 days, the expression of CXCR5 was slightly decreased in the EBV-infected cells compared with cells kept in medium (Fig. 1). A further decrease was found on day 7, and by day 14 EBV-infected B cells no longer expressed CXCR5. The expression of CCR7 was examined at similar time-points. After 2 days, there was a minor decrease in CCR7 expression in the EBV-infected cells (Fig. 1). However, after 7 days CCR7 was strongly downregulated in infected cells and at day 14, CCR7 could not be detected on the cell surface. Approximately 30% of the cells were EBNA2-positive after 2 days and 70% after 7 days.
Figure 1.
CXCR5 and CCR7 expression after Epstein–Barr virus (EBV) infection of tonsillar B cells. The surface expression was monitored at 2, 7 and 14 days. The Epstein–Barr nuclear antigen (EBNA) expression in cell cultures was approximately 30% and 70% at these time-points and at day 14 the culture contained very few uninfected cells. The bold line represents the specific staining and the thin line represents the staining with isotype-matched control. MFI = mean fluorescence intensity. Representative data of three independent experiments are shown.
Reduced migration towards the ligands for CXCR5 and CCR7 in EBV-infected cells
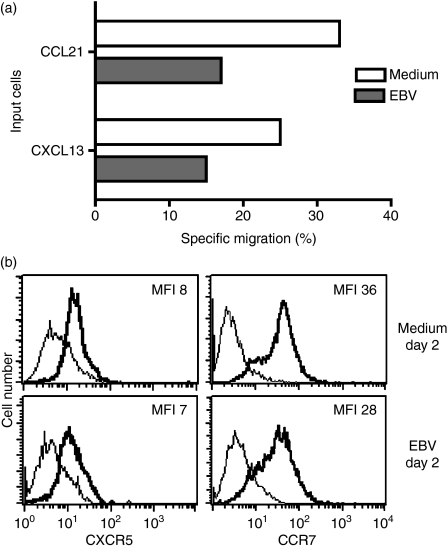
To investigate whether the reduced expression of CXCR5 corresponded to a reduction in ligand-induced migration, a transmigration assay was performed on cells harvested after 2 days. Migration towards the ligand for CXCR5, CXCL13, was reduced in the EBV-infected cells compared with control cells (15% versus 25% specific migration, P = 0·09) (Fig. 2a) although the expression of CXCR5 was similar on the cell surface of infected cells compared with control cells (Fig. 2b). The expression of CCR7 showed only minor changes upon EBV infection (Fig. 2b), but the EBV-infected cells migrated less (17% versus 33% specific migration, P = 0·08) towards CCL21, one of the ligands for CCR7 (Fig. 2a).
Figure 2.
Chemotactic response of tonsillar B cells to CXCL13 and CCL21. (a) Tonsillar B cells were infected with Epstein–Barr virus (EBV) and the chemotactic response induced by CXCL13 and CCL21 in infected and control cells was compared after 2 days in a 4-hr Transwell migration assay. The result is presented as percentage of cells that migrated to the ligand compared with the input population after subtraction of background migration. In the CXCR5 experiment, 50% of the infected population expressed Epstein–Barr nuclear antigen (EBNA) whereas in the CCR7 experiment 40% of the cells contained EBNA. (b) CXCR5 and CCR7 expression at time of the migration assay. The bold line represents the specific staining and the thin line the isotype-matched control. MFI = mean fluorescence intensity. Representative data of two independent experiments are shown.
CCR7 is differently expressed after EBV infection depending on the B-cell compartment
The reduced level of CCR7 in tonsillar B cells has not previously been described. On the contrary, the expression of EBNA2 in cell lines has been associated with the upregulation of CCR7.21,22 Therefore, we decided to examine the expression of CCR7 on ER/EB2-5 cells where the expression of EBNA2 can be regulated by culturing cells in the presence or absence of oestrogen19 and on peripheral B cells after EBV infection in vitro.
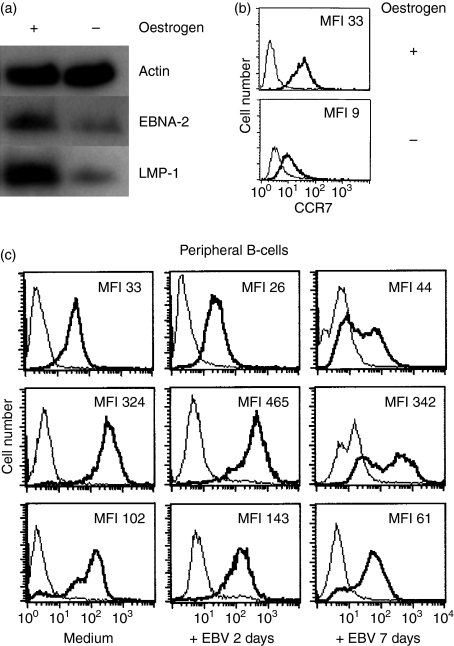
After withdrawal of oestrogen, the cell surface expression of CCR7 decreased (Fig. 3a), with the subsequent reduction in EBNA2 (Fig. 3b) when compared with the proliferating ER/EB2-5 cells. In the ER/EB2-5 system, the expression of EBNA2 was clearly associated with CCR7 cell surface expression. In peripheral B cells, the profile of CCR7 expression after in vitro EBV infection showed a different pattern compared with the one found in tonsillar B cells. After 7 days of infection, CCR7 could still be detected at a high level on the cell surface (Fig. 3c). At this time-point, an average of 75% of cells were EBNA2 positive.
Figure 3.
Changes in CCR7 expression in ER/EB2-5 cells after oestrogen withdrawal. (a) CCR7 expression in proliferating ER/EB2-5 cells and ER/EB2-5 cells which have been cultivated in the absence of oestrogen for 3 days. The bold line represents the specific staining and the thin line the isotype-matched control. MFI = mean fluorescence intensity. (b) Western blot analysis of Epstein–Barr nuclear antigen 2 (EBNA2) and latent membrane protein 1 (LMP1). Actin was blotted as a loading control. Representative data of three independent experiments are shown. (c) CCR7 expression on peripheral B cells after in vitro infection with Epstein–Barr virus (EBV) for 2 and 7 days (n = 3). Approximately 75% of the cells were EBNA2 positive in the cultures on day 7. MFI = mean fluorescence intensity.
Overall gene expression changes induced by EBV in human tonsillar B cells
To further assess the chemokines and chemokine receptors in EBV-infected and uninfected tonsillar B cells, the messenger RNA (mRNA) expression of these molecules was analysed using gene expression profiling after 2 and 7 days (as above). Infection with EBV induced the expression of several chemokines and chemokine receptors compared with uninfected cells from the same donor (Table 1). Two molecules were found to be upregulated at the mRNA level at both time-points. CCR9, a receptor that is important for homing to mucosal tissue,24 was increased approximately threefold in the EBV-infected samples compared with uninfected tonsillar B cells of the same origin. C5AR1, the receptor for complement C5a, was overexpressed (10·7-fold and 5·0-fold increase, respectively) in the EBV-infected cells at both time-points. After 48 hr incubation, we found that one of the CCR7 ligands, CCL19, was upregulated. The chemokine CCL20 (ligand for CCR6), which is induced by the EBV-encoded LMP1 gene,18 was also increased (4·1-fold) in EBV-infected tonsillar B cells at 2 days. Several chemokines mainly attracting T cells such as CCL11, CCL24, CCL1 and CCL13 were also upregulated after 7 days.
Table 1.
Overexpressed genes in Epstein–Barr virus (EBV)-infected tonsillar B cells compared with uninfected cells
| EBV + 48 hr | EBV + 7 days | ||
|---|---|---|---|
| CCR9 | 2·91 | CCR9 | 2·61 |
| C5AR1 | 10·7 | C5AR1 | 5·0 |
| CCL20 | 4·1 | CCL11 | 7·4 |
| CCL19 | 2·9 | CCL24 | 6·5 |
| CCR2 | 3·1 | CCL1 | 3·0 |
| CMTM | 4·4 | CCL13 | 3·2 |
| SDF2 | 3·7 | ||
| CCL5 | 4·9 | ||
| CXCR3 | 2·6 | ||
| PPBP | 2·9 | ||
| SLIT | 3·3 | ||
Fold increase.
EBV infection also induced a decrease in mRNA levels for certain chemokines and chemokine receptors (Table 2). At 2 days no genes were decreased compared with uninfected control cells but after 7 days there were more differences. CXCR5 and CCR6 were less expressed at this time-point (0·4-fold and 0·1-fold decreases, respectively). We could not detect a decrease in CCR7 mRNA expression. The orphan receptor GPR31, the CCR2-ligand CCL2 and the CXCR2-ligand CXCL2 were also less expressed at this time-point in the EBV-infected B cells.
Table 2.
Underexpressed genes in Epstein–Barr virus (EBV)-infected tonsillar B cells compared with uninfected cells
| EBV + 7 days | |
|---|---|
| BLR1/CXCR5 | 0·361 |
| CCR6 | 0·08 |
| CCL2 | 0·10 |
| CXCL2 | 0·12 |
| GPR31 | 0·21 |
| CCRL2 | 0·3 |
| VHL | 0·37 |
After 48 hr, no significant changes were found in the EBV-infected tonsillar B cells compared with medium.
Fold decrease.
Discussion
The present study extends the studies of chemokine and chemokine receptor expression during primary EBV infection. We have focused the study on CCR7 and CXCR5, two chemokine receptors that are important for entry and migration into lymphoid tissue25 and they were compared after EBV infection of primary tonsillar B cells in vitro.
Early after infection (2 days) the EBV-infected cells showed a slight reduction in the cell surface expression of CXCR5 that decreased further over time. In the microarray, decreased mRNA expression for CXCR5 was also detected after 7 days. Low CXCR5 expression has previously been reported in EBV-immortalized B-cell lines.17 However, the downmodulation of CXCR5 was not linked to EBNA2 or LMP1 expression17,22 because the mRNA level of CXCR5 did not change after stable transfection of either EBNA2 or LMP1 into the human lymphoma line BJAB. It is possible that the reduced CXCR5 expression in EBV-infected cells reflects their activated plasmablast-like state, because plasmablasts were shown to express CXCR5 at low levels.10,11
CCR7 expression on the cell surface of EBV-infected tonsillar B cells decreased after 7 days of culturing. This was unexpected because CCR7 has previously been shown to be upregulated by EBV in different cell lines,19,21,22 such as BL41 and the ER/EB2-5 via oestrogen-mediated activation of EBNA226 and this was also confirmed by us. In addition, CCR7 expression on EBV-infected peripheral B cells showed a slower decrease in cell surface expression over time compared with tonsillar B cells. This may suggest that baseline CCR7 expression is differently regulated in B cells depending on origin and in B-cell lines immortalized by EBV.22,27 Transcriptional analysis of different B-cell compartments has revealed a higher CCR7 mRNA expression in peripheral B cells compared with tonsillar B cells.28
The chemokine receptors CXCR5 and CCR7 play an essential role in B-cell trafficking in lymphoid tissue25,29 and for microanatomic organization of lymphoid tissue. CXCR5–CXCL13 interactions direct the activated B cells into the follicle and CCR7–CCL21 guide the cell to the B-cell–T-cell border. CXCR5, together with CXCR4, is involved in the GC dark and light zone organization.30 Consequently, reduced expression of the CXCR5 and CCR7 chemokine receptors in the EBV-infected tonsillar B cells may interfere with the migration of the infected cell, as we also show in this study.
Migration towards CCL21 was impaired in EBV-infected cells although the surface expression of CCR7 remained similar to that of the uninfected controls. Interestingly, it has recently been shown that CCL19-induced and CCL21-induced migration may be impaired even in the presence of cell surface CCR7.31 Otero et al. showed that distinct, and separate motifs of the CCR7 receptor regulate receptor internalization and chemotaxis; where the intracellular tail of the receptor mediates signal transduction of chemotaxis.
Furthermore, when analysing EBNA2 in non-migrating cells, EBNA2 was more abundant in the non-migrating cells, also indicating that EBV impairs chemokine-induced migration (data not shown). Interestingly, one of the most important regulators of G-coupled chemokine receptors is the family of regulators of G-protein signalling (RGS) proteins. The expression of the protein RGS-1 is induced by EBNA2 and RGS-13 is downregulated by EBV-encoded genes27 and so may interfere with chemotaxis as already shown for CXCL13 and CCL21.32 In addition, EBV encodes a gene, BILF1 (G-protein coupled receptor) that may heterodimerize with human chemokine receptors and alter the signalling properties of these receptors.33 The biological consequence of reduced migration could be that EBV-infected cells are excluded from the B-cell follicle.
A proposed model of the establishment of EBV latency suggests that the infected B cell follows the same path as the antigen-activated B cell, which proliferates, enters the follicle and expands to form a germinal centre (reviewed in ref. 7). However, in tonsils from infectious mononucleosis patients the EBV-infected B cells are detected mainly in the interfollicular regions and do not migrate to the germinal centres.6,34 On the other hand, an even distribution of EBER-positive cells in both the germinal centre and the extrafollicular area has also been reported.35 In the LMP1 transgenic mouse model, however, mice produce high-affinity IgG1 despite disrupted germinal centre formation.36 This may be explained by the recent finding of activation-induced cytidine deaminase, an enzyme necessary for immunoglobulin somatic hypermutation and class switch recombination,37 in both extrafollicular large proliferating B cells and germinal centre B cells.38 Taken together, extrafollicular expansion and differentiation of EBV-carrying B cells may also occur in vivo.
The microarray data show that EBV induces the expression of a number of genes. The chemokine receptor CCR9 (upregulated at both time-points) is involved in homing to mucosal tissue. It has been shown that IgA+ antibody-producing cells express CCR9 at a high level in mucosal lymphoid tissues.12,24 The ligand for CCR9, TECK/CCL25, is produced by mucosal epithelial cells in tonsil tissue and, may therefore induce the homing of EBV-infected IgA+ B cells to the tonsil.39 In addition, high expression of C5AR1 (CD88) mRNA (the receptor for the complement C5a)40 was found. The receptor CD88 mediates chemotaxis of human tonsillar B cells and in tissue sections from human tonsils, CD88+ B cells are mainly localized to the interfollicular and subepithelial areas.41 This may suggest that EBV increases the transcription of genes important for B-cell homing to mucosal lymphoid tissue.
Epstein–Barr virus also influences the level of mRNA expression for chemokines and chemokine receptors associated with T-cell immune responses. The inflammatory chemokine CCL20 was upregulated after 48 hr and CCL20 has been shown to be chemotactic for the majority of T regulatory cells in blood.42 A recent study showed that EBNA1 upregulates CCL20 secretion in primary Hodgkin’s lymphoma cells, as well as in cell lines. Interestingly, the tumour cells secreted CCL20, which attracted T regulatory cells in vitro.43
There was also a set of genes less expressed after EBV infection including CXCL2 (ligand for CXCR2). Upon influenza virus infection, CXCL2 is produced as part of the host response and CXCL2 mediates proper recruitment of cytotoxic T cells44 to the site of infection. The changes in the expression of CCL20 and CXCL2 may be a mechanism by which the virus evades host immune responses locally in the tonsil. In infectious mononucleosis tonsils, EBV-specific cytotoxic T cells are poorly represented compared with the fraction of cells found in blood during acute infection.45
In conclusion, we have demonstrated that primary EBV infection of tonsillar B cells leads to a reduced cell surface expression of CCR7 and CXCR5, as well as to altered expression of several chemokine receptors and chemokines. Overall, a picture is emerging where primary EBV infection alters the expression of several chemokine receptors and as a consequence, the EBV-infected B cell may be excluded from the B-cell follicles and will be retained in the interfollicular areas of the tonsil.
Acknowledgments
This study was supported by grants received from the Swedish Children Cancer Foundation, the Regione Autonoma della Sardegna, Cagliari, Italy and Cancerfonden (Sweden). The Cancer Research Institute and Concern Foundation for Cancer Research also supported this study. Financial support was also provided through the regional agreement between Stockholm County Council and the Karolinska Institute. We thank Mia Löwbeer for excellent technical assistance.
Acknowledgments
The authors have no conflicting financial interests.
References
- 1.Ehlin-Henriksson B, Zou J-Z, Klein G, Ernberg I. Epstein–Barr virus genomes are found predominantly in IgA-positive B cells in the blood of healthy carriers. Int J Cancer. 1999;83:50–4. doi: 10.1002/(sici)1097-0215(19990924)83:1<50::aid-ijc10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Rickinson AM, Kieff E. Epstein–Barr virus. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott, Williams, Wilkins; 2001. p. 2. [Google Scholar]
- 3.Pegtel DM, Middeldorp J, Thorley-Lawson DA. Epstein–Barr virus infection in ex vivo tonsil epithelial cell cultures of asymptomatic carriers. J Virol. 2004;78:12613–24. doi: 10.1128/JVI.78.22.12613-12624.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niedobitek G, Agathanggelou A, Herbst H, Whitehead L, Wright DH, Young LS. Epstein–Barr virus (EBV) infection in infectious mononucleosis: virus latency, replication and phenotype of EBV-infected cells. J Pathol. 1997;182:151–9. doi: 10.1002/(SICI)1096-9896(199706)182:2<151::AID-PATH824>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Laichalk LL, Hochberg D, Babcock GJ, Freeman RB, Thorley-Lawson DA. The dispersal of mucosal memory B cells: evidence from persistent EBV infection. Immunity. 2002;16:745–54. doi: 10.1016/s1074-7613(02)00318-7. [DOI] [PubMed] [Google Scholar]
- 6.Kurth J, Spieker T, Wustrow J, Strickler GJ, Hansmann LM, Rajewsky K, Küppers R. EBV-infected B cells in infectious mononucleosis: viral strategies for spreading in the B cell compartment and establishing latency. Immunity. 2000;13:485–95. doi: 10.1016/s1074-7613(00)00048-0. [DOI] [PubMed] [Google Scholar]
- 7.Thorley-Lawson DA. Epstein–Barr virus; exploiting the immune system. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Huang J, Wu FY, Liao G, Hutt-Fletcher L, Hayward SD. Regulation of expression of the Epstein–Barr virus BamHI-A rightward transcripts. J Virol. 2005;79:1724–33. doi: 10.1128/JVI.79.3.1724-1733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–59. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama T, Hieshima K, Izawa D, Tatsumi Y, Kanamaru A, Yoshie O. Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J Immunol. 2003;170:1136–40. doi: 10.4049/jimmunol.170.3.1136. [DOI] [PubMed] [Google Scholar]
- 11.Hargreaves DC, Hyman PL, Lu TT, et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194:45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams IR. Chemokine receptors and leukocyte trafficking in the mucosal immune system. Immunol Res. 2004;29:283–92. doi: 10.1385/IR:29:1-3:283. [DOI] [PubMed] [Google Scholar]
- 13.Nicholas J. Human gammaherpesvirus cytokines and chemokine receptors. J Interferon Cytokine Res. 2005;25:373–83. doi: 10.1089/jir.2005.25.373. [DOI] [PubMed] [Google Scholar]
- 14.Paulsen SJ, Rosenkilde MM, Eugen-Olsen J, Kledal TN. Epstein–Barr virus-encoded BILF1 is a constitutively active G protein-coupled receptor. J Virol. 2005;79:536–46. doi: 10.1128/JVI.79.1.536-546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clay C, Rodrigues D, Harvey D, Leutenegger C, Esser U. Distinct chemokine triggers and in vivo migratory paths of fluorescein dye-labeled T lymphocytes in acutely simian immunodeficiency virus SIVmac251-infected and uninfected macaques. J Virol. 2005;79:13759–68. doi: 10.1128/JVI.79.21.13759-13768.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller A, Gerard C, Schaller M, Gruber A, Humbles A, Lukacs N. Deletion of CCR1 attenuates pathophysiologic responses during respiratory syncytial virus infection. J Immunol. 2006;176:2562–7. doi: 10.4049/jimmunol.176.4.2562. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama T, Fujisawa R, Izawa D, Hieshima K, Takada K, Yoshie O. Human B cells immortalized with Epstein–Barr virus upregulate CCR6 and CCR10 and downregulate CXCR4 and CXCR5. J Virol. 2002;76:3072–7. doi: 10.1128/JVI.76.6.3072-3077.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okudaira T, Yamamoto K, Kawakami H, et al. Transactivation of CCL20 gene by Epstein–Barr virus latent membrane protein 1. Br J Haematol. 2006;132:293–302. doi: 10.1111/j.1365-2141.2005.05877.x. [DOI] [PubMed] [Google Scholar]
- 19.Spender L, Lucchesi W, Bodelon G, et al. Cell target genes of Epstein–Barr virus transcription factor EBNA-2: induction of the p55alpha regulatory subunit of PI3-kinase and its role in survival of EREB2.5 cells. J Gen Virol. 2006;87:2859–67. doi: 10.1099/vir.0.82128-0. [DOI] [PubMed] [Google Scholar]
- 20.Chen A, Zhao B, Kieff E, Aster JC, Wang F. EBNA-3-B and EBNA-3C-regulated cellular genes in Epstein–Barr-virus-immortalized lymphoblastoid cell lines. J Virol. 2006;80:10139–50. doi: 10.1128/JVI.00854-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maier S, Santak M, Mantik A, Grabusic K, Kremmer E, Hammerschmidt W, Kempkes B. A somatic knock-out of CBF1 in a human B-cell line reveals that induction of CD21 and CCR7 by EBNA-2 is strictly CBF1 dependent and that downregulation of immunoglobulin M is partially CBF1 independent. J Virol. 2005;79:8784–92. doi: 10.1128/JVI.79.14.8784-8792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maier S, Staffler G, Hartmann A, et al. Cellular target genes of Epstein–Barr virus nuclear antigen 2. J Virol. 2006;80:9761–71. doi: 10.1128/JVI.00665-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehlin-Henriksson B, Mowafi F, Klein G, Nilsson A. Epstein–Barr virus infection negatively impacts the CXCR4-dependent migration of tonsillar B cells. Immunology. 2006;117:379–85. doi: 10.1111/j.1365-2567.2005.02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pabst O, Ohl L, Wendland M, Wurbel MA, Kremmer E, Malissen B, Förster R. Chemokine receptor CCR9 contributes to the localization of plasma cells to the small intestine. J Exp Med. 2004;199:411–6. doi: 10.1084/jem.20030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okada T, Ngo VN, Ekland EH, Förster R, Lipp M, Littman DR, Cyster JG. Chemokine requirements for B cell entry to lymph nodes and Peyer’s patches. J Exp Med. 2002;196:65–75. doi: 10.1084/jem.20020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgstahler R, Kempkes B, Steube K, Lipp M. Expression of the chemokin receptors BLR2EB11 is specifically transactivated by Epstein–Barr virus nuclear antigen 2. Biochem Biophys Res Commun. 1995;215:737–43. doi: 10.1006/bbrc.1995.2525. [DOI] [PubMed] [Google Scholar]
- 27.Cahir-McFarland E, Carter K, Rosenwald A, Giltnane J, Henrickson S, Staudt L, Kieff E. Role of NF-kappa B in cell survival and transcription of latent membrane protein 1-expressing or Epstein–Barr virus latency III-infected cells. J Virol. 2004;78:4108–19. doi: 10.1128/JVI.78.8.4108-4119.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein U, Tu Y, Stolovitzky GA, et al. Transcriptional analysis of the B cell germinal center reaction. Proc Natl Acad Sci U S A. 2003;100:2639–44. doi: 10.1073/pnas.0437996100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller G, Höpken UE, Lipp M. The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol Rev. 2003;195:117–35. doi: 10.1034/j.1600-065x.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 30.Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5:943–52. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 31.Otero C, Eisele PS, Schaeuble K, Groettrup M, Legler DF. Distinct motifs in the chemokine receptor CCR7 regulate signal transduction, receptor trafficking and chemotaxis. J Cell Sci. 2008;121:2759–67. doi: 10.1242/jcs.029074. [DOI] [PubMed] [Google Scholar]
- 32.Reif K, Cyster JG. RGS molecule expression in murine B lymphocytes and ability to down regulate chemotaxis to lymphoid chemokines. J Immunol. 2000;164:4720–9. doi: 10.4049/jimmunol.164.9.4720. [DOI] [PubMed] [Google Scholar]
- 33.Vischer HF, Nijmeijer S, Smit MJ, Leurs R. Viral hijacking of human receptors through heterodimerization. Biochem Biophys Res Commun. 2008;377:93–7. doi: 10.1016/j.bbrc.2008.09.082. [DOI] [PubMed] [Google Scholar]
- 34.Kurth J, Hansmann M-L, Rajewsky K, Kuppers R. Epstein–Barr virus-infected B cells expanding in germinal centers of infectious mononucleosis patients do not participate in the germinal center reaction. Proc Natl Acad Sci USA. 2003;100:4730–5. doi: 10.1073/pnas.2627966100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi R, Takeuchi H, Sasaki M, Hasegawa M, Hirai K. Detection of Epstein–Barr virus infection in the epithelial cells and lymphocytes of non-neoplastic tonsils by in situ hybridization and in situ PCR. Arch Virol. 1998;143:803–13. doi: 10.1007/s007050050332. [DOI] [PubMed] [Google Scholar]
- 36.Uchida J, Yasui T, Takaoka-Shichijo Y, Muraoka M, Kulwichit W, Raab-Traub N, Kikutani H. Mimicry of CD40 signals by Epstein–Barr virus LMP1 in B lymphocyte responses. Science. 1999;286:300–3. doi: 10.1126/science.286.5438.300. [DOI] [PubMed] [Google Scholar]
- 37.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–63. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 38.Cattoretti G, Büttner M, Shaknovich R, Kremmer E, Alobeid B, Niedobitek G. Nuclear and cytoplasmic AID in extrafollicular and germinal center B cells. Blood. 2006;107:3967–75. doi: 10.1182/blood-2005-10-4170. [DOI] [PubMed] [Google Scholar]
- 39.Bourges D, Wang CH, Chevaleyre C, Salmon H. T and IgA B lymphocytes of the pharyngeal and palatine tonsils: differential expression of adhesion molecules and chemokines. Scand J Immunol. 2004;60:338–50. doi: 10.1111/j.0300-9475.2004.01479.x. [DOI] [PubMed] [Google Scholar]
- 40.Boshra H, Li J, Peters R, Hansen J, Matlapudi A, Oriol Sunyer J. Cloning, expression, cellular distribution, and role in chemotaxis of a C5a receptor in rainbow trout: the first identification of a C5a receptor in a nonmammalian species. J Immunol. 2004;172:4381–90. doi: 10.4049/jimmunol.172.7.4381. [DOI] [PubMed] [Google Scholar]
- 41.Ottonello L, Corcione A, Tortolina G, et al. rC5a directs the in vitro migration of human memory and naive tonsillar B lymphocytes: implications for B cell trafficking in secondary lymphoid tissues. J Immunol. 1999;162:6510–7. [PubMed] [Google Scholar]
- 42.Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS. The majority of human peripheral blood CD4+ CD25high Foxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol. 2006;177:4488–94. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- 43.Baumforth KR, Birgersdotter A, Reynolds GM, et al. Expression of the Epstein–Barr virus-encoded Epstein–Barr virus nuclear antigen 1 in Hodgkin’s lymphoma cells mediates up-regulation of CCL20 and the migration of regulatory T cells. Am J Pathol. 2008;173:195–204. doi: 10.2353/ajpath.2008.070845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piqueras B, Connolly J, Freitas H, Palucka AK, Banchereau J. Upon viral exposure, myeloid and plasmacytoid dendritic cells produce 3 waves of distinct chemokines to recruit immune effectors. Blood. 2006;107:2613–8. doi: 10.1182/blood-2005-07-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hislop AD, Kuo M, Drake-Lee AB, et al. Tonsillar homing of Epstein–Barr virus-specific CD8+ T cells and the virus-host balance. J Clin Invest. 2005;115:2546–55. doi: 10.1172/JCI24810. [DOI] [PMC free article] [PubMed] [Google Scholar]