Abstract
To investigate the phenotypic and migrational properties of oral mucosal dendritic cells (OMDCs), fluorescein isothiocyanate (FITC) was painted onto mouse buccal mucosa and the expression patterns of functional molecules in FITC-bearing migrating DCs within the regional lymph nodes (RLNs) were analysed. We found three distinct subpopulations of migrating OMDCs within the RLNs: CD11chi CD207− (F1), CD11cint/lo CD207− (F2) and CD11cint/lo CD207+ (F3). The F1 DCs reached the RLNs earlier (after 24 hr) but diminished immediately. Additionally, F1 DCs expressed high levels of CD11b. The F2 DCs migrated continuously to the RLNs and maintained the highest ratio of all three fractions. The F3 DCs migrated slowly to the RLNs and demonstrated a late peak at 96 hr. In addition, F3 DCs showed the highest CD205 expression levels of all three subsets. All fractions of migrating OMDCs expressed CD80, CD86 and major histocompatibility complex class II at high levels, suggesting that all OMDCs are in a mature stage and have the potential for antigen presentation. All migrating OMDCs lacked CD8α expression. Taken together, our results indicate that the lack of CD207 is one factor that identifies submucosal DCs. Both F1 and F2 DCs lack CD207; F1 DCs are resident and F2 DCs are newly recruited following FITC application. The F3 DCs, which express CD207, are mucosal Langerhans cells that migrate later. The identification of OMDC subsets should facilitate further studies investigating the functional roles of each fraction.
Keywords: dendritic cells, migration, mucosal immunity, oral immunology, regional lymph nodes
Introduction
The internal surfaces of the body are covered with two distinct types of mucosae.1 Type I mucosal surfaces, which include the intestine, lung and nasal cavity, are covered by a single epithelial layer with absorptive and respiratory functions. In contrast, type II mucosal surfaces are covered by stratified squamous epithelium. Type II surfaces share many common features with the skin and provide physical protective barriers as a first line of defence. Oral mucosa, the cornea and vagina have type II mucosal surfaces. Two distinct types of dendritic cells (DCs) are found in type II mucosa. As with the skin, Langerhans cells (LCs) reside within the epithelial layers, whereas submucosal DCs (i.e. interstitial DCs), which are the counterpart of dermal DCs in the skin, reside beneath the basement membrane of the mucosal epithelium. In general, DCs recognize pathogen-derived antigens, induce innate immunity at local surfaces, and then migrate into the regional lymph nodes (RLNs) via afferent lymphatic vessels to present antigens to T cells.1–4 During this process, DCs alter multiple properties including antigen capture, endocytosis, migration, antigen processing and antigen presentation by upregulating and downregulating the expression of unique receptors and cytokines.2,3
Although LCs exist in an immature state within the epidermis of the skin, they display a mature phenotype in the LNs.2,4 Traditionally, LCs have been thought to play a dominant role in the induction of contact hypersensitivity (CH). Recent studies using genetically LC-disrupted mice, however, have shown less involvement of LCs and provide evidence for the more active involvement of dermal DCs.5–8 CD207/Langerin, a C-type lectin endocytic receptor, is highly expressed on LCs9,10 and is essential for Birbeck granule formation, but not for LC function.11 Recent reports have demonstrated the existence of CD207+ dermal DCs, which appear to be responsible for CH responses.12,13 Therefore, the actual involvement of two types of DCs in cutaneous immunity remains controversial.
Little is known regarding the features of oral mucosal DCs (OMDCs). Studies using sublingual immunotherapy in patients with allergic rhinitis and asthma suggest tolerogenic properties of OMDCs, but precise functional analyses have not been performed.14,15 Studies have demonstrated that topical application of hapten onto buccal mucosa induces both the migration of DCs and hapten-specific T-cell responses.16,17 Comparative experiments between oral mucosa and skin applications have reported that oral mucosa sensitization induces clearly impaired CH responses. These responses, however, can be explained by either the lower dose of antigen concentrations used or the lower number of LCs present at the mucosal site. These responses do not appear to be a result of the different antigen-presenting capacities of DCs.17,18 In addition, these studies did not discriminate between the functions of submucosal DCs and LCs.17,19,20 Recently, Mascarell et al.21 performed more detailed analyses of OMDCs. These studies identified four subsets of OMDCs: CD207+ LCs in the mucosa, a major population of CD11b+ CD11c− and CD11b+ CD11c+ myeloid DCs at the mucosal/submucosal interface, and B220+ 120G8+ plasmacytoid DCs in the submucosa. The last three of these subsets showed antigen-processing and antigen-presenting abilities, but only CD11b+ CD11c+ myeloid and plasmacytoid DCs induced interferon-γ/interleukin-10 (IFN-γ/IL-10)-producing CD4+ T cells exhibiting suppressive properties, suggesting that these OMDCs have tolerogenic properties. However, DCs alter their function between the oral mucosa and the RLNs following migration. Therefore, to investigate the precise roles of OMDCs in T-cell-mediated immunity, migrating OMDCs in the RLNs should be examined. For this purpose, the identification of migrating OMDCs in the RLNs is necessary. As a first step, we investigated the phenotypic and migrational properties of OMDCs in the RLNs. We applied fluorescein isothiocyanate (FITC) as hapten onto buccal mucosa and investigated the histological changes in OMDCs at mucosal sites. We then analysed FITC-bearing DCs in the RLNs using flow cytometry. Comparative ear skin painting experiments were also performed to examine differences between OMDCs and cutaneous DCs.
Materials and methods
Mice
Female BALB/c mice were purchased from Japan SLC (Hamamatsu, Japan) and used at 6–10 weeks of age. All animal procedures were reviewed and approved by the Animal Care and Use Committee of Tokyo Medical and Dental University.
FITC painting
The FITC was dissolved in a 1 : 1 (vol/vol) acetone : dibutyl phthalate solution before painting.22,23 For painting of the buccal mucosa, Vaseline was applied around the mouth while the mouse was under anaesthesia to prevent the FITC solution from reaching the perioral skin tissues.16 After the buccal mucosa was swabbed with cotton buds, 15 μl of a 1% FITC solution was applied to both sides of the buccal mucosa. Mucosal surfaces were then air-dried for 1 min. For ear skin painting, 30 μl of a 0·5% FITC solution was applied onto each side of the ears.
Immunohistochemistry
Buccal mucosal tissues from intact mice or FITC-painted mice after 2 and 6 hr were surgically removed, embedded in Tissue-Tek (Sakura, Tokyo, Japan), frozen and stored at −80° until use. Cryostat sections (5 μm thickness) were fixed in absolute acetone and subjected to enzymatic immunohistochemistry as described previously.23 Briefly, after blocking, sections were incubated with primary monoclonal antibodies (mAbs) against major histocompatibility complex (MHC) class II [M5/114, rat immunoglobulin G2b (IgG2b)], CD207 (eBioL31, rat IgG2a), CD205 (NLDC-145, rat IgG2a), or isotype control rat immunoglobulins, and then were incubated with biotinylated anti-rat IgG (Vector Laboratories, Burlingame, CA). All incubation steps, excluding those for CD205, were performed in a microwave processor (MI-77; Azumaya, Tokyo, Japan). A mAb against CD205 was incubated at 4° overnight. The avidin–biotin–peroxidase complex (ABC) system (Vectastain Elite Universal ABC Kit; Vector Laboratories) was used according to the manufacturer’s instructions. Sections were visualized with the substrate diaminobenzidine (DAB; Merck, Darmstadt, Germany) and counterstained with haematoxylin. Digital images were obtained using an inverted microscope and camera system (IX71 and Pro600ES-D; Olympus, Tokyo, Japan).
Isolation of LN cells
Mandibular and cervical (auricular) LNs from buccal mucosa-painted mice and cervical LNs from ear skin-painted mice were collected from RLNs at 24, 48, 72 and 96 hr after painting. For isolating a single-cell suspension of LN cells, the collected LNs were digested with Type I collagenase (400 U/ml; Sigma, St Louis, MO) at 37° for 30 min. Ethylenediaminetetraacetic acid at the final concentration of 5 mm was added for an additional 5 min at room temperature to disrupt T-cell–DC complexes.
Flow cytometry
Cells were preincubated with supernatants for anti-CD16/32 (2.4G2) mAb and then stained with fluorochrome-conjugated or biotinylated mAbs. Monoclonal antibodies against CD11c (N418), CD11b (M1/70), CD8α (53-6·7), CD86 (GL-1), CD80 (16-10A1), MHC class II (M5/114.15.2), CD205 (NLDC-145), F4/80 and CD207 (eBioL31) were used. All FITC-, phycoerythrin-, allophycocyanin-, peridinin chlorophyll protein–carbocyanin 5.5 (PerCP-Cy5.5)-conjugated or biotinylated mAbs were obtained from eBiosciences (San Diego, CA) or BD-Pharmingen (San Diego, CA). For the biotinylated mAb, PerCP-Cy5.5-streptavidin (BD-Pharmingen) was used. After cell surface staining, intracellular staining for CD207 was performed as described previously.24 Stained cells were analysed on a FACSCalibur using the cellquest software (BD Biosciences, San Jose, CA).
Results
Rapid recruitment of circulating DCs into submucosal areas after FITC painting
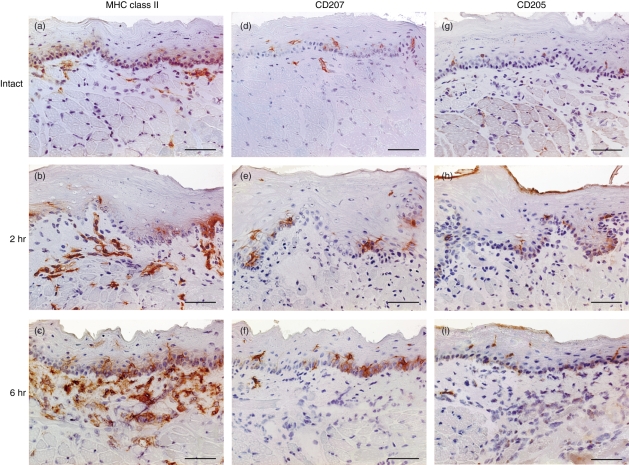
Fluorescein isothiocyanate has been commonly used for monitoring skin-derived DCs in RLNs and evaluating hapten-specific immune responses in murine CH models.22 In this study, we used FITC to examine DCs in the oral mucosa. The FITC was painted onto buccal mucosa and changes in DCs were analysed using immunohistochemistry. In the buccal mucosa from intact mice, MHC class II+ cells with dendritic morphology were located within both the epithelium and subepithelium (Fig. 1a). Two hours after FITC painting, the number and intensity of MHC class II+ cells in the submucosal layer markedly increased (Fig. 1b). These levels were further enhanced at 6 hr (Fig. 1c). The MHC class II+ cell numbers decreased at 24 hr (data not shown). In contrast, the number of MHC class II+ cells within the epithelium did not vary at any time-point, although the signal intensity and cell size increased gradually and reached a maximum at 24 hr (data not shown). Application with vehicle alone had no discernible effects (data not shown). CD207/Langerin and CD205/DEC205 are representative C-type lectin receptors that are expressed on DCs.9,10,25 CD205 is a crucial endocytic receptor expressed in DCs with high phagocytic potency.25,26 CD207+ cells were observed in the epithelium, but few CD207+ cells were seen in the subepithelium (Fig. 1d–f). Following FITC painting, no clear changes in CD207+ cell number were observed; however, a marked enlargement and elongation of CD207+ cells within the epithelium was seen, which was similar to the changes in MHC class II+ cells. CD205+ cells were also abundant within the epithelial layer, although the signal was weak (Fig. 1g). The intensity of CD205+ cells within the epithelium gradually increased after FITC application (Fig. 1h,i). These results suggest that FITC painting of the buccal mucosa induces a rapid morphological change in pre-existing subepithelial DCs. Additionally, new recruitment of DCs into the subepithelium at earlier time-points (2 hr) was observed with a consistent enhancement up to 6 hr. Painting with FITC also induced a late morphological change in resident CD207+ CD205+ Langerhans cells within the epithelium.
Figure 1.
Kinetic changes in major histocompatibility complex (MHC) class II, CD207, and CD205 expression within the buccal mucosa after fluorescein isothiocyanate (FITC) painting. Cryostat sections of buccal mucosa from intact mice (a, d, g) and mice 2 hr (b, e, h), 6 hr (c, f, i), and 24 hr (data not shown) after FITC painting were stained with anti-MHC class II (a–c), anti-CD207 (d–f), or anti-CD205 (g–i) monoclonal antibodies (with an appropriate control antibody). Representative sections are shown. Scale bars = 50 μm.
Migration of OMDCs to RLNs
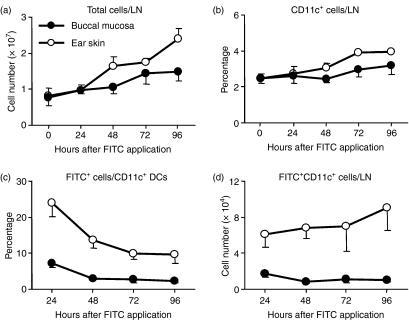
Antigen-captured DCs at the skin and mucosa migrate to the RLNs to present antigens to T cells. To investigate the migrational capacity and kinetics of OMDCs, we monitored FITC-carrying DCs from both the oral mucosa and ear skin to the RLNs using flow cytometry. The total number of cells in the RLNs following painting (both buccal mucosa and ear skin) gradually increased and this increase continued until 96 hr; however, the increased level observed with buccal mucosa painting was clearly impaired compared with that after ear skin painting (Fig. 2a). Intact submandibular and cervical LNs contained approximately 2% of CD11c+ DCs and the percentage of CD11c+ cells gradually increased after painting until 96 hr for both buccal mucosa and ear skin painting (Fig. 2b). The increased levels observed with buccal mucosa painting were consistently impaired. When FITC-bearing DCs within LN DCs were analysed, a peak was observed at 24 hr with both types of painting (Fig. 2c). Levels then sharply decreased at 48 hr and were maintained until 96 hr. The percentage of FITC+ DCs in buccal mucosa-painted mice at 24 hr was approximately threefold lower than that in ear skin-painted mice. Buccal mucosa painting did not increase the numbers of FITC-bearing DCs in the RLNs, whereas FITC-bearing DCs as a result of ear skin painting gradually increased until 96 hr (Fig. 2d). The final difference between buccal mucosa and ear skin painting was over eightfold. These results demonstrate that FITC-painting induces not only migration of DCs into the RLNs, but also the migration of other lymphocytes, that are presumably circulating via blood vessels. The migration of OMDCs and cutaneous DCs following local painting showed similar kinetics, although a clear difference in cell number was observed.
Figure 2.
Changes in regional lymph node (RLN) cells after fluorescein isothiocyanate (FITC) painting. Mandibular and/or cervical LN cells from intact and painted mice 24, 48, 72 and 96 hr after FITC application were counted and stained with allophycocyanin-conjugated anti-CD11C or with the appropriate fluorochrome-conjugated control antibody. The stained cells were analysed by flow cytometry. Total cell number per one LN is shown (a). An electronic gate was placed on either lymphocytes or CD11c+ lymphocytes and then the percentages of CD11c+ or FITC+ cells were evaluated (b–d). Total numbers of FITC+ CD11c+ cells were calculated from total LN cell number and the percentage of FITC+ CD11c+ cells. Values are the mean ± SD from each group of three mice. Data are representative of two independent experiments.
Distinct subsets of migrating OMDCs
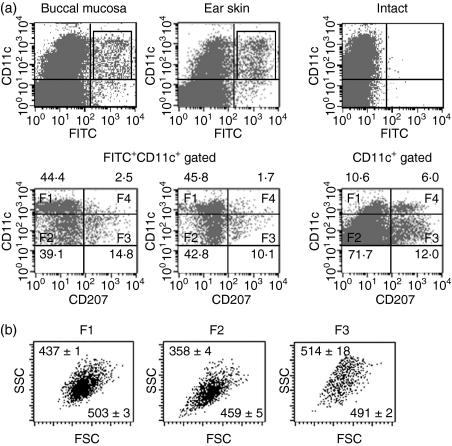
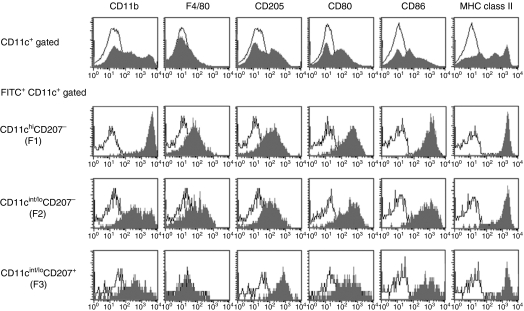
Both LCs and dermal DCs possess migrational capacity following antigenic challenge, but the kinetics of these responses differ.7,27 We examined more detailed phenotypes of FITC-carrying migratory DCs within RLNs. As shown in Fig. 3(a), based on differences in CD11c and CD207 expression, we divided FITC+ CD11c+ migratory DCs from the buccal mucosa and ear skin into four fractions: F1, CD11chi CD207−; F2, CD11cint/lo CD207−; F3, CD11cint/lo CD207+; and F4, CD11chi CD207+. In RLNs at 24 hr, F1 and F2 were the major subsets observed within migrating DCs, followed by the F3 in both paintings. F4 cells were hardly detected within FITC-carrying migrating DCs. When cervical LNs from intact mice were analysed, F2 DCs were the major fraction. Additionally, intact LN DCs contained a substantial percentage (∼ 6%) of F4 cells. Three fractions of migrating DCs (F1, F2 and F3) did not express CD8α, whereas F4 DCs expressed high levels of CD8α (data not shown). When forward scatter (FSC) and side scatter (SSC) profiles for each fraction were analysed, we found that the FSC and SSC profiles for F2 DCs were relatively lower in both the buccal mucosa-painted (Fig. 3b) and ear skin-painted (data not shown) RLNs.
Figure 3.
Identification of different subsets of migrating dendritic cells (DCs). Regional lymph node (RLN) cells were obtained as described in Fig. 2. Cells were stained with allophycocyanin-conjugated anti-CD11c and phycoerythrin-conjugated anti-CD207 monoclonal antibodies or with the appropriate control immunoglobulins and analysed by flow cytometry. (a) The expressions of CD11c and fluorescein isothiocyanate (FITC) are shown as dotted plots (four-decade log scale) (upper panels). An electronic gate was placed on either FITC− CD11c+ or FITC+ CD11c+ cells, and then the expression of CD11c and CD207 are also shown as dotted plots (lower panels). Markers were positioned to include > 98% of cells stained with control immunoglobulins in the lower left quadrant. Other maker lines were placed to separate CD11chi and CD11clo cells (lower panels). CD11chi CD207− cells (F1), CD11cint/lo CD207− cells (F2), CD11cint/loCD207+ cells (F3) and CD11chi CD207+ cells (F4) were defined based on the expression levels of CD11c and CD207. Values are the mean percentages ± SD from each group of three mice. Representative profiles at 24 hr after painting are shown. (b) An electronic gate was placed on F1, F2, or F3 FITC+ lymphocytes and then forward scatter (FSC) and side scatter (SSC) profiles are shown as dotted plots. Values are the mean FSC and SSC from each group of three mice. Representative profiles at 24 hr are shown.
Different subsets of OMDCs show different migrational kinetics to RLNs
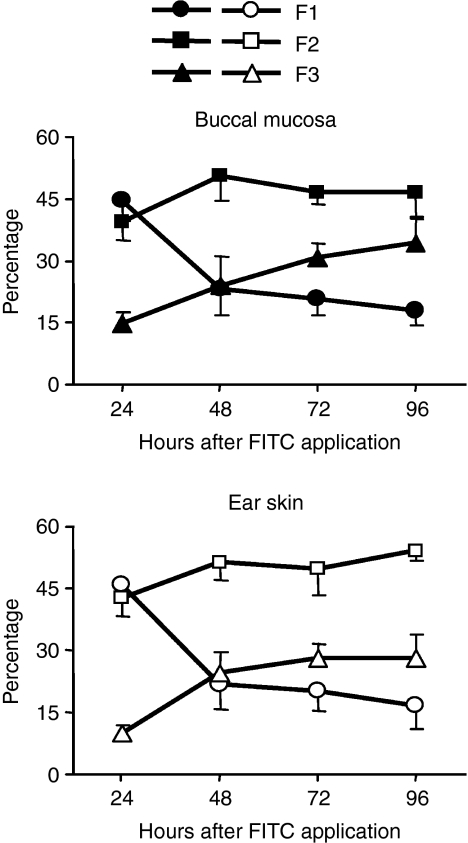
Next, we analysed the kinetic changes of each migrating DC fraction (Fig. 4). In buccal mucosa-painted mice, the percentage of F1 cells peaked at 24 hr and then rapidly decreased. In contrast, F2 percentages were maintained at high ratios at all time-points up to 96 hr. The percentage of F3 DCs was lowest at 24 hr and then gradually increased until 96 hr. Similar results were obtained for migrating DCs induced by ear skin painting. These results demonstrate that the three fractions of hapten-captured DCs in both buccal mucosa and ear skin migrate to the RLNs with distinct kinetics.
Figure 4.
Each dendritic cell (DC) subset shows distinct migratory kinetics. Regional lymph node (RLN) cells were obtained, cells were stained, and each fraction was identified as described in Fig. 3. The percentages of CD11chi CD207− (F1), CD11cint/lo CD207− (F2) and CD11cint/lo CD207+ (F3) cells within FITC+ CD11c+ DCs from RLNs at the indicated time-points are shown. Values are the mean ± SD from each group of three mice. Representative data from two independent experiments are shown. FITC, fluorescein isothiocyanate.
Phenotypic differences between the three subsets of migrating OMDCs
Some DCs migrating from the skin express myeloid markers, F4/80 and CD11b.28,29 We examined these myeloid antigens on migratory DCs from the buccal mucosa. F1 DCs expressed markedly higher levels of CD11b compared with other DCs, and F2 DCs contained both high- and intermediate-level cells (Fig. 5). This was the only unique difference in antigenic phenotypes between F1 and F2 DCs. Both F1 and F2 cells expressed low levels of F4/80 and intermediate levels of CD205. In addition, both fractioned cells expressed similar levels of the CD80, CD86 and MHC class II molecules crucial for antigen presentation. The staining profiles of CD80 and CD86 in unfractioned RLN-DCs revealed that the level of CD80 and CD86 expression in F1 and F2 cells was markedly high. CD11cint/lo CD207+ F3 DCs also expressed similar levels of CD80, CD86 and MHC class II as compared with F1 and F2 DCs. F3 DCs expressed the highest levels of CD205 and intermediate levels of CD11b. No clear differences were observed in each subset of migrating DCs between buccal mucosa and ear skin (data not shown).
Figure 5.
Comparison of antigenic phenotypes among three dendritic cell (DC) subsets. Regional lymph node (RLN) cells 24 hr after buccal mucosa painting were stained with allophycocyanin-conjugated anti-CD11c, phycoerythrin-conjugated anti-CD207 and phycoerythrin-conjugated anti-CD80, anti-CD86, anti-major histocompatibility complex class II, biotinylated anti-CD205, anti-CD11b monoclonal antibodies, or the appropriate control immunoglobulin. Biotinylated monoclonal antibodies were followed by peridinin chlorophyll protein–carbocyanin 5·5–streptavidin. Samples were analysed by flow cytometry. Patterns of CD207 and CD11c expression under the FITC+ CD11c+ or CD11c+ cell gate are shown in Fig. 3. An electronic gate was placed on CD11chi CD207− (F1), CD11cint/lo CD207− (F2) or CD11cint/lo CD207+ (F3) cells, and the expression levels of respective antigens are displayed as histograms (shaded histograms) with the control histograms nearest the ordinate (open histograms) (four-decade log scales). Data shown are representative of three to five results. FITC, fluorescein isothiocyanate.
Discussion
We demonstrated that hapten-bearing, migrating DCs from the buccal mucosa within the RLNs are divided into at least three subpopulations: CD11chi CD207− (F1), CD11cint/lo CD207− (F2), and CD11cint/lo CD207+ (F3). All fractions of migrating DCs express CD80, CD86 and MHC class II at highest levels compared with the expression of these molecules in whole LN DCs, which suggests that they are in a mature state and have the potential for antigen presentation. However, the migrational kinetics and relative cell numbers among subpopulations following hapten application showed distinct differences. F1 DCs migrated quickly to the RLNs, but levels diminished immediately. F2 DCs migrated at a constant rate to the RLNs and maintained high ratios. F3 DCs migrated slowly to the RLN and peak levels were observed at 3–4 days.
In this study, we performed the majority of the experiments in parallel with ear skin painting and we observed similar results in antigenic phenotypes and migrational kinetics between ear skin and buccal mucosa painting. One observed difference was that the cell numbers of FITC-carrying migrating DCs from the ear skin were approximately threefold higher than those from the buccal mucosa at a maximum migration point of 24 hr. One reason for this observed variation may have been the difference in painted surface area. For ear skin painting, the total surface area was approximately fivefold larger than that for oral mucosa painting. Although the distribution (cell numbers/same length of basal cell membrane) of MHC class II+ (CD207+) resident LCs in the steady state for ear skin was approximately twofold higher (data not shown), LCs are not the major source of migrating DCs (described below). It is also possible that the unique conditions existing in oral mucosa lower the migratory property of OMDCs. Further studies are now underway on this issue.
Our immunohistochemical staining of buccal mucosa showed that CD207+ cells are abundant in the epithelium and are barely present in the subepithelium. In addition, levels did not increase after hapten application, despite the enlargement and elongation of cells. Within LNs, we found two fractions of CD207+ DCs: CD11chi CD207+ (F4) and CD11cint/lo CD207+ (F3). Intact LN cells contained a substantial ratio of CD11chi CD207+ (F4) cells; however, FITC-carrying migrating DCs hardly contained this fraction for both oral mucosa and ear skin painting. In contrast to F3 DCs, F4 DCs expressed high levels of CD8α. F4 DCs did not express F4/80 and expressed low levels of CD11b, CD205, CD80, CD86 and MHC class II (data not shown). We (data not shown) and other groups27,30 also observed similar results in the RLNs by epicutaneous painting. The phenotypes observed for F4 DCs indicate that they are in an immature state. CD11chi CD207+ CD8+ DCs in LNs have been proposed to be ‘blood-derived’ lymphoid DCs that may correspond to cross-presentation.7,27,30,31 Therefore, we propose that CD11chi CD207+ DCs (F4) are blood-derived resident LN DCs that are distinct from LCs migrating via afferent lymph vessels. In contrast, CD11cint/lo CD207+ DCs (F3) and CD11clo-hi CD207− DCs (F1 and F2) are migrating mucosal LCs and submucosal DCs from buccal mucosa, respectively. LCs and dermal DCs have been previously reported to continuously emigrate to cutaneous RLNs, even in the steady state.7 Therefore, that the intact LN cells contained these fractions of DCs at considerable ratios is not surprising (Fig. 3). Our kinetic experiments, which examined FITC-carrying DCs, demonstrated that F3 DCs show delayed migration to RLNs. Additionally, peak migration was observed at 3–4 days after both skin and mucosa painting. Similar results have been reported in earlier studies using a skin painting model with a fluorescent dye32 and CD207-EGFP knockin mice.7 Recent reports have demonstrated the existence of CD207+ dermal DCs showing both earlier migration and involvement in CH responses.12,13 We also observed some CD207+ cells in the subepithelium of intact buccal mucosa. Therefore, we cannot negate the possibility that F3 contains such submucosal/dermal CD207+ DCs in addition to genuine LCs.
We found that CD207− migrating non-LC DCs can be further divided into two subpopulations based on the expression levels of CD11c: CD11chi CD207− (F1) and CD11cint/lo CD207− (F2). Both fractions of cells expressed comparable, moderate levels of CD205, low levels of F4/80, and high levels of CD80, CD86 and MHC class II. However, several different characteristics were observed. First, F2 DCs showed the lowest FSC and SSC profiles among the three DC fractions (F1–F3). Second, most F1 cells expressed CD11b at high levels, although F2 DCs contained both high- and intermediate-level cells. Third, F1 migration reached a maximum within the initial 24 hr and then quickly decreased, while migration of F2 occurred persistently over the entire experimental period up to 96 hr (Fig. 3). In addition, immunohistological analyses showed that FITC application induced a rapid recruitment of MHC class II+CD207− cells in the submucosal area at earlier time-points (2–6 hr). Finally, the number of newly recruited cells quickly increased over the number of resident submucosal DCs (Fig. 1). Taken together, our results indicate that the origins of F1 and F2 cells may be resident submucosal DCs and newly recruited submucosal DCs via blood vessels following painting, respectively. FITC application may induce a rapid migration of resident submucosal DCs to the subepithelium near the site of FITC painting. These submucosal DCs may be able to uptake antigens and reach the RLNs in the shortest amount of time. However, since the number of resident submucosal DCs was limited, their concentrations decreased quickly. Therefore, newly recruited DCs (via blood vessels) may replace resident submucosal DCs. Expression levels of CD11c and CD11b within CD207− migrating DCs seem to be positively correlated and F1 cells expressed higher levels of CD86 assessed by mean fluorescence intensity. Cytokines such as granulocyte–macrophage colony-stimulating factor, Fms-like tyrosine kinase-3 and tumour necrosis factor-α may directly and indirectly affect expression levels of CD11b and CD11c.33,34 The antigenic properties of newly recruited submucosal DCs (F2) may be affected by sojourn time and site-specific cytokine conditions in the submucosa. The difference between F1 and F2 cells may come from the difference in the length of time that the cells reside in the submucosa.
In the present study, we found that all migrating oral DCs, including F1, F2 and F3, expressed high levels of CD80, CD86 and MHC class II. Although these three molecules are essential for antigen presentation, the differential profiles of additional positive and negative costimulatory molecules such as CD40, OX40L, 4-1BBL, glucocorticoid-induced tumour necrosis factor receptor-related receptor ligand, B7-H1 and B7-DC, or cytokine expression may affect various functions of DCs.2,3 This may result in differential production of T-cell effector cytokines and T-cell subset generation. Further studies are required to clarify their precise role in inducing antigen-specific T-cell-mediated immune responses. Recent observations suggest that dermal DCs rather than LCs play a crucial role in inducing cutaneous immune responses.7,8 Therefore, it will be of great interest to compare the function of these three subsets of OMDCs and the differences between oral mucosa and skin migrating DCs. Future studies in our laboratory will address these issues.
Acknowledgments
This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan. We thank Drs Jian Zhang and Patcharee Ritprajak for technical assistance.
Acknowledgments
The authors have no financial conflict of interest.
References
- 1.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 4.Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 5.Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, Clausen BE. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–76. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal Langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–20. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Kissenpfennig A, Henri S, Dubois B, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–54. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Fukunaga A, Khaskhely NM, Sreevidya CS, Byrne SN, Ullrich SE. Dermal dendritic cells, and not Langerhans cells, play an essential role in inducing an immune response. J Immunol. 2008;180:3057–64. doi: 10.4049/jimmunol.180.5.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 10.Takahara K, Omatsu Y, Yashima Y, et al. Identification and expression of mouse angerin (CD207) in dendritic cells. Int Immunol. 2002;14:433–44. doi: 10.1093/intimm/14.5.433. [DOI] [PubMed] [Google Scholar]
- 11.Kissenpfennig A, Ait-Yahia S, Clair-Moninot V, et al. Disruption of the langerin/CD207 gene abolishes Birbeck granules without a marked loss of Langerhans cell function. Mol Cell Biol. 2005;25:88–99. doi: 10.1128/MCB.25.1.88-99.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–31. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–56. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frew AJ. Sublingual immunotherapy. N Engl J Med. 2008;358:2259–64. doi: 10.1056/NEJMct0708337. [DOI] [PubMed] [Google Scholar]
- 15.Novak N, Haberstok J, Bieber T, Allam JP. The immune privilege of the oral mucosa. Trends Mol Med. 2008;14:191–8. doi: 10.1016/j.molmed.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Ahlfors E, Czerkinsky C. Contact sensitivity in the murine oral mucosa. I. An experimental model of delayed-type hypersensitivity reactions at mucosal surfaces. Clin Exp Immunol. 1991;86:449–56. doi: 10.1111/j.1365-2249.1991.tb02952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okamura T, Morimoto M, Yamane G, Takahashi S. Langerhans’ cells in the murine oral mucosa in the inductive phase of delayed type hypersensitivity with 1-chloro-2, 4-dinitrobenzene. Clin Exp Immunol. 2003;134:188–94. doi: 10.1046/j.1365-2249.2003.02277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Wilsem E, van Hoogstraten I, Breve J, Zaman Y, Kraal G. In vivo antigen presentation capacity of dendritic cells from oral mucosa and skin draining lymph nodes. Adv Exp Med Biol. 1994;355:63–7. doi: 10.1007/978-1-4615-2492-2_11. [DOI] [PubMed] [Google Scholar]
- 19.Van Wilsem EJ, Van Hoogstraten IM, Breve J, Scheper RJ, Kraal G. Dendritic cells of the oral mucosa and the induction of oral tolerance. A local affair. Immunology. 1994;83:128–32. [PMC free article] [PubMed] [Google Scholar]
- 20.Eriksson K, Ahlfors E, George-Chandy A, Kaiserlian D, Czerkinsky C. Antigen presentation in the murine oral epithelium. Immunology. 1996;88:147–52. doi: 10.1046/j.1365-2567.1996.d01-647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mascarell L, Lombardi V, Louise A, et al. Oral dendritic cells mediate antigen-specific tolerance by stimulating TH1 and regulatory CD4+ T cells. J Allergy Clin Immunol. 2008;122:603–9. doi: 10.1016/j.jaci.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 22.Macatonia SE, Edwards AJ, Knight SC. Dendritic cells and the initiation of contact sensitivity to fluorescein isothiocyanate. Immunology. 1986;59:509–14. [PMC free article] [PubMed] [Google Scholar]
- 23.Ritprajak P, Hashiguchi M, Azuma M. Topical application of cream-emulsified CD86 siRNA ameliorates allergic skin disease by targeting cutaneous dendritic cells. Mol Ther. 2008;16:1323–30. doi: 10.1038/mt.2008.91. [DOI] [PubMed] [Google Scholar]
- 24.Sakurai J, Ohata J, Saito K, et al. Blockade of CTLA-4 signals inhibits Th2-mediated murine chronic graft-versus-host disease by an enhanced expansion of regulatory CD8+ T cells. J Immunol. 2000;164:664–9. doi: 10.4049/jimmunol.164.2.664. [DOI] [PubMed] [Google Scholar]
- 25.Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–5. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 26.Henri S, Vremec D, Kamath A, et al. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–8. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- 27.Douillard P, Stoitzner P, Tripp CH, et al. Mouse lymphoid tissue contains distinct subsets of langerin/CD207 dendritic cells, only one of which represents epidermal-derived Langerhans cells. J Invest Dermatol. 2005;125:983–94. doi: 10.1111/j.0022-202X.2005.23951.x. [DOI] [PubMed] [Google Scholar]
- 28.Larsen CP, Steinman RM, Witmer-Pack M, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172:1483–93. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinlich G, Heine M, Stossel H, et al. Entry into afferent lymphatics and maturation in situ of migrating murine cutaneous dendritic cells. J Invest Dermatol. 1998;110:441–8. doi: 10.1046/j.1523-1747.1998.00161.x. [DOI] [PubMed] [Google Scholar]
- 30.Cheong C, Idoyaga J, Do Y, et al. Production of monoclonal antibodies that recognize the extracellular domain of mouse langerin/CD207. J Immunol Methods. 2007;324:48–62. doi: 10.1016/j.jim.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allan RS, Waithman J, Bedoui S, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–62. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100:1734–41. [PubMed] [Google Scholar]
- 33.Daro E, Butz E, Smith J, Teepe M, Maliszewski CR, McKenna HJ. Comparison of the functional properties of murine dendritic cells generated in vivo with Flt3 ligand, GM-CSF and Flt3 ligand plus GM-SCF. Cytokine. 2002;17:119–30. doi: 10.1006/cyto.2001.0995. [DOI] [PubMed] [Google Scholar]
- 34.Sundquist M, Wick MJ. TNF-alpha-dependent and -independent maturation of dendritic cells and recruited CD11cintCD11b+ cells during oral Salmonella infection. J Immunol. 2005;175:3287–98. doi: 10.4049/jimmunol.175.5.3287. [DOI] [PubMed] [Google Scholar]