Abstract
Although some studies have shown that the cell penetrating peptide (CPP) TAT can enter a variety of cell lines with high efficiency, others have observed little or no transduction in vivo or in vitro under conditions mimicking the in vivo environment. The mechanisms underlying TAT-mediated transduction have been investigated in cell lines, but not in primary brain cells. In this study we demonstrate that transduction of a green fluorescent protein (GFP)-TAT fusion protein is dependent on glycosaminoglycan (GAG) expression in both the PC12 cell line and primary astrocytes. GFP-TAT transduced PC12 cells and did so with even higher efficiency following NGF differentiation. In cultures of primary brain cells, TAT significantly enhanced GFP delivery into astrocytes grown under different conditions: 1) monocultures grown in serum-containing medium; 2) monocultures grown in serum-free medium; 3) cocultures with neurons in serum-free medium. The efficiency of GFP-TAT transduction was significantly higher in the monocultures than in the cocultures. The GFP-TAT construct did not significantly enter neurons. Experimental modulation of GAG content correlated with alterations in TAT transduction in PC12 cells and astrocyte monocultures grown in the presence of serum. In addition, this correlation was predictive of TAT-mediated transduction in astrocyte monocultures grown in serum free medium and in coculture. We conclude that culture conditions affect cellular GAG expression, which in turn dictates TAT-mediated transduction efficiency, extending previous results from cell lines to primary cells. These results highlight the cell-type and phenotype-dependence of TAT-mediated transduction, and underscore the necessity of controlling the phenotype of the target cell in future protein engineering efforts aimed at creating more efficacious CPPs.
Keywords: cell penetrating peptide, protein transduction domain, intracellular protein delivery, TAT, flow cytometry, glycosaminoglycans
INTRODUCTION
A growing number of protein therapeutics are being developed, however the vast majority of these proteins suffer from an inability to cross the plasma membrane and enter cells, thereby greatly diminishing their therapeutic potential (Trehin and Merkle 2004). Methods such as microinjection, electroporation, viral and nonviral vectors, and lipid encapsulation have associated undesirable characteristics such as low transfer efficiency, limited cell targeting, damaging effects to cell membranes, and/or unwanted immunoresponse (Sakurai et al. 2007). Engineering an efficient vehicle for transmembrane delivery, therefore, would have wide-spread applications.
CPPs are short, mainly basic peptides that have the ability to cross the plasma membrane and enter cells (Chauhan et al. 2007; Dietz and Bahr 2004; Joliot and Prochiantz 2004; Temsamani and Vidal 2004; Trehin and Merkle 2004; Vives 2005). At active concentrations, most CPPs have negligible effects on membrane integrity (Suzuki et al. 2002) and can be fused to heterologous proteins for delivery across cell membranes (Nagahara et al. 1998). As opposed to viral vectors and cationic lipid complexes, engineered CPP fusion proteins require little processing and can be produced as a single protein construct. In addition, CPPs may have a broad tissue distribution (Schwarze et al. 1999) and, because the delivered cargo is the fully-functional, therapeutic construct, CPP-delivered cargoes can be immediately active.
Although our understanding of neurodegenerative mechanisms in central nervous system (CNS) disorders, traumatic brain injury, and ischemia continues to increase, drug delivery to the CNS remains a major obstacle to therapeutic development (Begley 2004; Pardridge 2005). Entry of compounds to the brain is tightly regulated as there is limited substance distribution along extracellular fluid-flow pathways, and fluid is constantly cleared by CNS-specific lymphatics (Huynh et al. 2006). Even if these in vivo brain delivery barriers could be overcome, the difficult task of penetrating the cell membrane remains. Non-viral gene transfer into post-mitotic cells, including primary cortical and hippocampal neurons, has proven to be inefficient (Washbourne and McAllister 2002). Therefore, there is a critical need for the development of a more efficient method of transducing brain cells, in particular.
In a landmark study, the TAT protein, a well studied CPP, was reported to deliver the active enzyme β-galactosidase to all tissues in the mouse, including the brain, following intraperitoneal injection (Schwarze et al. 1999). Subsequently, several other groups have used CPPs to deliver functional neuroprotective cargos, such as the anti-apoptotic proteins Bcl-2 and Bcl-xL, into the brain in in vivo animal models (Asoh et al. 2002; Cao et al. 2002; Yin et al. 2006), as well as into in vitro cultures of primary neurons (Asoh et al. 2002; Cao et al. 2002; Soane and Fiskum 2005) and hippocampal brain slices (Matsushita et al. 2001).
While early evidence suggested that TAT peptides could transduce the cell membranes of a variety of cell types in vitro with almost 100% efficiency (Asoh et al. 2002; Cao et al. 2002; Nagahara et al. 1998), much of this data has been called into question in light of more recent experiments. Specifically, Richard et al. have convincingly demonstrated that the use of fixation prior to fluorescent microscopic analysis and/or a lack of trypsinization before flow cytometric analysis produce spurious results (Richard et al. 2003). Many of the previously mentioned successful in vitro studies used immunohistochemistry to confirm cell transduction, which includes a fixation step following CPP transduction (Asoh et al. 2002; Cao et al. 2002; Matsushita et al. 2001; Nagahara et al. 1998). Although many of these constructs provided neuroprotection both in vitro and in vivo, studies have shown that Bcl-2 is neuroprotective in hippocampal slice cultures when simply added to the medium (Panizzon et al. 1998).
The controversy of CPP-mediated transduction extends to non-brain cells as well. CPP-mediated transduction has been successful in cell lines such as Jurkat (Ho et al. 2001; Nagahara et al. 1998; Wender et al. 2000), HeLa (Suzuki et al. 2002; Violini et al. 2002), and HEK293 (Niesner et al. 2002), however poor or no CPP-mediated cargo delivery has been observed in other cell lines such as MDCK epithelial cells (Kramer and Wunderli-Allenspach 2003; Violini et al. 2002) and CaCo-2 colonic carcinoma cells (Violini et al. 2002), both of which are thought to be morphologically and biochemically representative of primary cells. Similar poor cell transduction has been observed in studies of muscle cells in vivo (Caron et al. 2001). Moreover, several of the previously published successful in vivo results, including those of Schwarze et al. (1999) and Cao et al. (2002) could not be repeated, even in some cases using the same construct (Cai et al. 2006). As such, there is an unresolved controversy regarding the ability of TAT to cross plasma membranes and whether CPP studies performed on one cell type can translate to other cell types or even the same cell type under different environmental conditions.
We hypothesized that variability in the amount of cell surface proteoglycan may contribute to some of the variability in TAT-mediated transduction efficiency between cell lines and between various primary cells both in vivo and in vitro. Although the exact mechanism by which TAT crosses the plasma membrane has been debated, it is commonly believed that the process involves an electrostatic interaction with cell surface proteoglycans, in particular heparan sulfate (Bugatti et al. 2007; Duchardt et al. 2007; Kumarasuriyar et al. 2007; Nakase et al. 2007; Poon and Gariepy 2007; Tyagi et al. 2001; Vives 2003; Wadia et al. 2004); these conclusions have all been drawn based on studies of TAT-mediated transduction into cell lines. The amount of proteoglycan on the surface of a cell varies depending on cell type, location and function (David 1993; Warda et al. 2006), even within a single organ like the brain (Costa et al. 2007). Herein, we extend previous results from cell lines to cultures of primary brain cells. To test this hypothesis, we used flow cytometry to quantify TAT-mediated delivery efficiency in undifferentiated and differentiated PC12 cells, monocultures of primary astrocytes grown in either a serum-containing medium or a serum-free medium, and cocultures of primary neurons and astrocytes grown in a serum-free medium.
We observed significant differences in overall transduction efficiency between different cell types and for a given cell type under distinct culture conditions. Furthermore, we found that the different culture conditions affected the glycosaminoglycan (GAG) content of the cells, and, within a single cell type, GAG content was positively correlated with efficiency of TAT-mediated GFP transduction. Elimination of cell-surface GAG by trypsin treatment reduced TAT transduction whereas enhancement of GAG production increased transduction. We conclude that TAT-mediated protein delivery is strongly dependent on cell type and growth environment, which in turn affect GAG content. Our results indicate that, while it may be an efficient delivery vehicle for cells with high GAG content, TAT may be an inefficient vehicle for many types of primary cells and tissues with low GAG content. Our results also highlight the pitfalls of extrapolating transduction efficiencies from cell-lines to primary cells, or even from primary cells grown in one environment to the same cells in a different environment, when engineering novel delivery vehicles.
MATERIALS AND METHODS
Vectors
The GFP gene was encoded in a pRSET-S65T vector (Yang et al. 1996) containing a 6-histidine tag at the NH2-terminus of the GFP gene. The 14 amino acid TAT sequence (NGYGRKKRRQRRRG) was inserted onto C-terminus of the GFP gene to create the pGFP-TAT expression vector, as described previously (Gao et al. 2009).
Expression and purification of recombinant proteins
As described in our previous work (Gao et al. 2009), the GFP and GFP-TAT proteins were expressed in BL21 competent E. coli (Sigma-Aldrich, St. Louis, MI) using the pRSET-S65T and pGT vectors, respectively. The proteins were purified using a HisTrap crude FF 5 ml column in an FPLC apparatus (GE Healthcare, Uppsala, Sweden). The concentration of the purified proteins was determined by their absorbance at 280nm using 0.6766(mg/mL)−1 cm−1 and 0.6737(mg/mL)−1 cm−1 as extinction coefficients (Gill and von Hippel 1989) for GFP and GFP-TAT, respectively. Construct molecular weights were verified by MALDI-TOF mass spectrometry.
Cell culture
PC12 cells (ATCC, Manassas, VA) were plated onto poly-L-lysine coated 24-well plates at a density of 100,000 cells per well and grown in F12K medium (ATCC, Manassas, VA) supplemented with 15% horse serum and 2.5% newborn calf serum for approximately 5 days until confluent. Differentiation was achieved by treating PC12 cells with 100ng/mL 2.5S nerve growth factor (NGF, Invitrogen, Carlsbad, CA) for 7 days.
Astrocyte cultures and mixed cortical cell cultures were obtained from the cortices of 8–10 day old Sprauge-Dawley rat pups. All procedures involving animals were approved by the Columbia University IACUC. For cocultures, cortices were minced, digested with papain, triturated, and plated at a density of 100,000 cells/coverslip onto poly-D-lysine/laminin coated coverslips (Becton Dickinson, San Jose, CA). The cultures were fed every 2 days with Neurobasal medium (Neurobasal-A medium, 5% B27 Supplement, 1mM glutamine, 5mg/ml D-glucose) and grown for approximately 7 days. The distribution of neurons and astrocytes in the cocultures was verified by immunocytochemistry for GFAP to detect astrocytes, and β-III tubulin and Thy-1 to detect neurons.
Astrocyte monocultures were prepared from minced cortices that were digested with papain, triturated and plated into T75 tissue culture flasks. After an hour, the flasks were vigorously shaken to remove non-adherent cells, and the media was discarded resulting in a pure astrocyte culture. The remaining astrocytes were grown in a serum-containing medium (DMEM, 10% heat-inactivated newborn calf serum, 1mM glutamine) for 2 weeks. The cells were then trypsinized and replated onto uncoated, tissue culture 24-well plates at a density of 36,000 cells per well and grown for another week in either serum-containing medium or Neurobasal. The purity of astrocyte monocultures was verified by GFAP immunocytochemistry.
Quantification of protein transduction into PC12 cells
To examine differences in GFP transduction efficiency between undifferentiated and differentiated PC12 cells, the cells were incubated with 100µg/mL GFP or GFP-TAT for 4 hours. The optimal concentration was determined from previously calculated dose-response curves (Gao et al. 2009). Cells were washed twice with PBS, trypsinized for 5 minutes, and resuspended in 300µL PBS. The trypsinization step was essential to remove any surface-bound protein, which could cause an overestimation of GFP transduction (Richard et al. 2003). Cells were kept on ice until analysis using either an LSRII or a FACSCanto II flow cytometer (Becton Dickinson, San Jose, CA). The percent increase in geometric mean fluorescence (excitation 488nm, emission 530/30nm) above background was calculated to account for autofluorescence. A total of 30,000 events per sample were counted. Statistical differences between GFP and GFP-TAT transduction, as well as between the GFP-TAT transduction of undifferentiated and differentiated PC12 cells were calculated using Student’s t-tests with p<0.05. Equality of variance was confirmed using the Levene test and, if necessary, data was log transformed to ensure a normal distribution prior to statistical analysis.
Quantification of protein transduction into primary brain cells
Cocultures and astrocyte monocultures were incubated with 100µg/mL GFP or GFP-TAT for 4 hours. Cells were washed twice in PBS, trypsinized for 5 minutes and resuspended in 300µL PBS. To quantify GFP transduction by cell type in cocultures, cells were incubated with mouse anti-Thy1 (Abcam, Cambridge, MA), to identify neurons, fixed with 4% formaldehyde and then incubated with rabbit anti-GFAP (Sigma-Aldrich, St. Louis, MO) to identify astrocytes. Alexa-350 and Alexa-647 conjugated secondary antibodies (Invitrogen, Carlsbad, CA) were used to detect the Thy1 and GFAP primary antibodies, respectively. All antibody and fixation steps were performed on ice.
Cocultures and astrocyte monocultures were analyzed for both GFP transduction and cell-type using either an LSRII or a FACSCanto II flow cytometer (Becton Dickinson, San Jose, CA). GFAP was detected using a 633nm excitation and 660/20nm emission, while Thy1 was detected using a 405nm excitation and 450/50 emission. GFAP+ and Thy1+ gates were created based on control cells that had also been stained for the appropriate antibodies, but were not treated with GFP or GFP-TAT. GFP and GFP-TAT transduction into cells positive for GFAP or Thy1 was quantified by calculating the percent increase in geometric mean fluorescence in the green channel (excitation 488nm, emission 530/30nm) above that of untreated cells, to account for autofluorescence. A total of 10,000 events per sample were counted. Statistical differences between GFP and GFP-TAT transduction were determined independently for the neurons and each of the three astrocyte culture conditions using Student’s t-tests. Comparisons between the GFP-TAT transduction of the three astrocyte conditions were performed using one-way ANOVA followed by Bonferroni post-hoc tests. Equality of variance was confirmed using the Levene test and, if necessary, data was log transformed to ensure a normal distribution prior to statistical analysis.
Quantification of GAG
To determine cellular GAG content, cells were washed with PBS, incubated in 500µL of lysis buffer (0.1M sodium acetate buffer, pH 5, containing 5mM EDTA, 5mM cysteine HCl, and 0.6U papain/mL) overnight at 37°C, sonicated, and then analyzed using the 1,9-dimethylmethylene blue (DMMB, Sigma-Aldrich, St. Louis, MO) dye-binding assay (Farndale et al. 1986). GAG content was normalized to total DNA content using the Quant-iT Picogreen assay (Invitrogen, Carlsbad, CA).
The role of GAG in GFP-TAT transduction
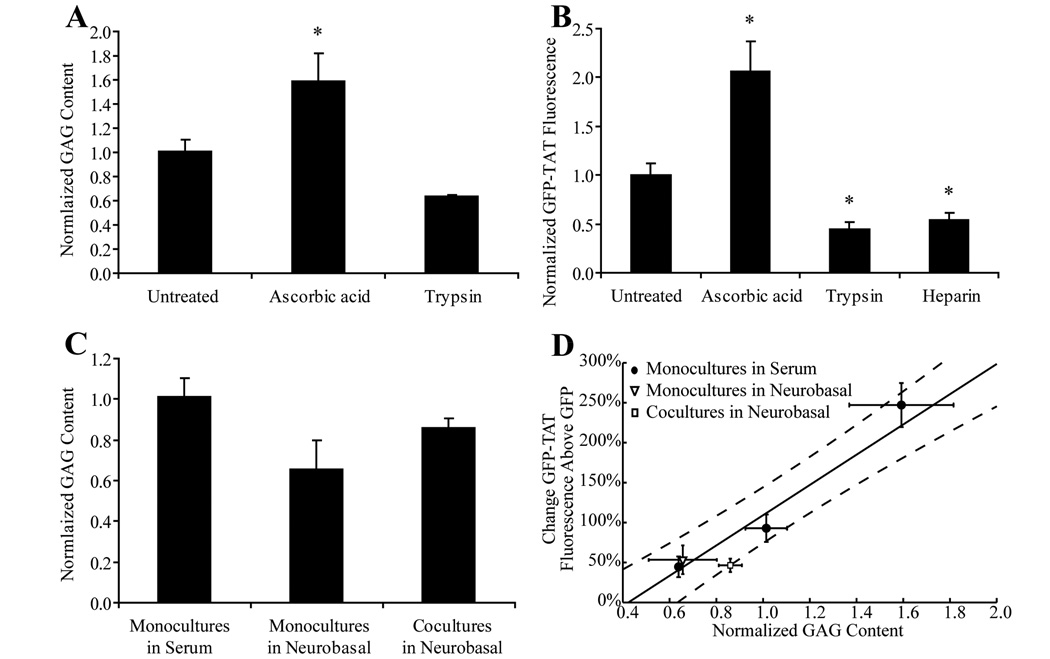
To further discern the role of GAG in GFP-TAT transduction, GAG content was experimentally altered. Differentiation of PC12 cells with NGF, as described above, has been shown previously to increase heparan sulfate proteoglycan expression (Katoh-Semba et al. 1990). Ascorbic acid has been shown to promote extracellular matrix production in cells such as fibroblasts (Kao et al. 1990). In the brain, ascorbic acid plays a role in brain development, and has been shown to induce cell differentiation and proliferation (Lee et al. 2003; Yu et al. 2004). We hypothesized that ascorbic acid may increase GAG content in astrocytes, and this was confirmed using the DMMB assay. 250µM ascorbic acid was added to the astrocytes every two days for one week prior to the addition of GFP or GFP-TAT.
Cell-surface proteoglycans were removed by treating undifferentiated PC12 cells or serum-grown astrocytes with trypsin for 30 minutes. Following the trypsin treatment, the cells were incubated in media for 30 minutes prior to the addition of GFP or GFP-TAT. A decrease in GAG content was confirmed using the DMMB assay. The GAG/DNA content of the experimentally GAG-modulated PC12 cells and serum-grown astrocyte monocultures was normalized to the untreated condition, and statistically compared using a Dunnett post-hoc comparison using the untreated condition as a control.
To confirm the importance of electrostatic interactions at the cell surface, in particular with heparan sulfate proteoglycans, cells were pretreated with 100µg/mL heparin (Sigma-Aldrich, St. Louis, MO) for one hour. Heparin has a structure that is similar to heparan sulfate, and if GFP-TAT does electrostatically interact with heparan sulfate, heparin should act as a competitive inhibitor and decrease GFP-TAT transduction (Tyagi et al. 2001). The heparin was left on the cells during the GFP or GFP-TAT treatment.
The geometric mean green fluorescence (excitation 488nm, emission 530/30nm) was measured for each GAG-modifying treatment and was normalized to either untreated undifferentiated PC12 cells or untreated astrocyte monocultures grown in serum. Statistical differences were determined using one-way ANOVA followed by a Dunnett post-hoc comparison to the control condition. Equality of variance was confirmed using the Levene test and, if necessary, data was log transformed prior to statistical analysis to preserve homoscedacity.
A straight line was fit to plots of TAT transduction versus GAG content for PC12 cells and for astrocyte monocultures grown in serum medium. To account for errors in both variables, the line was fit using a weighted least squares algorithm (Krystek and Anton 2007). Confidence bands were calculated using the methods of Giordano (1999).
RESULTS
Protein construct and purification
In this study, the TAT CPP sequence (NGYGRKKRRQRRRG) was fused to the carboxy terminus of the GFP protein which contained an N-terminal polyhistidine purification tag. As opposed to previously reported PolyHis-TAT-GFP constructs (Han et al. 2001; Kilic et al. 2002; Kilic et al. 2003; Lea et al. 2003; Park et al. 2002; Vazquez et al. 2003; Yang et al. 2002), our construct allowed for the purification domain (polyhistidine) and the CPP domain (TAT) to be separated by the GFP protein, which may allow the TAT peptide to be more accessible to participate in cellular transduction by preventing potential confounding interactions by the polyhistidine purification tag. In addition, the GFP and GFP-TAT fusion proteins were purified by Ni2+ affinity chromatography under native conditions so that refolding of the protein was unnecessary. Many of the studies involving chimeric GFP-TAT constructs report purification under denaturing conditions (Han et al. 2001; Kilic et al. 2002; Kilic et al. 2003; Lea et al. 2003; Vazquez et al. 2003) while some report purification under both denaturing and native conditions (Park et al. 2002; Ryu et al. 2003; Yang et al. 2002).
Analysis of the purified GFP and GFP-TAT proteins by SDS-PAGE showed apparent homogeneity of the expressed products (Figure 1). Molecular weights obtained by MALDI-TOF mass spectrometry, 31,313 Da for GFP and 33,194 Da for GFP-TAT, compared well to the calculated theoretical molecular weights of 31,113 Da and 33,144 Da.
Fig. 1.
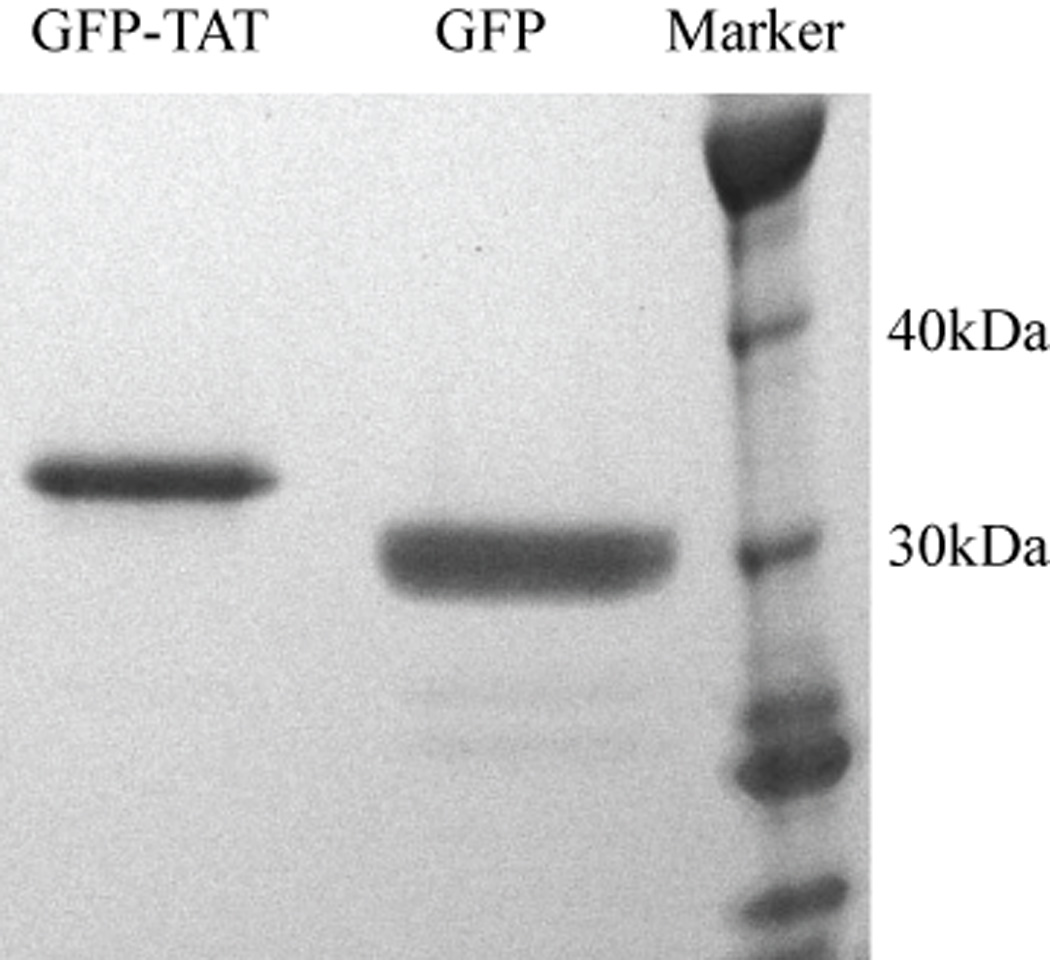
SDS-PAGE analysis of GFP and GFP-TAT purity. Samples were run on a 4–12% NuPAGE Bis-Tris gel under denaturing conditions. The gel was stained with SimplyBlue Safe Stain and shows apparent homogeneity of the expressed products. The construct molecular weights compare well to the calculated theoretical molecular weights of 31,113 Da and 33,144 Da for GFP and GFP-TAT, respectively.
Quantification of protein transduction into PC12 cells
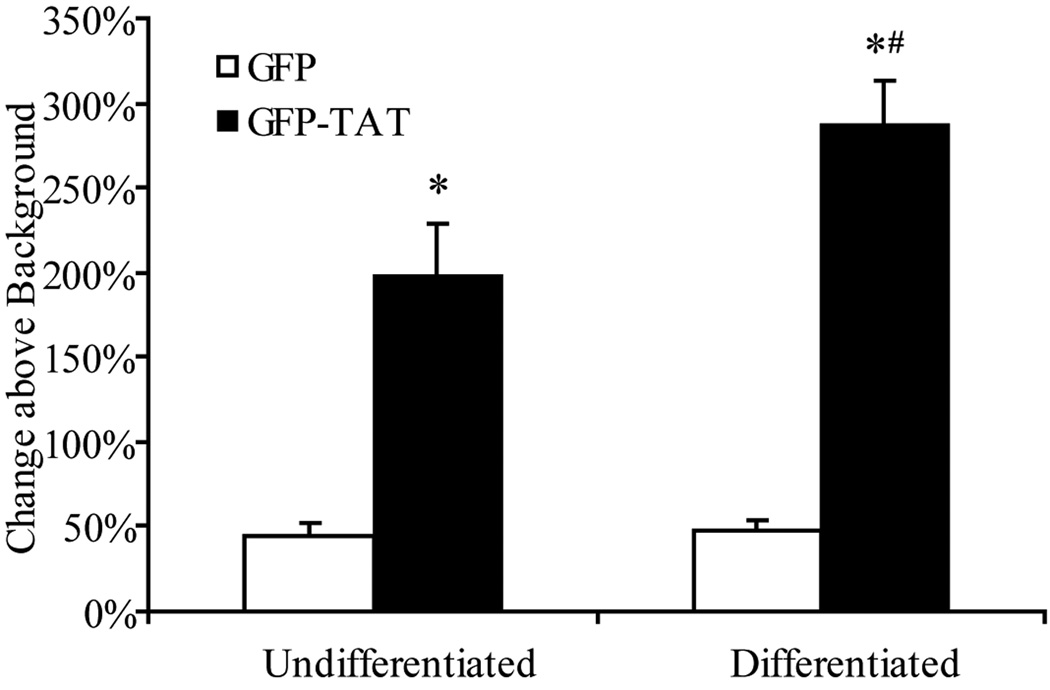
Significant GFP-TAT transduction, in comparison to GFP alone, was measured into both undifferentiated (NGF-) and differentiated (NGF+) cells (Figure 2). In addition, overall GFP-TAT transduction into differentiated cells was significantly (p<0.05) more efficient than into undifferentiated cells, indicating that the cell’s phenotype may influence the transduction of GFP-TAT.
Fig. 2.
Transduction of GFP and GFP-TAT into differentiated and undifferentiated PC12 cells. Undifferentiated PC12 cells and differentiated (NGF-treated) PC12 cells were incubated in 100 µg/mL GFP or GFP-TAT for 4 hours, and transduction was quantified using flow cytometry. Results are expressed as the percent increase in geometric mean fluorescence compared to untreated control cells, to account for background autofluorescence (n>3, error bars: ±SEM). TAT significantly enhanced GFP transduction into both undifferentiated and differentiated PC12 cells compared to GFP alone. *Significance from Student’s t-test (p<0.05). Overall GFP-TAT transduction was significantly greater in differentiated cells. #Significance from Student’s t-test (p<0.05).
Quantification of protein transduction into primary brain cells
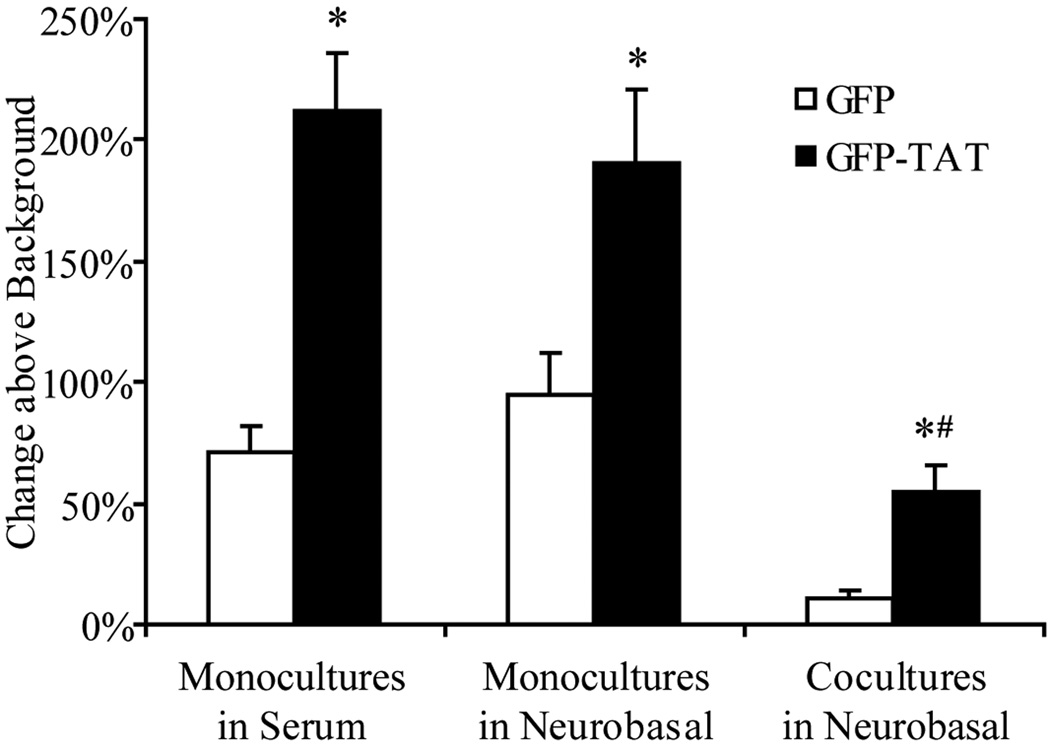
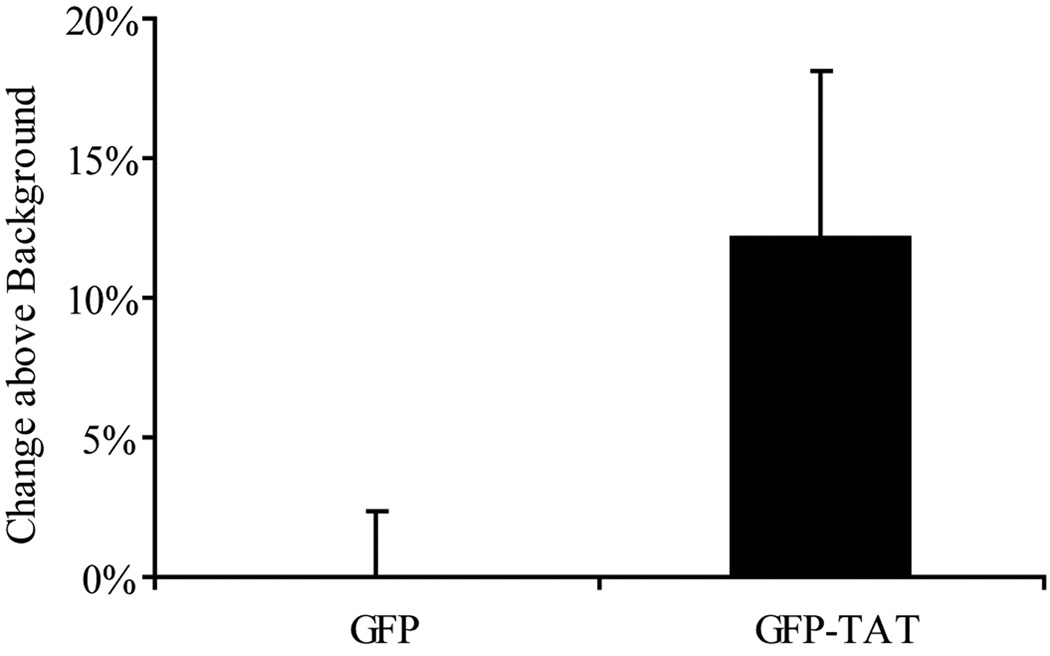
TAT significantly (p<0.05) enhanced GFP delivery into astrocyte monocultures grown in serum-containing medium, astrocyte monocultures grown in serum-free Neurobasal medium, and astrocytes in coculture (Figure 3). In addition, total GFP-TAT transduction into the monocultures was significantly greater than into astrocytes in coculture. GFP-TAT delivery into neurons was not significant (Figure 4). Together, these data imply that GFP-TAT transduction is dependent not only on cell type, but also on the cell’s phenotypic state affected by environment.
Fig. 3.
Transduction of GFP and GFP-TAT into astrocytes. Astrocytes in monoculture, grown either in serum-containing medium or serum-free Neurobasal medium, and astrocytes in coculture with neurons were incubated in 100 µg/mL GFP or GFP-TAT for 4 hours, and transduction was quantified using flow cytometry. Cell type was confirmed by GFAP immunofluorescence after trypsinization. The percent increase in geometric mean fluorescence compared to untreated control cells was calculated, to account for background autofluorescence (n>6, error bars: ±SEM). TAT significantly enhanced GFP transduction into all three astrocyte cultures compared to GFP. *Significance from Student’s t-test (p<0.05). Overall GFP-TAT transduction into cocultures was significantly less than into astrocyte monocultures grown in serum medium and serum-free medium. #Significance from Bonferroni post-hoc test (p<0.05).
Fig. 4.
Transduction of GFP and GFP-TAT into neurons. Neurons in cocultures were incubated in 100 µg/mL GFP or GFP-TAT for 4 hours, and transduction was quantified using flow cytometry. Cell type was confirmed by Thy1 immunofluorescence after trypsinization. The percent increase in geometric mean fluorescence of Thy1+ cells compared to untreated control cells was calculated, to account for background autofluorescence (n>6, error bars: ±SEM). TAT did not significantly enhance GFP transduction into neurons, compared to GFP alone.
Role of GAG in transduction of PC12 cells
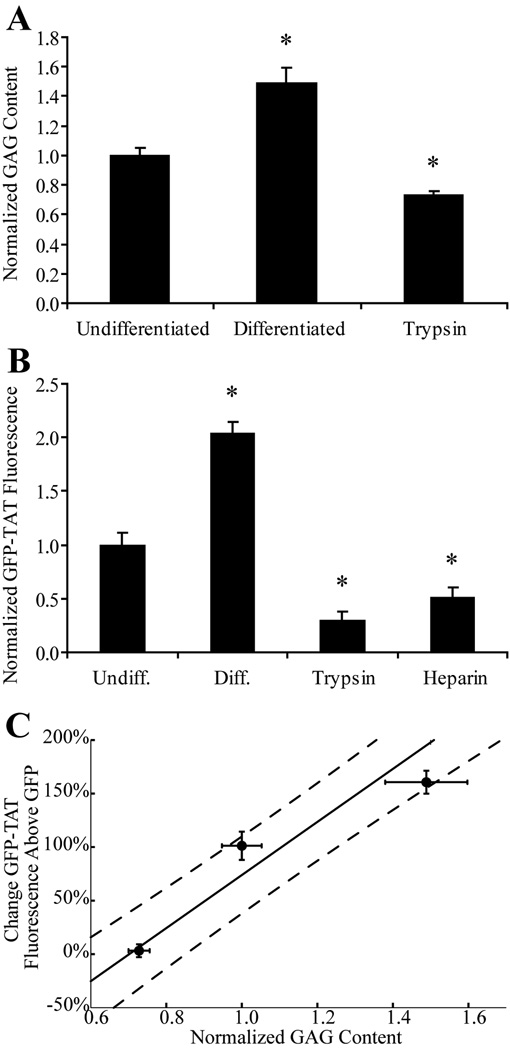
NGF treatment significantly increased the GAG content of PC12 cells (Figure 5a), and also caused significant increases in GFP-TAT transduction (Figure 5b). Trypsin treatment significantly decreased GAG content and caused significant decreases in TAT transduction. Heparin also significantly decreased TAT transduction. (Figure 5b). The relationship between GAG content and increased TAT transduction, compared to GFP alone, for NGF-treated, untreated and trypsin-treated PC12 cells was linearly correlated (Figure 5c). These results confirm that surface GAGs contribute to the ability of TAT to increase GFP transduction in PC12 cells and that the electrostatic interaction can be competitively blocked by a negatively charged, soluble molecule like heparin.
Fig. 5.
Dependence of GFP-TAT transduction on PC12 cell GAG content. (A) The average µg of GAG / ng of DNA, normalized to the average µg of GAG / ng of DNA in undifferentiated cultures is shown for undifferentiated PC12 cells treated to experimentally alter GAG content (n>4, error bars: ±SEM). There was a significant increase in GAG with differentiation, and a significant decrease in GAG with trypsin treatment. (B) Geometric mean fluorescence of GFP-TAT was normalized to undifferentiated PC12s (n>4, error bars: ±SEM). GFP-TAT transduction significantly increased in NGF-treated PC12 cells, and significantly decreased in both trypsin and heparin treated cells. *Significance from Dunnett post-hoc comparison to the control, undifferentiated condition. (C) Percent increase in GFP-TAT transduction, compared to GFP alone, showed a positive correlation with GAG content (n>4, three data points correspond to trypsin-treated, untreated, and NGF-treated PC12 cells, error bars: ±SEM), indicating that GAG content influences the ability of TAT to enhance GFP transduction.
Role of GAG in transduction of primary brain astrocytes
Ascorbic acid treatment of astrocyte monocultures grown in serum medium significantly increased the GAG content of astrocytes, while trypsin treatment decreased GAG (Figure 6a). The ascorbic acid-treated cells had a significant increase in GFP-TAT transduction and both trypsin- and heparin-treated cells had a significant decrease (Figure 6b). As with the PC12 cells, the experimentally GAG-modulated astrocytes showed a linear correlation between GAG content and increased GFP-TAT transduction, compared to GFP alone (Figure 6d).
Fig. 6.
Dependence of GFP-TAT transduction on astrocyte GAG content. (A) The average µg of GAG / ng of DNA, normalized to the average µg of GAG / ng of DNA in serum monocultures is shown for serum-grown astrocyte monocultures treated to experimentally alter GAG content (n>4, error bars: ±SEM). There was a significant increase in GAG content with ascorbic acid treatment. Trypsin treatment decreased GAG content. (B) GFP-TAT transduction was quantified in astrocyte monocultures grown in serum-containing medium as well as in monocultures treated with ascorbic acid, trypsin, or heparin. Geometric mean fluorescence was normalized to astrocyte monocultures grown in serum (n>4, error bars: ±SEM). GFP-TAT transduction was significantly increased in ascorbic acid treated astrocytes, and significantly decreased in both trypsin and heparin treated cells. (C) Modifying the growth environment of astrocytes altered the cellular GAG content. Both removal of serum and coculture with neurons resulted in a decrease in GAG. *Significance from Dunnett post-hoc comparison to the control, undifferentiated condition. (D) Percent increase in GFP-TAT transduction, compared to GFP alone, showed a positive correlation with GAG content in astrocyte monocultures grown in serum that were experimentally treated to modulate GAG (●, three data points correspond to trypsin-treated, untreated and ascorbic acid-treated astrocytes grown in serum-containing medium). Both the astrocyte monocultures grown in serum-free medium (▽) and cocultures (□) fell within the confidence bounds of the regression formed by the monocultures grown in serum, indicating that the ability of TAT to increase transduction of GFP in astrocytes correlated with GAG content.
Both the cocultures and astrocyte monocultures grown in serum-free, Neurobasal medium showed decreased GAG in comparison to astrocyte monocultures grown in serum-containing medium (Figure 6c). The cocultures and astrocyte monocultures grown in Neurobasal fall within the confidence bounds of the percent TAT transduction increase-GAG regression line calculated using data from the experimentally GAG-modulated astrocyte monocultures (Figure 6d). This indicated that for all astrocytes, GAG content correlated with the ability of TAT to increase GFP transduction.
DISCUSSION
In this study we determined for the first time that GAG content affects TAT-mediated delivery in primary astrocytes, and this relationship was confirmed in the PC12 cell line. Furthermore, we found that commonly employed cell culture conditions can drastically alter TAT transduction through the unforeseen consequence of altering GAG expression. Our results help to explain conflicting results in literature surrounding TAT-mediated transduction and highlight the crucial need for using a physiologic culture system in protein engineering efforts aimed at creating improved CPP sequences.
We initially hypothesized that the discrepancy in TAT-mediated delivery efficiency in previous publications may have been due to culture condition and/or phenotypic state. Because mitotic activity is often required for DNA transfection (Mortimer et al. 1999; Wilke et al. 1996), it was possible that TAT transduction would be more efficient in dividing cells. However, for the PC12 cells, our GFP-TAT fusion protein translocated into differentiated (non-mitotic) PC12 cultures significantly more efficiently than into undifferentiated, dividing cells (Greene and Tischler 1976). These findings were consistent with those of Matsushita et al., who reported limited CPP-mediated GFP delivery to PC12 cells in the undifferentiated state and high transduction efficiency after differentiation (Matsushita et al. 2001). In our study, a significant enhancement in transduction efficiency with NGF treatment was measured (Figure 2). In PC12 cells, NGF treatment enhances the expression of heparan sulfate proteoglycans (Katoh-Semba et al. 1990) which have been shown to be essential for the initial electrostatic interaction between TAT and the plasma membrane before internalization (Bugatti et al. 2007; Duchardt et al. 2007; Kumarasuriyar et al. 2007; Nakase et al. 2007; Poon and Gariepy 2007; Tyagi et al. 2001; Vives 2003; Wadia et al. 2004). We showed that NGF treatment increased the GAG content by almost 50%, and resulted in a significant increase in TAT transduction (Figure 5). Conversely, the trypsin treatment, which eliminates surface GAG (Rapraeger et al. 1986), reduced the GAG content by almost 30% and significantly decreased TAT transduction. Pretreatment with soluble heparin, which competitively blocks TAT from binding to cell surface GAG (Tyagi et al. 2001), also resulted in a decrease in TAT transduction. Our results agree with previous studies and confirm that in PC12 cells, TAT interactions with GAG play an important role in TAT-mediated transduction.
To our knowledge, this dependence of TAT transduction on GAG expression has not been validated for primary cells. Extending these results to primary cells is critical as protein engineering efforts often attempt to create novel CPPs designed for in vivo use. Our results showed a positive correlation between experimentally manipulated GAG content and TAT-mediated transduction in primary astrocytes (Figure 6). Proteoglycan content was increased by treating astrocytes with ascorbic acid, which resulted in an increase in TAT-mediated transduction. Conversely, trypsin treatment reduced GAG content and decreased TAT-mediated transduction. Pretreatment with free heparin decreased TAT-mediated transduction by over 50%, confirming the importance of electrostatic interactions. From these results, we determined a quantitative correlation between GAG content and TAT-mediated transduction efficiency in primary cells (Figure 6d).
Serum is normally excluded from the parenchyma, therefore neurons and astrocytes grown in serum-free conditions may be more phenotypically like quiescent astrocytes in vivo compared to astrocyte monocultures grown in serum-containing medium, which have been used as models of reactive gliosis (Audouy et al. 1999). The removal of serum from astrocyte cultures has been shown to shift astrocytes into a post-mitotic state (Chou and Langan 2003; Langan and Slater 1991), and we showed that the removal of serum also decreased GAG expression and decreased TAT transduction (Figure 2 and Figure 6). In addition, the influence of neurons on astrocytes and vice versa could induce different phenotypes in these cell types under coculture conditions, as has been shown previously for other brain cell types in vitro (Duport et al. 1998; Nakagawa et al. 2007). In the cocultures, however, both neuronal and astrocytic GAG content was measured together and the individual contribution of each cell type could not be determined. The astrocyte monocultures grown in serum-free medium and cocultures showed reduced GFP-TAT transduction, both overall in comparison to background (Figure 3) and in comparison to GFP alone. The latter agrees with the predicted relationship between GAG and TAT transduction efficiency for astrocyte monocultures grown in serum developed using trypsin-treated, untreated and ascorbic acid-treated astrocytes (Figure 6d). These results indicate that cellular GAG content correlates with TAT transduction efficiency, and for our cultures of primary brain cells, culture conditions affect GAG expression, and therefore TAT transduction. Our study, however, only examines the transduction of a single CPP and a single cargo. CPP transduction has been shown to be dependent not only on the CPP sequence (Hallbrink et al. 2001; Mueller et al. 2008), but also on the attached cargo (Tunnemann et al. 2006), therefore extrapolation of these results to the transduction of other protein constructs should be done with caution.
In studies targeting the brain, several groups have successfully demonstrated CPP-mediated delivery of functional neuroprotective cargos both in vivo (Asoh et al. 2002; Cao et al. 2002; Yin et al. 2006) and in vitro (Asoh et al. 2002; Cao et al. 2002; Matsushita et al. 2001; Soane and Fiskum 2005). Recently, however, several in vitro and in vivo studies have not reported the same success (Cai et al. 2006; Sengoku et al. 2004). Surprisingly, several recent studies have even reported limited TAT transduction into commonly used, non-brain cell lines such as HeLa and HEK293 cells (Koppelhus et al. 2002; Mueller et al. 2008). All of these results add to the controversy as to whether CPPs such as TAT can efficiently enter a wide variety of cells and indicate that environmental factors play an important role in the efficiency of TAT transduction.
Our results support the hypothesis that while TAT can enter most cell lines, the level of GAG expression determines transduction efficiency in both the PC12 cell line and primary astrocytes. Furthermore, even with the same type of cell, such as astrocytes or PC12 cells, culture conditions affect GAG content, which in turn affects TAT-mediated transduction. There is a critical need for efficient and safe protein delivery vehicles to the brain. A reengineering of TAT may therefore be necessary to optimize protein transduction into a particular target cell-type under a specific environmental condition by reducing the dependence on this initial electrostatic interaction. Using a protein engineering process such as directed evolution, novel CPPs could be developed with these optimal cell targeting and cell penetrating properties. While the goal may be to generate novel CPPs with specific in vivo targets, the initial screening processes could be done in vitro due to the volume of cells needed and the ease of analysis. Our results highlight the critical necessity of using a physiologic in vitro screen during these protein engineering efforts so that results can be translated to primary cells in culture, as well as cells in vivo.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Lance Kam (Columbia University, New York, NY) for the gift of the pRSET-S65T vector. This work was supported by the Brain Trust (BM and SB), NIH R21 MH080024 (BM and SB), and an NSF Graduate Research Fellowship (MJS).
The abbreviations used are
- CNS
central nervous system
- CPP
cell penetrating peptide
- DMEM
Dulbecco’s Modified Eagle’s Medium
- GAG
glycosaminoglycan
- GFP
green fluorescent protein
- HEK
Human Embryonic Kidney
- IPTG
isopropyl-β-D-thiogalactopyranoside
- MDCK
Madin-Darby Canine Kidney Cells
- MALDI-TOF
Matrix Assisted Laser Desorption/Ionization Time-of-Flight
- NGF
neural growth factor
- PBS
phosphate buffered saline
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
REFERENCES
- Asoh S, Ohsawa I, Mori T, Katsura K, Hiraide T, Katayama Y, Kimura M, Ozaki D, Yamagata K, Ohta S. Protection against ischemic brain injury by protein therapeutics. Proc Natl Acad Sci U S A. 2002;99(26):17107–17112. doi: 10.1073/pnas.262460299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audouy S, Mallet J, Privat A, Gimenez y, Ribotta M. Adenovirus-mediated suicide gene therapy in an in vitro model of reactive gliosis. Glia. 1999;25(3):293–303. doi: 10.1002/(sici)1098-1136(19990201)25:3<293::aid-glia9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Begley DJ. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther. 2004;104(1):29–45. doi: 10.1016/j.pharmthera.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Bugatti A, Urbinati C, Ravelli C, De Clercq E, Liekens S, Rusnati M. Heparin-mimicking sulfonic acid polymers as multitarget inhibitors of human immunodeficiency virus type 1 Tat and gp120 proteins. Antimicrob Agents Chemother. 2007;51(7):2337–2345. doi: 10.1128/AAC.01362-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai SR, Xu G, Becker-Hapak M, Ma M, Dowdy SF, McLeod HL. The kinetics and tissue distribution of protein transduction in mice. Eur J Pharm Sci. 2006;27(4):311–319. doi: 10.1016/j.ejps.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Cao G, Pei W, Ge H, Liang Q, Luo Y, Sharp FR, Lu A, Ran R, Graham SH, Chen J. In Vivo Delivery of a Bcl-xL Fusion Protein Containing the TAT Protein Transduction Domain Protects against Ischemic Brain Injury and Neuronal Apoptosis. J Neurosci. 2002;22(13):5423–5431. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron NJ, Torrente Y, Camirand G, Bujold M, Chapdelaine P, Leriche K, Bresolin N, Tremblay JP. Intracellular delivery of a Tat-eGFP fusion protein into muscle cells. Mol Ther. 2001;3(3):310–318. doi: 10.1006/mthe.2001.0279. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Tikoo A, Kapur AK, Singh M. The taming of the cell penetrating domain of the HIV Tat: myths and realities. J Control Release. 2007;117(2):148–162. doi: 10.1016/j.jconrel.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou RC, Langan TJ. In vitro synchronization of mammalian astrocytic cultures by serum deprivation. Brain Res Brain Res Protoc. 2003;11(3):162–167. doi: 10.1016/s1385-299x(03)00043-6. [DOI] [PubMed] [Google Scholar]
- Costa C, Tortosa R, Domenech A, Vidal E, Pumarola M, Bassols A. Mapping of aggrecan, hyaluronic acid, heparan sulphate proteoglycans and aquaporin 4 in the central nervous system of the mouse. J Chem Neuroanat. 2007;33(3):111–123. doi: 10.1016/j.jchemneu.2007.01.006. [DOI] [PubMed] [Google Scholar]
- David G. Integral membrane heparan sulfate proteoglycans. FASEB J. 1993;7(11):1023–1030. doi: 10.1096/fasebj.7.11.8370471. [DOI] [PubMed] [Google Scholar]
- Dietz GP, Bahr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol Cell Neurosci. 2004;27(2):85–131. doi: 10.1016/j.mcn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R, Brock R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic. 2007;8(7):848–866. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Duport S, Robert F, Muller D, Grau G, Parisi L, Stoppini L. An in vitro blood-brain barrier model: cocultures between endothelial cells and organotypic brain slice cultures. Proc Natl Acad Sci U S A. 1998;95(4):1840–1845. doi: 10.1073/pnas.95.4.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Gao S, Simon MJ, Morrison B, 3rd, Banta S. Bifunctional chimeric fusion proteins engineered for DNA delivery: Optimization of the protein to DNA ratio. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbagen.2009.01.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SC, von Hippel PH. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989;182(2):319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- Giordano JL. On reporting uncertainties of the straight-line regression parameters. European Journal of Physics. 1999;(5):343–349. [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallbrink M, Floren A, Elmquist A, Pooga M, Bartfai T, Langel U. Cargo delivery kinetics of cell-penetrating peptides. Biochim Biophys Acta. 2001;1515(2):101–109. doi: 10.1016/s0005-2736(01)00398-4. [DOI] [PubMed] [Google Scholar]
- Han K, Jeon MJ, Kim SH, Ki D, Bahn JH, Lee KS, Park J, Choi SY. Efficient intracellular delivery of an exogenous protein GFP with genetically fused basic oligopeptides. Mol Cells. 2001;12(2):267–271. [PubMed] [Google Scholar]
- Ho A, Schwarze SR, Mermelstein SJ, Waksman G, Dowdy SF. Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo. Cancer Res. 2001;61(2):474–477. [PubMed] [Google Scholar]
- Huynh GH, Deen DF, Szoka FC., Jr Barriers to carrier mediated drug and gene delivery to brain tumors. J Control Release. 2006;110(2):236–259. doi: 10.1016/j.jconrel.2005.09.053. [DOI] [PubMed] [Google Scholar]
- Joliot A, Prochiantz A. Transduction peptides: from technology to physiology. Nat Cell Biol. 2004;6(3):189–196. doi: 10.1038/ncb0304-189. [DOI] [PubMed] [Google Scholar]
- Kao J, Huey G, Kao R, Stern R. Ascorbic acid stimulates production of glycosaminoglycans in cultured fibroblasts. Exp Mol Pathol. 1990;53(1):1–10. doi: 10.1016/0014-4800(90)90020-e. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Oohira A, Kashiwamata S. Changes in glycosaminoglycans during the neuritogenesis in PC12 pheochromocytoma cells induced by nerve growth factor. J Neurochem. 1990;55(5):1749–1757. doi: 10.1111/j.1471-4159.1990.tb04965.x. [DOI] [PubMed] [Google Scholar]
- Kilic E, Dietz GP, Hermann DM, Bahr M. Intravenous TAT-Bcl-Xl is protective after middle cerebral artery occlusion in mice. Ann Neurol. 2002;52(5):617–622. doi: 10.1002/ana.10356. [DOI] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Dietz GP, Bahr M. Intravenous TAT-GDNF is protective after focal cerebral ischemia in mice. Stroke. 2003;34(5):1304–1310. doi: 10.1161/01.STR.0000066869.45310.50. [DOI] [PubMed] [Google Scholar]
- Koppelhus U, Awasthi SK, Zachar V, Holst HU, Ebbesen P, Nielsen PE. Cell-dependent differential cellular uptake of PNA, peptides, and PNA-peptide conjugates. Antisense Nucleic Acid Drug Dev. 2002;12(2):51–63. doi: 10.1089/108729002760070795. [DOI] [PubMed] [Google Scholar]
- Kramer SD, Wunderli-Allenspach H. No entry for TAT(44–57) into liposomes and intact MDCK cells: novel approach to study membrane permeation of cell-penetrating peptides. Biochim Biophys Acta. 2003;1609(2):161–169. doi: 10.1016/s0005-2736(02)00683-1. [DOI] [PubMed] [Google Scholar]
- Krystek M, Anton M. A weighted total least-squares algorithm for fitting a straight line. Measurement Science & Technology. 2007;18(11):3438–3442. [Google Scholar]
- Kumarasuriyar A, Dombrowski C, Rider DA, Nurcombe V, Cool SM. A novel use of TAT-EGFP to validate techniques to alter osteosarcoma cell surface glycosaminoglycan expression. J Mol Histol. 2007;38(5):435–447. doi: 10.1007/s10735-007-9136-z. [DOI] [PubMed] [Google Scholar]
- Langan TJ, Slater MC. Quiescent astroglia in long-term primary cultures re-enter the cell cycle and require a non-sterol isoprenoid in late G1. Brain Res. 1991;548(1–2):9–17. doi: 10.1016/0006-8993(91)91099-m. [DOI] [PubMed] [Google Scholar]
- Lea NC, Buggins AG, Orr SJ, Mufti GJ, Thomas NS. High efficiency protein transduction of quiescent and proliferating primary hematopoietic cells. J Biochem Biophys Methods. 2003;55(3):251–258. doi: 10.1016/s0165-022x(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Lee JY, Chang MY, Park CH, Kim HY, Kim JH, Son H, Lee YS, Lee SH. Ascorbate-induced differentiation of embryonic cortical precursors into neurons and astrocytes. J Neurosci Res. 2003;73(2):156–165. doi: 10.1002/jnr.10647. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Tomizawa K, Moriwaki A, Li ST, Terada H, Matsui H. A high-efficiency protein transduction system demonstrating the role of PKA in long-lasting long-term potentiation. J Neurosci. 2001;21(16):6000–6007. doi: 10.1523/JNEUROSCI.21-16-06000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer I, Tam P, MacLachlan I, Graham RW, Saravolac EG, Joshi PB. Cationic lipid-mediated transfection of cells in culture requires mitotic activity. Gene Ther. 1999;6(3):403–411. doi: 10.1038/sj.gt.3300837. [DOI] [PubMed] [Google Scholar]
- Mueller J, Kretzschmar I, Volkmer R, Boisguerin P. Comparison of cellular uptake using CPPs in 4 different cell lines. Bioconjug Chem. 2008;19(12):2363–2374. doi: 10.1021/bc800194e. [DOI] [PubMed] [Google Scholar]
- Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA, Dowdy SF. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med. 1998;4(12):1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Deli MA, Nakao S, Honda M, Hayashi K, Nakaoke R, Kataoka Y, Niwa M. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol. 2007;27(6):687–694. doi: 10.1007/s10571-007-9195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase I, Tadokoro A, Kawabata N, Takeuchi T, Katoh H, Hiramoto K, Negishi M, Nomizu M, Sugiura Y, Futaki S. Interaction of arginine-rich peptides with membrane-associated proteoglycans is crucial for induction of actin organization and macropinocytosis. Biochemistry. 2007;46(2):492–501. doi: 10.1021/bi0612824. [DOI] [PubMed] [Google Scholar]
- Niesner U, Halin C, Lozzi L, Gunthert M, Neri P, Wunderli-Allenspach H, Zardi L, Neri D. Quantitation of the tumor-targeting properties of antibody fragments conjugated to cell-permeating HIV-1 TAT peptides. Bioconjug Chem. 2002;13(4):729–736. doi: 10.1021/bc025517+. [DOI] [PubMed] [Google Scholar]
- Panizzon KL, Shin D, Frautschy S, Wallis RA. Neuroprotection with Bcl-2(20–34) peptide against trauma. Neuroreport. 1998;9(18):4131–4136. doi: 10.1097/00001756-199812210-00024. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Ryu J, Kim KA, Lee HJ, Bahn JH, Han K, Choi EY, Lee KS, Kwon HY, Choi SY. Mutational analysis of a human immunodeficiency virus type 1 Tat protein transduction domain which is required for delivery of an exogenous protein into mammalian cells. J Gen Virol. 2002;83(Pt 5):1173–1181. doi: 10.1099/0022-1317-83-5-1173. [DOI] [PubMed] [Google Scholar]
- Poon GM, Gariepy J. Cell-surface proteoglycans as molecular portals for cationic peptide and polymer entry into cells. Biochem Soc Trans. 2007;35(Pt 4):788–793. doi: 10.1042/BST0350788. [DOI] [PubMed] [Google Scholar]
- Rapraeger A, Jalkanen M, Bernfield M. Cell surface proteoglycan associates with the cytoskeleton at the basolateral cell surface of mouse mammary epithelial cells. J Cell Biol. 1986;103(6 Pt 2):2683–2696. doi: 10.1083/jcb.103.6.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278(1):585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- Ryu J, Han K, Park J, Choi SY. Enhanced uptake of a heterologous protein with an HIV-1 Tat protein transduction domains (PTD) at both termini. Mol Cells. 2003;16(3):385–391. [PubMed] [Google Scholar]
- Sakurai H, Kawabata K, Sakurai F, Nakagawa S, Mizuguchi H. Innate immune response induced by gene delivery vectors. Int J Pharm. 2007 doi: 10.1016/j.ijpharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285(5433):1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Sengoku T, Bondada V, Hassane D, Dubal S, Geddes JW. Tat-calpastatin fusion proteins transduce primary rat cortical neurons but do not inhibit cellular calpain activity. Exp Neurol. 2004;188(1):161–170. doi: 10.1016/j.expneurol.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Soane L, Fiskum G. TAT-mediated endocytotic delivery of the loop deletion Bcl-2 protein protects neurons against cell death. J Neurochem. 2005;95(1):230–243. doi: 10.1111/j.1471-4159.2005.03359.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Futaki S, Niwa M, Tanaka S, Ueda K, Sugiura Y. Possible existence of common internalization mechanisms among arginine-rich peptides. J Biol Chem. 2002;277(4):2437–2443. doi: 10.1074/jbc.M110017200. [DOI] [PubMed] [Google Scholar]
- Temsamani J, Vidal P. The use of cell-penetrating peptides for drug delivery. Drug Discov Today. 2004;9(23):1012–1019. doi: 10.1016/S1359-6446(04)03279-9. [DOI] [PubMed] [Google Scholar]
- Trehin R, Merkle HP. Chances and pitfalls of cell penetrating peptides for cellular drug delivery. Eur J Pharm Biopharm. 2004;58(2):209–223. doi: 10.1016/j.ejpb.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Tunnemann G, Martin RM, Haupt S, Patsch C, Edenhofer F, Cardoso MC. Cargo-dependent mode of uptake and bioavailability of TAT-containing proteins and peptides in living cells. Faseb J. 2006;20(11):1775–1784. doi: 10.1096/fj.05-5523com. [DOI] [PubMed] [Google Scholar]
- Tyagi M, Rusnati M, Presta M, Giacca M. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J Biol Chem. 2001;276(5):3254–3261. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- Vazquez J, Sun C, Du J, Fuentes L, Sumners C, Raizada MK. Transduction of a functional domain of the AT1 receptor in neurons by HIV-Tat PTD. Hypertension. 2003;41(3 Pt 2):751–756. doi: 10.1161/01.HYP.0000047878.13793.41. [DOI] [PubMed] [Google Scholar]
- Violini S, Sharma V, Prior JL, Dyszlewski M, Piwnica-Worms D. Evidence for a plasma membrane-mediated permeability barrier to Tat basic domain in well-differentiated epithelial cells: lack of correlation with heparan sulfate. Biochemistry. 2002;41(42):12652–12661. doi: 10.1021/bi026097e. [DOI] [PubMed] [Google Scholar]
- Vives E. Cellular uptake [correction of utake] of the Tat peptide: an endocytosis mechanism following ionic interactions. J Mol Recognit. 2003;16(5):265–271. doi: 10.1002/jmr.636. [DOI] [PubMed] [Google Scholar]
- Vives E. Present and future of cell-penetrating peptide mediated delivery systems: "is the Trojan horse too wild to go only to Troy?". J Control Release. 2005;109(1–3):77–85. doi: 10.1016/j.jconrel.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10(3):310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- Warda M, Toida T, Zhang F, Sun P, Munoz E, Xie J, Linhardt RJ. Isolation and characterization of heparan sulfate from various murine tissues. Glycoconj J. 2006;23(7–8):555–563. doi: 10.1007/s10719-006-7668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, McAllister AK. Techniques for gene transfer into neurons. Curr Opin Neurobiol. 2002;12(5):566–573. doi: 10.1016/s0959-4388(02)00365-3. [DOI] [PubMed] [Google Scholar]
- Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc Natl Acad Sci U S A. 2000;97(24):13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Fortunati E, van den Broek M, Hoogeveen AT, Scholte BJ. Efficacy of a peptide-based gene delivery system depends on mitotic activity. Gene Ther. 1996;3(12):1133–1142. [PubMed] [Google Scholar]
- Yang TT, Cheng L, Kain SR. Optimized codon usage and chromophore mutations provide enhanced sensitivity with the green fluorescent protein. Nucleic Acids Res. 1996;24(22):4592–4593. doi: 10.1093/nar/24.22.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ma J, Song Z, Wu M. HIV-1 TAT-mediated protein transduction and subcellular localization using novel expression vectors. FEBS Lett. 2002;532(1–2):36–44. doi: 10.1016/s0014-5793(02)03624-4. [DOI] [PubMed] [Google Scholar]
- Yin W, Cao G, Johnnides MJ, Signore AP, Luo Y, Hickey RW, Chen J. TAT-mediated delivery of Bcl-xL protein is neuroprotective against neonatal hypoxic-ischemic brain injury via inhibition of caspases and AIF. Neurobiol Dis. 2006;21(2):358–371. doi: 10.1016/j.nbd.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Yu DH, Lee KH, Lee JY, Kim S, Shin DM, Kim JH, Lee YS, Oh SK, Moon SY, Lee SH. Changes of gene expression profiles during neuronal differentiation of central nervous system precursors treated with ascorbic acid. J Neurosci Res. 2004;78(1):29–37. doi: 10.1002/jnr.20220. [DOI] [PubMed] [Google Scholar]