Abstract
Gliotoxin is a member of the epipolythiodioxopiperazine class of toxins and is both the major and the most potent toxin produced by Aspergillus fumigatus. Since the discovery of the putative gliotoxin biosynthetic 12-gene cluster in the genome of A. fumigatus, five different laboratories have attempted to determine the role of this toxin in the virulence of A. fumigatus. The genes in the cluster that have been disrupted to study the pathobiological importance of gliotoxin include gliZ that encodes a transcription factor and gliP that encodes a nonribosomal peptide synthase. Two of the five laboratories have reported gliotoxin to be an important virulence determinant of A. fumigatus, while the other three laboratories have shown it to be unimportant. Comparisons of the data generated among the five laboratories revealed that the immunosuppressive regimen used for mice was the key factor that contributed to the observed disparity. Regardless of either the mouse strains used or the route of infection, immunosuppression with a combination of cyclophosphamide and corticosteroids (neutropenic mice) showed gliotoxin to be unimportant. The mice immunosuppressed with corticosteroids alone, however, revealed that gliotoxin is an important virulence determinant of A. fumigatus. These studies indicate that the neutropenic mice model is inadequate to reveal the pathobiological importance of fungal secondary metabolites in invasive pulmonary aspergillosis.
Keywords: gliotoxin, virulence determinants, immunosuppressive regimen, invasive aspergillosis
Introduction
Gliotoxin is an epipolythiodioxopiperazine (ETP) class of toxin produced by Aspergillus fumigatus and has been suspected as one of the most likely virulence determinants among various secondary metabolites produced by the species. A. fumigatus is the most frequent cause of invasive aspergillosis worldwide and over 90% of the strains isolated from invasive aspergillosis cases in tertiary-care cancer centers were found to be gliotoxin producers [1]. Furthermore, the amount of toxin produced by A. fumigatus was substantially higher than the amount produced by other less frequent species of pathogenic Aspergillus including A. terreus and A. flavus [1].
In 2004, Gardiner and Howlett discovered a 12-gene cluster in the A. fumigatus genome sequence that resembles the gene cluster responsible for the synthesis of sirodesmin, an ETP toxin in Leptosphaera maculans [2]. Since gliotoxin is also an ETP toxin, they proposed it to be the putative gliotoxin biosynthetic gene cluster. Similar ETP types of gene clusters were subsequently found in many other fungi belonging to Ascomycetes [3]. The discovery of the putative gliotoxin biosynthetic gene cluster in A. fumigatus allowed us for the first time to determine whether this gene cluster was indeed responsible for gliotoxin biosynthesis and whether the toxin plays any role in the pathobiology of A. fumigatus. Five laboratories have embarked on the functional analysis of this gene cluster by disrupting either the gene encoding the nonribosomal peptide synthase gliP [4–7] or the gene encoding the transcriptional factor gliZ [8] in the A. fumigatus genomic sequencing strains Af293 and CEA10, or the clinical isolate B-5233. Results have shown that this 12-gene cluster is indeed responsible for gliotoxin production. However, results of the animal experiments were mixed regarding the importance of gliotoxin as a virulence determinant. Three laboratories found gliotoxin to be unimportant while reports from the remaining two laboratories showed it to be important for virulence of A. fumigatus. Comparisons between the reports from the five laboratories [4–8] that investigated the function of gli genes clearly revealed that the animal host immune status was the key factor responsible for the contradiction. In this mini-review, we sought to summarize the significance of gliotoxin in the pathobiology of A. fumigatus.
The pathogenic species of Aspergillus that produce gliotoxin
The most common species of Aspergillus that cause invasive aspergillosis world-wide are A. fumigatus, A. terreus, A. flavus and A. niger [9]. Although all the species produce gliotoxin, not every strain is a gliotoxin producer [1]. There are several reports that cite the frequency of toxin producing strains that have been isolated from the environment versus clinical specimens from patients. Santos et al. [10] reported that only 11% of the A. fumigatus strains isolated from moldy silos on Terceira Island in the Azores were gliotoxin producers. In contrast, 93% of the A. fumigatus strains (n =40) isolated between 1998 and 2003 from respiratory and tissue samples of cancer patients in MD. Anderson Cancer Center, Houston, Texas, USA were gliotoxin producers compared to 75% and 25% of A. niger (n =9) and A. terreus (n =27) strains, respectively. Surprisingly, only 4% of the A. flavus (n =18) strains produced gliotoxin. Furthermore, the concentrations of gliotoxin produced by A. fumigatus were significantly higher than those of other species [1].
Kosalec and Pepeljnjak [11] reported very different frequencies of gliotoxin producing A. fumigatus strains recovered from immunocompromised patients with various diseases in the hematology unit at the University of Zagreb in Croatia. Of the 50 clinical isolates, only 18% produced gliotoxin, while no environmental strains of an equivalent number screened were found to produce the toxin. Although the data are fragmentary, the results suggest that the frequency of gliotoxin producing A. fumigatus strains varies depending on the geographic region and/or type of patients that yielded the fungal strains. It appears, however, that the frequency of finding gliotoxin producing A. fumigatus strains is higher among clinical isolates than among environmental isolates.
The Ascomycete species that produce secondary metabolites, especially of ETP, are largely ubiquitous fungi such as Penicillium, Trichoderm, Leptosphaera and others besides Aspergillus [3]. Secondary metabolites may render the species more fit for survival in all types of soil throughout the world. The recent report by Rohlfs and colleagues has shown that the fungivorous springtails, a soil insect, preferred to consume conidia of laeA mutant over wild type conidia of A. nidulans [12]. Although A. nidulans does not produce gliotoxin, it produces an array of toxic secondary metabolites controlled by the global regulator, laeA. LaeA also regulates secondary metabolism in A. fumigatus. Deletion of laeA in A. fumigatus not only resulted in the abolition of gliotoxin production but attenuated fungal virulence [13,14]. Unlike ubiquitous fungi, no genomic evidence of ETP-like gene clusters were found in endemic species such as Coccidioides immitis, Histoplasma capsulate [3] and Blastomyces dermatitidis [15], which are confined to a certain environmental niche. Considering the presence of 2,000–18,000 different genomes per gram of soil [16], it is easy to understand how fungi not producing secondary metabolites would be at a disadvantage for competition with other organisms in soil.
Gliotoxin exerts detrimental effects on mammalian cells in vitro
Gliotoxin is a dipeptide characterized by the presence of a disulfide bridge across the piperazine ring [17]. The disulfide bridge allows the cross linking with proteins via cysteine residues and generate deleterious reactive oxygen species (ROS) through the redox cycling between the reduced and oxidized form. This mechanism of ROS generation is believed to be responsible for the toxicity of gliotoxin [18,19]. The toxin is known to be immunosuppressive: inhibits phagocytosis [20,21], inhibits the transcription factor NF-κB thereby blocking inflammatory response and cytokine production [22] and blocks mast cell degranulation [23]. Furthermore, the toxin is known to cause apoptotic cell death in professional immune cells such as macrophages and monocytes [24,25], as well as in non-immune cells such as EL4 thymoma cells [6] or mouse embryonic fibroblast (MEF) cells [26]. Recent work by Pardo et al. [26] demonstrated that gliotoxin directly activated the proapoptotic Bcl-2 family member Bak, a constitutive mitochondrial protein in MEF cells. The ROS generated as a result was reported to facilitate the release of cytochrome c and apoptosis inducing factors from mitochondria, leading to caspase activation, as well as other events that mediate cell death [26]. Unlike the effects of gliotoxin observed in macrophages or monocytes, neutrophils from healthy humans are reported to be resistant to gliotoxin-mediated apoptosis in vitro [27]. Neutrophil functions, however, were significantly blunted by gliotoxin, with suppressed ROS production and impaired phagocytic capacity. Methylprednisone, a corticosteroid commonly used to suppress immunity in the stem cell transplant recipient, reversed the suppression of ROS production by Neutrophils. These results suggest that gliotoxin inhibits important neutrophil functions in normal individuals, while increasing PMN-mediated inflammation in immunocompromised hosts; this differential effect may contribute to tissue destruction in patients with invasive aspergillosis [27].
In contrast to helvasin or tremogerin, other known secondary metabolites produced by A. fumigatus, gliotoxin inhibited oxidative burst of human neutrophils [28]. Gliotoxin also causes damage to the ciliated respiratory epithelium in vitro and this property might assist A. fumigatus in the colonization of the respiratory mucosa [29]. Furthermore, Nierman and colleagues have recently shown by the genome-wide gene expression profile analysis that gliotoxin genes are upregulated in germlings during initiation of infection in mice [30]. All of these findings implicate gliotoxin in playing a role in guarding A. fumigatus in the host environment.
Functional studies on gliotoxin biosynthetic genes
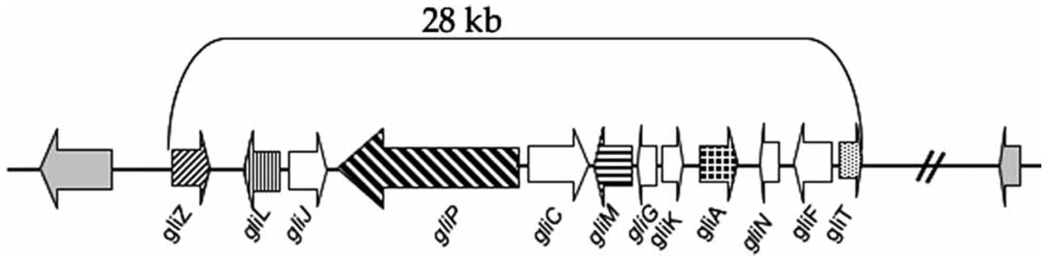
Using a comparative genomics approach, Gardiner and Howlett et al. [2] discovered the putative cluster of 12 genes involved in gliotoxin biosynthesis (Fig. 1) in A. fumigatus. Putative ETP gene clusters similar to that of A. fumigatus are also found in the genomes of other pathogenic aspergilli, such as A. terreus and A. flavus [3]. The gene cluster in A. terreus is identical to the gliotoxin gene cluster in A. fumigatus except for the absence in the former of the gene for the methyl transferase gliN in Fig. 1 [Howlett, B. Personal communication]. The gliotoxin gene cluster of A. fumigatus spanned 28 kb in the genome of the A. fumigatus strain Af293 and contained eight genes homologous to those in the gene cluster responsible for the synthesis of sirodesmin in L. maculans. Based on the sequence of the gene cluster and the time course of gliotoxin production, Gardiner et al. [2] proposed a pathway for the synthesis of the toxin with six enzymatic steps. The cluster is composed of genes encoding a putative zinc finger transcription factor (gliZ), an aminocyclo-propane carboxylic acid synthase (gliL), a dipeptidase (gliJ), a peptide synthase (gliP), two cytochrome p450 monooxygenases (gliC and gliF), an O-methyltransferase (gliM), a glutathione S-transferase (gliG), a hypothetical protein (gliK), a transporter (gliA), a methyltransferase (gliN) and a thioredoxin reductase (gliT). Conclusive evidence that the 12-gene cluster is responsible for gliotoxin synthesis was obtained by the functional studies of gliZ and or gliP in three strains of A. fumigatus Af293, B-5233 and CEA10. The gliZ gene controls gene expression of the remaining 11 genes in the cluster [8] while gliP encodes a multimodular nonribosomal peptide synthase that catalyzes the condensation of serine and phenylalanine, the first step of the pathway making diketopiperazine scaffold of the toxin [31]. The evidence that the gene cluster is indeed responsible for gliotoxin synthesis include: (i) Deletion of either gliP or gliZ in the strain Af293 and gliP deletion in the strains CEA10 and B-5233 abolished synthesis of the toxin. Reconstitution of the deletants with their respective wild type genes restored the production of gliotoxin to wild type level [5–7]; (ii) Deletion of gliZ in the strain Af293 resulted in the absence of gene expression in the remaining 11 genes of the cluster [8]; and (iii) Over expression of gliZ in the strain Af293 enhanced the production of gliotoxin above the wild type level [8]. These results confirmed that the 12-gene cluster is indeed responsible for the biosynthesis of gliotoxin.
Fig. 1.
Genomic organization of the 12 gli-gene cluster responsible for gliotoxin biosynthesis in Aspergillus fumigatus.
Pathobiological significance of gliotoxin in A. fumigatus
Deletants of either the gliP or gliZ gene showed no difference in morphology or growth rate compared to wild type strains except that mutants produced no gliotoxin [5–8]. These results were expected since, unlike primary metabolism, the secondary metabolism, which includes the biosynthesis of gliotoxin, does not affect growth of the organism. Secondary metabolism that is associated with fungal development, such as polyketide synthesis, is commonly associated with sporulation or pigmentation of spores [32 and, reviewed in 33]. Interestingly, deletion of gliP in Af293 caused a down regulation of gene expression in the 12-gene cluster. Addition of exogenous gliotoxin restored the gene expression level suggesting that gliotoxin regulates its own production [7]. In vitro analysis showed that culture filtrates from gliotoxin-negative mutants failed to inhibit oxidative burst in human neutrophils [6], failed to cause apoptosis or cell detachment in various mammalian cells [6,8] and also failed to inhibit mast cell degranulation [7].
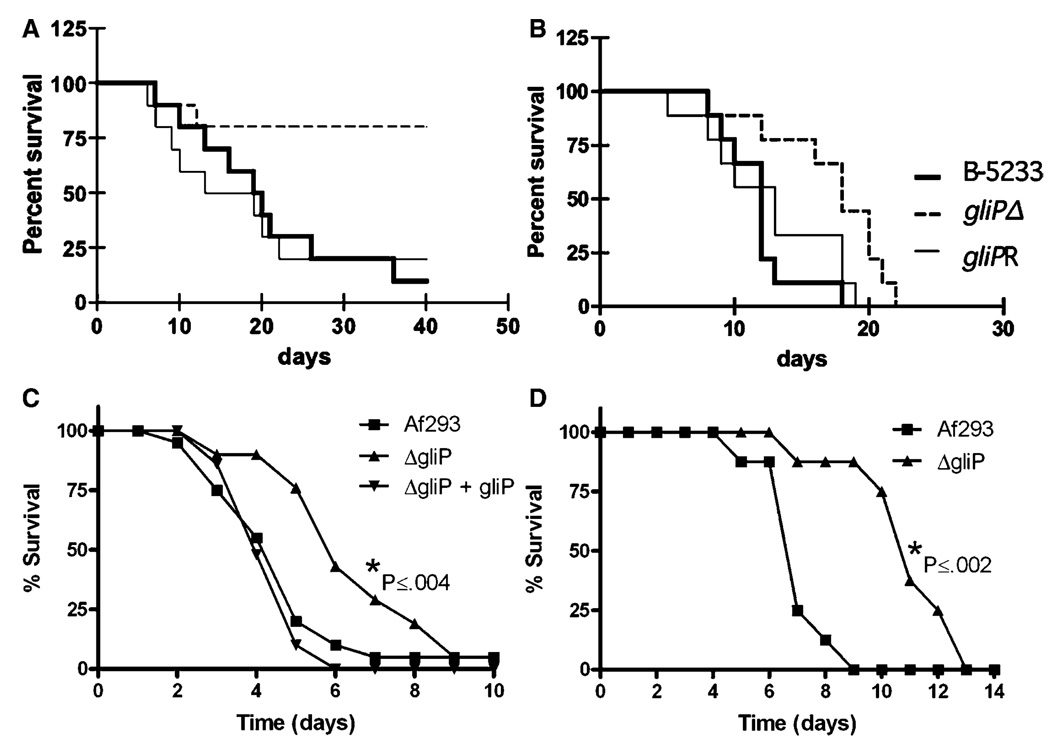
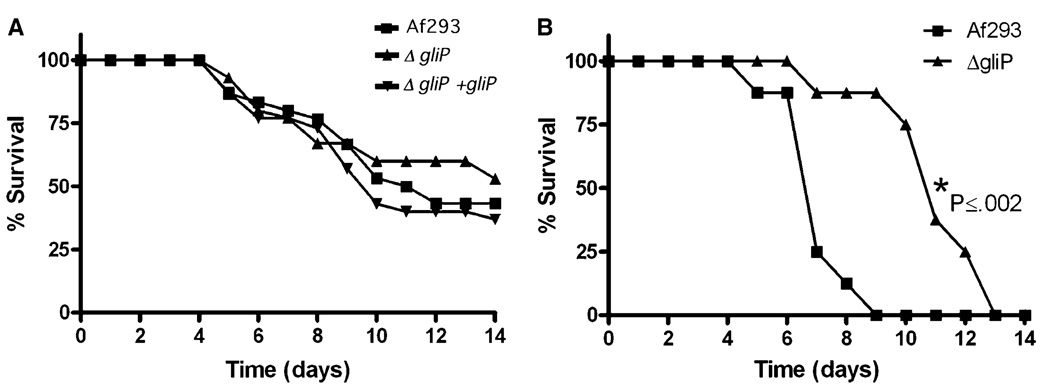
The importance of gliotoxin in virulence of A. fumigatus in mice, however, was equivocal. Five laboratories used various mouse strains that were either immunosuppressed by the administration of cyclophosphamide plus cortisone acetate [4,5,7,8] or cortisone acetate alone [4,6] in order to study the effect of gliotoxin on virulence of the three A. fumigatus strains (Table 1). The two laboratories that showed gliotoxin to be an important virulence determinant used mice that were immunosuppressed only with cortisone acetate. Mice infected with gliPΔ strains survived significantly longer than those infected with wild type or gliP reconstituted strains [4,6], as shown in Fig. 2. Histopathological analysis of lung sections confirmed the attenuated virulence of gliPΔ strains in 129/Sv mice [6]. Interestingly, the fungal burden of gliPΔ strain in the lung of BALB/c mice was not lower than those of the wild type or reconstituted strain [4]. Fragmentation of neutrophils at the infection foci, however, was much more pronounced in the lungs of mice infected with wild type or reconstituted strains than the gliPΔ strain [4]. The other three laboratories that showed no effect of the toxin in invasive infection invariably used neutropenic mice that resulted from immunosuppression by combination of cyclophosphamide and cortisone acetate. All succumbed to aspergillosis by the wild type and gliPΔ or gliZΔ strains at a similar rate. This suggested that one of the important targets of gliotoxin is neutrophils and as a result, gliotoxin is less important for A. fumigatus virulence in neutropenic mice that lack one of the toxin’s targets. The survival data shown in Fig. 3 directly support this notion.
Table 1.
Role of gliotoxin in pathobiology of Aspergillus fumigatus studied in five laboratories.
| Strain background |
Gene deleted | Mouse strains | Immunosuppressive regimen | Inoculation method |
Virulence | Reference |
|---|---|---|---|---|---|---|
| Af293 | gliP | ICR | Cyclophosphamide+Cortisone | Inhalation | No effect | [7] |
| Af293 | gliP | BALB/C | Cyclophosphamide+Cortisone | Inhalation | No effect | [4] |
| Af293 | gliP | BALB/C | Cortisone | Inhalation | Attenuated | [4] |
| CEA10 | gliP | BALB/C | Cyclophosphamide+Cortisone | Intranasal | No effect | [5] |
| B-5233 | gliP | BALB/C | Cortisone | Intranasal | Attenuated | [6] |
| B-5233 | gliP | 129/Sv | Cortisone | Intranasal | Attenuated | [6] |
| Af293 | gliZ | ICR | Cyclophosphamide+Cortisone | Intranasal | No effect | [8] |
Fig. 2.
Virulence of the wild type, gliPΔ and reconstituted strains of Aspergillus fumigatus inoculated into two different mouse strains immunosuppressed with cortisone acetate. (A) and (B) Survival of 129/Sv (A) and BALB/c (B) mice infected intranasally with conidia of B-5233, gliPΔ and gliPR (gliPΔ + gliP) strains (from Sugui et al. 2007) [6]. (C) Survival of BALB/c mice infected intranasally with conidia of Af293, gliPΔ and gliPΔ + gliP. (D) Survival of BALB/c mice infected by inhalation of conidia from the strains Af293 and gliP deletant (C and D from Spikes et al. 2008) [4].
Fig. 3.
Survival of BALB/c mice infected by inhalation of Af293 and its gli strains. (A) Mice immunosuppressed by combination of cyclophosphamide and cortisone acetate showing no virulence difference between the strains. (B) Mice immunosuppressed by cortisone acetate alone showing statistically significant difference in virulence between the wild type and gliP deletant (from Spikes et al. 2008) [4].
With these results, one should ask about the clinical relevance of the gliotoxin effect in the cortisone-treated mouse model of invasive aspergillosis. Invasive aspergillosis occurs most commonly in neutropenic patients [34]. However, invasive aspergillosis is being increasingly reported in allogeneic stem cell transplant (SCT) recipients who have been treated with immunosuppressive regimens, most commonly corticosteroids, because of the graft-versus host disease [35–38]. These patients are no longer neutropenic but susceptible to invasive fungal infection. Gliotoxin would play an important role in the pathogenesis of A. fumigatus in these patients.
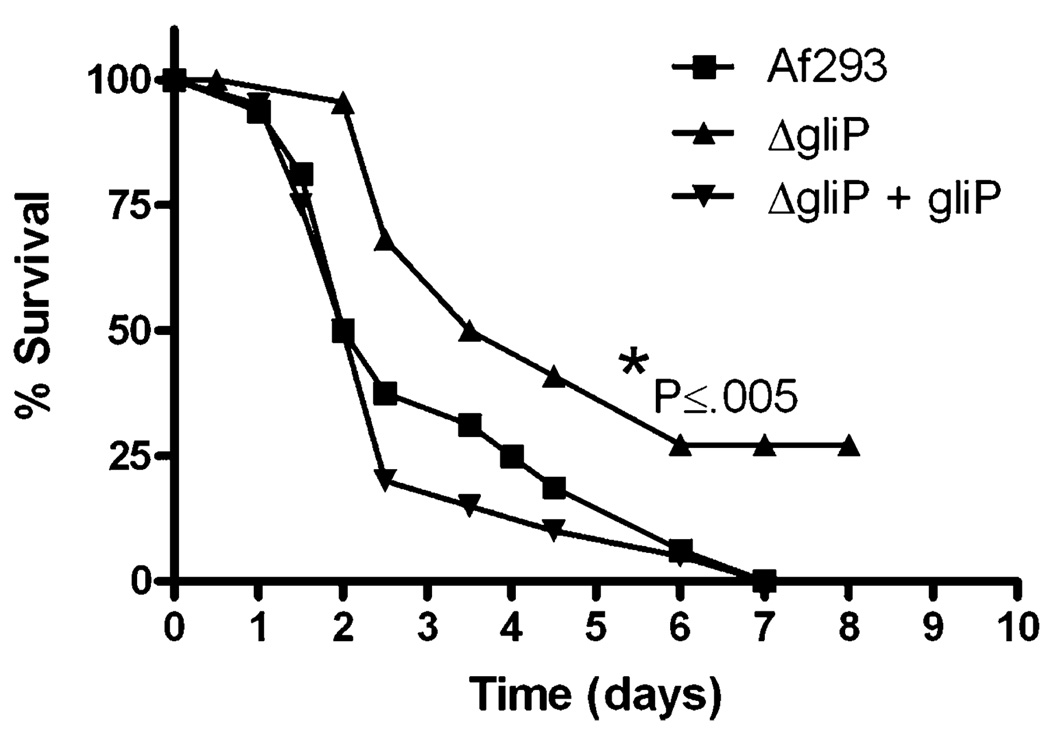
In addition to mice immunosuppressed by cortisone acetate, pathobiological importance of gliotoxin was also demonstrated in Drosophila melanogaster [4]. Toll-deficient D. melanogaster was significantly less susceptible to gliPΔ of the A. fumigatus Af293 strain than the wild type or the reconstituted strain (Fig. 4). This suggests that Toll-deficient D. melanogaster is useful model for the assessment of the pathobiological importance of fungal secondary metabolites.
Fig. 4.
Toll deficient Drosophila melanogaster infected with Af293 and its derivative gli strains showing attenuated virulence in gliP deletant compared to the wild type and gliP deletant strain reconstituted with the wild type gliP gene (from Spikes et al. 2008) [4].
Conclusions
Gliotoxin is an important factor contributing to the virulence of A. fumigatus in mouse model. Its pathobiological importance, however, has only been demonstrated in non-neutropenic mice suggesting that gliotoxin plays an important role in the pathogenesis of aspergillosis in patients immunosuppressed, but not neutropenic. The disparate result published on the importance of gliotoxin as a virulence determinant was neither due to differences in the fungal strains, the inoculation method, nor the differences in the mouse strain used. It is clear that the immune status of the mice used was the key factor which contributed to the controversy. Mice immunosuppressed by a combination of cyclophosphamide and cortisone acetate should not be considered as the standard model applicable for every situation in which the potential virulence determinants are being evaluated.
Acknowledgments
This study was supported by funds from the intramural program of the National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Footnotes
Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article maybe used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Lewis RE, Wiederhold NP, Lionakis MS, Prince RA, Kontoyiannis DP. Frequency and species distribution of gliotoxin-producing Aspergillus isolates recovered from patients at a tertiary-care cancer center. J Clin Microbiol. 2005;43:6120–6122. doi: 10.1128/JCM.43.12.6120-6122.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardiner DM, Howlett BJ. Bioinformatic and expression analysis of the putative gliotoxin biosynthetic gene cluster of Aspergillus fumigatus. FEMS Microbiol Lett. 2005;248:241–248. doi: 10.1016/j.femsle.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 3.Patron NJ, Waller RF, Cozijnsen AJ, et al. Origin and distribution of epipolythiodioxopiperazine (ETP) gene clusters in filamentous ascomycetes. BMC Evol Biol. 2007;7:174–188. doi: 10.1186/1471-2148-7-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spikes S, Xu R, Nguyen CK, et al. Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J Infect Dis. 2008;197:479–486. doi: 10.1086/525044. [DOI] [PubMed] [Google Scholar]
- 5.Kupfahl C, Heinekamp T, Geginat G, et al. Deletion of the gliP gene of Aspergillus fumigatus results in loss of gliotoxin production but has no effect on virulence of the fungus in a low-dose mouse infection model. Mol Microbiol. 2006;62:292–302. doi: 10.1111/j.1365-2958.2006.05373.x. [DOI] [PubMed] [Google Scholar]
- 6.Sugui JA, Pardo J, Chang YC, et al. Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot Cell. 2007;6:1562–1569. doi: 10.1128/EC.00141-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramer RA, Jr, Gamcsik MP, Brooking RM, et al. Disruption of a nonribosomal peptide synthetase in Aspergillus fumigatus eliminates gliotoxin production. Eukaryot Cell. 2006;5:972–980. doi: 10.1128/EC.00049-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bok JW, Chung D, Balajee SA, et al. GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect Immun. 2006;74:6761–6768. doi: 10.1128/IAI.00780-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon-Chung KJ, Bennett JE. Medical Mycology. Philadelphia: Lea & Febiger; 1992. [Google Scholar]
- 10.dos Santos VM, Dorner JW, Carreira F. Isolation and toxigenicity of Aspergillus fumigatus from moldy silage. Mycopathologia. 2003;156:133–138. doi: 10.1023/a:1022996911563. [DOI] [PubMed] [Google Scholar]
- 11.Kosalec I, Pepeljnjak S. Mycotoxigenicity of clinical and environmental Aspergillus fumigatus and A. flavus isolates. Acta Pharm. 2005;55:365–375. [PubMed] [Google Scholar]
- 12.Rohlfs M, Albert M, Keller NP, Kempken F. Secondary chemicals protect mould from fungivory. Biol Lett. 2007;3:523–525. doi: 10.1098/rsbl.2007.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bok JW, Balajee SA, Marr KA, et al. LaeA, a regulator of morphogenetic fungal virulence factors. Eukaryot Cell. 2005;4:1574–1582. doi: 10.1128/EC.4.9.1574-1582.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugui JA, Pardo J, Chang YC, et al. Role of laeA in the regulation of alb1, gliP, conidial morphology, and virulence in Aspergillus fumigatus. Eukaryot Cell. 2007;6:1552–1561. doi: 10.1128/EC.00140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blastomyces dermatitidis. St Louis, MO: Genome Sequencing Center, Washington University School of Medicine; 2008. Available from the website: http://genome.wustl.edu/tools/blast/ [Google Scholar]
- 16.Daniel R. The metagenomics of soil. Nat Rev Microbiol. 2005;3:470–478. doi: 10.1038/nrmicro1160. [DOI] [PubMed] [Google Scholar]
- 17.Trown PW, Bilello JA. Mechanism of action of gliotoxin: elimination of activity by sulfhydryl compounds. Antimicrob Agents Chemother. 1972;2:261–266. doi: 10.1128/aac.2.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waring P, Beaver J. Gliotoxin and related epipolythiodioxopiperazines. Gen Pharmacol. 1996;27:1311–1316. doi: 10.1016/s0306-3623(96)00083-3. [DOI] [PubMed] [Google Scholar]
- 19.Gardiner DM, Waring P, Howlett BJ. The epipolythiodioxopiperazine (ETP) class of fungal toxins: distribution, mode of action, functions and biosynthesis. Microbiology. 2005;151:1021–1032. doi: 10.1099/mic.0.27847-0. [DOI] [PubMed] [Google Scholar]
- 20.Mullbacher A, Eichner RD. Immunosuppression in vitro by a metabolite of a human pathogenic fungus. Proc Natl Acad Sci USA. 1984;81:3835–3837. doi: 10.1073/pnas.81.12.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eichner RD, Al Salami M, Wood PR, Mullbacher A. The effect of gliotoxin upon macrophage function. Int J Immunopharmacol. 1986;8:789–797. doi: 10.1016/0192-0561(86)90016-0. [DOI] [PubMed] [Google Scholar]
- 22.Pahl HL, Krauss B, Schulze-Osthoff K, et al. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-kappaB. J Exp Med. 1996;183:1829–1840. doi: 10.1084/jem.183.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niide O, Suzuki Y, Yoshimaru T, Inoue T, Takayama T, Ra C. Fungal metabolite gliotoxin blocks mast cell activation by a calcium- and superoxide-dependent mechanism: implications for immunosuppressive activities. Clin Immunol. 2006;118:108–116. doi: 10.1016/j.clim.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Waring P, Eichner RD, Mullbacher A, Sjaarda A. Gliotoxin induces apoptosis in macrophages unrelated to its antiphagocytic properties. J Biol Chem. 1988;263:18493–18499. [PubMed] [Google Scholar]
- 25.Stanzani M, Orciuolo E, Lewis R, et al. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxinmediated apoptosis of monocytes. Blood. 2005;105:2258–2265. doi: 10.1182/blood-2004-09-3421. [DOI] [PubMed] [Google Scholar]
- 26.Pardo J, Urban C, Galvez EM, et al. The mitochondrial protein Bak is pivotal for gliotoxin-induced apoptosis and a critical host factor of Aspergillus fumigatus virulence in mice. J Cell Biol. 2006;174:509–519. doi: 10.1083/jcb.200604044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orciuolo E, Stanzani M, Canestraro M, Galimberti S, Carulli G, Lewis R, Petrini M, Komanduri KV. Effects of Aspergillus fumigatus gliotoxin and methylprednilosone on human neutrophils: implications for the pathogenesis of invasive aspergillosis. J Leukocyte Biol. 2007;82:839–848. doi: 10.1189/jlb.0207090. [DOI] [PubMed] [Google Scholar]
- 28.Tsunawaki S, Yoshida LS, Nishida S, Kobayashi T, Shimoyama T. Fungal metabolite gliotoxin inhibits assembly of the human respiratory burst NADPH oxidase. Infect Immun. 2004;72:3373–3382. doi: 10.1128/IAI.72.6.3373-3382.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amitani R, Taylor G, Elezis EN, et al. Purification and characterization of factors produced by Aspergillus fumigatus which affect human ciliated respiratory epithelium. Infect Immun. 1995;63:3266–3271. doi: 10.1128/iai.63.9.3266-3271.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nierman WC, Fedorova N. 3rd Advances Against Aspergillosis. Miami Beach, Florida: 2008. Subtelomeric diversity as a major force in evolution of Aspergillus secondary metabolism and virulence pathways; p. 77. [Google Scholar]
- 31.Balibar CJ, Walsh CT. GliP, a multimodular nonribosomal peptide synthetase in Aspergillus fumigatus, makes the diketopiperazine scaffold of gliotoxin. Biochem. 2006;45:15029–15038. doi: 10.1021/bi061845b. [DOI] [PubMed] [Google Scholar]
- 32.Tsai H-F, Chang YC, Wheeler MH, Kwon-Chung KJ. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 1999;181:6469–6477. doi: 10.1128/jb.181.20.6469-6477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calvo AM, Wilson RA, Bok JW, Keller NP. Relationship between secondary metabolism and fungal development. J Leukocyte Biol. 2002;66:447–459. doi: 10.1128/MMBR.66.3.447-459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latge LP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grow WB, Moreb JS, Roque D, et al. Late onset of invasive aspergillus infection in bone marrow transplant patients at a university hospital. Bone Marrow Transplant. 2002;29:15–19. doi: 10.1038/sj.bmt.1703332. [DOI] [PubMed] [Google Scholar]
- 36.Ribaud P, Chastang C, Latge JP, et al. Survival and prognostic factors of invasive aspergillosis after allogeneic bone marrow transplantation. Clin Infect Dis. 1999;28:322–330. doi: 10.1086/515116. [DOI] [PubMed] [Google Scholar]
- 37.Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100:4358–4366. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]
- 38.Jantunen E, Anttila VJ, Ruutu T. Aspergillus infections in allogeneic stem cell transplant recipients: have we made any progress? Bone Marrow Transplant. 2002;30:925–929. doi: 10.1038/sj.bmt.1703738. [DOI] [PubMed] [Google Scholar]