Abstract
Environmental isolates of the fungus Rhizopus have been shown to harbor a bacterial endosymbiont (Burkholderia) that produces rhixozin, a plant mycotoxin. We sought to define the role of rhizoxin production by endosymbionts in the pathogenesis of mucormycosis. Endosymbiotic bacteria were identified by polymerase chain reaction in 15 (54%) of 28 clinical isolates of Zygomycetes, with 33% of the bacterial strains showing ≥87% identity to Burkholderia 16S rDNA. The presence of rhizoxin in myclial extracts from fungi harboring bacteria was confirmed by high-performance liquid chromatography analysis. However, fungal strains with or without endosymbionts did not differ in their ability to cause endothelial cell injury in vitro, nor did antibiotic-mediated eradication of endosymbionts and rhizoxin production decrease the virulence of fungal strains in mice or flies. In summary, although bacterial endosymbiosis is widely detected in clinical isolates of Zygomycetes, including Rhizopus oryzae strains, we found no evidence that bacterial endosymbionts and rhizoxin contribute to the pathogenesis of mucormycosis in the models studied.
Mucormycoses are infections caused by fungi belonging to the order Mucorales of the class Zygomycetes [1]. Rhizopus oryzae is the most common organism isolated from patients with mucormycosis and is responsible for 60%–80% of all mucormycosis cases [2, 3]. The standard therapy for invasive mucormycosis includes reversal of predisposing factors (if possible), widespread surgical debridement, and antifungal therapy [2, 4, 5]. In the absence of surgical removal of the infected focus, antifungal therapy alone is rarely curative [2, 5]. Even when surgical debridement is combined with antifungal therapy, the mortality rate associated with mucormycosis exceeds 50% [2]. In patients with prolonged neutropenia and in those with disseminated disease, mortality approaches 90%–100% [6–8]. Equally alarming are recent data demonstrating a striking increase in the incidence of mucormycosis [7–10]. Because of its increasing incidence, unacceptably high mortality, and the extreme morbidity of highly disfiguring surgical therapy, it is imperative to look for new therapeutic modalities to treat mucormycosis.
Some Rhizopus species, especially the plant pathogen R. microsporus [11] and R. chinensis [12], are known for their ability to produce the mycotoxin rhizoxin, an antimitotic macrocyclic polyketide metabolite. Recent studies have demonstrated that rhizoxin is not biosynthesized by Rhizopus itself but rather by an intracellular, symbiotic bacterium of the genus Burkholderia [13]. This bacterium is sensitive to antibiotics belonging to the fluoroquinolone family. For example, production of rhizoxin was completely abrogated when Rhizopus was grown in medium containing 40 µg/mL ciprofloxacin [13]. This novel finding raises the possibility that Burkholderia may contribute to the pathogenesis of mucormycosis and that fluoroquinolones might be beneficial in combination therapy of mucormycosis.
In the present study, we sought to define the role of bacterial endosymbionts in the pathogenesis of mucormycosis. We screened for the presence of endosymbiotic bacteria in Zygomycetes isolates from patients with mucormycosis and determined the effect of the endosymbiotic bacteria on the pathogenesis of mucormycosis in vitro and in vivo.
METHODS
Organisms and culture conditions
Twenty-eight clinical isolates of Rhizopus or Mucor species were collected from a variety of medical centers in the United States. Two positive control strains, including Rhizopus species ATCC 20577 and R. microsporus ATCC 62417 (which has been previously reported to harbor Burkholderia [13, 14]), were obtained from the American Type Culture Collection. Organisms were grown on potato dextrose agar (PDA) for 4 days at 37°C. To render the fungus free of bacteria, isolates were grown on PDA containing 60 µg/mL ciprofloxacin. The sporangiospores used for inoculations in virulence experiments were collected in endotoxin-free PBS containing 0.01% Tween 80, washed with PBS, and counted with a hemocytometer to prepare the final inocula. The effect of ciprofloxacin treatment on fungal growth was determined by comparing the radial growth of bacteria-free fungi to their corresponding parent strains after plating of 1 × 102 spores/5 µL on PDA plates.
Screening for bacterial endosymbionts
To detect the presence of bacterial endosymbionts, universal primers amplifying bacterial 16S rDNA were used to amplify a 1.5-kb fragment from genomic DNA samples extracted from Zygomycetes. DNA samples were obtained from 100–200 mg of filtered mycelium placed in screw-cap tubes with glass beads (425–600 µm; Sigma) and 500 µL of Southern base (87.5 g/L NaCl and 20 g/L NaOH). The mycelia were broken by use of a FastPrep device (Thermo Scientific) for 30 s at speed 4.0 and were neutralized with 500 µL of Southern neutralizer (87.5 g/L NaCl and 121.1 g/L Tris base [pH 8.0]). The mixture was centrifuged for 10 min, and 250 µL of the supernatant was removed and added to 1.25 mL of binding (PB) buffer (QIAquick PCR Purification Kit [Qaigen]) to purify the DNA of impurities, in accordance with the manufacturer’s recommendation. The primers used to amplify the 16S rDNA were as follows: forward primer, 5'-CCGAATTCGTCGACAACAGAGTTTGATCCTGGCTCAG-3'; and reverse primer, 5'-CCCGGGATCCAAGCTTACGGCTACCTTGTTACGACTT- 3' [13]. Samples that were positive for 16S rDNA were further tested for the presence of the ketosynthase (KS) domain present in polyketide synthase genes involved in the biosynthesis of rhizoxin, using the following degenerate primers to amplify a 0.7-kb fragment: forward primer, 5'-MGNGARGCNNWNSMNATGGAYCCNCARCANMG- 3'); and reverse primer, 5'-GGRTCNCCNARNSWNGTNCCNGTNCCRTG- 3' [15]. Finally, the following primers amplifying the housekeeping gene actin (0.6 kb) were used as a control: forward primer, 5'-GTCTTTCCTTCTATTGTTGGTC-3'; and reverse primer, 5'-CCATCAGGAAGTTCATAAGAC-3'. PCR conditions were 33 cycles at 96°C for 1 min, 52°C for 1 min, and 72°C for 2 min, for both reactions. Representative PCR products of 16S rDNA were purified using a Qiagen extraction kit and cloned into the pGEM-T easy vector (Promega) for sequence verification.
High-performance liquid chromatography (HPLC) analysis of rhizoxin production
Rhizopus mycelia were grown in 100 mL of rhizoxin-inducing medium (1% corn starch, 0.5% glycerol, 1% gluten meal, 1% dried yeast, 1% corn, and 1% CaCO3 at pH 6.5) [14] with or without ciprofioxacin at 60 µg/mL for 4 days. Collected mycelia were extracted overnight with ethyl acetate (1:1 vol/vol), and the organic phase was separated, dried with sodium sulphate anhydrous, and concentrated under pressure [13]. The dried sample was redissolved in 250 µL of methanol and analyzed by HPLC based on the method described by Graham et al [16]. Fifty microliters of standard or sample was separated on a Gemini C18 column (150 × 4.6 mm, 5 µm; Phenomenex), using a mobile phase of 45% acetonitrile in 0.01 mol/L phosphate buffer (pH 7.0) at a flow rate of 1 mL/min. The UV detector was set at 310 nm, and peaks were identified on the basis of reference standards. Quanititation utilized peak area in comparison to a known concentration of rhizoxin standard (Sigma). Samples were assayed in duplicate, and the results averaged. The lower limit of detection was 0.2 µg rhizoxin/mL.
Endothelial cell injury assay
Endothelial cells were obtained from human umbilical cord veins and were maintained by a modification of the method of Jaffe et al. [17]. The cells were harvested using collagenase and were grown in M-199 medium (Gibco) enriched with 10% fetal bovine serum (Intergen), 10% defined bovine calf serum (Hyclone), l-glutamine, penicillin, and streptomycin. Second-passage cells were grown to confluency in 96-well tissue culture plates (Costar) on fibronectin (Collaborative Biomedical Products). All incubations were done in 5% CO2 at 37°C. The reagents were tested for endotoxin using a chromogenic limulus amebocyte lysate assay (Bio Whittaker), and the endotoxin concentrations were <0.01 IU/mL. Endothelial cell collection was approved by the Institutional Review Board at the Los Angeles Biomedical Research Institute at the Harbor-UCLA Medical Center.
Endothelial cell injury was quantified using a chromium-release (51Cr) assay [18]. Briefly, endothelial cells grown in 96-well tissue culture plates containing detachable wells were incubated with Na2 51CrO4 (ICN) in M-199 medium (1 µCi/well) for 16 h. On the day of the experiment, the unincorporated 51Cr was aspirated, and the wells were washed twice with warmed Hanks’ balanced salt solution (Irvine Scientific). Endothelial cells were infected with fungal spores (8 × 104) suspended in 150 µL of RPMI 1640 medium (Irvine Scientific) supplemented with glutamine and 10% pooled human serum (PHS; Sigma). Spontaneous 51Cr release was determined by incubating endothelial cells in RPMI 1640 medium supplemented with glutamine and 10% PHS without fungal spores. After 6 h of incubation at 37°C in a 5% CO2 incubator, 50% of the medium was aspirated from each well and transferred to glass tubes, and the cells were manually detached and placed into another set of tubes. The amount of 51Cr in the aspirate and the detached well was determined by gamma counting. The total amount of 51Cr incorporated by endothelial cells in each well equaled the sum of the radioactive counts per minute of the aspirated medium plus the radioactive counts of the corresponding detached wells. After the data were corrected for variations in the amount of tracer incorporated in each well, the percentage of specific endothelial cell release of 51Cr was calculated by the following formula: [(experimental release × 2) − (spontaneous release × 2)]/[total incorporation − (spontaneous release × 2)]. Each experimental condition was tested in triplicate with endothelial cells collected from different umbilical cords in 3 separate experiments.
Murine model
For in vivo infection, male BALB/c mice (≥20 g) were rendered diabetic by a single intraperitoneal injection of 210 mg/kg streptozotocin in 0.2 mL of citrate buffer 10 days before fungal challenge [19]. Glycosuria and ketonuria were confirmed in all mice 7 days after streptozotocin treatment. Diabetic ketoacidotic mice were infected with fungal spores by tail vein injection, with a target inoculum of 1 × 104 spores. To confirm the inoculum, dilutions were streaked on PDA plates containing 0.1% Triton X-100, and colonies were counted after a 24-h incubation period at 37°C. The primary efficacy end point was time to death.
To determine whether antibiotic treatment would alter the virulence of mucormycosis, diabetic ketoacidotic mice infected with Rhizopus species ATCC 20577 (reported to harbor Burkholderia [13]) were treated with intravenous liposomal amphotericin B (LAmB) at 5 mg/kg/day in 5% dextrose water (the LAmB dose was chosen on the basis of previously established low efficacy as monotherapy [20], thereby enabling statistical detection of the potentially enhanced efficacy of combination therapy), ciprofloxacin (administered by oral gavage at 80 mg/kg twice a day in 5% dextrose water), or a combination of both drugs. Placebo mice received 5% dextrose water. Treatment began 24 h after infection and continued for 4 consecutive days.
All procedures involving mice were approved by the Institutional Animal Use and Care Committee at the Los Angeles Biomedical Research Institute at the Harbor-UCLA Medical Center, following the National Institutes of Health guidelines for animal housing and care.
Fly model
Wild-type (OregonR) fruit flies were infected with fungal spores by the method of Lemaitre et al. [21]. Briefly, fruit flies were injected with a thin sterile needle previously dipped in a concentrated solution of 5 × 107 spores of each Zygomycetes isolate. Flies that died within 3 h of infection ( <5%) as a result of complications from the injection procedure were excluded from the survival analysis. After infection, flies were housed at 29°C and transferred daily into fresh vials. For ciprofloxacin treatment, specific vials were prepared by mixing a standard concentration (2 mg/mL) of ciprofloxacin with regular fly food, as described elsewhere [22]. In pilot experiments, ciprofloxacin was found to be nontoxic to the flies (data not shown). Flies were housed in empty vials for 6–8 h to starve them and then transferred into vials containing ciprofloxacin mixed fly food (2 mg/mL). After 24 h, the flies were infected with each Zygomycetes isolate as described above and were transferred daily into fresh ciprofloxacin-containing vials for 8 days at 29°C. Survival was assessed daily until day 8 after infection in all experiments. Each experiment was performed at least in triplicate, using 25 female flies that were 2–4 days old.
Statistical analysis
Endothelial cell injury was compared using the nonparametric Mann-Whitney U test. Kaplan-Meier curves were compared pairwise by the log-rank test. Differences for which P ≤ .05 were considered significant.
RESULTS
Wide association of bacterial endosymbionts with clinical isolates of Zygomycetes
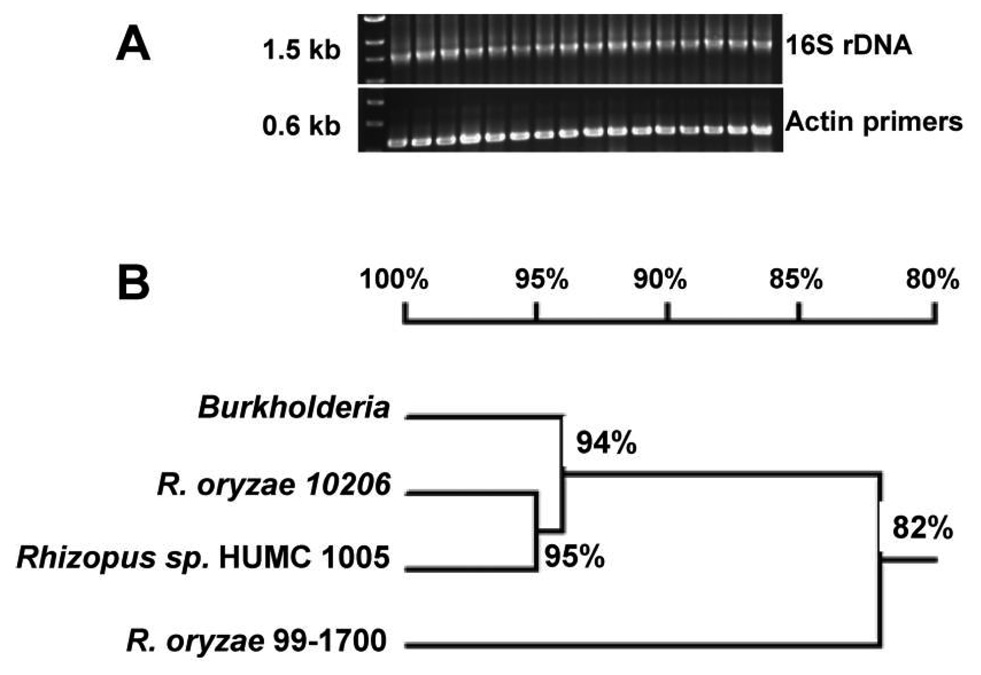
We screened 28 clinical isolates of Zygomycetes as well as the 2 American Type Culture Collection Rhizopus species previously reported to harbor Burkholderia (positive control) [13] for the presence of bacterial endosymbionts by use of primers specific for 16S rDNA. The expected 1.5-kb band representing the 16S rDNA fragment (figure 1A) was amplified from 15 isolates (54% of the total number of isolates). DNA sequencing of 3 representative PCR amplification products from R. oryzae 99–1700, R. oryzae type I NRRL 10206, and Rhizopus species HUMC 1005 demonstrated 87%, 90%, and 93% homology, respectively, to the published Burkholderia 16S rDNA sequence (figure 1B).
Figure 1.
Wide presence of bacterial symbiosis in Zygomycetes. A, Polymerase chain reaction of genomic DNA extracted from Zygomycetes, demonstrating the presence of bacterial endosymbionts. B, Dendrogram showing the close homology of 16S bacterial rDNA extracted from multiple Rhizopus species with 16S rDNA of Burkholderia.
Harboring of rhizoxin-producing Burkholderia by R. oryzae
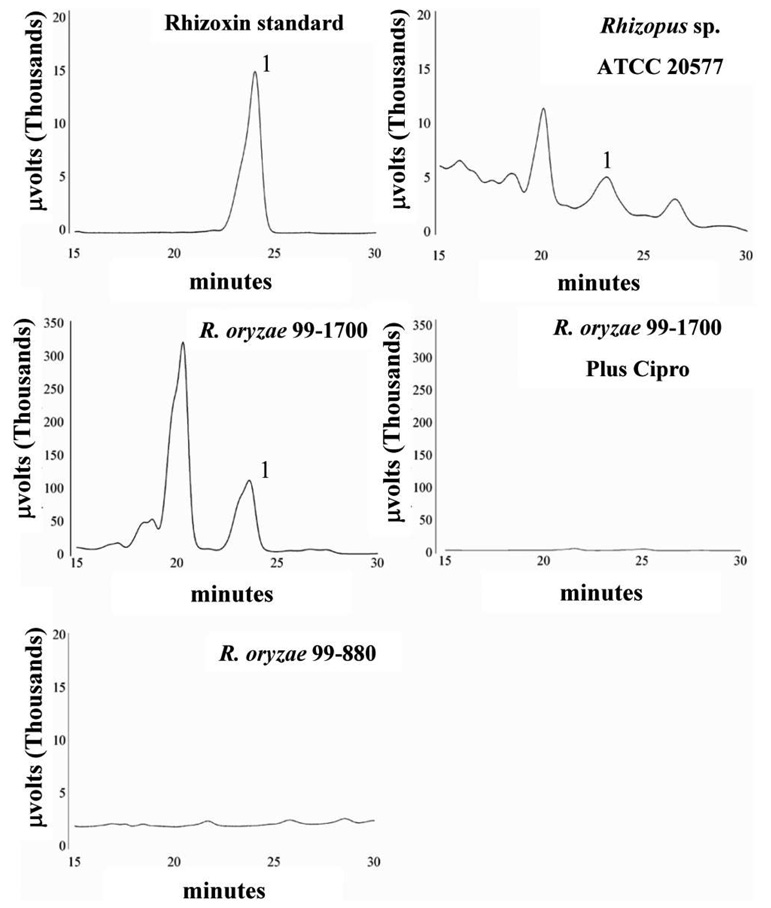
R. oryzae strains harboring Burkholderia were screened by PCR for the presence of the KS gene involved in the synthesis of rhizoxin. Five of the 15 isolates that were positive for a 16S rDNA band amplified a KS-specific band, including 4 R. oryzae strains and 1 unspeciated Rhizopus strain. Furthermore, to confirm that these R. oryzae strains harbored rhizoxin-producing Burkholderia, we grew these fungi in rhizoxin-inducing medium and analyzed mycelial extracts using HPLC. Mycelial extracts of all R. oryzae strains from which a KS band was amplified contained rhizoxin (figure 2). Indeed, among all samples tested, R. oryzae 99–1700 had the highest concentration of rhizoxin, with almost a 30-fold increase compared with the positive control strain, Rhizopus species ATCC 20577 (table 1). As expected, HPLC analysis of mycelial extracts of R. oryzae 99–880 (a strain that amplified the 16S rDNA but not the KS band) did not demonstrate the presence of rhizoxin (figure 2 and table 1). Furthermore, addition of ciprofloxacin to the medium abrogated or significantly reduced rhizoxin production by all fungi, indicating that the toxin was produced by the bacterial symbiont (figure 2 and table 1). In addition to rhizoxin, the bacterial symbiont also produced putative rhizoxin derivatives (e.g., 2,3-deoxyrhizoxin, seco-2,3-deoxyrhizoxin, and nor-2,3-deoxyrhizoxin) that have been previously isolated from Rhizopus organisms [23].
Figure 2.
High-performance liquid chromatography profiles of representative culture extracts monitored for the detection of rhizoxin. The rhizoxin peak is marked with 1. Other peaks likely represent putative rhizoxin derivatives [13]. “Plus cipro” denotes treatment of the culture with 60 µg/mL ciprofloxacin to eliminate the symbiotic bacteria.
Table 1.
Concentrations of rhizoxin extracted from Rhizopus mycelia growing in rhizoxin-inducing medium.
| Rhizoxin concentration, µg/mL | ||
|---|---|---|
| Sample | Without ciprofloxacin |
With ciprofloxacina |
| R. oryzae 99–1700 | 73.3 | 1.7 |
| R. oryzae type I NRRL 10206 | 6.7 | ND |
| R. oryzae 99–880 | ND | ND |
| Rhizopus species HUMC 1005 | 4.0 | ND |
| Rhizopus species ATCC 20577 | 2.5 | 0.4 |
NOTE. Rhizoxin concentration was measured by high-performance liquid chromatography analysis. The lower limit of detection of this assay is 0.2 µg/mL rhizoxin. ND, none detected.
Ciprofloxacin (60 µg/mL) was included in the culture supernatant to eliminate bacterial symbionts.
No contribution of bacterial endosymbiosis to endothelial cell injury induced by Zygomycetes
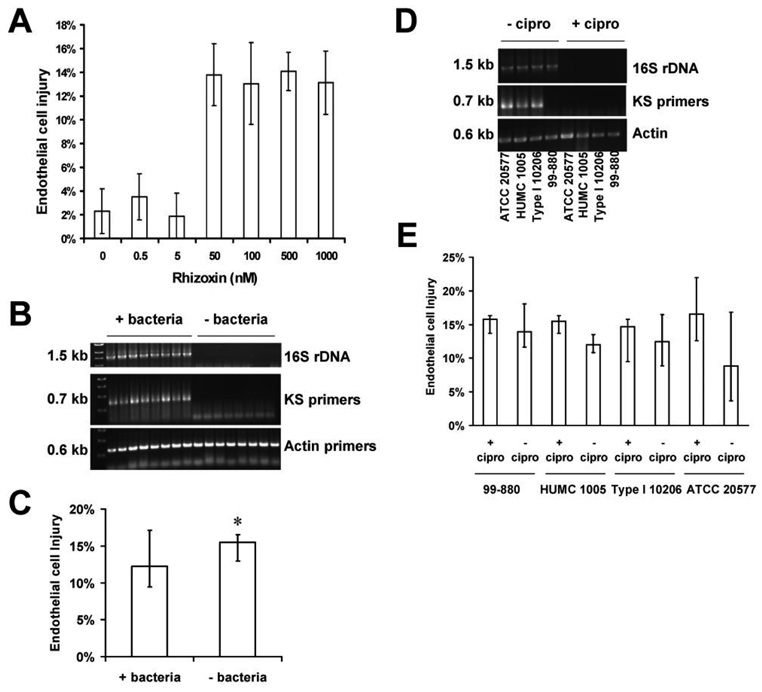
We confirmed that pure rhizoxin toxin at concentrations ≥50 nmol/L is capable of inducing endothelial cell injury (figure 3A). However, we could not detect any difference in endothelial cell injury caused by clinical isolates of Zygomycetes that harbored bacteria versus those that did not (median damage, 12.2% for Zygomycetes that harbored bacteria vs. 15.5% for bacteria-free isolates; P > .05) (figure 3B and 3C). Furthermore, eradication of bacteria by means of ciprofloxacin, confirmed by the lack of 16S rDNA and KS bands after antibiotic exposure (figure 3D), had no effect on the ability of selected Rhizopus isolates to cause endothelial cell injury (figure 3E). Of note, ciprofloxacin treatment had no effect on the growth of the isolates as determined by radial growth rate on PDA (data not shown). These data suggest that endosymbiotic bacteria do not contribute to the pathogenesis of mucormycosis in vitro.
Figure 3.
Results of endothelial cell injury experiments. Although rhizoxin causes damage to endothelial cells, endothelial cell injury caused by agents of mucormycosis was neither affected by the presence of bacterial endosymbionts nor altered by bacterial eradication with ciprofloxacin treatment. A, Demonstration that pure rhizoxin toxin at concentrations ≥50 nmol/L is capable of inducing endothelial cell injury. Purified rhizoxin (Sigma) was incubated with endothelial cells for 6 h, and injury to endothelial cells was measured using our 51Cr-release assay on a 96-well plate [18]. B, Polymerase chain reaction of genomic DNA extracted from Zygomycetes, demonstrating the presence of rhizoxin-producing bacterial endosymbionts in some Rhizopus isolates but not others. C, Pooled results for endothelial cell injury caused by Rhizopus that harbor bacteria (+ bacteria) and bacteria-free Rhizopus (n = 8 in each arm). D, Eradication of bacteria by means of ciprofloxacin. Bacteria-harboring Rhizopus strains were rendered free of bacteria by treating the fungus with ciprofloxacin at 60 µg/mL, and the absence of bacteria was verified by a lack of amplificatio of 1.6 kb of 16S rDNA. E, No effect of ciprofloxacin treatment on the ability of selected Rhizopus isolates to cause endothelial cell injury. The bacteria-free organisms generated in panel D were compared with their corresponding parent strains that harbored bacteria with respect to their ability to cause endothelial cell injury (n = 8). Data are displayed as medians plus interquartile ranges. *P > .05, compared with Rhizopus-harboring bacteria.
No contribution of bacterial endosymbiosis to mucormycosis pathogenesis in vivo
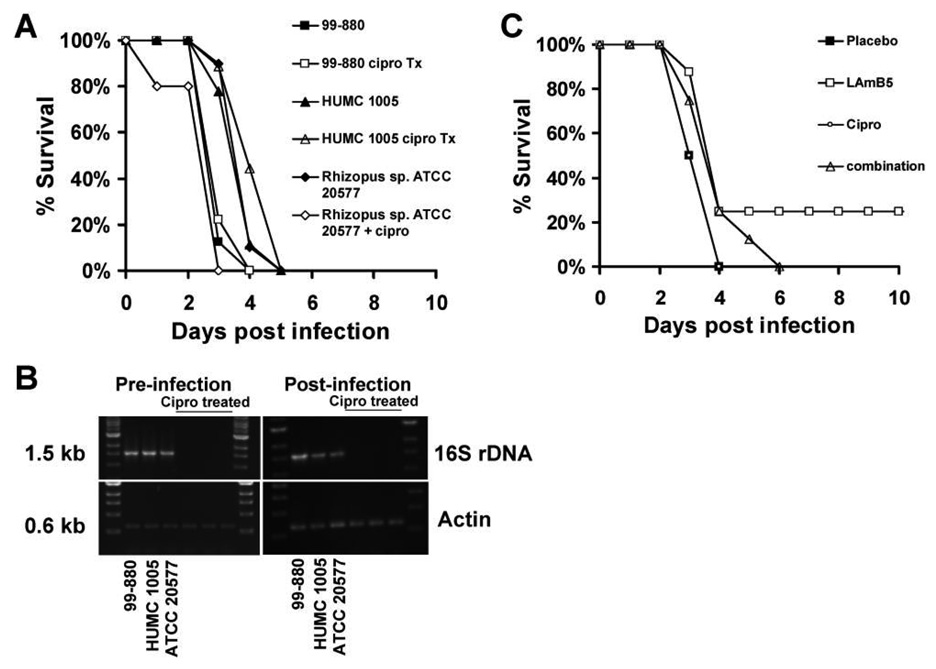
To investigate the contribution of bacterial symbiosis to the pathogenesis of mucormycosis in vivo, we used our established diabetic ketoacidotic mouse model. The virulence of fungal strains positive for 16S rDNA, including 2 rhizoxin producers (R. oryzae HUMC 1005 and Rhizopus species ATCC 20577), was compared to the virulence of their corresponding bacteria-free isolates after ciprofloxacin treatment. There was no difference in the survival of mice infected with bacteria-free fungi or their corresponding parent strains (figure 4A). This lack of difference in virulence cannot be attributed to the regrowth of the bacteria in vivo in the mouse, because PCR of fungal colonies recovered from killed animals confirmed the lack of bacterial endosymbionts in ciprofloxacin-treated strains (figure 4B).
Figure 4.
No effect of the eradication of bacteria from Rhizopus by ciprofloxacin treatment on fungal virulence in diabetic ketoacidotic mice. A, Survival of mice (n = 8 per group) infected with 1 of 3 bacteria-free Rhizopus strains or their corresponding parent strains at 1 × 104 spores per mouse. B, Polymerase chain reaction demonstrating the presence of bacteria in non–ciprofloxacin-treated Rhizopus and the absence of bacteria in ciprofloxacin-treated Rhizopus, both before infection and after fungal strains were retrieved from expired mice. C, Survival of mice (n = 8 per group) infected with 1 × 104 spores of Rhizopus species ATCC 20577 and treated with liposomal amphotericin B (LAmB) at 5 mg/kg/day, ciprofloxacin at 80 mg/kg twice a day, or a combination of both drugs. Treatment began 24 h after infection and continued for 4 consecutive days.
Additionally, we infected diabetic ketoacidotic mice with Rhizopus species ATCC 20577 harboring Burkholderia [13, 14] and treated with LAmB, ciprofloxacin, or a combination of both drugs. Ciprofloxacin treatment did not enhance the survival of mice infected with R. microsporus (figure 4C).
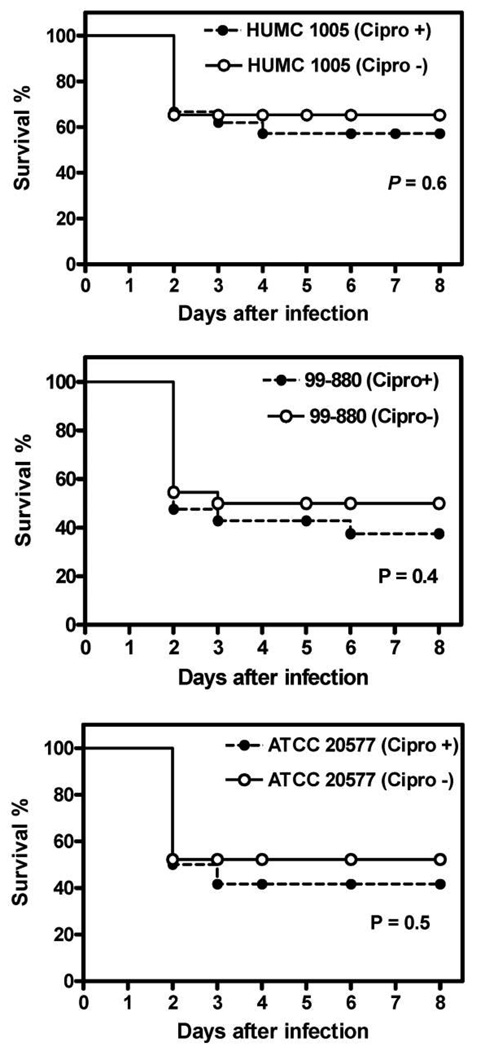
Finally, we compared the virulence of bacteria-free Zygomycetes to their corresponding parent strains in a fly model of mucormycosis. Similar to our mouse data, there was no difference in survival time of flies infected with 3 independent bacteria-free fungal strains versus those infected with the corresponding parent strains (figure 5). Furthermore, ciprofloxacin did not affect the virulence of Rhizopus in flies.
Figure 5.
No effect of the eradication of bacteria from Rhizopus by ciprofloxacin treatment on fungal virulence in a fly model of infection. Shown is the survival of Drosophila flies infected with 1 of 3 bacteria-free Rhizopus strains (as proved by lack of amplification of rDNA) or their corresponding parent strains. Each experiment was conducted at least in triplicate, using 25 female flies.
DISCUSSION
A hallmark of mucormycosis infections is the virtually uniform presence of extensive angioinvasion with subsequent vessel thrombosis and extensive tissue necrosis. It has been hypothesized that the extensive tissue necrosis is related to the presence of toxin(s) in Zygomycetes, such as rhizoxins and rhizonin A and B [11]. Indeed, plant-pathogenic fungi have been reported to produce rhizoxins [11, 12], and rhizoxin has been shown to inhibit the proliferation of cultured human endothelial cells [24]. R. oryzae, which is responsible for the majority of clinical cases of mucormycosis, has not previously been reported to produce rhizoxins. However, recent reports of rhizoxin production by endosymbiotic Burkholderia within R. microsporus [13, 14], which is also a human pathogen, have suggested that rhizoxin production might have clinical relevance.
Our results demonstrate that association with bacterial endosymbionts is widespread among clinical isolates of Zygomycetes. Although we did not attempt to distinguish between internalized bacteria and fungal-associated bacteria, previous reports have confirmed that rhizoxin-producing Burkholderia are contained within the hyphae of Rhizopus species [13]. Furthermore, we found that rhizoxin-producing Burkholderia strains are frequently detected in clinical isolates of R. oryzae and other Rhizopus species. Nevertheless, we found no evidence that endosymbiotic bacteria and rhizoxin contribute to the pathogenesis of mucormycosis in the models studied, because (1) in vitro endothelial cell injury did not differ between ciprofloxacin-induced, bacteria-free Rhizopus species and their corresponding bacteria-harboring parent strains; (2) bacteria-free Rhizopus species demonstrated similar virulence to their corresponding bacteria-harboring parent strains in a diabetic ketoacidotic mouse model, nor did ciprofloxacin treatment have any effect on murine mucormycosis caused by Rhizopus species ATCC 20577, a positive control strain known to harbor endosymbionts that produce rhizoxin [13]; and (3) bacteria-free Rhizopus species demonstrated similar virulence to their corresponding bacteria-harboring parent strains in a fly model of mucormycosis.
We cannot rule out an effect of rhizoxin in a sinonasal model of infection. Furthermore, our results do not preclude the possibility that the tissue necrosis seen in patients with mucormycosis is caused by a toxin associated with the fungus. Indeed, in a previous study we reported that clinical isolates of R. oryzae could injure human umbilical vein endothelial cells in vitro irrespective of their viability and that the damage was caused by a cell-surface substance [25].
Rhizoxin exerts its effect by binding to β-tubulin, which results in inhibition of mitosis and causes cell cycle arrest [11, 14]. In addition to being responsible for the abnormal swelling of rice seedling roots in rice seedling blight disease [13], rhizoxin has been found to arrest mitosis in many other eukaryotic cells, including solid and hematologic tumors [26, 27]. Because of its potent antimitotic effect, rhizoxin has undergone extensive clinical trails as a potential antitumor drug candidate [28, 29]. In the present study, we found that one of the R. oryzae clinical isolates that harbors Burkholderia, strain 99–1700, had an almost 30-fold increase in rhizoxin production compared with the control strain, Rhizopus species ATCC 20577, which was originally used for isolating the rhizoxin-producing Burkholderia [13]. Additionally, the recent finding that production of rhizoxin from a pure culture of an endosymbiotic Burkholderia rhizoxina was elevated by >10-fold [30] raises the possibility of further increasing the production of rhizoxin from the bacterium endosymbiotically associated with strain 99–1700. This enhanced secretion of rhizoxin could be used for the sustainable production of rhizoxin for commercial purposes.
In summary, we have demonstrated that R. oryzae clinical isolates harbor endosymbiotic Burkholderia, which produce the toxin rhizoxin. We have also found no evidence that bacterial endosymbiosis and, in particular, rhizoxin secretion contribute to the pathogenesis of mucormycosis in the models studied.
Acknowledgments
Financial support: This work was supported by the Public Health Service (grants R01 AI063503 and R21 AI064716 to A.S.I). B.J.S. is supported by the Public Health Service (grant K08 AI060641), an American Heart Beginning Grant-in-Aid (0665154Y), and a Liu Young Investigator in Biomedical Research Award. K.J.K.-C. is supported by funds from the intramural program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Research described in this article was conducted in part at the research facilities of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
Footnotes
Potential conflicts of interest: none reported.
Presented in part: 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, 17-20 September 2007 (abstract B-1443).
References
- 1.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301. doi: 10.1128/cmr.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon-Chung KJ, Bennett JE. Medical mycology. Philadelphia: Lea & Febiger; 1992. Mucormycosis; pp. 524–559. [Google Scholar]
- 3.Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47:364–371. doi: 10.1086/589857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugar AM. Agent of mucormycosis and related species. In: Mandell G, Bennett J, Dolin R, editors. Principles and practices of infectious diseases. 4th ed. New York: Churchill Livingstone; 1995. pp. 2311–2321. [Google Scholar]
- 5.Ibrahim AS, Edwards JEJ, Filler SG. Zygomycosis. In: Dismukes WE, Pappas PG, Sobel JD, editors. Clinical mycology. New York: Oxford University Press; 2003. pp. 41–251. [Google Scholar]
- 6.Husain S, Alexander BD, Munoz P, et al. Opportunistic mycelial fungal infections in organ transplant recipients: emerging importance of non-Aspergillus mycelial fungi. Clin Infect Dis. 2003;37:221–229. doi: 10.1086/375822. [DOI] [PubMed] [Google Scholar]
- 7.Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 8.Kontoyiannis DP, Wessel VC, Bodey GP, Rolston KV. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis. 2000;30:851–856. doi: 10.1086/313803. [DOI] [PubMed] [Google Scholar]
- 9.Gleissner B, Schilling A, Anagnostopolous I, Siehl I, Thiel E. Improved outcome of zygomycosis in patients with hematological diseases? Leuk Lymphoma. 2004;45:1351–1360. doi: 10.1080/10428190310001653691. [DOI] [PubMed] [Google Scholar]
- 10.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 11.Jennessen J, Nielsen KF, Houbraken J, et al. Secondary metabolite and mycotoxin production by the Rhizopus microsporus group. J Agric Food Chem. 2005;53:1833–1840. doi: 10.1021/jf048147n. [DOI] [PubMed] [Google Scholar]
- 12.White JD, Blakemore PR, Green NJ, et al. Total synthesis of rhizoxin D, a potent antimitotic agent from the fungus Rhizopus chinensis. J Org Chem. 2002;67:7750–7760. doi: 10.1021/jo020537q. [DOI] [PubMed] [Google Scholar]
- 13.Partida-Martinez LP, Hertweck C. Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature. 2005;437:884–888. doi: 10.1038/nature03997. [DOI] [PubMed] [Google Scholar]
- 14.Partida-Martinez LP, de Looss CF, Ishida K, et al. Rhizonin, the first mycotoxin isolated from the zygomycota, is not a fungal metabolite but is produced by bacterial endosymbionts. Appl Environ Microbiol. 2007;73:793–797. doi: 10.1128/AEM.01784-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He J, Hertweck C. Iteration as programmed event during polyketide assembly: molecular analysis of the aureothin biosynthesis gene cluster. Chem Biol. 2003;10:1225–1232. doi: 10.1016/j.chembiol.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Graham MA, Bissett D, Setanoians A, et al. Preclinical and phase I studies with rhizoxin to apply a pharmacokinetically guided dose-escalation scheme. J Natl Cancer Inst. 1992;84:494–500. doi: 10.1093/jnci/84.7.494. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins: identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toenjes KA, Munsee SM, Ibrahim AS, Jeffrey R, Edwards JE, Jr, Johnson DI. Small-molecule inhibitors of the budded-to-hyphal-form transition in the pathogenic yeast Candida albicans. Antimicrob Agents Chemother. 2005;49:963–972. doi: 10.1128/AAC.49.3.963-972.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibrahim AS, Avanessian V, Spellberg B, Edwards JE., Jr Liposomal amphotericin B, and not amphotericin B deoxycholate, improves survival of diabetic mice infected with Rhizopus oryzae. Antimicrob Agents Chemother. 2003;47:3343–3344. doi: 10.1128/AAC.47.10.3343-3344.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim AS, Gebremariam T, Fu Y, Edwards JE, Jr, Spellberg B. Combination echinocandin-polyene treatment of murine mucormycosis. Antimicrob Agents Chemother. 2008;52:1556–1558. doi: 10.1128/AAC.01458-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemaitre B B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 22.Chamilos G, Lionakis MS, Lewis RE, et al. Drosophila melanogaster as a facile model for large-scale studies of virulence mechanisms and antifungal drug efficacy in Candida species. J Infect Dis. 2006;193:1014–1022. doi: 10.1086/500950. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki S, Namikoshi M, Kobayashi H, et al. Studies on macrocyclic lactone antibiotics. VIII. Absolute structures of rhizoxin and a related compound. J Antibiot (Tokyo) 1986;39:424–429. doi: 10.7164/antibiotics.39.424. [DOI] [PubMed] [Google Scholar]
- 24.Aoki K, Watanabe K, Sato M, Ikekita M, Hakamatsuka T, Oikawa T. Effects of rhizoxin, a microbial angiogenesis inhibitor, on angiogenic endothelial cell functions. Eur J Pharmacol. 2003;459:131–138. doi: 10.1016/s0014-2999(02)02853-4. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim AS, Spellberg B, Avanessian V, Fu Y, Edwards JE., Jr Rhizopus oryzae adheres to, is phagocytosed by, and damages endothelial cells in vitro. Infect Immun. 2005;73:778–783. doi: 10.1128/IAI.73.2.778-783.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuruo T, Oh-hara T, Iida H, et al. Rhizoxin, a macrocyclic lactone antibiotic, as a new antitumor agent against human and murine tumor cells and their vincristine-resistant sublines. Cancer Res. 1986;46:381–385. [PubMed] [Google Scholar]
- 27.Takahashi M, Iwasaki S, Kobayashi H, et al. Studies on macrocyclic lactone antibiotics. XI. Anti-mitotic and anti-tubulin activity of new antitumor antibiotics, rhizoxin and its homologues. J Antibiot (Tokyo) 1987;40:66–72. doi: 10.7164/antibiotics.40.66. [DOI] [PubMed] [Google Scholar]
- 28.Hanauske AR, Catimel G, Aamdal S, et al. The EORTC Early Clinical Trials Group. Phase II clinical trials with rhizoxin in breast cancer and melanoma. Br J Cancer. 1996;73:397–399. doi: 10.1038/bjc.1996.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLeod HL, Murray LS, Wanders J, et al. Multicentre phase II pharmacological evaluation of rhizoxin: eortc early clinical studies (ECSG)/pharmacology and molecular mechanisms (PAMM) groups. Br J Cancer. 1996;74:1944–1948. doi: 10.1038/bjc.1996.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scherlach K, Partida-Martinez LP, Dahse HM, Hertweck C. Antimitotic rhizoxin derivatives from a cultured bacterial endosymbiont of the rice pathogenic fungus Rhizopus microsporus. J Am Chem Soc. 2006;128:11529–11536. doi: 10.1021/ja062953o. [DOI] [PubMed] [Google Scholar]