Abstract
Although protein kinase C (PKC) plays a key role in ischemic preconditioning (IPC), the actual mechanism of that protection is unknown. We recently found that protection from IPC requires activation of adenosine receptors during early reperfusion. We, therefore, hypothesized PKC might act to increase the heart’s sensitivity to adenosine. IPC limited infarct size in isolated rabbit hearts subjected to 30-min regional ischemia/2-h reperfusion and IPC’s protection was blocked by the PKC inhibitor chelerythrine given during early reperfusion revealing involvement of PKC at reperfusion. Similarly chelerythrine infused in the early reperfusion period blocked the increased phosphorylation of the protective kinases Akt and ERK1/2 observed after IPC. Infusing phorbol 12-myristate 13-acetate (PMA), a PKC activator, during early reperfusion mimicked IPC’s protection. As expected, the protection triggered by PMA at reperfusion was blocked by chelerythrine, but surprisingly it was also blocked by MRS1754, an adenosine A2b receptor–selective antagonist, suggesting that PKC was somehow facilitating signaling from the A2b receptors. NECA [5′-(N-ethylcarboxamido) adenosine], a potent but not selective A2b receptor agonist, increased phosphorylation of Akt and ERK1/2 in a dose-dependent manner. Pretreating hearts with PMA or brief preconditioning ischemia had no effect on phosphorylation of Akt or ERK1/2 per se, but markedly lowered the threshold for NECA to induce their phosphorylation. BAY 60-6583, a highly selective A2b agonist, also caused phosphorylation of ERK 1/2 and Akt. MRS1754 prevented phosphorylation induced by BAY 60-6583. BAY 60-6583 limited infarct size when given to ischemic hearts at reperfusion. These results suggest that activation of cardiac A2b receptors at reperfusion is protective, but because of the very low affinity of the receptors endogenous cardiac adenosine is unable to trigger their signaling. We propose that the key protective event in IPC occurs when PKC increases the heart’s sensitivity to adenosine so that endogenous adenosine can activate A2b-dependent signaling.
Keywords: adenosine A2b receptors, BAY 60-6583, NECA, preconditioning, protein kinase C
In ischemic preconditioning (IPC) brief periods of sublethal ischemia/reperfusion cause the heart to become resistant to infarction during a subsequent episode of ischemia/reperfusion. Signaling in IPC begins with activation of several Gi-coupled receptors and their signals are believed to converge on protein kinase C (PKC), which plays a key role in IPC’s protection. Although the importance of PKC in IPC has been well established, it is unknown how it confers that protection.
Hausenloy et al. [1] noted protection from IPC is exerted early in reperfusion following the lethal ischemic insult and requires activation of phosphatidylinositol 3-OH kinase (PI3-K) and extracellular signal-regulated protein kinase (ERK) at that time. Postconditioning with multiple brief reperfusion/ischemia cycles immediately following the ischemic insult protects the heart and, like IPC, also requires activation of PI3-K and ERK during reperfusion to elicit protection [2,3]. Accumulated evidence implicates both PKC and adenosine receptors in that kinase activation. Binding of endogenous adenosine to its receptors in early reperfusion is a requirement for both IPC [4] and postconditioning [2,5] to limit infarction. Our findings using selective adenosine receptor antagonists suggested that the receptor involved might be A2b. We recently found that in situ rabbit hearts can be protected by a left atrial infusion of the PKC activator phorbol 12-myristate 13-acetate (PMA) in the first minutes of reperfusion and that this protection was blocked by MRS 1754, an adenosine A2b receptor antagonist [2]. Furthermore PKC appeared to be upstream of the adenosine receptors since protection from the adenosine agonist 5′-(N-ethylcarboxamido)adenosine (NECA) given at reperfusion was not affected by the PKC blocker chelerythrine [2]. These two observations suggested PKC activation at reperfusion alone was sufficient to produce the protected phenotype. Because high concentration of an adenosine agonist at reperfusion can mimic IPC, it would appear that PKC somehow acted to augment the heart’s adenosine receptor signaling. This is the reverse of the typical situation in which occupancy of receptors leads to activation of PKC.
If PKC is located upstream of adenosine receptors, how can it modulate their response? One possibility is that PKC activity increases adenosine release in preconditioned myocardium. Kitakaze and colleagues [6] reported PKC increases cardiac 5′-nucleotidase activity which generates adenosine by dephosphorylation of AMP. However, direct adenosine measurements by Schulz et al. [7] indicated the adenosine level in ischemically preconditioned pig myocardium following a lethal ischemic insult was actually lower than that in non-preconditioned hearts. Adenosine levels were also lower in ischemically preconditioned rabbit [8] and rat [9] hearts during ischemia. An attractive alternative hypothesis is that PKC might increase the heart’s sensitivity to adenosine, either at the receptor or somewhere along its signal transduction pathway. That would be a particularly attractive hypothesis if signaling from A2b receptors is responsible since they normally have a very low affinity and might not be occupied with endogenous adenosine levels even during ischemia.
The present study investigated the hypothesis that PKC protects by increasing the heart’s sensitivity to adenosine. We first tested whether direct activation of PKC at reperfusion could limit infarction in isolated rabbit hearts and whether that involved adenosine receptors. We next examined the effect of PKC activation on the heart’s sensitivity to adenosine by measuring phosphorylation of PI3-K’s downstream target, Akt, and of ERK1/2 in response to an adenosine agonist. We chose the A2b-potent, but non-selective, agonist NECA because it has been shown to be very protective when given at reperfusion and, unlike adenosine, it is not rapidly metabolized in the tissue. We also tested a new, highly selective A2b receptor agonist, BAY 60-6583.
METHODS
Infarct size study in isolated rabbit hearts
Surgical preparation
Briefly, New Zealand White rabbits were anesthetized with pentobarbital sodium (30 mg/kg i.v.) and ventilated with 100% oxygen [10]. A suture was passed around a coronary arterial branch. The excised heart was perfused on a Langendorff apparatus with Krebs-Henseleit bicarbonate buffer bubbled with 95% O2/5% CO2 to a pH of 7.35–7.45 at 38°C. A fluid-filled latex balloon measured pressure in the left ventricle.
Experimental protocol
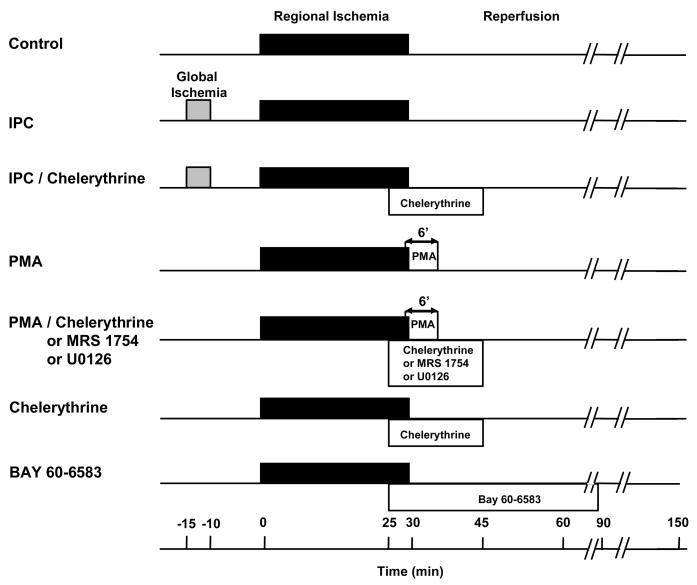
Hearts of 9 experimental groups underwent 30-min coronary branch occlusion/2-h reperfusion (Fig 1). Control hearts had no other intervention. IPC hearts were preconditioned with 5-min global ischemia/10-min reperfusion. In group 3 IPC hearts received chelerythrine (2.8 μM) for 20 min starting 5 min before reperfusion. The fourth group received PMA (0.05 nM) from 1 min before to 5 min after reperfusion. In groups 5–7 hearts were co-treated with PMA and either chelerythrine (2.8 μM), the adenosine A2b-selective antagonist MRS 1754 (20 nM), or the MEK1/2 and, therefore, ERK1/2 inhibitor U0126 (0.5 μM) for 20 min starting 5 min before reperfusion. The eighth group received only chelerythrine for 20 min starting 5 min before reperfusion. The ninth group received an infusion of 300nM BAY 60-6583, a highly selective A2b adenosine agonist, for 60 min starting 5 min before reperfusion.
Figure 1.
Infarct protocols. Abbreviations: IPC = ischemic preconditioning, PMA = phorbol 12-myristate 13-acetate
Measurement of risk zone and infarct size
At the end of experiments the coronary artery was reoccluded, and fluorescent microspheres were infused to demarcate the non-fluorescent risk zone. The heart was cut into 2-mm-thick slices which were incubated in 1% triphenyltetrazolium chloride to stain noninfarcted tissue. Areas of infarct and risk zone were determined by planimetry of each slice and volumes calculated. Infarction was expressed as a percentage of the region at risk.
Biochemical Studies
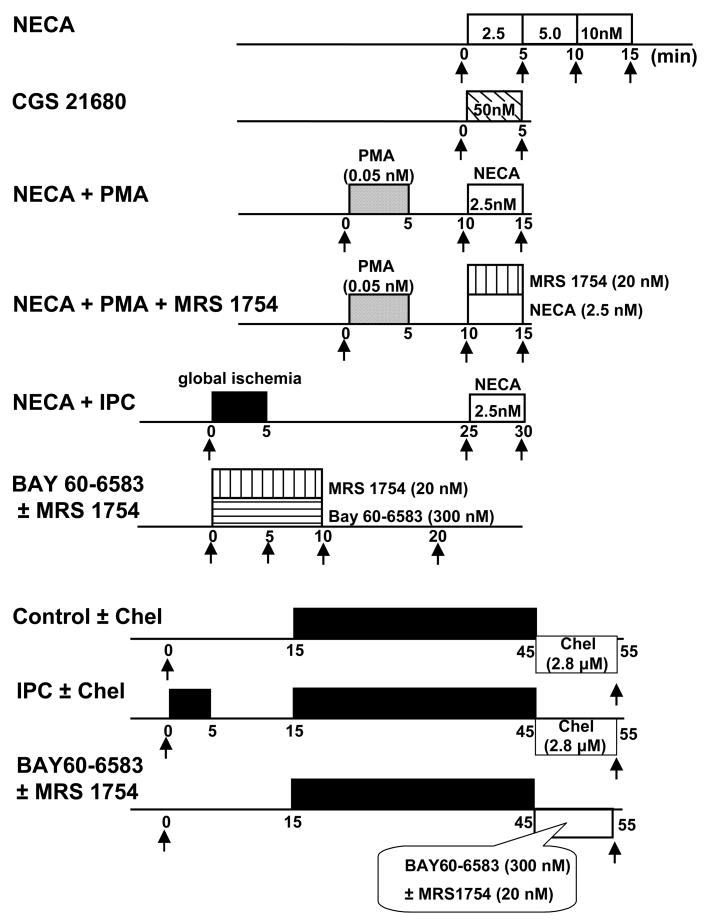
Phospho-Akt and phospho-ERK1/2 were measured in thirteen additional groups of isolated rabbit hearts. Transmural left ventricular biopsies weighing ~25 mg were obtained serially at times indicated by arrows in Fig. 2 and frozen in liquid nitrogen within 1 s of excision. In the first group, hearts were treated for 5 min apiece with stepwise increases in NECA concentration to produce a dose response curve. In the second group, the selective adenosine A2a agonist CGS 21680 (50nM) was infused for 5 min. In the third group, PMA (0.05 nM) was administered for 5 min followed by 5 min of washout before treatment for 5 min with a subthreshold dose of NECA as determined in group 1 (2.5nM). In group 4, PMA and NECA were again infused and MRS 1754 (20 nM) was added concomitantly with NECA. In the fifth group, hearts were preconditioned with 5-min global ischemia/20-min reperfusion before treatment with NECA. In two additional groups biopsies were obtained from hearts at baseline before any intervention, after 5 and 10 min of treatment with 300nM BAY 60-6583 in the presence or absence of MRS 1754, and again after 10 min of washout. Biopsies were also obtained from non-preconditioned and IPC hearts exposed to global ischemia, some of which were treated with chelerythrine started at reperfusion and continued for 10 min. Finally hearts exposed to 30 min of global ischemia were treated with BAY 60-6583 for 10 min following reperfusion. In some of these hearts MRS 1754 was co-infused with the adenosine agonist. As indicated in Fig. 2 the left ventricle was biopsied just before the preconditioning ischemia or at a comparable time in non-preconditioned hearts and again 10 min after the end of the 30-min period of ischemia.
Figure 2.
Biochemical protocols. Arrows indicate times of biopsies of left ventricle. Abbreviations: see Fig. 1, NECA = 5′-(N-ethylcarboxamido)adenosine, Chel = chelerythrine
Myocardium was homogenized with a Polytron in Cell Lysis Buffer supplemented with PMSF and centrifuged for 15 min at 13,000 g. Samples were diluted in Laemmli sample buffer and 20 μg of total protein were added per lane on 10% SDS-polyacrylamide gel. After electrophoresis proteins were transferred to a nitrocellulose membrane which was probed with monoclonal antibodies for phospho-Akt (Ser473) and phospho-ERK1/2 (Thr202/Thr204).
Adenosine A1, A2a, and A2b assay
CHO cells recombinantly expressing human A1, A2a or A2b receptors and a CRE-luciferase construct as read-out system for intracellular cAMP modulation were grown in 384-well plates for 2 days at 37°C in an atmosphere enriched with 5% CO2. Cells were incubated for 4 h at 37°C with increasing concentrations of BAY 60-6583 and additionally with 1 μM forskolin in cells expressing A1 receptors. Cells were lysed with Triton X100 buffer and luciferin was added. Relative light units (RLU) were measured in a CCD camera system over 30 sec.
Adenosine A3-Gα16 Assay
The assay is performed with a permanently transfected CHO luc9AQ adenosine A3-Gα16 pcDNA3 cell line in DMEM-F12/10% FCS incubated at 37° C in air enriched with 5% CO2. On the day of testing the medium was discarded and replaced by CAFTY/2 mM Ca2+/11 μM coelenterazine. After 3 h of incubation cells were used for bioluminescent measurement of intracellular Ca2+ release caused by A3 receptor stimulation by the specific A3 agonist IB-MECA. Compounds were tested for agonistic effects after 10 minutes of preincubation by the addition of an EC50 concentration of IB-MECA (~ 300 nM) followed by addition of 15 μM ATP. Agonists should decrease the effects of both IB-MECA and ATP.
Statistics
All data are expressed as mean±SEM. One-way analysis of variance (ANOVA) with Student-Newman-Keuls post hoc test was performed on baseline hemodynamics, infarct size, and western blot band density. Temporal differences in hemodynamic variables in any given group, the dose-response of NECA-induced phosphorylation of Akt, and phosphorylation response of Akt and ERK to BAY 60-6583 were analyzed with one-way repeated measures ANOVA with Tukey post hoc testing. P<0.05 was considered significant.
RESULTS
BAY 60-6583
The non-purine A2b-selective adenosine agonist BAY 60-6583 was developed by Bayer HealthCare in Germany. EC50 values of BAY 60-6583 are 3–10 nM for human A2b and >10 μM for A1 and A2a receptors [11]. BAY 60-6583 had no agonistic effect in the adenosine A3-Gα16 assay up to a concentration of 10 μM (data not shown).
Hemodynamics
No group differences in baseline heart rates were observed, but the chelerythrine group had modestly lower left ventricular developed pressure while PMA+chelerythrine group had higher coronary flow than Control group prior to drug treatment (Table 1). Otherwise PMA and antagonists had little effect on hemodynamics. Developed pressure and coronary flow in all groups were significantly lower than baseline during coronary artery occlusion with partial recovery during reperfusion.
Table 1.
Hemodynamics
| Baseline |
25′ Occlusion |
30′ Occlusion |
15′ Reperfusion |
30′ Reperfusion |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR bpm |
LVDP mmHg |
CF ml/min/g |
HR bpm |
LVDP mmHg |
CF ml/min/g |
HR bpm |
LVDP mmHg |
CF ml/min/g |
HR bpm |
LVDP mmHg |
CF ml/min/g |
HR bpm |
LVDP mmHg |
CF ml/min/g |
|
| Control | 214 ± 8 | 122 ± 4 | 9.0 ± 0.4 | 205 ± 13 | 55 ± 10* | 4.7 ± 0.8* | 219 ± 11 | 83 ± 4* | 6.0 ± 0.5* | ||||||
| IPC | 202 ±6 | 119 ± 2 | 9.0 ± 0.6 | 220 ± 0* | 43 ± 4* | 4.5 ± 0.4* | 220 ± 0* | 73 ± 5* | 5.7 ± 0.8* | ||||||
| IPC + Chelerythrine | 205 ± 4 | 123 ± 2 | 9.9 ± 0.3 | 207 ± 9 | 48 ± 4* | 5.0 ± 0.4* | 217 ± 3 | 53 ± 6* | 6.3 ± 0.6* | 212 ± 7 | 58 ± 6* | 8.1 ± 0.5* | 217 ± 3 | 90 ± 8* | 8.8 ± 0.5 |
| Chelerythrine | 230 ± 4 | 108 ± 2† | 9.3 ± 0.1 | 220 ± 8 | 45 ± 3* | 5.2 ± 0.6* | 218 ± 9 | 49 ± 3* | 5.9 ± 0.8* | 218 ± 10 | 67 ± 4* | 7.3 ± 0.7* | 218 ± 8 | 74 ± 4* | 7.0 ± 0.3* |
| PMA | 208 ± 4 | 123 ± 3 | 9.6 ± 0.4 | 198 ± 8 | 51 ± 6* | 5.4 ± 0.3* | 220 ± 0 | 81 ± 4* | 5.9 ± 0.3* | ||||||
| PMA + Chelerythrine | 204 ± 8 | 122 ± 4 | 11.7 ± 0.8† | 216 ± 0 | 53 ± 5* | 7.5 ± 0.5* | 210 ± 6 | 53 ± 4* | 7.2 ± 0.2* | 209 ± 5 | 87 ± 3* | 8.4 ± 1.0* | 212 ± 3 | 76 ± 3* | 7.8 ± 0.7* |
| PMA + MRS 1754 | 212 ± 12 | 118 ± 2 | 9.6 ± 0.4 | 198 ± 12 | 53 ± 4* | 5.8 ± 0.3* | 218 ± 9 | 54 ± 5* | 5.9 ± 0.3* | 228 ± 8 | 78 ± 5* | 6.5 ± 0.4* | 228 ± 8 | 77 ± 5* | 7.3 ± 0.4* |
| PMA + U0126 | 211 ± 4 | 118 ± 3 | 10.0 ± 0.5 | 196 ± 9 | 46 ± 3* | 5.5 ± 0.5* | 190 ± 10 | 43 ± 5* | 4.7 ± 0.7* | 209 ± 6 | 71 ± 8* | 5.7 ± 1.0* | 209 ± 6 | 76 ± 9* | 5.5 ± 0.7* |
| BAY 60-6583 | 229 ± 11 | 108 ± 4 | 10.4 ± 0.3 | 212 ± 13 | 42 ± 4* | 5.2 ± 0.4* | 214 ± 12 | 44 ± 3* | 5.4 ± 0.5* | 216 ± 14 | 80 ± 5* | 6.9 ± 0.1* | 211 ± 13 | 82 ± 5* | 6.7 ± 0.2* |
Mean±SEM
Abbreviations: CF, coronary flow; HR, heart rate; IPC, ischemic preconditioning; LVDP, left ventricular developed pressure; PMA, phorbol 12-myristate 13-acetate
Statistical significance:
p<0.05 between experimental points and baseline data;
p<0.05 between Control and experimental groups
Infarct size
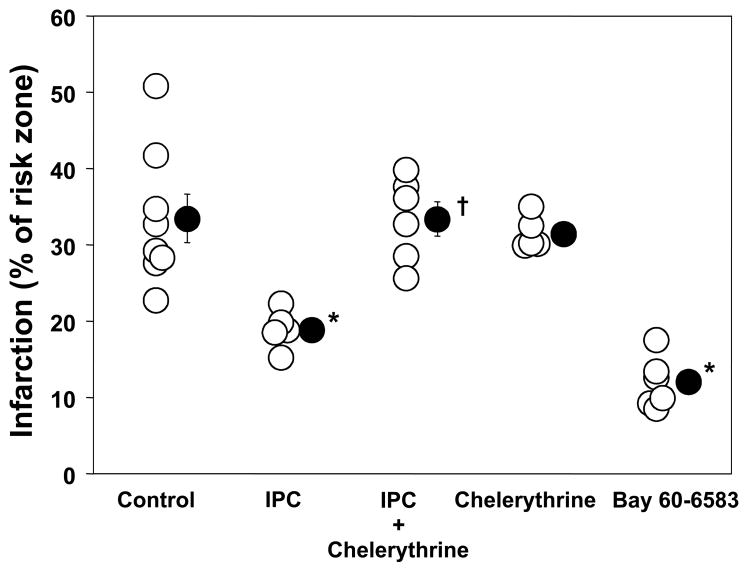
There was no significant difference in heart weight or risk zone volume among groups (Table 2). In Control hearts 33.4±3.2% of risk zone infarcted and this was significantly reduced to 18.9±1.1% by IPC (Fig. 3). When chelerythrine was begun after 25 min of ischemia IPC’s protection was abolished (33.4±2.2% infarction, p<0.05 vs. IPC; Fig. 3). Chelerythrine had no effect on infarction in non-IPC hearts.
Table 2.
Infarction
| n | Body Weight (kg) | Heart Weight (g) | Risk Zone Volume (cm3) | Infarct Volume (cm3) | I/R (%) | |
|---|---|---|---|---|---|---|
| Control | 8 | 2.2 ± 0.0 | 6.7 ± 0.3 | 1.12 ± 0.09 | 0.39 ± 0.06 | 33.4 ± 3.2 |
| IPC | 5 | 2.2 ± 0.1 | 6.8 ± 0.4 | 1.14 ± 0.08 | 0.22 ± 0.02 | 18.9 ± 1.1* |
| IPC + Chelerythrine | 6 | 2.2 ± 0.1 | 6.7 ± 0.2 | 1.21 ± 0.05 | 0.41 ± 0.04 | 33.4 ± 2.2 |
| Chelerythrine | 5 | 2.1 ± 0.1 | 6.4 ± 0.1 | 1.16 ± 0.07 | 0.37 ± 0.03 | 31.5 ± 1.0 |
| PMA | 7 | 2.4 ± 0.0* | 7.1 ± 0.2 | 1.04 ± 0.05 | 0.18 ± 0.02 | 16.6 ± 1.5* |
| PMA + Chelerythrine | 5 | 2.1 ± 0.0 | 6.5 ± 0.1 | 1.18 ± 0.16 | 0.35 ± 0.13 | 35.5 ± 4.9 |
| PMA + MRS 1754 | 6 | 2.4 ± 0.0 | 6.9 ± 0.2 | 1.12 ± 0.06 | 0.34 ± 0.05 | 30.2 ± 2.9 |
| PMA + U0126 | 6 | 2.2 ± 0.1 | 7.0 ± 0.3 | 1.11 ± 0.10 | 0.34 ± 0.07 | 28.4 ± 4.2 |
| BAY 60-6583 | 6 | 2.1 ± 0.0 | 6.8 ± 0.1 | 1.11 ± 0.07 | 0.13 ± 0.02* | 11.9 ± 1.4* |
Mean±SEM
Abbreviations: see Table 1; I/R, infarction as a % of risk zone; n, number of animals
Statistical significance of difference between experimental and Control groups:
p<0.05.
Figure 3.
Effect of ischemic preconditioning (IPC) and administration of a protein kinase C inhibitor chelerythrine during early reperfusion on myocardial infarct size as a percentage of the risk zone. BAY 60-6583 an A2b-selective adenosine agonist given at reperfusion also reduced infarct size. Open circles represent individual experiments while closed circles depict group mean±SEM. IPC’s anti-infarct effect was blocked by chelerythrine (2.8 μM). *p<0.05 vs. control, †p<0.05 vs. IPC
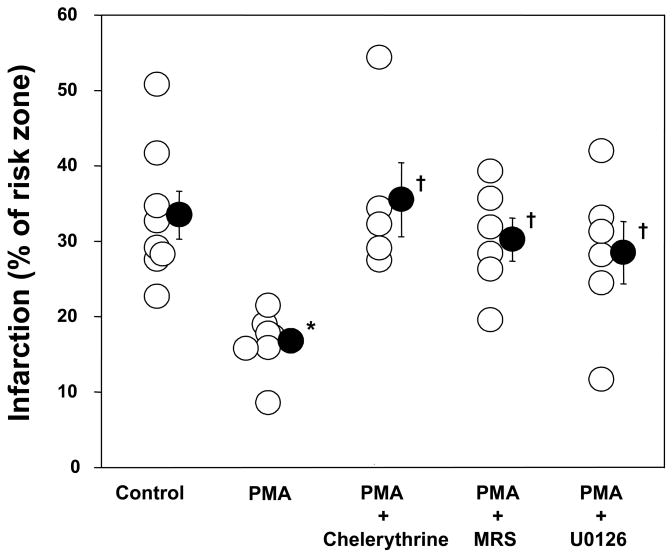
PMA at reperfusion reduced infarction to 16.6±1.5% (p<0.05 vs. Control; Fig. 4). This protection was blocked by chelerythrine (35.5±4.9% infarction, p<0.05 vs. PMA) confirming a PKC mechanism. Co-infusion of MRS 1754, an adenosine A2b receptor-selective antagonist, abolished PMA’s protection (30.2±2.9% infarction, p<0.05 vs. PMA) as did U0126 (28.4±4.2% infarction, p<0.05 vs. PMA). Neither MRS 1754 nor U0126 given at reperfusion has any effect on infarction in non-IPC hearts [4]. Finally, the highly A2b-selective BAY 60-6583 was equipotent with IPC at limiting infarct size (Fig. 3).
Figure 4.
Effect of phorbol 12-myristate 13-acetate (PMA, 0.05 nM) and inhibitors on myocardial infarct size as a percentage of the risk zone. Open circles represent individual experiments while closed circles depict group mean±SEM. PMA at reperfusion reduced infarct size. A protein kinase C inhibitor chelerythrine, an adenosine A2b receptor-selective antagonist MRS 1754 (20 nM), and a MEK and therefore ERK1/2 inhibitor U0126 (0.5 μM) abolished PMA’s protection. *p<0.05 vs. control, †p<0.05 vs. PMA
Biochemical studies
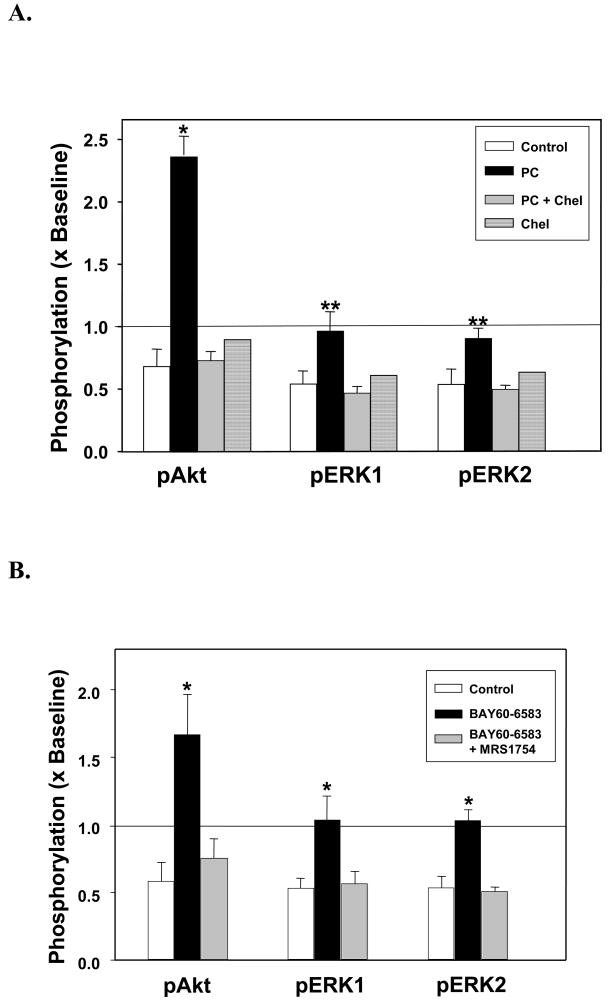
IPC significantly increased phosphorylation of the activation sites for Akt and ERK1/2 (Fig. 5A). Chelerythrine completely blocked this response to IPC, while chelerythrine alone had no effect. Panel B of Fig 5 shows ischemic hearts reperfused with the A2b agonist BAY 60-6583. Akt and ERK were phosphorylated at their activation sites by an amount similar to that seen with IPC (Fig. 5A). That activation was completely blocked by MRS 1754.
Figure 5.
Summary data of changes in phosphorylation of Akt (Ser473) and ERK1/2 (Thr202/Thr204) in hearts following 30 min of global ischemia and 10 min of reperfusion. Phosphorylation is normalized to that in a preischemic sample. (A) Phosphorylation of Akt and ERK in Control hearts (n=4) and hearts preconditioned (PC) with 5 min of global ischemia preceding the 30-min index ischemia with (n=4) or without (n=4) chelerythrine (Chel, 2.8 μM) during the 10 min of reperfusion. Measurements were also made in a non-preconditioned heart treated only with chelerythrine during reperfusion. *p<0.001 and **p<0.05 vs. Control and PC + Chel. (B) Phosphorylation of Akt and ERK in hearts after 10 min of reperfusion in Control hearts, hearts treated with BAY 60-6583 alone during reperfusion or hearts treated with BAY 60-6583 + MRS 1754. *p<0.05 vs. Control and BAY 60-6583+ MRS 1754.
NECA increased both Akt (Fig. 6) and ERK1/2 (data not shown) phosphorylation in a dose-dependent manner. 2.5 nM NECA was below threshold for increasing phosphorylation of either Akt or ERK1/2. However, the A2a-selctive agonist CGS 21680 whose Kd is 16 nM for rabbit A2a receptors [12] caused no phosphorylation of either Akt (Fig. 6) or ERK (data not shown). The phosphorylation status of either Akt or ERK1/2 was not altered after treatment of hearts with PMA or brief ischemia (IPC) (data not shown). However, after treatment with PMA or IPC a subthreshold dose of NECA (2.5 nM) now markedly increased phosphorylation of all 3 kinases. The PMA-induced increase in NECA sensitivity was blocked by MRS 1754. Representative western blots for Akt phosphorylation are shown in Fig. 7A, while summary phosphorylation data for all three kinases are presented in Figs. 7B–D).
Figure 6.
Graph of Akt phosphorylation (Ser473) as a % of phospho-Akt at baseline after 5 min of either 2.5, 5.0 or 10 nM NECA infusion in escalating doses in 4 isolated hearts. A representative western blot of one of these hearts is seen at the top of the figure. Phospho-Akt significantly increased after infusion of 5.0 and 10 nM NECA, whereas 2.5 nM NECA did not change phosphorylation status of Akt. The A2a-selective agonist CGS 21680 at a concentration 3 times its Kd had no effect on Akt phosphorylation. *p<0.05 vs. baseline
Figure 7.
(A) Representative western blots of bands for phospho-Akt (Ser473) in control hearts, hearts treated with phorbol 12-myristate 13-acetate (PMA, 0.05 nM) alone, PMA + MRS 1754, or hearts exposed to brief preconditioning ischemia. The left-hand column presents phospho-Akt bands before infusion of the subthreshold dose of NECA, while the right-hand column presents bands after NECA treatment. Phosphorylation increased after NECA administration only in hearts pre-treated with either PMA or IPC. MRS 1754 blocked any increase. (B) Phospho-Akt (Ser473) and (C and D) phospho-ERK1/2 (Thr202/Thr204) as % of levels measured just before treatment with NECA. All hearts in panels B–D were treated for 5 min with the subthreshold dose of 2.5 nM NECA. NECA had no effect in untreated hearts. After pretreatment with PMA the subthreshold dose of NECA now caused a robust phosphorylation of Akt and ERK1/2. NECA had no effect on phosphorylation when co-infused with a selective A2b receptor antagonist MRS 1754 in PMA treated hearts. Brief ischemia (IPC) also enabled the subthreshold dose of NECA to phosphorylate Akt and ERK1/2. Each bar is mean±SEM of 4 observations. *p<0.05 vs. control, #p<0.05 vs. PMA
Although NECA is a potent A2b agonist, it is not selective and can activate other adenosine receptor subtypes. NECA’s ability to increase phosphorylation in PMA-treated hearts was completely abolished by co-infusion of MRS 1754 which supports but still does not prove that NECA stimulated phosphorylation through an adenosine A2b receptor. Interestingly, similar to PMA, an IPC protocol also lowered the threshold for NECA-induced phosphorylation. Fig. 8 shows that infusion of BAY 60-6583, a highly selective A2b receptor agonist, also significantly increased Akt and ERK phosphorylation by an amount similar to that seen with NECA, and MRS 1754 blocked that increase.
Figure 8.
(A) Effect of 300nM BAY 60-6583 on Akt and ERK phosphorylation in serial biopsies from isolated rabbit hearts taken at baseline, after 5 and 10 min of drug administration, and again after 10 min of washout. BAY 60-6583 nearly doubled phosphorylation of each. (B) Phosphorylation of Akt and ERK1/2 was blocked by MRS 1754. *p<0.05, †p<0.01, ‡p<0.005, all vs Baseline
Discussion
These results confirm in an isolated heart model that protection from IPC depends on PKC activity at reperfusion and that pharmacological activation of PKC during early reperfusion mimics IPC’s cardioprotection. Again there was clear evidence that adenosine receptor activation occurred in response to PKC activation, the reverse of what is normally seen. Either direct PKC activation or an ischemic preconditioning protocol markedly lowered the adenosine agonist NECA’s threshold for phosphorylation of the protective kinases, PI3-K/Akt and ERK 1/2. These data support our hypothesis that PKC in IPC hearts protects by increasing the heart’s sensitivity to its endogenous adenosine in early reperfusion. Thus adenosine appears to have a dual role in IPC: binding to A1 receptors prior to the lethal ischemic insult acts as trigger to enter the protected state while binding to what appears to be the A2b receptor during reperfusion acts as mediator.
Infusion of PMA at reperfusion mimics IPC’s protection in open-chest rabbits and this protective effect is dependent on activation of adenosine receptors [2]. The present study now confirms these observations in an isolated heart model. Our finding that PMA’s protection was blocked by chelerythrine confirms a PKC mechanism. Solenkova et al. [4] observed that IPC’s anti-infarct effect depended on binding of adenosine receptors during reperfusion. Philipp et al. [2] and Kin et al. [5] both saw a requirement for adenosine receptor binding in postconditioning. IPC requires Akt and ERK activation at reperfusion [1,4], and we now show that PKC blockade with chelerythrine abolishes IPC’s phosphorylation of these survival kinases.
PMA’s protection requires ERK activation. Similarly, we had observed that the protective effect of the adenosine agonist NECA was aborted by the ERK inhibitors PD98059 and U0126 [13], indicating ERK is also an important signaling component for cardioprotection following adenosine receptor activation at reperfusion. Therefore, we conclude IPC, postconditioning and PMA likely all protect through similar pathways and that activation of PKC early in reperfusion is sufficient to duplicate the IPC phenotype.
Inagaki et al. [14,15] reported that specific inhibition of PKC-δ at reperfusion decreased infarct size. Our present observation that PKC activation at reperfusion confers protection reveals that while the δ isoform may be detrimental at reperfusion, one or more PKC isoforms must be protective at that time. We do not know which isoforms are activated by our low dose of PMA. It should be noted that in our pilot studies higher doses of PMA that would likely have recruited more PKC isoforms were not protective. Also inhibition of all isoforms with chelerythrine at reperfusion obviously blocks the one or more protective PKC isoforms that IPC must activate at reperfusion to confer its protection. It also blocks PMA’s protection verifying that PMA acted by activating PKC. Chelerythrine had no effect on infarction in non-preconditioned hearts. Interestingly, an ischemic preconditioning protocol had the same effect on sensitivity to NECA in these hearts as was seen with PMA. That observation bolsters our hypothesis that IPC increases the heart’s sensitivity to adenosine through activation of PKC.
Chelerythrine is a potent and highly selective PKC blocker. Unfortunately, it is also an adenosine receptor blocker with Kis of 5.7 μM and 37.6 μM for human A1 and A2a receptors, respectively [16]. We used 2.8 uM chelerythrine in this study which is close to the Ki for A2a receptors. While blockade of adenosine receptors could conceivably explain why chelerythrine blocked protection from ischemic preconditioning in the present study, the mimicking of IPC’s protection by PMA still implicates PKC as a direct trigger of that protection. Furthermore chelerythrine could not block NECA’s protection [2].
Pretreating hearts with PMA had no effect on phosphorylation of Akt or ERK1/2 in the absence of an adenosine agonist, but markedly lowered the threshold required for NECA to increase their phosphorylation. MRS 1754 blocked the ability of NECA to phosphorylate the kinases in PMA-treated hearts indicating that the pathway involved adenosine receptors, and most likely the A2b subtype. We are not the first to see this effect. In Jurkat cells PKC activation greatly enhances the cAMP response from NECA [17]. A similar effect was seen in retinal cells [18]. Because NECA increased cAMP the investigators concluded that it must have been an A2 receptor effect but did not differentiate between the two subtypes. In the retinal cells PKC activation potentiated the response to isproterenol and forskolin as well suggesting the effect was not at the adenosine receptor level. Trincavelli et al. [19] noted tumor necrosis factor-α, which among other things activates PKC, increased A2b receptor’s functional response to NECA in human astrocytoma cells; however, the phosphorylation of threonine residues in A2b receptors was unaffected again suggesting the effect is not at the receptor.
We have proposed that the A2b receptor may be the subtype responsible for adenosine-dependent protection in IPC [4] and postconditioning [2]. Two lines of evidence in this study support the A2b receptor subtype as the one responsible for protection at reperfusion. First, an antagonist with good selectivity for A2b receptors, MRS 1754, blocked NECA’s and PMA’s protection in our previous [2] as well as present study. MRS 1754’s exact pharmacology for rabbit receptors is unknown but reported Kis for MRS 1754 for human A1, A2a and A2b receptors are 403, 503 and 2.0 nM respectively [20]. In rats MRS showed less selectivity with a Ki for the A1 receptor of 17 nM [20]. Because rabbit A1 receptors have a high affinity for xanthine antagonists like those of rats [21], they may bind MRS1754 as well. However, selectivity against the A2a receptor was still good in the rat with a Ki of 612 nM [20].
The second line of evidence was that a highly A2b-selective agonist, BAY 60-6583, was equipotent with IPC in protecting these hearts. The fact that both NECA and BAY 60-6583 given at reperfusion can fully protect the isolated heart and can duplicate IPC’s activation of Akt and ERK indicates that the receptor and its protective signaling are intact in non-IPC hearts. However, we propose there is insufficient stimulation of those receptors by endogenous adenosine. Although BAY 60-6583 is highly selective for human A2b receptors [11], BAY 60-6583’s selectivity for rabbit receptors is unknown. However, in a previous study [22] we protected ischemic rabbit hearts with an intravenous injection of 10 ug/kg of BAY 60-6583 at reperfusion and it caused neither hypotension (an A2a effect) nor bradycardia (an A1 effect).
A2b receptors expressed in CHO cells reportedly cause activation of Akt and ERK [23] as do A2b receptors in human retinal endothelial cells [24], but a similar coupling to Akt and ERK has been seen in transfected CHO cells with all 4 known adenosine receptor subtypes [25]. Nevertheless when we activated Akt and ERK with the mixed agonist NECA, the A2b selective blocker MRS 1754 completely abolished the response indicating that only the A2b receptor was responsible for activating those kinases in rabbit myocardium.
There is evidence for other adenosine receptor subtypes being protective at reperfusion. Kin et al. [5] blocked protection from postconditioning with an A2a-selective antagonist, but because the test was done in an in situ heart model in which plasma concentration of the drug was unknown, an A2a-selective concentration may not have been present. Several papers report that highly selective A2a agonists can limit infarct size when infused at reperfusion [26–28]. All of those studies attributed the protection to an anti-inflammatory effect against neutrophils. Indeed, previously [29] and in studies reported here we were unable to limit infarct size with the highly selective A2a agonist CGS 21680 in the leukocyte-free isolated rabbit heart model. We were also unable to block protection from IPC in the rabbit heart with 8-(3-chlorostyryl)caffeine (CSC), a highly selective A2a receptor blocker [4]. Since IPC and A2b agonists are very effective in leukocyte-free systems, one likely explanation is that IPC causes substantial protection through a mechanism that does not involve inflammation, e.g., suppression of mitochondrial permeability transition pores. To further confuse the issue A3 receptors also reportedly protect when activated at reperfusion [30,31], but those receptors are Gi-coupled and the PKC enhancement of sensitivity to adenosine has so far only been reported in Gs-coupled systems.
Xu et al. [32] noted adenosine preserved Ψm in isolated rat cardiomyocytes during H2O2 challenge which was assumed to result from inhibition of permeability transition pores. Their studies suggested that the effect was mediated by A2a receptors as CGS 12680 mimicked it and CSC blocked adenosine’s effect. It should be noted, however, that Xu was unable to limit infarct size in isolated rabbit hearts with CGS 21680 but could with the mixed adenosine agonist AMP579 [29].
It is controversial whether A2b receptors are even expressed on cardiomyocytes. Yang et al. [33] replaced exon 1 of the A2b gene in transgenic mice with a reporter construct containing β-Gal, and they did not detect β-Gal expression in heart muscle indicating either there was no or a very low cardiac expression level. On the other hand, Morrison and colleagues [34] saw a positive inotropic response to NECA in A2a knockout mice suggesting that some A2b receptors must reside on cardiomyocytes.
Probably the strongest evidence supporting the A2b receptor as being important in ischemic preconditioning is provided in the recent study of Eckle et al. [11]. Using a genetic approach they examined the effects of ischemic preconditioning in mouse hearts with knockout of either A1, A2a, A2b, or A3 adenosine receptors. Ischemic preconditioning reduced infarction in all but the A2b-deficient mice. Of all the adenosine receptors, there was an absolute requirement for only the A2b receptor. Eckle at al. also tested BAY 60-6583 as a pretreatment and found that it protected wild type but not A2b knockout hearts. We extend their findings by showing that this A2b-selective agonist also protects in situ rabbit hearts when given only at reperfusion.
Conclusion
Protection in ischemically preconditioned hearts was found to depend on activation of PKC as well as occupation of adenosine receptors in the first minutes of reperfusion following the index ischemia. Furthermore activation of PKC by IPC was seen to potentiate the ability of an adenosine agonist to activate the survival kinases PI3-K and ERK. Our findings suggest PKC elicits cardioprotection by increasing the heart’s sensitivity to adenosine so that endogenous adenosine can activate the survival kinases during reperfusion. If the above scenario is correct, then the key protective event in preconditioning occurs when PKC raises the sensitivity of the heart to adenosine early in the reperfusion period.
Acknowledgments
This study was supported in part by grants HL-20648 and HL-50688 from the Heart, Lung and Blood Institute of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 2.Philipp S, Yang X-M, Cui L, Davis AM, Downey JM, Cohen MV. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res. 2006;70:308–314. doi: 10.1016/j.cardiores.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Yang X-M, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103–1110. doi: 10.1016/j.jacc.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 4.Solenkova NV, Solodushko V, Cohen MV, Downey JM. Endogenous adenosine protects preconditioned heart during early minutes of reperfusion by activating Akt. Am J Physiol. 2006;290:H441–H449. doi: 10.1152/ajpheart.00589.2005. [DOI] [PubMed] [Google Scholar]
- 5.Kin H, Zatta AJ, Lofye MT, Amerson BS, Halkos ME, Kerendi F, et al. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res. 2005;67:124–133. doi: 10.1016/j.cardiores.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Kitakaze M, Funaya H, Minamino T, Node K, Sato H, Ueda Y, et al. Role of protein kinase C-a in activation of ecto-5′-nucleotidase in the preconditioned canine myocardium. Biochem Biophys Res Commun. 1997;239:171–175. doi: 10.1006/bbrc.1997.7445. [DOI] [PubMed] [Google Scholar]
- 7.Schulz R, Post H, Vahlhaus C, Heusch G. Ischemic preconditioning in pigs: a graded phenomenon. Its relation to adenosine and bradykinin. Circulation. 1998;98:1022–1029. doi: 10.1161/01.cir.98.10.1022. [DOI] [PubMed] [Google Scholar]
- 8.Goto M, Cohen MV, Van Wylen DGL, Downey JM. Attenuated purine production during subsequent ischemia in preconditioned rabbit myocardium is unrelated to the mechanism of protection. J Mol Cell Cardiol. 1996;28:447–454. doi: 10.1006/jmcc.1996.0041. [DOI] [PubMed] [Google Scholar]
- 9.Harrison GJ, Willis RJ, Headrick JP. Extracellular adenosine levels and cellular energy metabolism in ischemically preconditioned rat heart. Cardiovasc Res. 1998;40:74–87. doi: 10.1016/s0008-6363(98)00123-0. [DOI] [PubMed] [Google Scholar]
- 10.National Research Council. Guide for the Care and Use of Laboratory Animals. 7. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- 11.Eckle T, Krahn T, Grenz A, Köhler D, Mittelbronn M, Ledent C, et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson KA, Stiles GL, Ji X-D. Chemical modification and irreversible inhibition of striatal A2a adenosine receptors. Mol Pharmacol. 1992;42:123–133. [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X-M, Krieg T, Cui L, Downey JM, Cohen MV. NECA and bradykinin at reperfusion reduce infarction in rabbit hearts by signaling through PI3K, ERK, and NO. J Mol Cell Cardiol. 2004;36:411–421. doi: 10.1016/j.yjmcc.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Inagaki K, Hahn HS, Dorn GW, II, Mochly-Rosen D. Additive protection of the ischemic heart ex vivo by combined treatment with d-protein kinase C inhibitor and e-protein kinase C activator. Circulation. 2003;108:869–875. doi: 10.1161/01.CIR.0000081943.93653.73. [DOI] [PubMed] [Google Scholar]
- 15.Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K-i, Bouley DM, et al. Inhibition of d-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108:2304–2307. doi: 10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- 16.Schulte G, Fredholm BB. Diverse inhibitors of intracellular signalling act as adenosine receptor antagonists. Cell Signal. 2002;14:109–113. doi: 10.1016/s0898-6568(01)00228-5. [DOI] [PubMed] [Google Scholar]
- 17.Nordstedt C, Kvanta A, Van der Ploeg I, Fredholm BB. Dual effects of protein kinase-C on receptor-stimulated cAMP accumulation in a human T-cell leukemia line. Eur J Pharmacol. 1989;172:51–60. doi: 10.1016/0922-4106(89)90044-8. [DOI] [PubMed] [Google Scholar]
- 18.Nash MS, Wood JPM, Osborne NN. Protein kinase C activation by serotonin potentiates agonist-induced stimulation of cAMP production in cultured rat retinal pigment epithelial cells. Exp Eye Res. 1997;64:249–255. doi: 10.1006/exer.1996.0214. [DOI] [PubMed] [Google Scholar]
- 19.Trincavelli ML, Marroni M, Tuscano D, Ceruti S, Mazzola A, Mitro N, et al. Regulation of A2B adenosine receptor functioning by tumour necrosis factor a in human astroglial cells. J Neurochem. 2004;91:1180–1190. doi: 10.1111/j.1471-4159.2004.02793.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y-C, Ji X-d, Melman N, Linden J, Jacobson KA. Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A2B adenosine receptors. J Med Chem. 2000;43:1165–1172. doi: 10.1021/jm990421v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takano H, Bolli R, Black RG, Jr, Kodani E, Tang X-L, Yang Z, et al. A1 or A3 adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circ Res. 2001;88:520–528. doi: 10.1161/01.res.88.5.520. [DOI] [PubMed] [Google Scholar]
- 22.Albrecht B, Krahn T, Philipp S, Rosentreter U, Cohen M, Downey J. Selective A2b receptor activation mimics postconditioning in a rabbit infarct model. Circulation. 2006;114(Suppl II):II-14–II-15. [Google Scholar]
- 23.Schulte G, Fredholm BB. The Gs-coupled adenosine A2B receptor recruits divergent pathways to regulate ERK1/2 and p38. Exp Cell Res. 2003;290:168–176. doi: 10.1016/s0014-4827(03)00324-0. [DOI] [PubMed] [Google Scholar]
- 24.Grant MB, Davis MI, Caballero S, Feoktistov I, Biaggioni I, Belardinelli L. Proliferation, migration, and ERK activation in human retinal endothelial cells through A2B adenosine receptor stimulation. Invest Ophthalmol Vis Sci. 2001;42:2068–2073. [PubMed] [Google Scholar]
- 25.Schulte G, Fredholm BB. Human adenosine A1, A2A, A2B, and A3 receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase 1/2. Mol Pharmacol. 2000;58:477–482. [PubMed] [Google Scholar]
- 26.Jordan JE, Zhao Z-Q, Sato H, Taft S, Vinten-Johansen J. Adenosine A2 receptor activation attenuates reperfusion injury by inhibiting neutrophil accumulation, superoxide generation and coronary endothelial adherence. J Pharmacol Exp Ther. 1997;280:301–309. [PubMed] [Google Scholar]
- 27.Glover DK, Riou LM, Ruiz M, Sullivan GW, Linden J, Rieger JM, et al. Reduction of infarct size and postischemic inflammation from ATL-146e, a highly selective adenosine A2A receptor agonist, in reperfused canine myocardium. Am J Physiol. 2005;288:H1851–H1858. doi: 10.1152/ajpheart.00362.2004. [DOI] [PubMed] [Google Scholar]
- 28.Schlack W, Schafer M, Uebing A, Schafer S, Borchard U, Thamer V. Adenosine A2-receptor activation at reperfusion reduces infarct size and improves myocardial wall function in dog heart. J Cardiovasc Pharmacol. 1993;22:89–96. doi: 10.1097/00005344-199307000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Xu Z, Downey JM, Cohen MV. AMP 579 reduces contracture and limits infarction in rabbit heart by activating adenosine A2 receptors. J Cardiovasc Pharmacol. 2001;38:474–481. doi: 10.1097/00005344-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Auchampach JA, Ge Z-D, Wan TC, Moore J, Gross GJ. A3 adenosine receptor agonist IB-MECA reduces myocardial ischemia-reperfusion injury in dogs. Am J Physiol. 2003;285:H607–H613. doi: 10.1152/ajpheart.01001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maddock HL, Mocanu MM, Yellon DM. Adenosine A3 receptor activation protects the myocardium from reperfusion/reoxygenation injury. Am J Physiol. 2002;283:H1307–H1313. doi: 10.1152/ajpheart.00851.2001. [DOI] [PubMed] [Google Scholar]
- 32.Xu Z, Park S-S, Mueller RA, Bagnell RC, Patterson C, Boysen PG. Adenosine produces nitric oxide and prevents mitochondrial oxidant damage in rat cardiomyocytes. Cardiovasc Res. 2005;65:803–812. doi: 10.1016/j.cardiores.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison RR, Talukder MAH, Ledent C, Mustafa SJ. Cardiac effects of adenosine in A2A receptor knockout hearts: uncovering A2B receptors. Am J Physiol. 2002;282:H437–H444. doi: 10.1152/ajpheart.00723.2001. [DOI] [PubMed] [Google Scholar]