Abstract
Objective
To determine in pediatric Duchenne (DMD) and Becker (BMD) muscular dystrophy or other dilated cardiomyopathies (ODCM) whether outcomes differ by diagnosis.
Background
Children with dilated cardiomyopathy are treated as a single undifferentiated group.
Methods
This cohort study of 128 children with DMD, 15 with BMD, and 312 with ODCM uses outcome measures of LV size and function, death, heart transplant, and death or transplant.
Results
At cardiomyopathy diagnosis, the DMD and BMD groups had similar mean ages (14.4 and 14.6 years), prevalence of CHF (30% and 33%), and LV fractional shortening (FS) z-scores (median, −5.2 for DMD and −6.7 for BMD). The BMD group had more severe mitral regurgitation (P=.05) and a higher mean LV end-diastolic dimension Z-score than the DMD group (2.9±1.5 vs. 1.2±1.9, P=.002). DMD group survival was lower than in BMD or ODCM groups (P=.06) at 5-years (57%, 100%, and 71% respectively). In BMD, 25% received cardiac transplants within 0.4 years of cardiomyopathy diagnosis. The combined DMD and BMD group had less LV dilation and a closer-to-normal LVFS at cardiomyopathy diagnosis than the ODCM group. After 2 years, LV dilation increased and LVFS did not change in the combined DMD and BMD group; for OCDM patients, LV dilation did not progress and LVFS improved.
Conclusions
Children with DMD and cardiomyopathy have a higher mortality. BMD has a high heart transplantation rate in the 5 years after diagnosis of cardiomyopathy. Serial echocardiography demonstrates a different disease course for DMD and BMD patients compared with ODCM patients.
Keywords: Muscular dystrophy, neuromuscular disease, cardiomyopathy, heart failure, pediatric, Duchenne, Becker
INTRODUCTION
Children with dilated cardiomyopathy have been treated as a single undifferentiated group. However, we have suspected that children with dystrophinopathies, comprising Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), and X-linked dilated cardiomyopathy have clinical cardiac characteristics at the time of cardiomyopathy diagnosis that differ from each other, as well as from children with other non-neuromuscular dilated cardiomyopathies (ODCM).1
The dystrophinopathies lead to alternating areas of myocyte hypertrophy, atrophy/necrosis and fibrosis with replacement of myocardium by connective tissue and fat, as well as generalized muscle weakness.2 Molecular studies have identified defects in the dystrophin gene or its regulation as the cause of these variant phenotypes, which have differing degrees of cardiac and skeletal muscle involvement.1,3 Skeletal muscle weakness, respiratory symptoms and electrocardiographic abnormalities4 start at a young age, which correlate with pathologic changes in the myocardium. Although cardiomyopathy is present on a cellular or histological level, echocardiographic abnormalities, clinical dilated cardiomyopathy and cardiovascular symptoms are usually delayed until the second decade of life in DMD and X-linked dilated cardiomyopathy and until the third decade of life in BMD. Cardiac involvement is present in about 90% of DMD and BMD patients but is the cause of death in about 20% of the DMD and 50% of the BMD patients.2,4
Until recently, children with DMD often died at 15 to 20 years of age from respiratory complications, congestive heart failure [CHF], or arrhythmias. With intensive respiratory care, however, many patients with DMD now live into their late 20s and older.5 Death from cardiac or respiratory failure typically occurs in the fifth decade in BMD.4 This increased longevity has made cardiac function and cardiovascular health an increasingly important part of evaluating and treating DMD.
The American Academy of Pediatrics (AAP) has recently noted that the time course for development of cardiomyopathy and diagnostic tests for cardiac dysfunction have not been well characterized in individuals affected by Duchenne or Becker muscular dystrophy, and has recommended that medical information be shared to maximize understanding of cardiomyopathy in these patients.5 This analysis is in response to these AAP recommendations.
The National Heart, Lung, and Blood Institute (NHLBI)-sponsored multi-center Pediatric Cardiomyopathy Registry (PCMR)6 includes extensive longitudinal data on children with DMD and BMD in whom cardiomyopathy has already developed, as well as information on children with dilated cardiomyopathy of different origins (ODCM). The purpose of this analysis was to describe the characteristics and outcomes of children with DMD or BMD, to determine how the two disorders differ from one another, and as a single group, how they differ from children with ODCM.
PATIENTS AND METHODS
The Pediatric Cardiomyopathy Registry
This report is based on PCMR data as of April 2005 on 3,045 cases (newborn to 18 years at diagnosis) who presented with cardiomyopathy to a pediatric cardiologist in 1990 or later. Each case is classified according to morphology as dilated, hypertrophic, restrictive, mixed, or other type of cardiomyopathy. Cases are further classified as “definite” if specified quantitative strict echocardiographic criteria of LV dilation and systolic dysfunction are met, if the pattern of cardiomyopathy conforms to a defined semi-quantitative pattern, if the diagnosis is confirmed by autopsy or tissue analysis, or if the investigator has other compelling evidence of CM.6 Fifteen clinical exclusion criteria are also applied before children are enrolled.6 Children with only a family history or sub-clinical evidence of cardiomyopathy are not included.
Data Collection
An outreach team with standardized training reviews patient charts to complete annual data collection until the patient undergoes heart transplantation, is deceased, or is no longer seen at the center.
The PCMR consists of two cohorts - prospective (diagnosis of cardiomyopathy in 1996 or later) and retrospective (diagnosis of cardiomyopathy between 1990 and 1995). Certain data have been collected only for the retrospective cohort (n=126, 28% of this analysis): medical therapies except for anti-congestive therapy, extended family history, and echocardiographic evidence of mitral and tricuspid regurgitation. A positive family history for a genetic syndrome is defined as first-degree relatives with a recognizable pattern of dysmorphic features, organ malformations, and/or disease symptoms caused by a genetic abnormality (this may include DMD or BMD).
Each of the 57 pediatric cardiac centers that contributed data to this report obtained Institutional Review Board approval with a waiver of consent for medical record abstraction and submission to the Registry.
Patients
Three groups were included in this analysis: DMD (n=128), BMD (n=15), and a control group of 312 cases with ODCM. All DMD and BMD cases had dilated cardiomyopathy. The ODCM group included 52 cases of myocarditis (24/52 with Dallas criteria met) and 260 cases of idiopathic dilated cardiomyopathy aged 10 to 18 years at the time of cardiomyopathy diagnosis. Therefore, the age range of the ODCM group was representative (i.e., within 2 SD) of that of the combined DMD and BMD group (mean±SD 14±2 years at cardiomyopathy diagnosis), as infants and young children who present with dilated cardiomyopathy have a very different disease course.7
Statistical Methods
Echocardiographic measurements were transformed into body surface area- or age-adjusted Z-scores relative to normal children.8 The distributions of categorical variables were compared using a Fisher exact test or the Mantel-Haenszel test for linear trend. Group comparisons of non-normally and normally distributed continuous variables were compared using Wilcoxon’s rank sum test, and Student’s t-test, respectively. Change scores and Z-scores were tested for difference from zero using a one-sample t-test or the Wilcoxon signed rank test.
Time to death, heart transplant, and the combined endpoint of death or transplant were estimated using the Kaplan-Meier method, and groups were compared with the log-rank and weighted log-rank (Gehan-Wilcoxon) tests. For mortality analyses, the follow-up times of children who underwent heart transplantation were censored at the time of transplant.
Alpha was set at 0.05, and all tests were two-tailed. All analyses were conducted using the Statistical Analysis System version 9.1 (SAS®, Cary, NC) and S-Plus 6.1 (Insightful Corporation, Seattle, WA).
RESULTS
Comparison of DMD and BMD Groups
The DMD and BMD groups had a similar age at cardiomyopathy diagnosis (14.4±2.3 vs. 14.6±2.0 years) and rates of CHF at diagnosis (30% vs. 33%). (Table 1).
Table 1.
Demographics, Medical History and Clinical Characteristics at the Diagnosis of Cardiomyopathy by Etiologic Classification in 455 Patients with Dilated Cardiomyopathy.
| Characteristic at the Diagnosis of Cardiomyopathy | DMD | BMD | DMD vs. BMD P-value | DMD and BMD combined | ODCM* | DMD and BMD vs. ODCM P-value |
|---|---|---|---|---|---|---|
| N | 128 | 15 | 143 | 312 | ||
| Male (%) | 100 | 100 | — | 100 | 62.2 | <.001 |
| Mean age ± SD, years | 14.4±2.3 | 14.6±2.0 | .70 | 14.4±2.3 | 14.0±2.2 | .06 |
| Median age, years | 14.8 | 14.5 | 14.8 | 14.1 | ||
| Race | .08 | <.001 | ||||
| White (%) | 74.6 | 53.3 | 72.3 | 52.9 | ||
| Black (%) | 13.5 | 20.0 | 14.2 | 28.9 | ||
| Hispanic (%) | 9.5 | 13.3 | 9.9 | 13.3 | ||
| Other (%) | 2.4 | 13.3 | 3.6 | 4.9 | ||
| CHF present (%) | 29.9 | 33.3 | .77 | 30.3 | 65.7 | <.001 |
| Retrospective cohort (%) | 26.6 | 40.0 | .36 | 28.0 | 27.6 | 1.00 |
| Anti-congestive therapy (%) | 68.0 | 60.0 | .57 | 67.2 | 79.8 | .006 |
| Anti-arrhythmic therapy (%)† | 20.6 (n=34) | 33.3 (n=6) | .60 | 22.5 (n=40) | 41.7 (n=84) | .05 |
| ACE inhibitor (%)† | 38.2 (n=34) | 33.3 (n=6) | 1.00 | 37.5 (n=40) | 70.2 (n=84) | <.001 |
| Calcium channel blocker (%)† | 2.9 (n=34) | 0.0 (n=6) | 1.00 | 2.5 (n=40) | 2.4 (n=84) | 1.00 |
| Beta-blocker (%)† | 0.0 (n=34) | 0.0 (n=6) | — | 0.0 (n=40) | 8.4 (n=83) | .095 |
DMD = Duchenne muscular dystrophy, BMD = Becker muscular dystrophy, CHF = congestive heart failure, ACE = angiotensin converting enzyme
ODCM includes patients aged 10 to 18 years at the diagnosis of cardiomyopathy with either myocarditis or a currently unidentified cause of cardiomyopathy.
Anti-arrhythmic, ACE inhibitor, calcium channel blocker and beta-blocker therapy data were only collected on retrospective cohort patients, who comprise 28% of the data set.
Echocardiographic Findings
At the time of cardiomyopathy diagnosis, the BMD group had larger LV end-diastolic and LV end-systolic dimension Z-scores than did the DMD group (Table 2), but similar LV fractional shortening (FS) and LV wall thickness. Although only qualitative echocardiographic data were available for 40 of the 143 children with DMD or BMD, the BMD group was more likely to have at least moderate mitral regurgitation (50% vs. 18% in DMD group, linear trend P=.05). Similarly, moderate tricuspid regurgitation was more common in the BMD group (17% vs. 0% in DMD group, linear trend P=.004). Forty-one BMD and DMD cases had follow-up echocardiograms. At 2 years following cardiomyopathy diagnosis, only LV end-diastolic dimension (EDD) reflected marked deterioration in the DMD+BMD group (mean±SD change in Z-score, 1.39±1.81, P=.003). All other change scores of the DMD+BMD group were in the direction of deterioration (Table 3), but did not achieve significance in this small sample.
Table 2.
Echocardiographic Characteristics at the Diagnosis of Cardiomyopathy by Etiologic Classification in 414 Patients with Dilated Cardiomyopathy and Echocardiographic Data.
| Characteristic at the Diagnosis of Cardiomyopathy | DMD | BMD | DMD vs. BMD P-value | DMD and BMD combined | ODCM* | DMD and BMD vs. ODCM P-value |
|---|---|---|---|---|---|---|
| N with Echocardiographic Data | 126 | 14 | 140 | 274 | ||
| Mitral regurgitation† | (n=34) | (n=6) | .09/.05‡ | (n=40) | (n=80) | .07 |
| [N=34,6,40,80] | .056‡ | |||||
| None (%) | 55.9 | 16.7 | 50.0 | 27.5 | ||
| Mild (%) | 26.5 | 33.3 | 27.5 | 45.0 | ||
| Moderate (%) | 17.7 | 50.0 | 22.5 | 25.0 | ||
| Severe (%) | 0.0 | 0. 0 | 0.0 | 2.5 | ||
| Tricuspid regurgitation† | (n=33) | (n=6) | .04/.004‡ | (n=39) | (n=79) | .006 |
| [N=33,6,39,79] | .001‡ | |||||
| None (%) | 90.9 | 50.0 | 84.6 | 54.4 | ||
| Mild (%) | 9.1 | 33.3 | 12.8 | 26.6 | ||
| Moderate (%) | 0.0 | 16.7 | 2.6 | 17.7 | ||
| Severe (%) | 0.0 | 0. 0 | 0.0 | 1.3 | ||
| LV EDD Z-score | 1.19±1.88 (n=90) | 2.94±1.50 (n=13) | .002 | 1.41±1.92 (n=103) | 3.37±2.29 (n=224) | <.001 |
| LV ESD Z-score | 2.65±2.29 (n=81) | 4.47±2.18 (n=11) | .02 | 2.86±2.34 (n=92) | 4.81±2.74 (n=195) | <.001 |
| Median LV fractional shortening Z- score (IQR) | −5.15 (−7.25, −3.23) | −6.74 (−9.70, −3.69) | .31 | −5.20 (−7.56, −3.23) | −7.80 (−9.44, −4.52) | <.001 |
| LV end-diastolic posterior wall thickness Z-score | −1.68±2.25 | −1.94±2.17 | .73 | −1.71±2.23 | −0.85±2.81 | .009 |
| [N=77,10,87,167] | ||||||
| LV end-diastolic septal wall thickness Z-score | −1.40±1.37 | −1.76±2.65 | .68 | −1.44±1.56 | −0.83±1.50 | .003 |
| [N=73,10,83,155] | ||||||
| LV mass Z-score | −0.52±1.82 (n=75) | 0.39±1.54 (n=10) | .14 | −0.41±1.81 (n=85) | 1.67±2.14 (n=167) | <.001 |
| Median LV end-diastolic posterior wall thickness to LVEDD ratio (IQR) | 0.14 (0.12, 0.16) (n=95) | 0.13 (0.09, 0.15) (n=10) | .13 | 0.14 (0.12, 0.16) (n=105) | 0.13 (0.10, 0.16) (n=207) | .03 |
DMD = Duchenne muscular dystrophy, BMD = Becker muscular dystrophy, ODCM = other dilated cardiomyopathy, LV = left ventricular, EDD = end-diastolic dimension, ESD = end-systolic dimension; IQR = inter-quartile range.
ODCM includes patients aged 10 to 18 years at the diagnosis of cardiomyopathy with either myocarditis or a currently unidentified cause of cardiomyopathy.
Mitral and tricuspid regurgitation data were only collected on retrospective cohort patients who comprise 28% of the dataset.
First P value is Fisher’s exact test and second is Mantel-Haenszel test for linear trend.
Statistics are mean±standard deviation unless otherwise noted.
Table 3.
Mean (±Standard Deviation) Change in Echocardiographic Characteristics from Diagnosis of Cardiomyopathy to 2 Years After Diagnosis
| Change in Z- Score† (Follow-up minus Baseline) | DMD | BMD | DMD vs. BMD P- value | DMD+BMD | ODCM* | DMD+BMD vs. ODCM P-value |
|---|---|---|---|---|---|---|
| Time between echocardiograms, years | 1.95± .73 (n=36) | 2.05± 061 (n=5) | .78 | 1.97±0.71 (n=41) | 1.87±0.54 (n=55) | .47 |
| LV EDD Z-score | 1.66*±1.86 (n=16) | 0.29±1.25 (n=4) | .18 | 1.39*±1.81 (n=20) | −0.22±1.70 (n=33) | .002 |
| LV ESD Z-score | 1.24±2.10 (n=13) | 0.07±1.40 (n=3) | .38 | 1.02±2.00 (n=16) | −0.72±2.35 (n=25) | .02 |
| LV fractional shortening Z-score | −0.51±2.92 (n=29) | −0.68±1.88 (n=4) | .91 | −0.53±2.79 (n=33) | 1.64*±3.76 (n=38) | .008 |
| LV End-diastolic posterior wall thickness Z-score | 0.89±2.64 (n=12) | 0.59*±0.02 (n=2) | .88 | 0.85±2.43 (n=14) | −0.19±4.08 (n=24) | .40 |
| LV End-diastolic septal wall thickness Z-score | 0.26±1.71 (n=10) | 1.29*±2.47 (n=2) | .48 | 0.44±1.76 (n=12) | −0.22±1.72 (n=20) | .31 |
| LV Mass Z-score | 1.11±2.19 (n=11) | 0.97±0.08 (n=2) | .93 | 1.09±2.00 (n=13) | −0.69±2.9 (n=23)2 | .06 |
Mean change in Z-score significantly differs from zero, P<.05.
Z-score adjusted for age (for LV fractional shortening) or body surface area (all other measurements).
LV = left ventricular
EDD = end-diastolic dimension
ESD = end-systolic dimension
Time to Death or Transplant after Cardiomyopathy Diagnosis
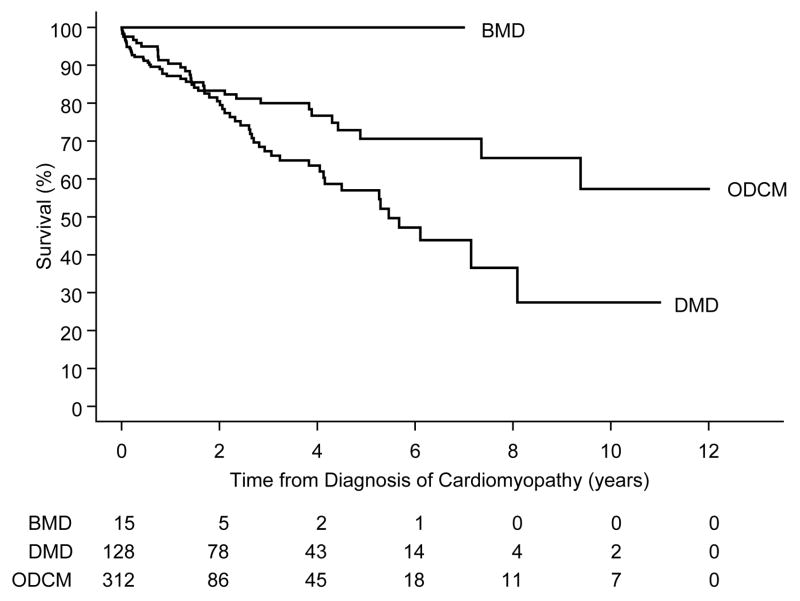
Forty-seven cases with DMD and none with BMD died (median follow-up time of non-transplanted survivors, 3.3 years). In the DMD group, 25% and 50% died within 2.4 years and 5.5 years after cardiomyopathy was diagnosed, respectively (Figure 1; log-rank P=.06). The striking difference in survival but lack of statistical significance was probably due to the shorter follow-up time of the BMD cases, many of whom underwent heart transplantation.
Figure 1.
Survival from time of diagnosis of cardiomyopathy, by diagnosis (P=.06).
DMD = Duchenne muscular dystrophy, BMD = Becker muscular dystrophy, ODCM = other dilated cardiomyopathy
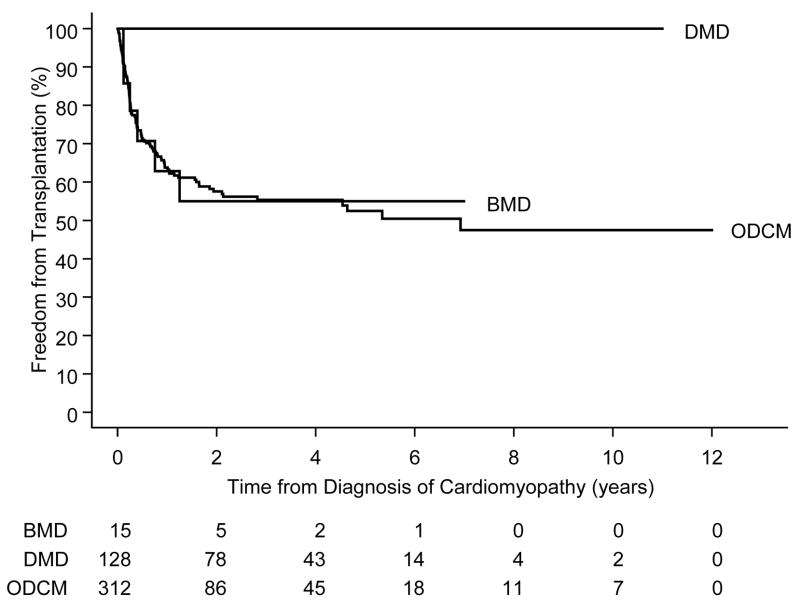
Six of the 15 BMD cases but none of the 128 in the DMD group underwent heart transplantation. In the BMD group, 25% underwent heart transplantation within 5 months after cardiomyopathy diagnosis (Figure 2). Because the DMD and BMD groups experienced roughly the same proportion of events overall, there was no difference in the composite endpoint (death or transplant) between the two groups (Figure 3; log-rank P=.25), but weighting earlier differences more heavily there was longer event-free survival in the DMD group (Gehan-Wilcoxon test, P=.03). The 5-year event-free rates were 55% (95% C.I. 28%–82%) and 57% (95% C. I. 46%–68%) for the children with DMD and BMD, respectively.
Figure 2.
Freedom from transplantation following diagnosis of cardiomyopathy, by diagnosis (P<.001).
DMD = Duchenne muscular dystrophy, BMD = Becker muscular dystrophy, ODCM = other dilated cardiomyopathy
Figure 3.
Freedom from death and transplantation following diagnosis of cardiomyopathy, by diagnosis (log-rank P=.25; and Gehan-Wilcoxon, P=.03).
DMD = Duchenne muscular dystrophy, BMD = Becker muscular dystrophy, ODCM = other dilated cardiomyopathy
Cause of Death
Sixty-four percent (30 of 47) of the deaths had a known cause. Three of the 30 deaths with a specified cause (10%) were due to ventricular arrhythmia, with one noted as sudden. Two of 30 were due to cardiogenic shock (7%). Five deaths (17%) were primarily respiratory in nature (two cardiopulmonary arrests secondary to airway obstruction, one pneumonitis, two respiratory failure). Twenty deaths were due to CHF, with respiratory failure noted as a secondary cause in all nine for whom respiratory failure was specifically queried. One of the 20 CHF deaths was also noted to be secondarily due to sepsis, one to liver stasis, and one to renal failure.
Medical Therapy
The use of digoxin and diuretics was similar for the DMD and BMD groups at cardiomyopathy diagnosis (68% vs. 60%, P=.57). The use of anti-arrhythmics, ACE inhibitors, and calcium channel blockers was less common (3%–38%) and similar in the two groups. No beta-blocker use was documented. Two years after cardiomyopathy diagnosis, 81% of DMD children and 57% of BMD children used digoxin and diuretics (P=.16 for DMD-BMD differences).
Comparison of DMD+BMD with ODCM Children & Adolescents
At presentation, the ODCM group was significantly more likely to have CHF (P<.001) and to receive anti-congestive therapy (P=.006) compared with the DMD+BMD group. The DMD+BMD group had a significantly higher familial incidence of genetic syndromes (defined to include muscular dystrophy) than did the ODCM group (41% vs. 3%, P<.001).
Echocardiographic Findings
At cardiomyopathy diagnosis, the DMD+BMD group had less dilation than the ODCM group (LVEDD Z-scores, 1.41±1.92 vs. 3.37±2.29, P<.001), less impaired LVFS (median Z-scores −5.20 vs. −7.80, P<.001), and closer-to-normal mean LV mass Z-scores (−0.41 ±1.81 vs. 1.67±2.14, P<.001) (Table 2). Median LV posterior wall thickness to EDD ratio was nearly identical for the DMD+BMD and ODCM groups.
LV end-diastolic and systolic dimensions increased over two years in the DMD+BMD group but not in the ODCM group (mean EDD change score 1.39±1.81 vs. −0.22±1.70, P=.002). LVFS did not change in the DMD+BMD group, but improved in the ODCM group (mean Z-score change −0.53±2.79 vs. 1.64±3.76, P=.008).
Time to Death or Transplant after Cardiomyopathy Diagnosis
Survival after cardiomyopathy diagnosis did not differ significantly between the ODCM group and the DMD+BMD group (log-rank P=.08). The 5-year survival rate was 71% (95% C.I. 61%–80%) in the ODCM group (Figure 1) and was 59% in the DMD+BMD group (95% C.I. 49%–70%).
Medical Therapy
At the time of cardiomyopathy diagnosis, anti-congestive therapy use was significantly higher in the ODCM group than in the DMD+BMD group (80% vs. 67%, P=.006). However, two years after diagnosis, the rates were similar (73% vs. 79%, P=.77). The ODCM group received anti-arrhythmic and ACE inhibition therapy twice as often at the time of cardiomyopathy diagnosis (P=.045 and <.001 respectively) compared with the DMD+BMD group. Calcium channel blockers and beta-blockers were rarely used at the time of diagnosis of cardiomyopathy.
DISCUSSION
Echocardiographic Findings
Patients with BMD do not usually experience marked cardiomyopathy as children, but when they do they present with a higher degree of mitral and tricuspid regurgitation, LV dilation, and CHF than do children with DMD. Because BMD children have fewer neuromuscular symptoms until they reach older ages, they may not be diagnosed until they present with CHF or cardiac symptoms.
Children and adolescents with ODCM had a higher incidence of CHF at the time of diagnosis than did those in the DMD+BMD group. The ODCM group included cases of myocarditis and acute cardiac decompensation at the time of cardiomyopathy diagnosis. Those with ODCM typically do not have other disorders that bring them to medical attention, so it is not surprising that they more often present with CHF than is found in children with DMD or BMD, who may undergo longitudinal echocardiographic surveillance for cardiomyopathy.
Medical Therapy
At the time of diagnosis, the DMD and BMD children had less LV dilation and higher LV FS, but thinner LV walls with lower LV mass than did the ODCM children. For both groups, the LV wall thickness to end-diastolic dimension is low, which may reflect a limited hypertrophic response of the LV. Children with DMD or BMD have ongoing cardiomyocyte loss, which is less likely in those with ODCM, except for those with active myocarditis. For many children with DMD or BMD there is a limitation of compensatory cardiomyocyte hypertrophy to keep up with progressive LV dilation in conjunction with the progressive loss of cardiomyocytes. These abnormalities in ventricular mechanics result in increased LV wall stress that may alone or in combination with progressive neuromuscular abnormalities be why children with DMD and BMD do poorly clinically over time. This may be particularly an issue for the small group of children with BMD who have well preserved skeletal muscle function but who develop CHF during adolescence. This thesis also suggests that we should look for new treatments that are distinct from the standard ones (beta-blockers and ACE inhibitors) now used that may reduce cardiomyocyte loss or LV dilation, or increase compensatory cardiomyocyte hypertrophy.9
ACE inhibitors and beta-blockers improve survival and clinical status in adults with DCM and are also recommended for children with dilated cardiomyopathy.10 These therapies are reported to have at least short-term benefit in this population, decreasing CHF symptoms and improving LV ejection fraction and neurohormonal state.11,12 When started early in the course of cardiac dysfunction, these therapies may also slow progression of cardiac abnormalities.13,14, In our DMD+BMD group, ACE inhibitors and beta-blockers were prescribed between 1990 and 2005 in fewer than 40% at the time of cardiomyopathy diagnosis, despite 31% having CHF symptoms. Educating caregivers about this consensus may improve long-term outcomes including quality of life in these patients.
Time to Death or Transplant after the Diagnosis of Cardiomyopathy
After diagnosis of cardiomyopathy, the DMD group had a higher mortality rate than the BMD and ODCM cohorts. The BMD group had a transplant rate that was higher than that of DMD patients and similar to that of the ODCM group. Children with BMD had a more rapid short-term decline in event-free survival than did children with DMD; with the six observed transplants in the 15 BMD patients occurring within two years of cardiomyopathy diagnosis.
Three factors influencing the difference in survival of patients with DMD and BMD are the level of physical activity, which is higher in patients with BMD; respiratory muscle function, which is better in patients with BMD; and suitability for transplantation. We observed no heart transplants in 128 DMD patients. Children with DMD have been successful recipients of heart transplants15, but their neuromuscular disease makes them less acceptable candidates than other children with cardiomyopathy. Therefore, the higher mortality rate we observed in children with DMD is in part due to this contraindication.
Myocardial Involvement and Genetics
In BMD, skeletal muscle involvement is considered to have a later onset than in DMD6, and therefore we speculate that cardiac muscle involvement may also have a later onset in BMD than in DMD. Although complications of cardiomyopathy are usually the cause of death in patients with BMD16, many BMD patients have a relatively late onset of cardiac symptoms.4
In this study, by definition, all children met the criteria for cardiomyopathy before adulthood. This sample of children may have more severe and possibly earlier-onset cardiomyopathy than the general population of neuromuscular disease patients. This difference may be the result of environmental effects or other cofactors, but it also raises the question of whether the genetic defect is different in this subset of children and therefore predisposes them to earlier onset of cardiomyopathy than other children with BMD or DMD. Jeffries et al. have reported mutation-dependent differences in the severity of cardiomyopathy in children with muscular dystrophy.13 In our cohort, there may be two separate groups of children with BMD, one with very rapid cardiac deterioration and one with much more stable LV function. Although the PCMR did not collect data about neuromuscular status, cardiomyopathy may have a high prevalence in children with sub-clinical BMD.17 Because the progression of cardiac disease may not parallel neuromuscular function in these children, we recommend cardiac evaluation in the young and mid-teenage years to identify those who are prone to early cardiac decompensation. These children may benefit from early medical therapy, or be identified as candidates for heart transplantation.
Limitations of the Study
Although PCMR data collection was standardized, it was obtained by chart review and complete family history and medical therapy data were only for a subset. Some comparisons between the DMD and BMD cohorts had low statistical power due to the small number of BMD patients.
The PCMR by design excluded children known to have dystrophinopathies but who did not manifest echocardiographic evidence of cardiomyopathy, as well as those who developed cardiomyopathy after age 18 years. Therefore, these data cannot be used to determine the natural history of DMD and BMD cardiomyopathy because much of the natural history occurs before the threshold for inclusion in the PCMR is reached. We must review data from children below the threshold for inclusion if we are to develop therapies to delay, ameliorate, or prevent progression to the point of irreversible injury.
Although our study is not a natural history study it represents one of the largest multi-center cohorts of these patients presenting to cardiologists and defines real-world clinical practice. As such, it validates the concerns raised by both the European Neuro Muscular Center (ENMC) and AAP that: (1) affected individuals do not come to the attention of the cardiac specialist until late in the disease process when then clinical manifestations of cardiac dysfunction become evident; (2) echocardiographic and electrocardiographic screening of affected individuals at diagnosis and serially thereafter as recommended is minimal; (3) the use of cardiac therapies to potentially alter the course of cardiac involvement in affected individuals is limited; and (4) the evaluation of more sophisticated tools (i.e., cardiac magnetic resonance for fibrosis detection18) to understand cardiovascular involvement in affected patients is currently limited but needed.4,5
Summary
We have shown that a subset of patients with DMD and BMD has clinically important cardiac dysfunction during childhood, and that BMD patients have more severe LV dilation and valvular regurgitation at the time of presentation with cardiomyopathy than do DMD patients. The mortality rate for DMD patients with cardiomyopathy is significantly worse than that of BMD patients (who often undergo transplant) and similarly aged myocarditis and idiopathic dilated cardiomyopathy patients; however, rates of cardiac medical therapy are lower in neuromuscular disease patients compared with patients with other types of dilated cardiomyopathy. As the importance of cardiac dysfunction in the morbidity and mortality of these children has been recognized, guidelines have been developed for their care.4,5 Practitioners need to be aware of both the problem of myocardial involvement in patients with DMD and BMD and the therapies available. Until etiology-specific genetic or cell-based therapies are developed, work in this area should concentrate on (1) implementing the complementary consensus recommendations for clinical care and research developed by neuromuscular specialists4 and cardiovascular specialists5 and (2) identifying these children with cardiomyopathy so currently available treatments can be utilized.19
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute/DHHS grant R01 HL53392, the Children’s Cardiomyopathy Foundation, and the Muscular Dystrophy Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cox GF, Kunkel LM. Dystrophies and heart disease. Curr Opin Cardiol. 1997;12:329–43. [PubMed] [Google Scholar]
- 2.Finsterer J, Stollberger C. The heart in human dystrophinopathies. Cardiology. 2003;99:1–19. doi: 10.1159/000068446. [DOI] [PubMed] [Google Scholar]
- 3.Towbin JA, Hejtmancik JF, Brink P, et al. X-linked dilated cardiomyopathy. Molecular genetic evidence of linkage to the Duchenne muscular dystrophy (dystrophin) gene at the Xp21 locus. Circulation. 1993;87:1854–65. doi: 10.1161/01.cir.87.6.1854. [DOI] [PubMed] [Google Scholar]
- 4.Bushby K, Muntoni F, Bourke JP. 107th ENMC International Workshop: the management of cardiac involvement in muscular dystrophy and myotonic dystrophy. 7th-9th June 2002, Naarden, the Netherlands. Neuromusc Disord. 2003;13:166–172. doi: 10.1016/s0960-8966(02)00213-4. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics, Section on Cardiology and Cardiac Surgery. Cardiovascular health supervision for individuals affected by Duchenne or Becker muscular dystrophy. Pediatrics. 2005;116:1569–1573. doi: 10.1542/peds.2005-2448. [DOI] [PubMed] [Google Scholar]
- 6.Grenier MA, Osganian SK, Cox GF, et al. Design and implementation of the North American Pediatric Cardiomyopathy Registry. Am Heart J. 2000;139(2 Pt 3):S86–95. doi: 10.1067/mhj.2000.103933. [DOI] [PubMed] [Google Scholar]
- 7.Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296(15):1867–76. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 8.Sluysman T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99(2):445–57. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 9.Dellefave LM, McNally EM. Cardiomyopathy in neuromuscular disorders. Prog Pediatr Cardiol. 2007;24:35–46. [Google Scholar]
- 10.International Society for Heart and Lung Transplantation. Practice guidelines for management of heart failure in children. J Heart Lung Transplant. 2004;23:1313–1333. doi: 10.1016/j.healun.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa Y, Bach JR, Minami R. Cardioprotection for Duchenne’s muscular dystrophy. Am Heart J. 1999;137:895–902. doi: 10.1016/s0002-8703(99)70414-x. [DOI] [PubMed] [Google Scholar]
- 12.Duboc D, Meune C, Lerebours G, Devaux JY, Vaksman G, Bécane HM. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol. 2005;45:855–7. doi: 10.1016/j.jacc.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 13.Jeffries JL, Eidem BW, Belmont JW, et al. Genetic predictors and remodeling of dilated cardiomyopathy in muscular dystrophy. Circulation. 2005;112:2799–2804. doi: 10.1161/CIRCULATIONAHA.104.528281. [DOI] [PubMed] [Google Scholar]
- 14.Rees W, Schuler S, Hummel M, Hetzer R. Heart transplantation in patients with muscular dystrophy associated with end-stage cardiomyopathy. J Heart Lung Transplant. 1993;12(5):804–7. [PubMed] [Google Scholar]
- 15.Tsirka AE, Trinkaus K, Chen SC, et al. Improved outcomes of pediatric dilated cardiomyopathy with utilization of heart transplantation. J Am Coll Cardiol. 2004;44:391–7. doi: 10.1016/j.jacc.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 16.Yazawa M, Ideda S, Owa M. A family of Becker’s progressive muscular dystrophy with severe cardiomyopathy. Eur Neurol. 1987;27:13. doi: 10.1159/000116122. [DOI] [PubMed] [Google Scholar]
- 17.Melacini P, Fanin M, Danieli GA, et al. Myocardial involvement is very frequent among patients affected with subclinical Becker’s muscular dystrophy. Circulation. 1996;94:3168–3175. doi: 10.1161/01.cir.94.12.3168. [DOI] [PubMed] [Google Scholar]
- 18.Silva MC, Meira ZM, Gurgel Giannetti J, et al. Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. J Am Coll Cardiol. 2007;49:1874–9. doi: 10.1016/j.jacc.2006.10.078. [DOI] [PubMed] [Google Scholar]
- 19.Lipshultz SE. Realizing optimal care for children with cardiovascular disease: Funding challenges and research approaches. Prog Pediatr Cardiol. 2005;20:71–90. [Google Scholar]