Abstract
Background
Anesthetic drugs administered to immature animals may cause neurohistopathologic changes and alterations in behavior. We studied association between anesthetic exposure prior to age 4 and the development of reading, written language and math learning disabilities (LD).
Methods
This was a population-based, retrospective birth cohort study. The educational and medical records of all children born to mothers residing in five townships of Olmsted County, Minnesota from 1976–1982 and who remained in the community at 5 years of age were reviewed to identify children with LD. Cox proportional hazards regression was used to calculate hazard ratios for anesthetic exposure as a predictor of LD, adjusting for gestational age at birth, gender, and birth weight.
Results
Of the 5,357 children in this cohort, 593 received general anesthesia before age 4. Compared to those not receiving anesthesia (N=4,764), a single exposure to anesthesia (N=449) was not associated with an increased risk of LD (hazard ratio =1.0, 95% CI 0.79–1.27). However, children receiving 2 anesthetics (N=100) or ≥3 anesthetics (N=44) were at increased risk for LD (hazard ratio =1.59, 95% CI 1.06–2.37, and hazard ratio =2.60, 95% CI 1.60–4.24, respectively). The risk for LD increased with longer cumulative duration of anesthesia exposure (expressed as a continuous variable) (P=0.016).
Conclusion
Exposure to anesthesia was a significant risk factor for the later development of LD in children receiving multiple, but not single anesthetics. We cannot determine whether anesthesia itself may contribute to LD, or whether the need for anesthesia is a marker for other unidentified factors that contribute to LD.
Introduction
Exposure to alcohol and some anesthetic and sedative drugs cause histopathological changes in the developing brains of animals.1–3 Implicated drugs include N-methyl-D-aspartate glutamate receptor antagonists (e.g. ketamine and nitrous oxide) and agents with gamma-aminobutyric acid-mimeticA properties (e.g. pentobarbital, diazepam, isoflurane, halothane, and propofol). Some studies suggest that even relatively brief single exposures triggers changes, especially when combinations of agents are employed.1–6 In one report, histological neurodegeneration was associated with a diminished capacity to retain learned behaviors.7 It is not known whether findings in rodent models can be extrapolated to humans, but emerging histologic data in non-human primates8 tends to confirm findings in rodents. These findings have engendered considerable concern among the Food and Drug Administration and others.5 Except for case series reporting developmental outcomes of critically-ill neonates and children undergoing repair of congenital heart disease,9–12 which have multiple limitations, there are no data that can yield insight into whether exposure to anesthesia and surgery during human development causes clinically relevant impairment in neural development.
One challenge to determining whether exposure to anesthesia and surgery in early life impairs neural development is defining relevant outcomes. Learning disabilities (LD) may be an appropriate outcome measure. Children with LDs experience problems with one or more of the basic psychological processes involved in understanding or in using spoken or written language, which may manifest itself in an imperfect ability to listen, think, speak, read, write, spell or to do mathematical calculations. Because learning disabilities are routinely sought on the basis of standardized individualized testing in educational settings, and because of the relatively high incidence rate of LD,13 this outcome is available in large populations of children.
The purpose of the present study was to determine if there was an association between exposure to anesthesia during the first four years of life and the development of any learning disability in a birth cohort of children in Olmsted County, Minnesota.
Materials and Methods
The Mayo Clinic and Olmsted Medical Center Institutional Review Boards (both Rochester, Minnesota, USA) approved this study. A birth cohort of children born in Rochester, MN identified in prior work by the authors13–16 formed the basis of the present study. All children (n = 8,548) born between January 1, 1976 and December 31, 1982 to mothers residing at the time of delivery in the five Olmsted County, Minnesota, townships (comprising Minnesota Independent School District No. 535, the Rochester public school system) were identified through computerized birth certificate information obtained from the Minnesota Department of Health, Division of Vital Statistics. To ascertain vital status (still living in Rochester, moved, or deceased), for each member of the birth cohort during the 1995–1996 school year, resources available from the Rochester Epidemiology Project,17 Minnesota Independent School District No. 535, the Reading Center/Dyslexia Institute of Minnesota, and the Minnesota Department of Health were utilized. Children who left Olmstead County before age 5 (i.e., moved or died, n = 2,830) were not included in the final study cohort. Through the Rochester Epidemiology Project, all diagnoses and surgical procedures recorded at all Rochester medical facilities are indexed for automated retrieval. This diagnostic index expedites retrieval of the unit (or dossier) medical record, which includes the history of all encounters in the hospital, community and ambulatory medical and social services, emergency department, outpatient clinics, and home visits as well as laboratory and psychological test results from birth until patients no longer reside in the community. The evaluation and instructional resources of Minnesota Independent School District No. 535, the school system for the city of Rochester, are of high quality, and the district has a long tradition of excellent care and management of children with all types of handicapping conditions, including LD. Through a contractual research agreement, all public (19 primary, 3 junior high, 3 high schools) and nonpublic (12 primary, 10 junior high, 4 high schools) schools gave permission to access their richly documented cumulative educational records for every child from this birth cohort. Under a second research agreement permission was obtained to access the resources of the privately owned Reading Center/Dyslexia Institute of Minnesota, the only private tutoring agency in the community during the years relevant to this study. The Reading Center/Dyslexia Institute of Minnesota files included a pool of some 3,000 evaluations and outcomes of tutorial instruction that spanned nearly 50 years. All of these records, including the results of all individually administered IQ (primarily age-appropriate Wechsler scales) and achievement (primarily Woodcock-Johnson tests) tests, medical, educational, and socioeconomic information were abstracted by trained personnel, using detailed data abstraction protocols.
Identification of Learning Disabilities
Learning disabilities (including reading, written language and math disabilities) were diagnosed using research criteria based on 3 formulas. In each of the following formulas, X is equal to the study subject’s IQ score, and Y represents the predicted standard score from the achievement test. The regression formula–Minnesota (RFM), Y<17.40+ 0.62X, is issued by the Minnesota Department of Education.18 Children classified as having LD by this formula had standard scores in academic achievement that were more than 1.75 SD below their predicted standard score from an individually administered measure of cognitive ability (IQ). The value 0.62 represents the correlation between IQ and achievement used in the formula from the state of Minnesota. The discrepancy (DS) nonregression method was used in Minnesota Independent School District No. 535 before 1989 and included the school years of the children in our birth cohort. By using this approach, differences between standard scores on measures of intelligence and aptitude and measures of test achievement that were believed to be important varied by grade as follows: (1) kindergarten-3rd grade, 15 or more standard score points difference, with achievement lower; (2) 4th–6 th grade, 19 or more points difference, achievement lower; and (3) 7th –12 th grade, 23 or more points difference. Finally, the low-achievement method (X≥80 (aptitude) and Y≤90 (achievement) represents a recent concept in identifying LD independent of measured cognitive ability, assuming that cognitive ability is at least in the low average range.19 Children meeting the criteria prior to age 19 for at least one of the three LDs (reading, written language, and math disabilities) using IQ and achievement scores obtained within the same calendar year were identified as LD cases regardless of presence or absence of any comorbid conditions.13
Identification of cohort members exposed to general anesthesia before age of 4
We identified all members of the birth cohort who underwent any type of surgery or diagnostic procedure requiring general anesthesia prior to their 4th birthday, using the Mayo Clinic Surgical Information Retrieval System and a similar resource for procedures performed at Olmsted County Medical Center. The choice of age range was based on the analogous developmental stage of animal models in which anesthetic effects on neurodevelopment have been shown.1,20 Procedures such as neonatal circumcision performed without anesthesia were excluded from review.
For the children undergoing anesthesia, the following information was abstracted: American Society of Anesthesiologists physical status (ASA PS) classification, type of surgery or procedure and urgency, total duration of anesthesia, number of anesthetic exposures, age(s) at which exposure occurred, anesthetic agents (inhalational, intravenous, sedatives), and co-morbidities (including syndromes that can be associated with mental retardation, congenital heart disease, and neurological diseases). For all children in the cohort (including those who did not receive anesthesia), sex, gestational age at birth, birth weight, APGAR (Activity, Pulse, Grimace, Appearance, Respiration) scores at 1 and 5 min (APGARs were available for children born 1980–1982), complications of pregnancy, complications of labor and delivery, the number of births (multiple or single), need for induced labor, as well as mother’s and father’s age and level of education (<12 years, 12 years, >12 years), were available from birth certificates.
Statistical analysis
The primary outcome for the current analysis was LD based on individually administered IQ and academic achievement test scores using any of the 3 standard formulas for determining the presence of reading written language or math LDs. The primary risk factor of interest for this investigation was exposure to general anesthesia under the age of 4. Preliminary analyses were performed to compare demographic, pregnancy and delivery, and parental characteristics between those who were exposed versus unexposed to general anesthesia under the age of 4 using the two-sample t-test (or rank sum test) for continuous variables and the chi-square test (or Fisher’s exact test) for categorical variables.
Individuals were followed from birth until the date they first met the LD criteria using any of the 3 standard formulas for determining the presence of reading, written language and math LDs. Cumulative incidence rates of LD were calculated according to the method of Kaplan and Meier with data censored at the initial occurrence of emigration, death, last follow-up date, or the age of 19 years. Proportional hazards regression was used to assess whether anesthetic exposure was a risk factor for LD. For these analyses, anesthetic exposure was quantified as any exposure to general anesthesia under the age of 4 (yes vs. no), the number of exposures to general anesthesia under the age of 4 (none, 1, 2, 3 or more), and the cumulative duration of exposure (treated as both a continuous variable and also categorically using 30-minute intervals). Both unadjusted and adjusted analyses were performed. In all cases, separate models were used to evaluate the different anesthesia exposure variables. The covariates that were included in the adjusted analyses include gestational age, sex, and birth weight. In the adjusted analysis, only those individuals for whom complete covariate information was available were included. Results were summarized using hazard ratio estimates and corresponding 95% confidence intervals. In all cases, two-tailed P values less than 0.05 were consideredto be statistically significant. Analyses were performed using SAS statistical software (Version 9.1; SAS Institute, Inc., Cary, NC).
Results
Between 1976 and 1982 there were 8,548 children born in the five Olmsted County, Minnesota townships comprising Minnesota Independent School District No. 535, and 5,718 of these children still resided in the community at 5 years of age. Of these, 19 individuals were diagnosed with severe mental retardation and were excluded, as were 342 patients who denied research authorization for the use of their medical records. Thus, 5,357 children are included in the present report. Of those, 593 underwent procedures requiring general anesthesia before age 4. In comparison to unexposed children, those exposed to anesthesia before age 4 had lower birth weight (P<0.001), lower gestational age (P = 0.001), and were more likely to be male (P < 0.001) (Table 1, and table, Supplemental Digital Content 1, which illustrates birth, maternal, and paternal characteristics). All these factors were subsequently used as adjustors in multivariate analysis. Children exposed to anesthesia also had mothers with higher levels of maternal education (P=0.039); however, this factor was not included as an adjustor in subsequent analysis because these data were missing for approximately 10% of children. APGAR scores were not different between two groups for those in whom data was available. The peripartum complications of those exposed or not to anesthesia were similar (Table 2 and table, Supplemental Digital Content 2, which illustrates peripartum complications from birth certificates.), with the exception that the mothers of those exposed to anesthesia experienced a slightly higher rate of peripartum hemorrhage and prolonged labor.
Table 1.
Birth, maternal, and paternal characteristics
| No Anesthesia (N=4,764) | Anesthesia (N=593) | ||||
|---|---|---|---|---|---|
| Characteristic | N* | Summary Statistics‡ | N* | Summary Statistics‡ | P |
| Sex | 4,764 | 593 | <0.001 | ||
| Female | 2,357 (49.5) | 207 (34.9) | |||
| Male | 2,407 (50.5) | 386 (65.1) | |||
| Birth Weight, g | 4,755 | 3,476 ± 531 | 592 | 3,396 ± 655 | <0.001 |
| < 2500 g | 185 (3.9) | 43 (7.3) | |||
| ≥2500 g | 4,570 (96.1) | 549 (92.7) | |||
| Gestational age, weeks | 4,467 | 40.0 ± 2.0 | 558 | 39.7 ± 2.6 | 0.001 |
| <32 | 25 (0.6) | 17 (3.0) | |||
| 32 to <37 | 254 (5.7) | 41 (7.3) | |||
| ≥37 | 4,188 (93.7) | 500 (89.6) | |||
| APGAR score at 1 min† | 1,446 | 7.9 ± 1.4 | 201 | 8.0 ± 1.3 | 0.275 |
| APGAR score at 5 min† | 1,447 | 9.1 ± 0.8 | 201 | 9.0 ± 0.8 | 0.163 |
| Number at birth | 4,764 | 593 | 0.237 | ||
| Single | 4,677 (98.2) | 578 (97.5) | |||
| Twins | 87 (1.8) | 15 (2.5) | |||
| Induction of labor | 4,739 | 590 | 0.286 | ||
| No | 3,729 (78.7) | 453 (76.8) | |||
| Yes | 1010 (21.3) | 137 (23.2) | |||
| Mother’s education | 4,356 | 536 | 0.039 | ||
| <12 years | 294 (6.7) | 21 (3.9) | |||
| 12 years | 1,482 (34.0) | 192 (35.8) | |||
| >12 years | 2,580 (59.2) | 323 (60.3) | |||
| Father’s education | 4,111 | 505 | 0.127 | ||
| <12 years | 195 (4.7) | 14 (2.8) | |||
| 12 years | 1,250 (30.4) | 160 (31.7) | |||
| >12 years | 2,666 (64.9) | 331 (65.5) | |||
| Mother’s age, years | 4,764 | 26.5 ± 4.7 | 593 | 26.8 ± 4.5 | 0.182 |
| Father’s age, years | 4,515 | 28.8 ± 5.4 | 568 | 29.0 ± 5.2 | 0.369 |
mean ± SD or N (%)
Number with information available for the given characteristic. APGAR (Activity, Pulse, Grimace, Appearance, Respiration) scores.
Data were available for 1980 – 1982 only.
Table 2.
Peripartum complications from birth certificates
| No Anesthesia (N=4,764) |
Anesthesia (N=593) |
P | |
|---|---|---|---|
| Characteristic | No. (%) | No. (%) | |
| Complication of pregnancy | |||
| Hemorrhage | 40 (0.8) | 11 (1.9) | 0.024 |
| Abnormalities of placenta and fetal membranes | 19 (0.4) | 2 (0.3) | 1.000 |
| Preeclampsia | 115 (2.4) | 19 (3.2) | 0.263 |
| Preeclampsia superimposed on preexisting hypertensive disease | 4 (<0.1) | 0 (0.0) | 1.000 |
| Eclampsia | 3 (<0.1) | 0 (0.0) | 1.000 |
| Complication of labor or delivery | |||
| Hemorrhage | 33 (0.7) | 6 (1.0) | 0.436 |
| Retained placenta (with or without hemorrhage) | 32 (0.7) | 3 (0.5) | 1.000 |
| Early postpartum hemorrhage | 28 (0.6) | 4 (0.7) | 0.775 |
| Fetopelvic disproportion | 91 (1.9) | 15 (2.5) | 0.346 |
| Dystocic position of fetus | 424 (8.9) | 48 (8.1) | 0.590 |
| Prolonged labor of other origin | 381 (8.0) | 73 (12.3) | <0.001 |
| Laceration of perineum | 149 (3.1) | 16 (2.7) | 0.705 |
| Umbilical cord complication | 54 (1.1) | 9 (1.5) | 0.416 |
N= number
The 593 children exposed to anesthesia underwent 875 procedures, with 449 (75.7%) having a single procedure (Table 3). Of the children exposed to anesthesia, 438 (74%) were classified as ASA PS 1 (Table 3). Types of surgeries are shown in Table 4. Most anesthetics included halothane (88%) and nitrous oxide (91%); 9% included ketamine (Table 5).
Table 3.
Patient and anesthesia characteristics (N=593)*
| Characteristic | N (%) |
|---|---|
| ASA physical status‡ | |
| 1 | 438 (73.9) |
| 2 | 124 (20.9) |
| 3 | 24 (4.0) |
| 4 | 7 (1.2) |
| Age at first exposure, years | |
| <1 | 195 (32.9) |
| 1 | 155 (26.1) |
| 2 | 131 (22.1) |
| 3 | 112 (18.9) |
| Anesthetic exposures (N) | |
| 1 | 449 (75.7) |
| 2 | 100 (16.9) |
| 3–4 | 31 (5.2) |
| 5–9 | 8 (1.3) |
| 10 or more | 5 (0.8) |
| Duration of anesthesia, minutes† | |
| Mean ± SD | 125 ± 227 |
| Median (IQR) | 75 (45, 120) |
ASA, American Society of Anesthesiologists; IQR, interquartile range. N, number; %, percentage; SD, standard deviation.
Data related to anesthesia characteristics were available for all patients; for all patients
For infants that underwent multiple anesthetics the highest ASA physical status is used. These 593 individuals underwent a total of 875 anesthetics (range 1 to 25 per person);
Data presented correspond to the cumulative total duration of anesthesia for each individual (N=593). The median duration per anesthetic (N=875) was 70 minutes (range 10 to 560).
Table 4.
Surgeries performed under the age of 4 (N=875)
| Overall | According to age at surgery | ||||
|---|---|---|---|---|---|
| < 1 year | 1 year | 2 year | 3 year | ||
| Type of Surgery, N (%) | N=875 | N=251 | N=246 | N=205 | N=173 |
| Cardiac | 15 (1.7) | 8 (3.2) | 6 (2.4) | 0 (0.0) | 1 (0.6) |
| Ear Nose and Throat | 229 (26.2) | 21 (8.4) | 57 (23.2) | 73 (35.6) | 78 (45.1) |
| General | 220 (25.1) | 114 (45.4) | 43 (17.5) | 35 (17.1) | 28 (16.2) |
| Neurosurgery | 9 (1.0) | 3 (1.2) | 3 (1.2) | 3 (1.5) | 0 (0.0) |
| Ophthalmology | 83 (9.5) | 23 (9.2) | 38 (15.4) | 9 (4.4) | 13 (7.5) |
| Oral Surgery | 10 (1.1) | 0 (0.0) | 5 (2.0) | 1 (0.5) | 4 (2.3) |
| Orthopedics | 115 (13.1) | 25 (10.0) | 43 (17.5) | 30 (14.6) | 17 (9.8) |
| Plastics | 56 (6.4) | 18 (7.2) | 21 (8.5) | 8 (3.9) | 9 (5.2) |
| Urology | 112 (12.8) | 29 (11.6) | 25 (10.2) | 36 (17.6) | 22 (12.7) |
| Other* | 26 (3.0) | 10 (4.0) | 5 (2.0) | 10 (4.9) | 1 (0.6) |
N, number;
Indicates diagnostic procedures, catheterization, angiography, and examination under anesthesia
Table 5.
Types of anesthetics used (N=875)*
| Variable | N (%) |
|---|---|
| Inhalational agents | |
| Isoflurane | 17 (1.9) |
| Halothane | 769 (87.9) |
| Enflurane | 14 (1.6) |
| Intravenous agents | |
| Sodium Thiopental | 46 (5.3) |
| Etomidate | 5 (0.6) |
| Ketamine | 77 (8.8) |
| Other | 32 (3.7) |
| Nitrous Oxide | 793 (90.7) |
| Diazepam | 12 (1.4) |
N, number; %, percentage
A total of 875 anesthetics were performed on 593 patients; Data related to anesthesia characteristics were available for all procedures
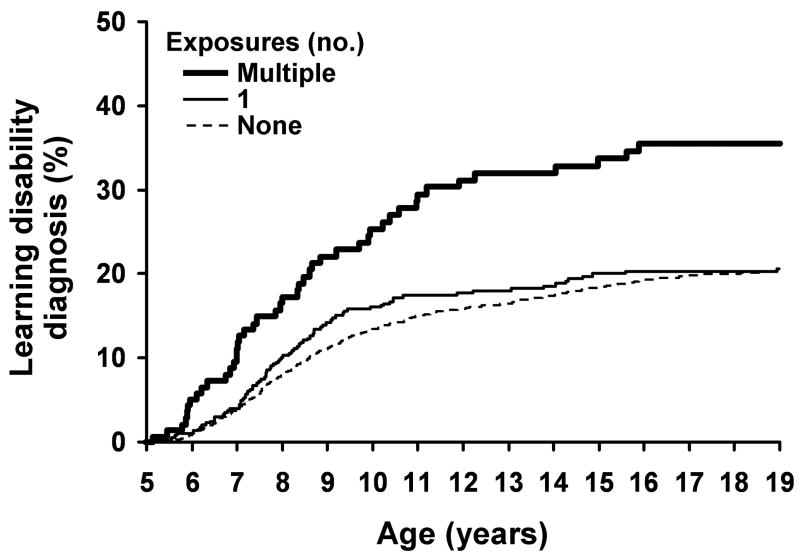
A total of 932 children developed a LD prior to age 19 as assessed by individually administered IQ and achievement tests scored using any of the 3 standard formulas [estimated cumulative incidence 20.5% (95% C.I. 19.3% to 22.7%)]. For both unadjusted and adjusted (for sex, birth weight, and gestational age) analyses, the risk for the development of LD (compared with children not exposed to anesthesia) increased (P < 0.001) with the number of exposures to anesthesia prior to age 4 (Table 6). The risk was not increased for the 449 children exposed to a single anesthetic (adjusted hazard ratio =1.00, 95% C.I. 0.79 to 1.27, Table 6). However, the risk was significantly increased for children exposed to 2 or more anesthetics (Table 6 and Figure 1). The estimated incidence of LD by age 19 was 20.0% (95% C.I. 18.8% to 21.3%) in those with no exposure to anesthesia, 20.4% (95% C.I. 16.3% to 24.3%) in those with single exposure, and 35.1% (95% C.I. 26.2% to 42.9%) in those with multiple exposures. The risk for LD was also increased with longer cumulative duration of anesthesia (P = 0.016 and P = 0.027 from adjusted analysis assessing cumulative duration of anesthesia as a continuous and categorical variable respectively; Table 6).
Table 6.
Effects of anesthetic exposures before the age of 4 on risk for developing learning disabilities.
| Unadjusted |
Adjusted* |
|||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% C.I. | P | Hazard Ratio | 95% C.I. | P | |
| Number of exposures | <0.001 | <0.001 | ||||
| 0 (N=4,764) | Reference | Reference | ||||
| 1 (N=449) | 1.05 | 0.84 to 1.32 | 1.00 | 0.79 to 1.27 | ||
| 2 (N=100) | 1.78 | 1.22 to 2.59 | 1.59 | 1.06 to 2.37 | ||
| 3 or more (N=44) | 2.50 | 1.55 to 4.04 | 2.60 | 1.60 to 4.24 | ||
| Total duration of anesthesia exposure | ||||||
| Continuous (per 30 min) | 1.02 | 1.00 to 1.03 | 0.011 | 1.02 | 1.00 to 1.03 | 0.016 |
| Categorical (30 minute intervals) | 0.004 | 0.027 | ||||
| No anesthesia (N=4,764) | Reference | Reference | ||||
| ≤30 minutes (N=95) | 0.93 | 0.56 to 1.55 | 0.94 | 0.56 to 1.60 | ||
| 31 to 60 minutes (N=135) | 0.80 | 0.51 to 1.26 | 0.74 | 0.46 to 1.20 | ||
| 61 to 90 minutes (N=135) | 1.50 | 1.06 to 2.14 | 1.40 | 0.97 to 2.02 | ||
| 91 to 120 minutes (N=87) | 1.45 | 0.94 to 2.24 | 1.36 | 0.89 to 2.10 | ||
| ≥120 minutes (N=141) | 1.65 | 1.19 to 2.29 | 1.56 | 1.11 to 2.19 | ||
| Any exposure | 0.014 | 0.067 | ||||
| No (N=4,764) | Reference | Reference | ||||
| Yes (N=593) | 1.27 | 1.05 to 1.53 | 1.20 | 0.99 to 1.46 | ||
C.I., confidence interval; N, number;
Adjusting for sex, birth weight (<2500, ≥2500) and gestational age (<32 weeks, 32 to <37 weeks, ≥37 weeks). Due to missing covariate information, only 5,020 individuals were included in the adjusted analysis.
Figure 1.
Cumulative percentage of learning disabilities diagnosis by the age of exposure shown separately for those that have 0, 1, or multiple anesthetic exposures under the age of 4.
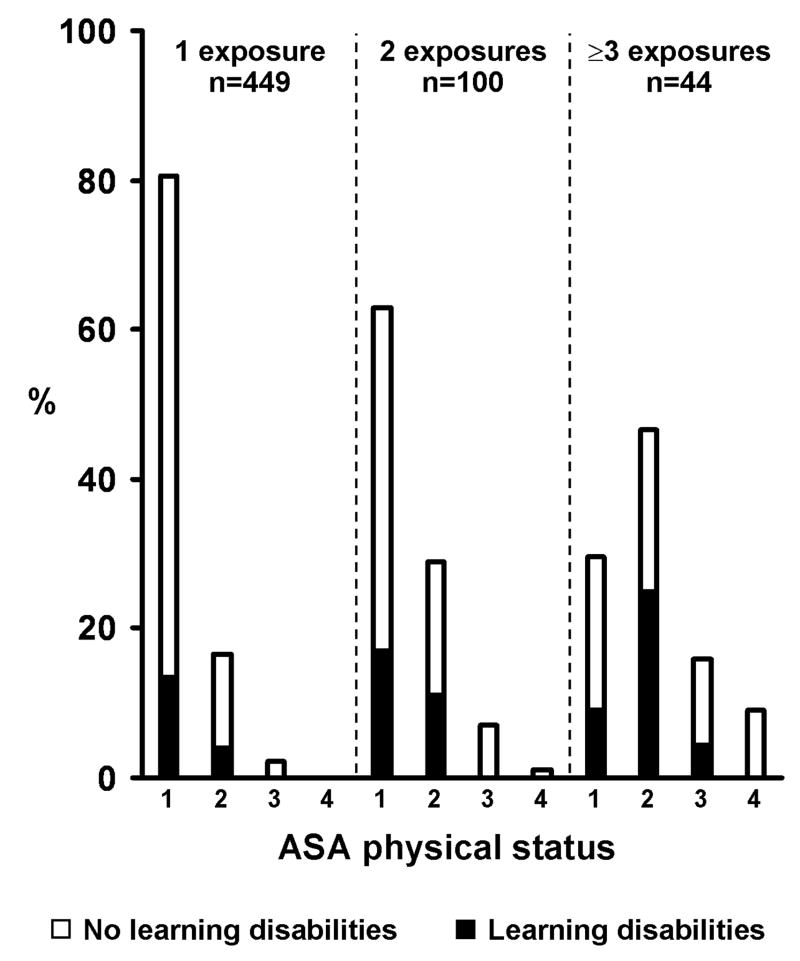
Children with multiple exposures to anesthesia were also more likely to be assigned a higher ASA PS compared to those with a single exposure, indicative of more severe comorbidity in the judgment of the anesthesia provider (Figure 2). However, when the analyses assessing the relationship between number of exposures and cumulative duration of exposures with development of LD were repeated after eliminating surgical patients with ASA PS ≥ 3 the findings were similar: anesthesia was a significant risk factor for the development of LD in children receiving multiple, but not single, anesthetics (data not shown). Detailed information regarding each of the 145 children who received multiple anesthetic exposures under the age of 4, including their medical diagnoses before and after age of 4, are provided in a web supplement (Table, Supplemental Digital Content 3, which illustrates diagnoses from the medical record in the 145 children who received multiple anesthetics prior to age 4.).
Figure 2.
Distribution of American Society of Anesthesiologists (ASA) physical status across patients with 1, 2 and ≥3 anesthetic exposures. Shading is used to indicate the percentage diagnosed with learning disability under the age of 19 versus not. For this presentation individuals who had incomplete follow-up are categorized based on information available through last follow-up prior to 19 years of age.
Discussion
In this population-based birth cohort, exposure to anesthesia prior to age 4 was a risk factor for the development of LD in children receiving multiple, but not single, anesthetics. The cumulative incidence of LD diagnosed by age 19 among those with repeated anesthetic exposures was almost twice as high (35.1%) compared to children not exposed to anesthesia (20.0%).
Late prenatal and early postnatal neural development is vulnerable to pharmacological and environmental influences.20–22 Exposure of immature animals to compounds with gamma-aminobutyric acid-mimetic receptor-agonist or N-methyl d-aspartate receptor-antagonist properties induces apoptotic degeneration of neurons in various brain regions.1–4,7,23 In particular, several drugs with sedative and anesthetic properties (including isoflurane, nitrous oxide, ketamine, benzodiazepines, halothane, and propofol) produce neurodegeneration when administered at sufficient doses and durations of exposure.7,8,23–26 In some animal models, these histological changes have been associated with impaired learning and memory assessed by water and radial arm mazes.7,27
Although these data have raised concerns and stimulated further research on the part of the Food and Drug Administration and others,5 it is not known whether exposure to anesthetics produces neuropathological or neurobehavioral sequelae in humans. A recent review28 summarized reports describing neurologic sequelae following surgery and anesthesia in children. Some studies of developmental outcomes in patients undergoing repair of congenital heart disease suggest neurologic impairment, although others do not.9–11,29–31 Similarly, studies comparing neurodevelopmental outcomes of neonates with patent ductus arteriosis and necrotizing enterocolitis managed either surgically or medically provide conflicting results.32–34 In the majority of these studies it was not possible to distinguish between potential effects of comorbidities, clinical characteristics and surgery from the effects of anesthesia, and the potential for selection bias is high.9,11,29,30,35,36
We examined a birth cohort originally created to study the incidence of learning disabilities in a population.13,15,37–39 This cohort provided several unique advantages. All these children resided in the same community, attended identified public and private schools, and received health care at one of two local facilities (Mayo Clinic and Olmsted County Medical Center), making it possible to review all available medical and educational records. These records, combined with rigorous definitions of LD, made it possible to perform a population assessment of a clinically-significant outcome that plausibly reflects the learning abnormalities observed in animal model post anesthesia. Nearly complete data available from birth records made it possible to control for potential confounding factors known to affect the frequency of LD (sex, gestational age, and birth weight). Complete anesthesia records were available for all procedures, and anesthetic technique was remarkably consistent. The distribution of surgical procedures reflected the population-based nature of the cohort, so is not weighted towards sicker patients undergoing more extensive procedures, as is the case with many studies at academic centers.
If exposure to anesthesia significantly affects neurodevelopment, there should be a dose-response relationship between exposure and a relevant outcome. We found evidence for such a dose-response relationship between anesthetic exposure and LD in two respects. First, risk was increased for children requiring multiple exposure (adjusted hazard ratio of 1.6 for two exposures, and 2.6 for ≥3 exposures, Table 6), but not for single exposures to anesthesia (adjusted hazard ratio of 1.0). Second, risk was increased for longer durations of anesthesia, reaching statistical significance for cumulative duration ≥ 120 min (Table 6). When anesthesia exposure was analyzed as a dichotomous variable (any exposure prior to age 4), exposure was a significant risk factor for LD in unadjusted (hazard ratio of 1.27) but not adjusted (hazard ratio of 1.20) analysis. The latter finding likely reflects the fact that the majority of children received only one exposure, which again was not associated with increased risk.
This study has several limitations. We cannot distinguish between potential effects of anesthesia itself and other factors associated with anesthesia such as the stress response to surgical injury. Perhaps most importantly, children requiring anesthesia may differ in important ways from those who do not, and such differences may affect risk for LD. Thus, we cannot exclude that requiring multiple anesthetics is a marker for conditions that increase LD risk, and that exposure to anesthetic drugs themselves is not causative. We adjusted for known factors (for which data were available) contributing to LD risk that differed between the groups with the exception of maternal education, because data was missing in a significant number of children. However, when analysis was repeated excluding children with missing data and including maternal education as a covariate, the qualitative results were the same (data not shown). Children requiring repeated procedures may have a higher burden of illness, which may increase risk for LD. For example, premature infants and children requiring repair of congenital heart defects may require more procedures. Indeed, children requiring multiple procedures were judged by their anesthesiologist to have more severe systemic disease, as indicated by higher ASA PS. We did not review the medical records of the children not requiring anesthesia, so cannot use medical diagnoses as covariates in the analysis – and ASA PS classification is not available in these children. However, among the 144 children receiving multiple anesthetics, LD was not more frequent in children with higher ASA status and among all children receiving anesthesia, LD frequency did not differ with ASA PS in univariate analysis (data not shown). Furthermore, the association of LD with repeated anesthetic exposure was still present when the analysis was repeated after eliminating surgical patients with ASA ≥ PS 3. These findings suggest that ASA PS was not associated with LD risk in our cohort, and that the increased frequency of LD observed in children receiving multiple anesthetics cannot be primarily attributed to those children with multiple medical problems.
The relative homogeneity of anesthetic technique is an experimental advantage, but conversely we cannot comment of the potential of anesthetics other than halothane26,40 and nitrous oxide 2,7 to cause neurodegeneration. Insufficient numbers of children received ketamine to perform a separate analysis for this drug.
In animal studies, there is a definite time window of vulnerability to the effects of anesthetic exposure (e.g., approximately 7 days after birth in rodents), thought to correspond to a period of maximal synaptogenesis.20,41 Thus, the ages chosen to study the effects of anesthetic exposure may be important. In humans, the period of synaptogenesis has been considered to extend through three years of age,20,41 which was the basis for our choice of the 4th birthday as the upper age limit to define anesthetic exposure. However, the correspondence between stages of human and animal neurodevelopment is controversial, and other authors suggest that the period corresponding to the time of greatest risk observed in animal models (e.g., approximately between 1–2 days before birth until 2 weeks after birth in rodents) is actually perinatal in humans (last month of gestation and first 6 months after birth).2 We repeated our analysis examining anesthetic exposure before the age of 2 (rather than age 4) on the risk of LD, and found similar results (data provided as figure, Supplemental Digital Content 4, which illustrates the cumulative percentage of learning disabilities by the age of exposure and table, Supplemental Digital Content 5, which illustrates the effects of anesthetic exposure before the age of 2 on risk for developing learning disabilities). We did not have sufficient numbers of cases to meaningfully examine a more restricted age range (e.g., infancy).
Another potential limitation is related to emigration from the original birth cohort of 8,548 children. Birth cohort studies can be biased due to migration from the community. For example, given the accessibility and quality of health care available in Rochester, children with a higher level of medical need may tend not to migrate and thus be overrepresented in the cohort. This could tend to bias the surgical group towards children with more severe illness. However, a previously published comparison of children who left the community before age 5 and those who stayed after age 5 (the usual age of school enrollment) indicates that the children included in the study are representative of the entire birth cohort.14 A further limitation related to the nature of this cohort is that in these years Rochester was a predominantly white, middle class community which may limit the generalization of these results to other populations.42
Finally, it remains to be determined whether LD is a relevant outcome measure for any potential neurotoxic effects of anesthesia in humans, recognizing that many other genetic, family, and socioeconomic factors may also impact LD. Based on the animal studies showing an association between the neurodegeneration caused by exposure to anesthetics and behavioral learning deficits,7 we argue that LD is a relevant endpoint in humans. It also remains to be determined whether the observed increase in the frequency of LD among children with multiple anesthetic exposures is specific to one type of LD (i.e., math, written language, and reading disabilities). For purposes of this analysis we chose a broad definition of LD to maximize the number of children with LD and thus the ability to detect effects. Analysis by specific type of LD would be of interest, but would be complicated by the overlap between types (i.e., some children have more than one type of LD), and the need for increased numbers of subjects to detect effect sizes of the magnitude noted in the current study. There are a wide variety of other neurodevelopmental outcomes that could be sought, but many require specialized testing that is difficult to administer in large population-based studies.
In conclusion, in this population-based birth cohort, exposure to anesthesia prior to age 4 was a significant risk factor for the later development of LD in children receiving multiple, but not single, anesthetics. These data cannot reveal whether exposure to anesthesia itself may contribute to the pathogenesis of LD, or whether the need for anesthesia is a marker for other unidentified confounding factors that contribute to LD. However, these results suggest that the possibility of potential adverse effects of repeated anesthetic exposures on human neurodevelopment cannot be excluded.
Supplementary Material
Acknowledgments
Funding Sources: All listed authors significantly contributed to the study design, data analysis, and discussion of results. Support was provided by the Department of Anesthesiology, College of Medicine, Mayo Clinic (Rochester, Minnesota, USA) and from research grants HD29745 and AR30582 from the National Institutes of Health (Bethesda, Maryland, USA).
We acknowledge the late Leonard T. Kurland, M.D. (Epidemiologist, Mayo Clinic, Rochester, Minnesota) for his vision in initiating the Rochester Epidemiology Project, and we thank Dr. Robert Colligan, PhD (Professor of Psychology, Mayo Clinic, Rochester, Minnesota) for sharing his knowledge and experience in the science of learning disability. We also thank Ms. Candice Klein (Clinical Research Coordinator, Mayo Clinic, Rochester, Minnesota), Ms. Peg Farrell (Registered Nurse, data abstractor) and other members of the Learning Disability team for data collection; Independent School District #535; and the Reading Center/Dyslexia Institute of Minnesota for their cooperation and collaboration. We would also like to thank data analyst, Mr. Andrew Hanson (statistical program analyst, Mayo Clinic Rochester, Minnesota), and Anthony Santamaria, M.D. for assistance in obtaining medical records from Olmsted County Medical Center, Rochester, Minnesota.
Footnotes
This work should be attributed to Department of Anesthesiology, Division of Epidemiology, Department of Pediatrics and Adolescent Medicine, and Division of Developmental and Behavioral Pediatrics.
References
- 1.Olney JW, Wozniak DF, Jevtovic-Todorovic V, Farber NB, Bittigau P, Ikonomidou C. Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathol. 2002;12:488–98. doi: 10.1111/j.1750-3639.2002.tb00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jevtovic-Todorovic V, Benshoff N, Olney JW. Ketamine potentiates cerebrocortical damage induced by the common anaesthetic agent nitrous oxide in adult rats. Br J Pharmacol. 2000;130:1692–8. doi: 10.1038/sj.bjp.0703479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olney JW, Young C, Wozniak DF, Jevtovic-Todorovic V, Ikonomidou C. Do pediatric drugs cause developing neurons to commit suicide? Trends Pharmacol Sci. 2004;25:135–9. doi: 10.1016/j.tips.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–4. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 5.Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007;104:509–20. doi: 10.1213/01.ane.0000255729.96438.b0. [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Sadovova N, Fu X, Schmued L, Scallet A, Hanig J, Slikker W. The role of the N-methyl-D-aspartate receptor in ketamine-induced apoptosis in rat forebrain culture. Neuroscience. 2005;132:967–77. doi: 10.1016/j.neuroscience.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 7.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slikker W, Jr, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP, Paule MG, Wang C. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–58. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 9.Bellinger DC, Rappaport LA, Wypij D, Wernovsky G, Newburger JW. Patterns of developmental dysfunction after surgery during infancy to correct transposition of the great arteries. J Dev Behav Pediatr. 1997;18:75–83. doi: 10.1097/00004703-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Bellinger DC, Wypij D, duDuplessis AJ, Rappaport LA, Jonas RA, Wernovsky G, Newburger JW. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385–96. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 11.Bellinger DC, Wypij D, Kuban KC, Rappaport LA, Hickey PR, Wernovsky G, Jonas RA, Newburger JW. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100:526–32. doi: 10.1161/01.cir.100.5.526. [DOI] [PubMed] [Google Scholar]
- 12.Wright M, Nolan T. Impact of cyanotic heart disease on school performance. Arch Dis Child. 1994;71:64–70. doi: 10.1136/adc.71.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katusic SK, Colligan RC, Barbaresi WJ, Schaid DJ, Jacobsen SJ. Incidence of reading disability in a population-based birth cohort, 1976–1982, Rochester, Minn. Mayo Clin Proc. 2001;76:1081–92. doi: 10.4065/76.11.1081. [DOI] [PubMed] [Google Scholar]
- 14.Katusic SK, Colligan RC, Barbaresi WJ, Schaid DJ, Jacobsen SJ. Potential influence of migration bias in birth cohort studies. Mayo Clin Proc. 1998;73:1053–61. doi: 10.4065/73.11.1053. [DOI] [PubMed] [Google Scholar]
- 15.Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Jacobsen SJ. Math learning disorder: incidence in a population-based birth cohort, 1976–82, Rochester, Minn. Ambulatory Pediatrics. 2005;5:281–9. doi: 10.1367/A04-209R.1. [DOI] [PubMed] [Google Scholar]
- 16.Katusic SK, Barbaresi WJ, Colligan RC, Weaver AL, Leibson CL, Jacobsen SJ. Case definition in epidemiologic studies of AD/HD. Ann Epidemiol. 2005;15:430–7. doi: 10.1016/j.annepidem.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Kurtland LT, Elverback LR, Nobrega FT. Population studies in Rochester and Olmstead County, Minnesota 1900–1968. Baltimore, MD: Johns Hopkins Press; 1970. [Google Scholar]
- 18.SLD Companion Manual. Revision Edition. Roseville: Minnesota Educational Series; 1998. [Google Scholar]
- 19.Fletcher J, Shaywitz S, Shankweiler D, Katz L, Liberman I, Stuebing K, Francis D, Fowler A, Shaywitz B. Cognitive profiles of reading disability: comparisons of discrepancy and low achievement definitions. J Educ Psychol. 1994;86:6–23. [Google Scholar]
- 20.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108 (Suppl 3):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Middaugh LD, Dow-Edwards D, Li AA, Sandler JD, Seed J, Sheets LP, Shuey DL, Slikker W, Jr, Weisenburger WP, Wise LD, Selwyn MR. Neurobehavioral assessment: a survey of use and value in safety assessment studies. Toxicol Sci. 2003;76:250–61. doi: 10.1093/toxsci/kfg211. [DOI] [PubMed] [Google Scholar]
- 22.Wang C, Slikker W., Jr Strategies and experimental models for evaluating anesthetics: effects on the developing nervous system. Anesth Analg. 2008;106:1643–58. doi: 10.1213/ane.ob013e3181732c01. [DOI] [PubMed] [Google Scholar]
- 23.Olney JW, Young C, Wozniak DF, Ikonomidou C, Jevtovic-Todorovic V. Anesthesia-induced developmental neuroapoptosis. Does it happen in humans? Anesthesiology. 2004;101:273–5. doi: 10.1097/00000542-200408000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Cattano D, Young C, Straiko MM, Olney JW. Subanesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesth Analg. 2008;106:1712–4. doi: 10.1213/ane.0b013e318172ba0a. [DOI] [PubMed] [Google Scholar]
- 25.Uemura E, Bowman RE. Effects of halothane on cerebral synaptic density. Exp Neurol. 1980;69:135–42. doi: 10.1016/0014-4886(80)90149-1. [DOI] [PubMed] [Google Scholar]
- 26.Uemura E, Ireland WP, Levin ED, Bowman RE. Effects of halothane on the development of rat brain: a golgi study of dendritic growth. Exp Neurol. 1985;89:503–19. doi: 10.1016/0014-4886(85)90002-0. [DOI] [PubMed] [Google Scholar]
- 27.Fredriksson A, Archer T, Alm H, Gordh T, Eriksson P. Neurofunctional deficits and potentiated apoptosis by neonatal NMDA antagonist administration. Behav Brain Res. 2004;153:367–76. doi: 10.1016/j.bbr.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 28.Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681–707. doi: 10.1213/ane.0b013e318167ad77. [DOI] [PubMed] [Google Scholar]
- 29.Hovels-Gurich HH, Seghaye MC, Dabritz S, Messmer BJ, von Bernuth G. Cognitive and motor development in preschool and school-aged children after neonatal arterial switch operation. J Thorac Cardiovasc Surg. 1997;114:578–85. doi: 10.1016/S0022-5223(97)70047-3. [DOI] [PubMed] [Google Scholar]
- 30.Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C. Neurodevelopmental status of newborns and infants with congenital heart defects before and after open heart surgery. J Pediatr. 2000;137:638–45. doi: 10.1067/mpd.2000.109152. [DOI] [PubMed] [Google Scholar]
- 31.Karl TR, Hall S, Ford G, Kelly EA, Brizard CP, Mee RB, Weintraub RG, Cochrane AD, Glidden D. Arterial switch with full-flow cardiopulmonary bypass and limited circulatory arrest: neurodevelopmental outcome. J Thorac Cardiovasc Surg. 2004;127:213–22. doi: 10.1016/j.jtcvs.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Surgery and the tiny baby: sensorineural outcome at 5 years of age. The Victorian Infant Collaborative Study Group. J Paediatr Child Health. 1996;32:167–72. doi: 10.1111/j.1440-1754.1996.tb00916.x. [DOI] [PubMed] [Google Scholar]
- 33.Simon NP, Brady NR, Stafford RL, Powell RW. The effect of abdominal incisions on early motor development of infants with necrotizing enterocolitis. Dev Med Child Neurol. 1993;35:49–53. doi: 10.1111/j.1469-8749.1993.tb11551.x. [DOI] [PubMed] [Google Scholar]
- 34.Chacko J, Ford WD, Haslam R. Growth and neurodevelopmental outcome in extremely-low-birth-weight infants after laparotomy. Pediatr Surg Int. 1999;15:496–9. doi: 10.1007/s003830050648. [DOI] [PubMed] [Google Scholar]
- 35.Blakely ML, Tyson JE, Lally KP, McDonald S, Stoll BJ, Stevenson DK, Poole WK, Jobe AH, Wright LL, Higgins RD. Laparotomy versus peritoneal drainage for necrotizing enterocolitis or isolated intestinal perforation in extremely low birth weight infants: outcomes through 18 months adjusted age. Pediatrics. 2006;117:e680–7. doi: 10.1542/peds.2005-1273. [DOI] [PubMed] [Google Scholar]
- 36.Lindahl H. Long-term prognosis of successfully operated oesophageal atresia-with aspects on physical and psychological development. Z Kinderchir. 1984;39:6–10. doi: 10.1055/s-2008-1044160. [DOI] [PubMed] [Google Scholar]
- 37.Barbaresi WJ, Katusic SK, Colligan RC, Pankratz VS, Weaver AL, Weber KJ, Mrazek DA, Jacobsen SJ. How common is attention-deficit/hyperactivity disorder? Incidence in a population-based birth cohort in Rochester, Minn. Arch Pediatr Adolesc Med. 2002;156:217–24. doi: 10.1001/archpedi.156.3.217. [DOI] [PubMed] [Google Scholar]
- 38.Barbaresi W, Katusic S, Colligan R, Weaver A, Pankratz V, Mrazek D, Jacobsen S. How common is attention-deficit/hyperactivity disorder? Towards resolution of the controversy: results from a population-based study. Acta Paediatr Suppl. 2004;93:55–9. doi: 10.1111/j.1651-2227.2004.tb03058.x. [DOI] [PubMed] [Google Scholar]
- 39.Katusic SK, Colligan RC, Beard CM, O’Fallon WM, Bergstralh EJ, Jacobsen SJ, Kurland LT. Mental retardation in a birth cohort, 1976–1980, Rochester, Minnesota. Amer J Ment Retard. 1996;100:335–44. [PubMed] [Google Scholar]
- 40.Uemura E, Levin ED, Bowman RE. Effects of halothane on synaptogenesis and learning behavior in rats. Exp Neurol. 1985;89:520–9. doi: 10.1016/0014-4886(85)90003-2. [DOI] [PubMed] [Google Scholar]
- 41.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 42.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.