Abstract
As a prerequisite for full clinical trials of pharmacological chaperone therapy (PCT) for Fabry disease we developed a rapid screening assay for enhancement of endogenous α-galactosidase A (α-Gal A) in patient-derived cells. We used a T-cell based system to screen 11 mutations causing Fabry disease for enhanceability using 1- deoxygalactonojirimycin (DGJ). When patient derived T-cells were grown in the presence of DGJ α-Gal A activity increased to more than 50% of normal in several mutations but was unaffected in others.
In addition to the mutation R301Q, reported previously, A97V, R112H, R112 C, A143T and L300P are enhanceable, but R356W, G132R, A143P, R220X and 30delG were not. The level of α-Gal A activity achieved provides a basis for the therapeutic trial of DGJ in patients with similarly enhanceable enzyme. This assay method has general utility in other disorders in assessing the degree of enhancement of activity of mutated proteins by PCT.
Introduction
Fabry disease is an X-linked systemic disorder caused by deficient activity of the lysosomal enzyme α-Gal A [1] that results in the storage of the globotriaosylceramide (Gb3) in a variety of organs and cell types, notably the endothelial cells and smooth muscle cells of blood vessels throughout the body [2]. Fabry patients present clinically with pain, angiokeratomas, cardiac disease, cerebrovascular stroke, progressive renal insufficiency, painful small-fiber neuropathy, and hypohidrosis and frequently die as a result of chronic renal failure. Classic Fabry patients have no detectable α-Gal A activity, whereas atypical variants have measurable residual α-Gal A activity resulting in milder and later-onset disease, frequently presenting with cardiac symptoms as the first indication of disease. Heterozygous females have an extremely variable presentation from asymptomatic to the full spectrum of manifestations as observed in the classical male patients including renal involvement [3;, 4].
Treatment options for a number of lysosomal storage disorders include enzyme replacement therapy (ERT), gene therapy and bone marrow transplantation. Although ERT has been approved for treatment of a number of lysosomal disorders including Gaucher disease, Fabry disease and others [5], success may be limited by the failure to deliver active enzyme to all cells and involved organs. ERT has been shown to be effective in non-neuronopathic Gaucher disease (Type 1) but has no therapeutic benefit for the treatment of neuronopathic disease. ERT for Fabry disease fails to decrease the risk of stroke, shows a slow response in cardiac muscle, and fails to remove accumulated Gb3 from podocytes in the kidney and from most of the blood vessel walls. Patients continue to develop vertigo and deafness [6]. Patients receiving ERT for Fabry disease suffer complications common to all intravenous drug administration procedures, as well as the development of immune reactions.
A small molecule that can be delivered orally and has the ability to reach all tissue types could provide significant therapeutic advantages over ERT. One such strategy has been the use of substrate reduction therapy (SRT) to decrease the substrate synthesis by the use of an inhibitor for the glucosyltransferase enzyme. SRT is approved for patients with non-neuropathic Gaucher disease type 1 [7] and in clinical trials for other lysosomal storage disorders [8].
An alternative strategy for the treatment of enzymatic and other protein defects is the use of pharmacological chaperone therapy (PCT), previously called chemical chaperone therapy, in which binding of a small active site specific chemical results in stabilization of the newly synthesized enzyme [9] permitting appropriate trafficking to the lysosome [9, 10 ]. Clinical trials of PCT for Fabry disease as well as other lysosomal storage diseases have begun [11; 12; 13], however published data exists for only three mutations causing Fabry disease, R301Q, Q279E and G328R [10, 14;, 15].
In order to apply PCT in clinical trials, a broadly applicable, fast, and efficient method for screening needs to be adopted. We report the results of a screening test for enhanceability of 11 different mutations in α-Gal A using a method for quickly generating a short-term T-cell culture that permits the testing of the enhancement of α-Gal A containing any of the various mutations responsible for Fabry disease but avoids the need to establish long-term fibroblast cultures or transfect and screen lymphoblast cultures [10, 16]. This system also provides a useful method for future studies on the mechanism of action or for screening of additional molecular chaperones for a variety of enzyme and protein deficiencies.
Materials and Methods
Reagents and suppliers are as follows: 1-deoxygalactonojirimycin (DGJ) from Cambridge Major Laboratories, Inc., Germantown, WI; 4-methylumbelliferyl-α-D-galactopyranoside (4MUG) from Research Products International, Mount Prospect, IL; N-acetylgalactosamine from Sigma Chemical Co., St. Louis, MO; RPMI-1640 medium, Dulbecco’s Modified Eagle’s medium (DMEM), and Phytohemagglutinin M (PHA) from Life Technology, Gaithersburg, MD; Cosmic calf serum (CCS) from Hyclone Laboratories, Logan, UT; Interleukin-2 (IL-2) from PreProTECH, Rocky Hill, NJ.
DGJ treatment of cultured cells
Mononuclear cells were isolated following the manufacturer’s procedure from fresh blood from male Fabry patients and normal controls, collected in a BD Vacutainer® CPT™ Cell Preparation Tube (Franklin Lakes, NJ) containing sodium citrate as anticoagulant. Selective expansion of T-cells from white blood cells was accomplished by growth in RPMI 1640 with 10% CCS, 50 ng/ml IL-2 and PHA (used at the lot specific recommendations for the concentration supplied by the supplier) at 37°C in 5% CO2. Typically, 4 to 10 days were required for establishing the T-cell cultures. Established cultures may be harvested and frozen in liquid nitrogen for later use. For the enzyme enhancement assay lymphocytes (~2.5 × 106) were grown in 5 ml culture medium for 3 days in an upright 50 ml tissue culture flask in the absence or presence of DGJ at 20 μM (except as noted in the figure legend). Fibroblast cultures, derived from skin biopsies of patients were grown in DMEM with 10% FBS. For enhancement assay, fibroblasts (~1.5 × 106) were grown for 3 days in 12 ml culture medium in a T75 tissue culture flask in the absence or presence of DGJ at 20 μM (except as noted).
α-Galactosidase A Assays
α-Gal A activity was determined by a standard fluorometric method [17] modified for a 96 well microplate format. Cells were rinsed twice with phosphate-buffered saline, sonicated for 10 seconds with citrate-phosphate (CP) buffer (29 mM citric acid/44 mM disodium phosphate, with 3 mg/ml sodium taurocholate, pH 4.4), centrifuged at 20,000 × g for 30 min at 4°C and the supernatant used for determination of α-Gal A activity. Aliquots of the supernatant were incubated at 37°C with 3.75 mM 4-methylumbelliferyl-α-D-galactopyranoside (4-MUG) in CP buffer without taurocholate, with 3 mg/ml bovine serum albumin (BSA) and 0.1 M N-acetylgalactosamine to inhibit N-acetylgalactosaminidase [18]. Released 4-methylumbelliferone was measured in a Wallac 1420 Victor3 ™ Fluorescence reader (Perkin Elmer, CA) with a 355 nm excitation and 460 nm emission filter set. One unit of enzyme activity catalyzes the hydrolysis of 1 nmole of 4-MUG per hour. At least three normal samples were tested concurrently with each patient sample to generate the daily average for normal activity.
Protein determination and Western Blot Analysis
Protein was determined using a Micro BCA Protein Assay kit (Pierce, Rockford, IL) using BSA as a standard. Absorbance at 562nm was measured using a 96-well microplate reader (Molecular Devices). For Western blots, proteins were separated electrophoretically using Novex Tris-glycine SDS-PAGE in 8–16% gradient gels (Invitrogen), transferred and developed using rabbit polyclonal antibody against α-gal A as described previously[19].
Results
Enhancement of α-Gal A in T-cells and in fibroblasts
We have successfully established cultures of a number of cell lines derived from normal controls and patients with Fabry disease using an established procedure for the growth and study of normal T lymphocytes from peripheral blood mononuclear cells[20]. When analyzed by fluorescence activated cell sorting, IL-2 and PHA stimulation results in 99% CD3-positive cells (a T-cell marker) with equal numbers of CD4-positive and CD4-negative cells (data not shown).
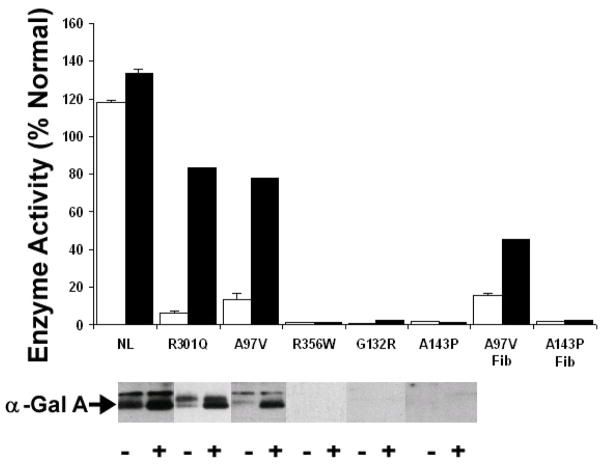
R301Q, reported previously to be enhanceable in lymphoblasts [10] showed a 12-fold increase in enzyme activity in the T-cell assay system, cultured as described here and incubated in the presence of 20 μM DGJ for 4 days (Figure 1). In cell cultures with the A97V and A143P mutations of α-Gal A, also incubated with 20 μM DGJ for 4 days, α-Gal A activity increased both in fibroblasts and in T-cells for the A97V mutation (Figure 1), but was unchanged in either cell type for the A143P mutation (Figure 1). The consistency of results for the mutations A97V, R301Q, A143P and R356W in fibroblasts, lymphoblasts and T-cells (Table 1) demonstrate that enhancement of an enzyme by a chemical chaperone such as DGJ is a property of the mutated protein and independent of cell-type.
Figure 1. Enhancement results of α-Gal A activity of A97V in T-cells and in fibroblast cells by DGJ.
T-cells (Panel A) or fibroblasts (Panel B) were cultured in the absence (open bars) or presence of 20 μM DGJ for four days then assayed for α-Gal A activity. Below the bar graph are shown the Western blots for each mutation. Westerns were developed using a rabbit polyclonal antiserum raised against the purified α-Gal A. The error bar indicates the standard deviation for three independent enhancement trials, assayed in triplicate for each specimen.
Table 1. Enhancement of α-Gal A by DGJ in Cells from Patients with Fabry disease.
Enhancement of α-Gal A activity by DGJ was compared in cultured T-cells (T) and fibroblasts (F) from patients with a variety of mutations. Mutations were based on the genotypes according to cDNA: GenBank accession number NM_000169.1 [21]. A143P*, A143P**, A143P*** are unrelated individuals. R356W* and R356W** are brothers. T-cells and fibroblasts were grown without or with 20 μM DGJ for three or four days as indicated. α-Gal A activity from freshly isolated white blood cells (WBC) without DGJ were shown for comparison only. Mean and standard deviation were calculated from the individual experiments, determined in triplicate. % Normal is calculated on a daily basis from the α-Gal A activity for all normals concurrently tested.
| Cell Type |
Group | Genotype | Mutation | Number of Replicates (n) |
Activity (%Normal) |
P value | Enhancement ratio of the Mean |
WBC Activity (% Normal) |
Phenotype | days of DGJ incubation |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| (− DGJ) | (+ DGJ) | (− DGJ) | |||||||||
| T | E | c.290C>T | A97V | 3 | 13.7 +/− 4.2 | 74.9 +/− 8.4 | 0.001 | 5.5 | 8.3 | np, c | 3 |
| T | E | c.902G>A | R301Q | 4 | 6.6 +/− 1.8 | 79.8 +/− 27 | 0.012 | 12 | 4.8 | r, c | 3 |
| T | E | c.335G>A | R112H | 3 | 3 +/− 1.5 | 59.9 +/− 17.2 | 0.029 | 20.3 | 2.2 | np, c | 3 |
| T | E | c.334C>T | R112C | 3 | 7.8 +3.9 | 49.0 + 9.7 | 0.0065 | 6.3 | 3 | ||
| T | E | c.427G>A | A143T | 4 | 30.6 + 4.6 | 68.8 + 11.4 | 0.0035 | 2.2 | np | 3 | |
| T | E | c.898C>T | L300P | 5 | 2.0 +/− 2.7 | 72.1 +/− 25.3 | 0.004 | 36.9 | np | 3 | |
| T | NE | c.1066C>T | R356W* | 4 | 0.01 +/− 0.02 | 0.43 +/− 0.85 | NE | NE | 0.2 | r, np | 3 |
| T | NE | c.394G>A | G132R | 3 | 1.3 +/− 1.1 | 2.1 +/− 0.4 | NE | NE | r, c, np | 3 | |
| T | NE | c.658C>T | R220X | 3 | 0.4 +/− 0.7 | 0.7 +/− 0.3 | NE | NE | 3.9 | s, c, r | 3 |
| T | NE | 30delG | 6 | 1.8 + 1.4 | 1.8 + 1.4 | NE | NE | 3 | |||
| T | NE | c.427G>C | A143P* | 2 | 1.6 +/− 1.3 | 1.0 +/− 1.1 | NE | NE | 0.3 | r, np | 3 |
| T | NE | c.427G>C | A143P** | 2 | 0.6 +/− 0.8 | 1.0 +/− 0.1 | NE | NE | s, np, r | 3 | |
| F | E | c.290C>T | A97V | 1 | 15.4 | 30.6 | 2 | 8.3 | np, c | 3 | |
| F | E | c.290C>T | A97V | 1 | 15.4 | 43.8 | 2.8 | 8.3 | np, c | 4 | |
| F | NE | c.427G>C | A143P* | 2 | 1.8 +/− 2.1 | 0.00 +/− 0.11 | NE | 1.2 | r, np | 4 | |
| F | NE | c.427G>C | A143P*** | 1 | ND | 1.84 | NE | 0.3 | r, np | 4 | |
| F | NE | c.1066C>T | R356W* | 1 | ND | ND | NE | 0.2 | r, np | 3 | |
| F | NE | c.1066C>T | R356W** | 1 | ND | ND | NE | np | 3 | ||
Abbreviations used are as follows. E: Enhanceable; NE: Non-Enhanceable; ND: Not detectable. i.e. value below minimum threshold of detection. The enhancement ratio is defined as the activity found in cells grown in the presence of DGJ divided by the basal activity. Abbreviated symptoms are as follows. c: cardiac; np: neuropathic pain; r: renal; s: stroke.
Enhancement assay conditions for T-cells
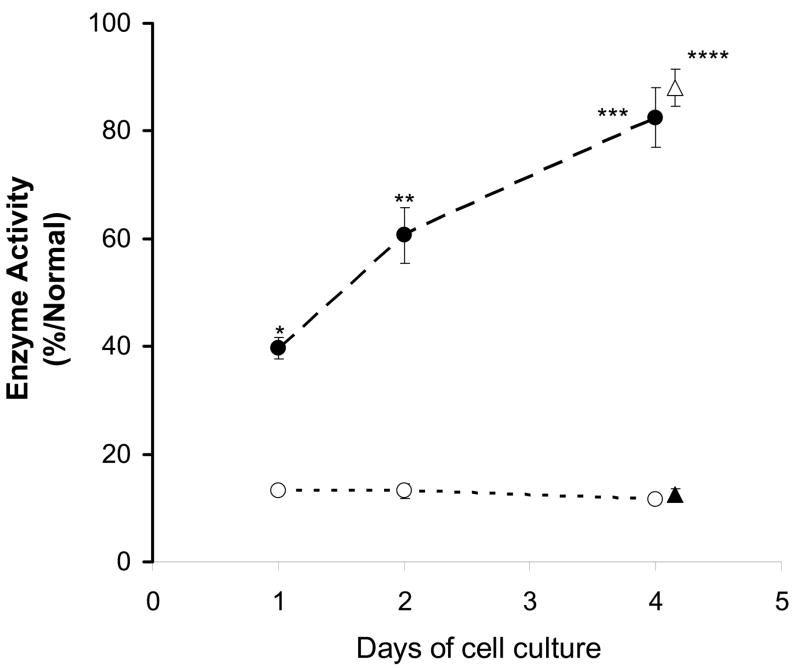
T-cells with the A97V mutation of α-Gal A were incubated without and with 20 μM DGJ for 1, 2, and 4 days, respectively and α-Gal A activity was compared to normal control values. With 20 μM DGJ added, A97V activity increased to about 40 % of normal after 1 day of incubation and continued to increase to 80 % of normal after 4 days of incubation (Figure 2). Changing media had no effect on DGJ enhancement (Figure 2). The results at 2 days and 4 days were qualitatively equivalent and easily distinguishable from the basal activity level for A97V (p < 0.001, Table 1). Thus, we chose a standard of 3 days incubation assay to decrease time required, to increase experimental flexibility and to avoid the necessity of changing media or splitting cells during an experiment. When A97V cells were tested 3 times using 20 μM DGJ for 3 days, the increase in activity ranged from 60 to 80% of normal.
Figure 2. Time course for enhancement of A97V.
T-cells bearing the A97V mutation in α-Gal A were cultured in the absence (○) or presence (●) of 20 μM DGJ for one to four days then assayed for α-Gal A activity as described in the text. Changing media after 2 days (▵ and ▲) resulted in no change in the enzyme activity. Statistical significance of the enhancement effect of DGJ compared with matching non-treated one for each day were shown; * for 1 day, p < 0.002; ** for 2 day, p < 0.004; *** for 4 day, p < 0.002; **** for 4 days with media changed, p < 0.0008; by Student’s t-test.
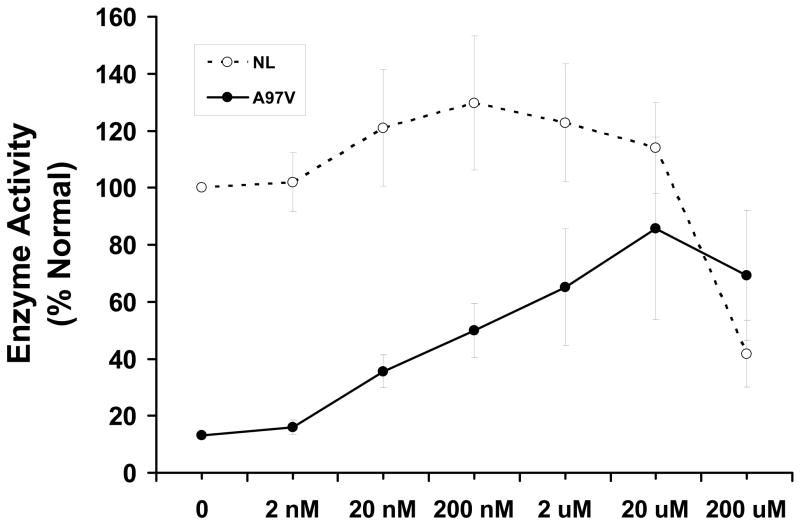
Dose dependence of DGJ in T-cells from three separate normal controls and A97V was evaluated over a range from 2 nM to 200 μM (Figure 3) and compared to basal activity in normal control cells assayed on the same day. α-Gal A activity increased from 2 to 200 nM in normal cells with a slight decline at higher concentrations. A97V cells showed a marked increase in activity up to 20 μM then declined at 200 μM. Activity measured in cells containing the R112H and R301Q mutations consistently increased up to a maximum of at least 50 % of normal controls at 20 μM DGJ and declined at 200 μM, (data not shown).
Figure 3. Effect of DGJ Concentration in the normal control and Fabry patient T-cells.
Fig. 3. T-cells from normal individuals (○; n=3) or from a Fabry patient with the A97V mutation (●; n=3) were incubated for 3 days with DGJ from 2 nM to 200 μM then assayed for α-Gal A activity. The results of three experiments on different days are shown.
Enhancement Screening Results
Enhancement of activity in T-cells from patients with Fabry disease with a variety of additional mutations permits segregation into enhanceable (E) and non-enhanceable (NE) groups (Table 1). Following incubation with DGJ, enhanceable mutations had activity greater than 25 % of normal controls whereas non-enhanceable had less than 2% of normal activity. In the enhanceable group are the mutations A97V, R301Q, R112H, R112C, A143T and L300P with observed activity up to 80% of normal. The L300P mutation, despite the introduction of a significant conformational change by the introduction of proline, increased from 2 % to 72 % of normal, which was the greatest change measured among the enhanceable mutations examined.
The non-enhanceable (NE) group included R356W, G132R, R220X, A143P and 30delG. Activity less than twice baseline variation is not reportable in our system and in some experiments α-Gal A activity both without or with DGJ did not meet this threshold. As expected, significant deletions in the peptide chain, either from a nonsense mutation, e.g. R220X, or a frameshift mutation, such as 30delG, were not enhanced by DGJ. Several single amino acid substitutions, A143P, R356W and G132R were also not enhanced in the presence of DGJ. No enhancement ratio (Table 1) was calculated for the non-enhanceable mutations because the α-Gal A activity in the presence of DGJ was less than 2 % of normal and therefore subject to significant error in measurement in addition to being of limited therapeutic potential.
Western Blotting
In T-cells, the band intensity seen on Western blots of cell homogenates (Figure 1, lower panel) and the α-Gal A activity showed excellent correlation. Band intensity was considerably increased by treatment with DGJ in normal control cells and those with the A97V and the R301Q mutation, while no increase was seen for R356W, G132R, and A143P. The enhanced protein appears to have shifted to a lower apparent molecular weight indicating maturation of the enzyme by passage from the endoplasmic reticulum, through the Golgi apparatus to the lysosome.
Discussion
Over 340 mutations have been reported in the GLA gene (MIM 300644) [4] that result in the development of Fabry disease (The Human Gene Mutation Database, www.hgmd.org). A critical step in determining which patients may benefit from PCT was the development of a rapid and reliable method to screen patient-derived cells for enhancement of α-Gal A activity by DGJ that we describe here.
We have demonstrated enhanced enzyme activity for each of the mutations R301Q, A97V, R112H, R112C, A143T and L300P. It is notable that the first five of these patients do not appear to have the classic Fabry phenotype. They share a milder disease presentation and higher enzyme activity in peripheral WBC’s (greater than 2 % of normal). These patients had a tendency to have cardiac disease with hypertrophic cardiomyopathy and conduction defects, strokes had not yet occurred, and renal disease progression had not become apparent until after the age of 40. The L300P patient, despite very low basal enzyme activity, presents only with neuropathic pain without angiokeratomas, renal or cardiac symptoms, but may develop additional symptoms with increasing age. The classification into cardiac variant or classic phenotype is not yet clear for this patient. The increase in activity for the L300P mutation from below 2 % of normal to the near normal range was unpredicted and shows the importance for screening all mutations, without any prescreening on the basis of measurable residual activity. DGJ appears to have potential benefit for patients with Fabry disease with several distinct mutations in addition to the previously reported R301Q and Q279E mutations [10]. The non-enhanceable group includes mutations R356W, G132R, A143P, R220X and 30delG. These are all classic Fabry patients that may have either a single amino acid mutation or a truncation. Not unexpectedly, nonsense mutations and frameshift mutations that result in premature termination were not enhanced by the presence of DGJ.
In all examples tested to date we have seen complete correlation between the enzyme activity and the amount of protein measured by Western blots. In the A143P example, in which the mutation occurs very close to the active site and may result in interference with an essential disulfide bridge, there is no evidence for an increase in inactive protein (Figure 1) following chaperone treatment. This might occur if the chaperone permitted increased stability of a non-functional protein.
From the paired mutations R112H and R112C and A143T and A143P we can predict that the location of the mutation alone does not determine enhanceability but the nature of the mutation also has a significant effect. The mutations R112H and R112C are both enhanceable although the first might be classified as a semi-conservative mutation (Arg replaced by His, retaining some positive charge) and the second non-conservative (loss of charge and introduction of a potentially reactive sulfhydryl group on the Cys). The mutation A143T, a conservative mutation with no change in charge, but with a slightly bulkier side chain, results in an enhanceable mutation whereas the A143P mutation does not. This might be due to the introduction of a significant conformational change due to the replacement of Ala by Pro in this mutation. Although establishment of a general mechanism requires many additional mutations to be mapped to the 3-dimensional structure of α-Gal A and is thus beyond the scope of the present study, the data presented here shows that the method is of great utility in that it allows the discrimination of patients responsive and unresponsive to PCT. Future studies are necessary to study the mechanism underlying selective enhanceability of some mutations but not others. The T-cell based assay described in this report provides a simple and rapid screening test to determine the degree of enhancement of enzymatic activity by PCT for each specific disease-causing mutation. T- cell cultures derived from fresh blood of normal control individuals and patients with Fabry disease can be used in an enhancement assay for α-Gal A in less than 2 weeks and enhancement was evident after 3 days in the growth media containing DGJ. Although the absence of appreciable storage of Gb3 in T-cells (unpublished data) prevents a measure of in vivo efficacy, we consider this limitation to be offset by the speed of obtaining requisite information and the absence of the requirement for establishing fibroblast cultures. This method can be used for chaperone based enhancement assays for other genetic diseases including glycosphingolipidoses, mucopolysaccharidoses and other lysosomal storage disorders in addition to other genetically based diseases, such as cystic fibrosis where maturation of the protein occurs in the ER.[9].
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke and a Collaborative Research and Development Agreement between Amicus Corporation and the Developmental and Metabolic Neurology Branch. We are indebted to our patients and volunteers who participated in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N Engl J Med. 1967;276:1163–1167. doi: 10.1056/NEJM196705252762101. [DOI] [PubMed] [Google Scholar]
- 2.Tabira T, Goto I, Kuroiwa Y, Kikuchi M. Neuropathological and biochemical studies in Fabry’s disease. Acta Neuropathol (Berl) 1974;30:345–354. doi: 10.1007/BF00697017. [DOI] [PubMed] [Google Scholar]
- 3.Desnick RJ, Ioannou YA, Eng CM. α-Galactosidase A Deficiency: Fabry Disease. In: Charles ALB, Scriver R, Valle David, Sly William S, Vogelstein Bert, Childs Barton, Kinzler Kenneth W, editors. The Metabolic and molecular bases of inherited disease. McGraw-Hill; New York: 2001. pp. 3733–3771. [Google Scholar]
- 4.McKusick VA. Mendelian inheritance in man. Baltimore: Johns Hopkins University Press; 1998. [Google Scholar]
- 5.Brady RO. Enzyme replacement for lysosomal diseases. Annu Rev Med. 2006;57:283–296. doi: 10.1146/annurev.med.57.110104.115650. [DOI] [PubMed] [Google Scholar]
- 6.Schiffmann R, Rapkiewicz A, Abu-Asab M, Ries M, Askari H, Tsokos M, Quezado M. Pathological findings in a patient with Fabry disease who died after 2.5 years of enzyme replacement. Virchows Arch. 2006;448:337–343. doi: 10.1007/s00428-005-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox T, Lachmann R, Hollak C, Aerts J, van Weely S, Hrebicek M, Platt F, Butters T, Dwek R, Moyses C, Gow I, Elstein D, Zimran A. Novel oral treatment of Gaucher’s disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet. 2000;355:1481–1485. doi: 10.1016/S0140-6736(00)02161-9. [DOI] [PubMed] [Google Scholar]
- 8.Jeyakumar M, Dwek RA, Butters TD, Platt FM. Storage solutions: treating lysosomal disorders of the brain. Nat Rev Neurosci. 2005 doi: 10.1038/nrn1725. [DOI] [PubMed] [Google Scholar]
- 9.Fan JQ. A contradictory treatment for lysosomal storage disorders: inhibitors enhance mutant enzyme activity. Trends Pharmacol Sci. 2003;24:355–360. doi: 10.1016/S0165-6147(03)00158-5. [DOI] [PubMed] [Google Scholar]
- 10.Fan JQ, Ishii S, Asano N, Suzuki Y. Accelerated transport and maturation of lysosomal alpha-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat Med. 1999;5:112–115. doi: 10.1038/4801. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda J, Suzuki O, Oshima A, Yamamoto Y, Noguchi A, Takimoto K, Itoh M, Matsuzaki Y, Yasuda Y, Ogawa S, Sakata Y, Nanba E, Higaki K, Ogawa Y, Tominaga L, Ohno K, Iwasaki H, Watanabe H, Brady RO, Suzuki Y. Chemical chaperone therapy for brain pathology in G(M1)-gangliosidosis. Proc Natl Acad Sci U S A. 2003;100:15912–15917. doi: 10.1073/pnas.2536657100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawkar AR, Cheng WC, Beutler E, Wong CH, Balch WE, Kelly JW. Chemical chaperones increase the cellular activity of N370S beta -glucosidase: a therapeutic strategy for Gaucher disease. Proc Natl Acad Sci U S A. 2002;99:15428–15433. doi: 10.1073/pnas.192582899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tropak MB, Reid SP, Guiral M, Withers SG, Mahuran D. Pharmacological enhancement of beta-hexosaminidase activity in fibroblasts from adult Tay-Sachs and Sandhoff Patients. J Biol Chem. 2004;279:13478–13487. doi: 10.1074/jbc.M308523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frustaci A, Chimenti C, Ricci R, Natale L, Russo MA, Pieroni M, Eng CM, Desnick RJ. Improvement in cardiac function in the cardiac variant of Fabry’s disease with galactose-infusion therapy. N Engl J Med. 2001;345:25–32. doi: 10.1056/NEJM200107053450104. [DOI] [PubMed] [Google Scholar]
- 15.Ishii S, Yoshioka H, Mannen K, Kulkarni AB, Fan JQ. Transgenic mouse expressing human mutant alpha-galactosidase A in an endogenous enzyme deficient background: a biochemical animal model for studying active-site specific chaperone therapy for Fabry disease. Biochim Biophys Acta. 2004;1690:250–257. doi: 10.1016/j.bbadis.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Yam GH, Zuber C, Roth J. A synthetic chaperone corrects the trafficking defect and disease phenotype in a protein misfolding disorder. Faseb J. 2005;19:12–18. doi: 10.1096/fj.04-2375com. [DOI] [PubMed] [Google Scholar]
- 17.Kusiak JW, Quirk JM, Brady RO. Purification and properties of the two major isozymes of alpha-galactosidase from human placenta. J Biol Chem. 1978;253:184–190. [PubMed] [Google Scholar]
- 18.Mayes JS, Scheerer JB, Sifers RN, Donaldson ML. Differential assay for lysosomal alpha-galactosidases in human tissues and its application to Fabry’s disease. Clin Chim Acta. 1981;112:247–251. doi: 10.1016/0009-8981(81)90384-3. [DOI] [PubMed] [Google Scholar]
- 19.Park J, Murray GJ, Limaye A, Quirk JM, Gelderman MP, Brady RO, Qasba P. Long-term correction of globotriaosylceramide storage in Fabry mice by recombinant adeno-associated virus-mediated gene transfer. Proc Natl Acad Sci U S A. 2003;100:3450–3454. doi: 10.1073/pnas.0537900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruscetti FW, Gallo RC. Human T-lymphocyte growth factor: regulation of growth and function of T lymphocytes. Blood. 1981;57:379–394. [PubMed] [Google Scholar]
- 21.Shabbeer J, Robinson M, Desnick RJ. Detection of alpha-galactosidase a mutations causing Fabry disease by denaturing high performance liquid chromatography. Hum Mutat. 2005;25:299–305. doi: 10.1002/humu.20144. [DOI] [PubMed] [Google Scholar]