Abstract
BACKGROUND CONTEXT
Most previous studies have investigated ligaments mechanical properties at slow elongation rates of less than 25 mm/s.
PURPOSE
To determine the tensile mechanical properties, at a fast elongation rate, of intact human cervical anterior and posterior longitudinal, capsular, and interspinous and supraspinous ligaments, middle-third disc, and ligamentum flavum.
STUDY DESIGN/SETTING
In vitro biomechanical study.
METHODS
A total of 97 intact bone-ligament-bone specimens (C2–C3 to C7-T1) were prepared from six cervical spines (average age: 80.6 years, range, 71 to 92 years) and were elongated to complete rupture at an average (SD) peak rate of 723 (106) mm/s using a custom-built apparatus. Non-linear force vs. elongation curves were plotted and peak force, peak elongation, peak energy, and stiffness were statistically compared (P<0.05) among ligament. A mathematical model was developed to determine the quasi-static physiological ligament elongation.
RESULTS
Highest average peak force, up to 244.4 and 220.0 N in the ligamentum flavum and capsular ligament, respectively, were significantly greater than in the anterior longitudinal ligament and middle-third disc. Highest peak elongation reached 5.9 mm in the intraspinous and supraspinous ligaments, significantly greater than in the middle-third disc. Highest peak energy of 0.57 J was attained in the capsular ligament, significantly greater than in the anterior longitudinal ligament and middle-third disc. Average stiffness was generally greatest in the ligamentum flavum and least in the intraspinous and supraspinous ligaments. For all ligaments, peak elongation was greater than average physiological elongation computed using the mathematical model.
CONCLUSIONS
Comparison of the present results with previously reported data indicated that high speed elongation may cause cervical ligaments to fail at a higher peak force and smaller peak elongation and may be stiffer and absorb less energy, as compared to a slow elongation rate. These comparisons may be useful to clinicians for diagnosing cervical ligament injuries based upon the specific trauma.
Keywords: Ligaments, Cervical Spine, Dynamic, Mechanical Properties
Introduction
Cervical spine injury can occur during automobile collisions, and diving, football, and equestrian accidents [1–3]. Intact cervical spine ligaments provide passive stability to the spinal column, protecting the spinal cord from injury [4]. For a certain trauma or load type, the specific function of each cervical spine ligament is dependent upon its specific anatomical location, orientation, geometry, and unique material composition. Main cervical spine ligaments inferior to the C2 vertebra, besides the intervertebral disc, include the anterior longitudinal ligament (ALL), posterior longitudinal ligament (PLL), capsular ligament (CL), ligamentum flavum (LF), and interspinous and supraspinous ligaments (ISL+SSL). The ALL and PLL, spanning the anterior and posterior aspects of vertebral bodies, respectively, have similar composition, with PLL consisting of approximately 67% collagen and 6% elastin fibers [5–8]. Located between vertebral bodies, the intervertebral disc consists of a central nucleus pulposus, which is viscous and jelly-like in the young, encased by annulus fibrosis fibers, having a high collagen composition with an increasing percentage of elastin near the cartilaginous endplates [4, 9]. The CLs, which encase facet joints, have an increasing percentage of elastin posteriorly near the LF [10]. The LF, the most elastic tissue in the human body, attaches at adjacent laminae bilaterally and consists of approximately 80% elastin and 20% collagen [11, 12]. The ISL, not present in all adult cervical spines, is composed of approximately 5% to 20% elastin [5, 13]. The SSL, the most posterior cervical ligament, has been described as histologically similar to and continuous with ISL, making it difficult to identify as a separate structure [14, 15]. The specific composition of each ligament, together with its cross-sectional area and length, determines its mechanical properties. Several previous biomechanical studies have investigated the tensile mechanical properties of human cervical spine ligaments at slow elongation rates, under 10 mm/s, and have reported peak force, peak elongation, stiffness, and energy absorbing capacity. Chazal et al [15] studied ALL and PLL and observed the highest peak force and elongation in the PLL. Pintar et al [16] and Myklebust et al [17] reported the average peak force and peak elongation of ALL, PLL, LF, CL, and ISL. CL achieved the highest peak force and elongation, ISL the lowest peak force, and ALL and PLL the lowest peak elongations. Yoganandan et al [18] found that the peak stress in ALL, PLL, and CL was generally higher than in LF and ISL. The peak strain in CL, LF, and ISL was generally higher than in ALL and PLL. Lastly, Przybylski et al [19] found the average peak force, stiffness, and energy of PLL were greater than those of ALL.
Few biomechanical studies have documented the tensile mechanical properties of human cervical spine ligaments at fast elongation rates [20–22]. Yoganandan et al [21] studied ALL and LF at 8.9, 25, 250 and 2500 mm/s. Peak force, stiffness, and energy absorbing capacity were found to increase with increased elongation rate, however no clear pattern emerged for peak elongation. Panjabi et al [20] investigated the mechanical properties of alar and transverse bone-ligament-bone preparations at 0.1 and 920 mm/s. Increased stiffness and decreased elongation and energy absorption were observed at 920 mm/s, as compared to 0.1 mm/s. Shim et al [22] studied alar and transverse ligaments and ALL, PLL, CL, LF, and ISL quasi-statically and at fast elongation rates between 10,000 and 12,000 mm/s. They found that the higher elongation rate caused increased ligament strength, but reduction in peak elongation.
While previous studies have investigated the mechanical properties of human cervical spine ligaments, primarily at slow elongation rates, no study has comprehensively investigated all ligaments at each spinal level at fast elongation rates. We hypothesize that there exist significant differences in the mechanical properties among the cervical spine ligaments. The purpose of the present study was to determine the tensile mechanical properties, at a fast elongation rate, of intact human cervical ALL, intervertebral disc, PLL, CL, LF, and ISL+SSL.
Materials and Methods
Overview
First, human cervical spine ligaments were tested in tension to failure at a fast elongation rate and non-linear force-elongation curves were generated. Peak force, peak elongation, peak energy, and stiffness were calculated. Subsequently, a simple mathematical model was developed to determine the quasi-static physiological ligament elongation.
Specimen Preparation
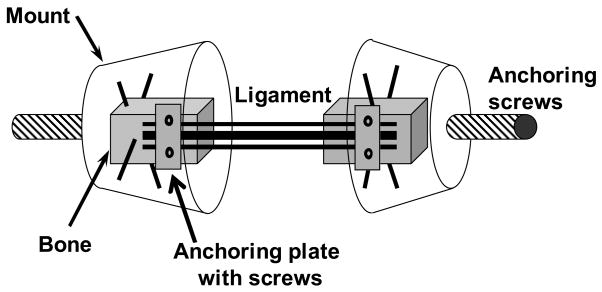
Six intact human cervical spine specimens (C2 to T1) were carefully dissected of all non-osteoligamentous soft tissues. The average age of the specimens was 80.6 years (range: 71 to 92 years). The specimens had no history of cervical spine injury or disease that could have affected the osteoligamentous structures. Specimens were divided into two equal groups: the first group was dissected into C2–C3, C4–C5, and C6–C7 functional spinal units (FSUs) while the second group was dissected into C3–C4, C5–C6, and C7–T1 FSUs. Each FSU was sectioned at the pedicles. Anterior elements were sectioned coronally into thirds to create ALL, middle-third disc (MTD), and PLL bone-ligament bone preparations, while posterior elements were appropriately sectioned to create CL, LF, and ISL+SSL preparations. Left and right CLs from each intervertebral level were prepared separately, while left and right LFs from each level were prepared as a single unit. Care was taken to isolate each ligamentous structure from surrounding tissue. Each preparation was then mounted for mechanical testing (Figure 1). To ensure rigid anchoring of bone within quick setting bondo mounts (Evercoat Z-Grip, Fibre Glass-Evercoat, Cincinnati, OH), two perpendicular thru-holes were drilled into each bone in which 19 gauge needles were inserted. Each mount contained an anchoring screw for subsequent attachment to the experimental apparatus. To increase the fixation of ALLs and PLLs to the bone, plastic plates were glued atop the ligament attachments and rigidly secured with machine screws. In total, 97 bone-ligament-bone specimens were prepared (Table 1).
Figure 1.
Schematic of bone-ligament-bone preparation. Anchoring plates ensured mid-substance tears during elongation.
Table 1. Sample sizes for bone-ligament-bone preparations.
Cervical ligaments included: anterior longitudinal ligament (ALL), middle-third disc (MTD), posterior longitudinal ligament (PLL), capsular ligament (CL), ligamentum flavum (LF), and interspinous and supraspinous ligaments (ISL+SSL).
| ALL | MTD | PLL | CL | LF | ISL+SSL | |
|---|---|---|---|---|---|---|
| C2–C3 | 2 | 3 | 3 | 3 | 2 | 1 |
| C3–C4 | 3 | 1 | 2 | 6 | 3 | 2 |
| C4–C5 | 2 | 2 | 3 | 5 | 2 | 1 |
| C5–C6 | 3 | 2 | 3 | 6 | 2 | 1 |
| C6–C7 | 1 | 1 | 3 | 6 | 3 | 2 |
| C7–T1 | 3 | 2 | 2 | 6 | 3 | 2 |
|
| ||||||
| Totals | 14 | 11 | 16 | 32 | 15 | 9 |
Experimental Apparatus
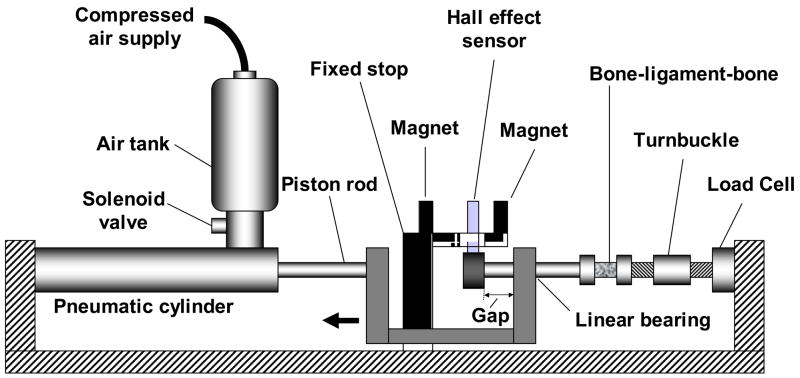
A custom apparatus was constructed to generate high speed elongation of the bone-ligament preparations (Figure 2) [23]. The apparatus consisted of a pneumatic cylinder (model 1.5 × 5 Allenair, Minneola, NY) supplied with compressed air via an air tank. Air flow from the tank to the pneumatic cylinder was controlled by a solenoid valve. A controlled gap in the system permitted the pneumatic piston to achieve sufficiently high speed prior to the onset of ligament elongation. Force was measured with a uni-axial load cell (667 N capacity, model LCCA-150, Omega, Stamford, CT). Elongation was measured using a Hall effect sensor (A3506LU, Allegro Microsystems, Worcester, Mass.) positioned between two magnets (13 × 13 × 5 mm, part no. PR28ES4187B, Dexter Magnetic, Billerica Mass.). The accuracy of the Hall effect sensor was 0.025 mm [24]. Prior to testing, the ligament was preloaded to 5 N tension, and this was defined as zero elongation. The force and elongation data were sampled at 6.3 kHz up to complete ligament rupture. The average (SD) peak ligament elongation rate was 723 (106) mm/s.
Figure 2.
Schematic of the experimental apparatus. Air flow was controlled via a solenoid valve and caused movement of the piston rod, and therefore ligament elongation. Force was measured by a load cell and elongation by a Hall effect sensor positioned between two magnets.
Data Analyses
No filtering of data was performed. Peak force was defined as the maximum force attained, while peak elongation was the elongation at the peak force. Peak energy was calculated by integrating the force between zero and peak elongation. To obtain ligament stiffness, each force-elongation curve was fitted to a second order polynomial and its derivative evaluated at 25, 50 and 75% of peak force. Average (SD) r2 was 0.97 (0.04).
Statistics
Data from each spinal level, C2–C3 to C7-T1, were combined for each ligament (Table 1). Single-factor, non-repeated measures ANOVA (P<0.05) and pair-wise Bonferroni post hoc tests were used to determine differences among ligaments in peak force, peak elongation, peak energy, and stiffness at 25, 50, and 75% of peak force. Adjusted P-values were computed based upon the number of post-hoc tests performed.
Average Physiological Ligament Elongations
To enable comparison of the present peak elongation to physiological elongation, a simple mathematical model was constructed using previously reported in vitro quantitative anatomy of cervical spine ligaments and vertebrae [14, 25], average in vivo normal intervertebral centers of rotation (CoRs) [26], and average in vitro physiological intervertebral rotations [27]. For each FSU in neutral posture, the average CoR and ligament origin and insertion coordinates were identified in an anatomical coordinate system, fixed to inferior endplate of the lower vertebral body. The upper vertebra was rotated about the CoR in flexion and extension to peak physiological rotation. Physiological ligament elongations were calculated as the differences in ligament lengths at maximum flexion for PLL, CL, LF, and ISL+SSL and at maximum extension for ALL and MTD, relative to the neutral posture lengths. Average physiological elongations were obtained for each ligament by averaging data from C2–C3 to C7-T1. The physiological ligament elongation range was defined as the average physiological elongation ±2 SD.
Results
The average physiological ligament elongations calculated using the mathematical model were largest at ISL+SSL, 3.6 mm, and LF, 2.3 mm (Table 2). MTD had the smallest physiological elongation of 0.3 mm.
Table 2. Physiological ligament elongations (mm).
Average physiological elongation and physiological elongation range (average ±2 SD) obtained using a mathematical model. The ligaments included the anterior longitudinal ligament (ALL), middle-third disc (MTD), posterior longitudinal ligament (PLL), capsular ligament (CL), ligamentum flavum (LF), and interspinous and supraspinous ligaments (ISL+SSL). The rotation direction used to obtain the physiological elongation is given for each ligament. See Methods for further details.
| Physiological Elongation | Rotation Direction | |
|---|---|---|
| ALL | 1.2 (0.6, 1.8) | Extension |
| MTD | 0.3 (0.1, 0.5) | Extension |
| PLL | 0.8 (0.2, 1.4) | Flexion |
| CL | 1.2 (0.4, 2.0) | Flexion |
| LF | 2.3 (0.9, 3.7) | Flexion |
| ISL+SSL | 3.6 (1.2, 6.0) | Flexion |
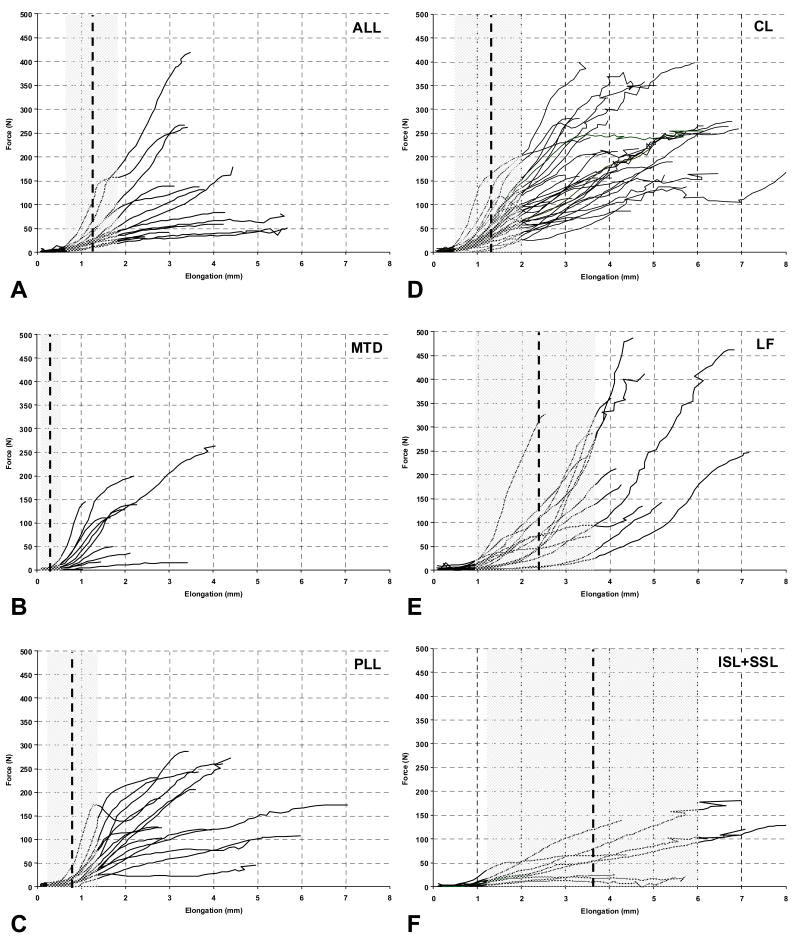
The dynamic force vs. elongation curves up to peak force displayed varying trends among ligaments (Figures 3A to 3F). For comparison, the average physiological ligament elongation with ±2 SD range is shown on each graph. The peak ligament elongation was generally greater than the physiological range, with the exception of LF and ISL+SSL, which had peak elongation in excess of the average physiological elongation.
Figure 3.
Ligament force vs. elongation including the average physiological elongation, shown by a vertical dashed line, and the physiological ligament elongation range (average ±2 SD), indicated by grey shading. The ligaments included A) anterior longitudinal ligament (ALL), B) middle-third disc (MTD), C) posterior longitudinal ligament (PLL), D) capsular ligament (CL), E) ligamentum flavum (LF), and F) interspinous and supraspinous ligaments (ISL+SSL).
The average peak force, elongation, and energy varied by ligament (Table 3A). The highest peak force of 244.4 N was attained in LF, followed by 220.0 N in CL. The peak forces in LF and CL were significantly greater than those in ALL, MTD, and ISL+SSL. The highest peak elongation of 5.9 mm was observed in ISL+SSL, followed by 4.9 mm in CL and 4.2 mm in LF and PLL. MTD had significantly less peak elongation than all ligaments. The highest peak energy of 0.57 J was attained in CL, followed by PLL, LF, and ISL+SSL, with values ranging from 0.33 to 0.36 J. The peak energy in CL was significantly greater than in ALL and MTD, while the peak energy in PLL significantly exceeded that in MTD.
Table 3. Mechanical properties of human cervical ligaments at 723 mm/s.
Average (SD) A) peak force (N), peak elongation (mm), peak energy (J), and B) stiffness (N/mm) at 25, 50, and 75% of peak force. The ligaments included anterior longitudinal ligament (ALL), middle-third disc (MTD), posterior longitudinal ligament (PLL), capsular ligament (CL), ligamentum flavum (LF), and interspinous and supraspinous ligaments (ISL+SSL). Significant differences (P<0.05) among ligaments are indicated in the column Significant. A blank entry indicates that no significant difference was observed.
| A) Peak Force, Elongation, and Energy | ||||||
|---|---|---|---|---|---|---|
| Peak Force | Significant | Peak Elongation | Significant | Peak Energy | Significant | |
| ALL | 137.9 (111.5) | CL,LF | 4.0 (1.0) | MTD | 0.25 (0.15) | CL |
| MTD | 115.6 (79.9) | CL,LF | 2.1 (0.9) | Every ligament | 0.12 (0.15) | PLL,CL |
| PLL | 163.7 (80.2) | 4.2 (1.5) | MTD | 0.33 (0.18) | MTD | |
| CL | 220.0 (83.7) | ALL,MTD,ISL+SSL | 4.9 (1.4) | MTD | 0.57 (0.30) | ALL,MTD |
| LF | 244.4 (143.0) | ALL,MTD | 4.2 (1.5) | MTD | 0.36 (0.25) | |
| ISL+SSL | 85.5 (67.6) | CL,LF | 5.9 (2.9) | MTD | 0.33 (0.39) | |
| B) Stiffness at 25%, 50%, and 75% of Peak Force | ||||||
|---|---|---|---|---|---|---|
| Stiffness at 25% | Significant | Stiffness at 50% | Significant | Stiffness at 75% | Significant | |
| ALL | 46.9 (38.8) | 49.9 (45.2) | 50.4 (53.4) | LF | ||
| MTD | 61.3 (38.8) | 81.4 (61.0) | 96.0 (79.1) | ISL+SSL | ||
| PLL | 71.6 (49.7) | 63.6 (41.4) | 53.0 (33.5) | LF | ||
| CL | 69.4 (34.3) | ISL+SSL | 65.1 (29.2) | 57.9 (28.9) | LF | |
| LF | 72.7 (44.0) | 98.8 (65.4) | ISL+SSL | 118.4 (82.9) | ALL,PLL,CL,ISL+SSL | |
| ISL+SSL | 22.1 (12.7) | CL | 21.3 (11.7) | LF | 19.9 (11.7) | MTD, LF |
LF was generally the stiffest, while ISL+SSL was generally the least stiff (Table 3B). At 25% of peak force, the highest stiffness of 72.7 N/mm was attained in LF. CL stiffness of 69.4 N/mm significantly exceeded ISL+SSL stiffness. At 50% of peak force, LF stiffness of 98.8 N/mm was significantly greater than in ISL+SSL. At 75% of peak force, LF stiffness of 118.4 N/mm was significantly greater than in ALL, PLL, CL, and ISL+SSL. MTD stiffness of 96.0 N/mm significantly exceeded ISL+SSL stiffness.
Discussion
The present study determined the tensile mechanical properties, at a fast elongation rate, of the anterior longitudinal ligament (ALL), middle-third disc (MTD), posterior longitudinal ligament (PLL), capsular ligament (CL), ligamentum flavum (LF), and interspinous and supraspinous ligaments (ISL+SLL). The ligaments were elongated to complete rupture and peak force, peak elongation, peak energy, and stiffness were determined from the force vs. elongation curves. The experimental design of the present study has several important advantages over those of previous studies [16–18, 21]. The present experimental methodology, using bone-ligament-bone preparations, optimized usage of scarce human cadaveric specimens. Rigid fixation of the ligament attachments within the mounts ensured mid-substance tears during elongation, avoiding ligament avulsion from the bone.
The limitations of the present study must be considered before interpreting the results. Since availability of young human cadaveric material is limited, the average age of the present specimens was 80.6 years. Although only six cervical spines were used, bone-ligament-bone preparations from all spinal levels were combined to maximize the sample size for each ligament (Table 1). The maximum number of samples per spinal level was six for CL and was three for all other ligaments, however difficulties in specimen preparation and mounting and lack of ISL+SSL in some spines [5] resulted in decreased sample sizes. In addition, every attempt was made to elongate each ligament along the direction of its fibers. However, this was difficult for MTD, CL and ISL+SSL due to anatomical constraints.
The present results may be compared with previously reported mechanical properties of human cervical spine ligaments obtained at fast elongation rates. We could find only two previous studies (Table 4A). Yoganandan et al [21] tested only ALL and LF at elongation rates of 250 and 2500 mm/s. Shim et al [22] tested alar and transverse ligaments and ALL, PLL, CL, LF, and ISL at elongation rates between 10,000 and 12,000 mm/s, however peak elongation and peak force data were presented only for CL, and alar and transverse ligaments. At both rates reported by Yoganandan et al [21], the peak force, elongation, and energy exceeded the corresponding present data (Table 3A), with the exception of the peak force in LF at 250 mm/s. Comparisons between our CL data (Table 3A) and the average data from Shim et al [22] indicated that peak force increased, while peak elongation decreased with increased elongation rate.
Table 4. Previously reported mechanical properties of human cervical ligaments.
Average (range) peak force (N), elongation (mm), and energy (J) at A) fast (≥ 250 mm/s) and B) slow (≤ 25 mm/s) elongation rates. The ligaments included anterior longitudinal ligament (ALL), intervertebral disc (IVD), posterior longitudinal ligament (PLL), capsular ligament (CL), ligamentum flavum (LF), and interspinous and supraspinous ligaments (ISL+SSL).
| A) Fast elongation rates (≥250 mm/s) | ||||
|---|---|---|---|---|
|
Peak Force |
Peak Elongation |
Peak Energy |
||
| ALL | 250 mm/s | 166.4a | 6.4a | 0.65a |
| 2500 mm/s | 349.5a | 6.3a | 1.23a | |
| LF | 250 mm/s | 181.5a | 6.3a | 0.63a |
| 2500 mm/s | 335.1a | 8.0a | 1.32a | |
| CL | 10,000 to 12,000 mm/s | 259.7 (140.5, 526.9)j | 2.6 (1.8, 3.6)j | |
| B) Slow elongation rates (≤ 25 mm/s) | |||
|---|---|---|---|
|
Peak Force |
Peak Elongation |
Peak Energy |
|
| ALL | 117.3 (8.0, 140.0)b,c,d,f,g | 5.8 (2.4, 8.1)b,c,f,g,i | 0.43 (0.14, 0.68)d,f,i |
| IVD | 569.0h | 11.1h | 4.00h |
| PLL | 114.7 (82.0, 186.0)b,c,d,g | 4.9 (2.4, 6.3)b,c,g,i | 0.22 (0.13, 0.31)d,i |
| CL | 130.2 (88.4, 224.0)a,b,c,e | 8.3 (5.8, 10.8)a,b,c,e,i | 1.50i |
| LF | 136.2 (81.0, 215.0)b,c,f | 7.8 (5.7, 8.9)b,c,f,i | 0.51 (0.35, 0.70)f,i |
|
ISL+SSL |
33.1 (32.0, 34.2)b,c |
7.0 (6.5, 7.3)b,c,i |
0.16i |
The present results may be compared with previously reported results obtained at slow elongation rates of less than 25 mm/s. Numerous studies have reported the peak force, elongation, and energy (Table 4B) using various experimental methodologies [15–19, 21, 28–30]. Comparisons between the present fast rate results to the slow rate results from the literature indicate that the average peak force increased while the peak elongation and energy absorbing capacity decreased with increased elongation rate (Tables 3A and 4B). However, exceptions included the peak force of MTD and the peak energy of PLL and ISL+SSL. Panjabi et al [20] observed similar peak elongation and energy vs. rate relationships, while testing cervical alar and transverse ligaments at slow (0.1 mm/s) and fast (920 mm/s) rates. Additionally, these researchers observed increased ligament stiffness due to increased elongation rate. Shim et al [22] documented increased ligament strength and decreased peak elongation with increased elongation rate. Thus, these cumulative findings suggest that during high speed elongation, cervical ligaments may fail at a higher peak force and smaller peak elongation and may be stiffer and absorb less energy, as compared to the slow elongation speed.
A few LF and ISL+SSL specimens of the present study failed within the physiological ligament elongation range (Figures 3E and 3F). If these failures were to occur in vivo, we believe that the cervical spine would not become unstable. The average peak elongations of LF and ISL+SSL (Table 3A) were between 1.6 and 1.8 times the corresponding average physiological elongations (Table 2). In contrast, the peak elongations of ALL, MTD, PLL, and CL were between 3.3 and 7.0 times the average physiological elongations. Thus, these ligaments which lie close to the intervertebral centers of rotation have a greater factor of safety and play a critical role in stabilizing the spinal column and protecting the neutral tissues from injury. These findings are supported by the results of a classic biomechanical study, which investigated increases in cervical spine mobility due to transection of ligamentous components [31]. Large increases in mobility were observed due to extension loading applied following sequential transection of components from anterior to posterior, indicating the importance of ALL and MTD in stabilizing the spine. In contrast, relatively small increases in mobility were observed due to flexion loading applied following sequential transection of components from posterior to anterior, indicating a less significant role for LF and ISL+SSL. LF and ISL+SSL contain a greater proportion of elastin fibers to enable large elongations during neck flexion, as compared to those in the vicinity of the intervertebral centers of rotation. The primary role of LF may be to protect the spinal cord from impingement during neck motion, while its contribution to spinal stability may be secondary.
The present study investigated the tensile mechanical properties, at a fast elongation rate, of human cervical ligaments including ALL, MTD, PLL, CL, LF, and ISL+SSL. These data may be used by clinicians to determine the strongest and stiffest cervical ligaments and by engineers for improving the biofidelity of mathematical models of the cervical spine. Comparison of the present results with previously reported data indicated that high speed elongation may cause cervical ligaments to fail at a higher peak force and smaller peak elongation and may be stiffer and absorb less energy, as compared to a slow elongation rate. These comparisons may be useful to clinicians for diagnosing cervical ligament injuries based upon the specific trauma.
Acknowledgments
Sources of Support: This research was supported by NIH Grant 1 RO1 AR45452 1A2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clarke KS. Epidemiology of athletic neck injury. Clin Sports Med. 1998;17:83–97. doi: 10.1016/s0278-5919(05)70063-6. [DOI] [PubMed] [Google Scholar]
- 2.Yoganandan N, Pintar FA, Haffner M, Jentzen J, Malman DJ, Weinshel SS, et al. Epidemiology and injury biomechanics of motor vehicle related trauma to the human spine. 1989 Society of Automotive Engineers paper No. 882438. [Google Scholar]
- 3.Torg JS, Guille JT, Jaffe S. Injuries to the cervical spine in American football players. J Bone Joint Surg Am. 2002;84-A:112–22. doi: 10.2106/00004623-200201000-00017. [DOI] [PubMed] [Google Scholar]
- 4.White AA, Panjabi MM. Clinical biomechanics of the spine. 2. Philadelphia: Lippincott; 1990. [Google Scholar]
- 5.Johnson RM, Crelin ES, White AA, 3rd, Panjabi MM, Southwick WO. Some new observations on the functional anatomy of the lower cervical spine. Clin Orthop Relat Res. 1975:192–200. doi: 10.1097/00003086-197509000-00027. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi K, Yabuki T, Kurokawa T, Seki H, Hogaki M, Minoura S. The anterior and the posterior longitudinal ligaments of the lower cervical spine. J Anat. 1977;124:633–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa H, Mikawa Y, Watanabe R. Elastin in the human posterior longitudinal ligament and spinal dura. A histologic and biochemical study. Spine. 1994;19:2164–9. doi: 10.1097/00007632-199410000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Tkaczuk H. Tensile properties of human lumbar longitudinal ligaments. Acta Orthop Scand. 1968;Suppl 115:1+. doi: 10.3109/ort.1968.39.suppl-115.01. [DOI] [PubMed] [Google Scholar]
- 9.Johnson EF, Chetty K, Moore IM, Stewart A, Jones W. The distribution and arrangement of elastic fibres in the intervertebral disc of the adult human. J Anat. 1982;135 (Pt 2):301–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Yahia LH, Garzon S. Structure on the capsular ligaments of the facet joints. Ann Anat. 1993;175:185–8. doi: 10.1016/s0940-9602(11)80179-2. [DOI] [PubMed] [Google Scholar]
- 11.Yahia LH, Garzon S, Strykowski H, Rivard CH. Ultrastructure of the human interspinous ligament and ligamentum flavum. A preliminary study. Spine. 1990;15:262–8. doi: 10.1097/00007632-199004000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Yong-Hing K, Reilly J, Kirkaldy-Willis WH. The ligamentum flavum. Spine. 1976;1:226–234. [Google Scholar]
- 13.Barros EM, Rodrigues CJ, Rodrigues NR, Oliveira RP, Barros TE, Rodrigues AJ., Jr Aging of the elastic and collagen fibers in the human cervical interspinous ligaments. Spine J. 2002;2:57–62. doi: 10.1016/s1529-9430(01)00167-x. [DOI] [PubMed] [Google Scholar]
- 14.Panjabi MM, Oxland TR, Parks EH. Quantitative anatomy of cervical spine ligaments. Part II. Middle and lower cervical spine. J Spinal Disord. 1991;4:277–85. doi: 10.1097/00002517-199109000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Chazal J, Tanguy A, Bourges M, Gaurel G, Escande G, Guillot M, et al. Biomechanical properties of spinal ligaments and a histological study of the supraspinal ligament in traction. J Biomech. 1985;18:167–76. doi: 10.1016/0021-9290(85)90202-7. [DOI] [PubMed] [Google Scholar]
- 16.Pintar FA, Myklebust JB, Yoganandan N, Maiman DJ, Sances A. Biomechanics of human spinal ligaments. In: Sances AS, Thomas DJ, Ewing CL, Larson SJ, Unterharnscheidt F, editors. Mechanics of Head and Spine Trauma. Goshen, NY: Alorny; 1986. pp. 505–527. [Google Scholar]
- 17.Myklebust JB, Pintar F, Yoganandan N, Cusick JF, Maiman D, Myers TJ, et al. Tensile strength of spinal ligaments. Spine. 1988;13:526–31. [PubMed] [Google Scholar]
- 18.Yoganandan N, Kumaresan S, Pintar FA. Geometric and mechanical properties of human cervical spine ligaments. J Biomech Eng. 2000;122:623–9. doi: 10.1115/1.1322034. [DOI] [PubMed] [Google Scholar]
- 19.Przybylski GJ, Carlin GJ, Patel PR, Woo SL. Human anterior and posterior cervical longitudinal ligaments possess similar tensile properties. J Orthop Res. 1996;14:1005–8. doi: 10.1002/jor.1100140623. [DOI] [PubMed] [Google Scholar]
- 20.Panjabi MM, Crisco JJ, 3rd, Lydon C, Dvorak J. The mechanical properties of human alar and transverse ligaments at slow and fast extension rates. Clin Biomech. 1998;13:112–120. doi: 10.1016/s0268-0033(97)00053-3. [DOI] [PubMed] [Google Scholar]
- 21.Yoganandan N, Pintar F, Butler J, Reinartz J, Sances A, Jr, Larson SJ. Dynamic response of human cervical spine ligaments. Spine. 1989;14:1102–10. doi: 10.1097/00007632-198910000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Shim VPW, Liu JF, Lee VS. A technique for dynamic tensile testing of human cervical spine ligaments. Experimental Mechanics. 2006;46:77–89. [Google Scholar]
- 23.Lydon C, Crisco J, Panjabi M, Galloway M. Effect of elongation rate on the failure properties of the rabbit anterior cruciate ligament. Clin Biomech (Bristol, Avon) 1995;10:428–433. doi: 10.1016/0268-0033(95)00019-2. [DOI] [PubMed] [Google Scholar]
- 24.Cholewicki J, Panjabi MM, Nibu K, Macias ME. Spinal ligament transducer based on a hall effect sensor. J Biomech. 1997;30:291–3. doi: 10.1016/s0021-9290(96)00137-6. [DOI] [PubMed] [Google Scholar]
- 25.Panjabi MM, Duranceau J, Goel V, Oxland T, Takata K. Cervical human vertebrae. Quantitative three-dimensional anatomy of the middle and lower regions. Spine. 1991;16:861–9. doi: 10.1097/00007632-199108000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Dvorak J, Panjabi MM, Novotny JE, Antinnes JA. In vivo flexion/extension of the normal cervical spine. J Orthop Res. 1991;9:828–34. doi: 10.1002/jor.1100090608. [DOI] [PubMed] [Google Scholar]
- 27.Ito S, Ivancic PC, Panjabi MM, Cunningham BW. Soft tissue injury threshold during simulated whiplash: a biomechanical investigation. Spine. 2004;29:979–87. doi: 10.1097/00007632-200405010-00006. [DOI] [PubMed] [Google Scholar]
- 28.Siegmund GP, Myers BS, Davis MB, Bohnet HF, Winkelstein BA. Mechanical evidence of cervical facet capsule injury during whiplash: a cadaveric study using combined shear, compression, and extension loading. Spine. 2001;26:2095–101. doi: 10.1097/00007632-200110010-00010. [DOI] [PubMed] [Google Scholar]
- 29.Winkelstein BA, Nightingale RW, Richardson WJ, Myers BS. The cervical facet capsule and its role in whiplash injury: a biomechanical investigation. Spine. 2000;25:1238–46. doi: 10.1097/00007632-200005150-00007. [DOI] [PubMed] [Google Scholar]
- 30.Yoganandan N, Pintar FA, Maiman DJ, Cusick JF, Sances A, Jr, Walsh PR. Human head-neck biomechanics under axial tension. Med Eng Phys. 1996;18:289–94. doi: 10.1016/1350-4533(95)00054-2. [DOI] [PubMed] [Google Scholar]
- 31.Panjabi MM, White AA, 3rd, Johnson RM. Cervical spine mechanics as a function of transection of components. J Biomech. 1975;8:327–36. doi: 10.1016/0021-9290(75)90085-8. [DOI] [PubMed] [Google Scholar]