Abstract
Damage induces remote occlusion of sieve tubes in Vicia faba by forisome dispersion, triggered during the passage of an electropotential wave (EPW). This study addresses the role of Ca2+ channels and cytosolic Ca2+ elevation as a link between EPWs and forisome dispersion. Ca2+ channel antagonists affect the initial phase of the EPW as well as the prolonged plateau phase. Resting levels of sieve tube Ca2+ of ∼50 nM were independently estimated using Ca2+-selective electrodes and a Ca2+-sensitive dye. Transient changes in cytosolic Ca2+ were observed in phloem tissue in response to remote stimuli and showed profiles similar to those of EPWs. The measured elevation of Ca2+ in sieve tubes was below the threshold necessary for forisome dispersion. Therefore, forisomes need to be associated with Ca2+ release sites. We found an association between forisomes and endoplasmic reticulum (ER) at sieve plates and pore-plasmodesma units where high-affinity binding of a fluorescent Ca2+ channel blocker mapped an increased density of Ca2+ channels. In conclusion, propagation of EPWs in response to remote stimuli is linked to forisome dispersion through transiently high levels of parietal Ca2+, release of which depends on both plasma membrane and ER Ca2+ channels.
INTRODUCTION
It has been shown that burning the tip of a Vicia faba leaf induces an electropotential wave (EPW) along the sieve tubes that triggers remote sieve tube occlusion by forisome dispersion and subsequent callose production (Furch et al., 2007). Both occlusion processes are known to require free Ca2+ for response in vitro (Colombani et al., 2004; Knoblauch et al., 2005). Hence, it was postulated that both occlusion mechanisms may be triggered by influx of Ca2+ ions during EPWs.
Long-distance communication by EPWs, initiated by sudden environmental changes, is well established in plants (Stankovic et al., 1998; Stahlberg et al., 2006; Grams et al., 2009). EPWs have been recorded in response to stimuli, such as wounding, cold, heat, and electrical shocks. For example, EPWs were detected after a distant heat stimulus (burning) in Vitis vinifera plants (Mancuso, 1999), Lupinus angustifolius shoots (Zawadzki and Trebacz, 1982), Solanum lycopersicum leaves (Rhodes et al., 1996), and leaf veins of V. faba (Furch et al., 2007). Fromm and Spanswick (1993) also recorded EPWs in response to electrical stimulation using microelectrodes impaled blindly into the stem phloem of Salix viminalis. These EPWs are thought to propagate mainly along the phloem, in particular along the sieve tubes (Samejima and Sibaoka, 1983; Fromm, 1991; Fromm and Spanswick, 1993; Fromm and Bauer, 1994; Rhodes et al., 1996).
The identity of ion channels involved in EPWs is not well characterized, not least because they embody features of both rapid transient action potentials (APs) and slow wave potentials or variation potentials (VPs). In plant APs, the initial depolarization is thought to involve activation of plasma membrane (PM) Ca2+ channels (cf. White, 2000; Demidchik and Maathuis, 2007), leading to Ca2+ influx, and Ca2+-dependent anion channels (Lunevsky et al., 1983; Felle and Zimmermann, 2007), leading to anion efflux. The recovery phase involves inwardly rectifying K+ channels (Lunevsky et al., 1983; Fromm and Lautner, 2007). In slow wave potentials or VPs, there may be activation of mechano-sensitive Ca2+ channels in response to hydraulic pressure waves (Stahlberg and Cosgrove, 1997) and additional shutdown of the PM H+-pump (Stahlberg et al., 2006), leading to prolonged depolarization (Fromm and Lautner, 2007).
The cell walls of sieve elements (SEs) may act as substantial extracellular Ca2+ reservoirs as the apoplast typically contains free Ca2+ concentrations in the order of 10−4 to 10−5 M (Kauss, 1987; Gilroy et al., 1993). Recruitment of Ca2+ from the apoplast implies the need for Ca2+ channels and Ca2+ pumps on the SE plasma membrane (SE-PM). There is some evidence from immunolabeling and fluorescent drug binding studies for nifedipine binding Ca2+ channels on the SE-PM of Nicotiana tabacum and Pistia stratiotes (Volk and Franceschi, 2000).
PM-mediated Ca2+ influx can be augmented by release of Ca2+ from internal Ca2+ stores, such as the endoplasmic reticulum (ER) and vacuole (Sanders et al., 2002). The Ca2+ concentration in vacuoles appears to be in the low millimolar range (Felle, 1988), with similar values reported for the ER lumen under resting conditions (Montero et al., 1995). Since SEs are devoid of vacuoles (e.g., van Bel, 2003), the ER is likely to be the main intracellular Ca2+ store in SEs (e.g., Sjolund and Shih, 1983). Indeed, there is a well-developed stacked sieve element reticulum (SER) that appears to be anchored to the SE-PM in clusters in V. faba (Ehlers et al., 2000) that could provide a substantial, localized Ca2+ reserve. Ca2+ release from and reuptake by ER requires the presence of Ca2+ channels and Ca2+ pumps on the ER membrane (Hong et al., 1999; Sanders et al., 2002).
Local influx of Ca2+ along the EPW pathway may trigger intracellular cascades. Sieve tube occlusion would be an example of such a local response provided that the Ca2+ gradient is steep enough and Ca2+ channel activity allows sufficient Ca2+ influx to trigger forisome dispersion and/or callose formation. However, widely divergent free Ca2+ concentrations have been reported for sieve tube sap, ranging from micromolar to millimolar (Fromm and Spanswick, 1993; Fromm and Bauer, 1994; Brauer et al., 1998; Knoblauch et al., 2005). In other plant cells, cytosolic free Ca2+ concentrations range from 10 to 200 nM and Ca2+ elevations during signaling tend to reach the high nanomolar range (Plieth et al., 1999; Trewavas, 1999; Plieth, 2001; Mithöfer and Mazars, 2002; Logan and Knight, 2003). Thus, reports on high free Ca2+ concentrations in the SE lumen are difficult to reconcile with established Ca2+ signaling paradigms.
Given the presumptive importance of Ca2+ homeostasis and signaling for sieve tube physiology, a detailed study on the resting level of Ca2+ in SE and Ca2+ dynamics during passage of EPWs is required, along with characterization of the possible Ca2+ stores and Ca2+ channels involved. Thus, we first tested the impact of various Ca2+ channel blockers on EPWs induced by distant stimuli and the associate forisome responses. Second, we measured the resting level of free Ca2+ in sieve tube sap using both Ca2+-selective electrodes and fluorescent Ca2+-sensitive dyes. Third, we followed changes in free Ca2+ concentration during EPWs in intact plants using fluorescent Ca2+ dyes and confocal laser scanning microscopy (CLSM). Finally, we characterized the intracellular distribution of Ca2+ channels over the SE using fluorescently tagged Ca2+ channel blockers and CLSM and the association of forisomes with SER clusters by transmission electron microscopy (TEM).
RESULTS
EPWs and Forisome Dispersion Are Sensitive to Ca2+ Channel Blockers
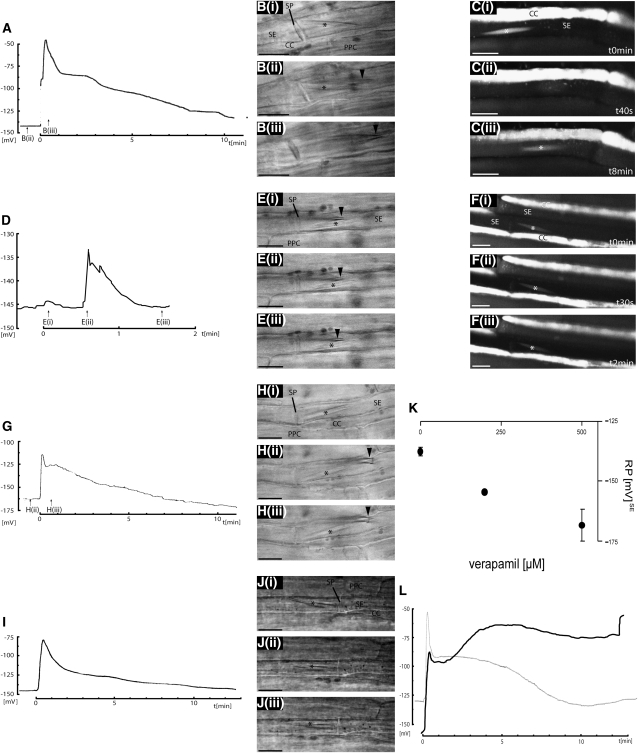
EPWs were recorded using microelectrodes impaled into sieve tubes simultaneously with observation of forisome responses. A wound stimulus, initiated by burning the leaf tip, triggered propagation of EPWs. Seconds after the remote stimulus, the SE-PM transiently depolarized from −140 to −50 mV (Figure 1A). The SE-PM partially repolarized after 60 s to a long-lasting plateau around −90 mV that then gradually returned to resting levels. The magnitude and duration of the plateau phase varied in each measurement before the resting potential was reestablished (n = 10). Prior to the stimulus, the spindle-shaped forisome was typically observed to be closely associated with the sieve plate [Figures 1B(i) to 1B(ii)]. As the peak of the EPW passed, the forisome dispersed shortly (5 to 10 s) after the initial membrane depolarization [Figure 1B(iii)]. To visualize the forisome dynamics more clearly, forisomes were labeled with 5-chloromethyleosin-diacetate (CMEDA)/5-chloromethylfluorescein-diacetate (CMFDA) and observed with CLSM (Figure 1C). Again, a burning stimulus rapidly induced forisome dispersion (n = 12) within ∼1 min [Figure 1C(ii)]. Forisomes subsequently recondensed after ∼8 to 10 min [Figure 1C(iii)], at the time when the membrane potential was approaching the resting state (Figure 1A). Depolarization of the PM alone in the absence of a wound stimulus by adding 100 mM KCl to a second phloem window 3 to 4 cm upstream from the observation window was not sufficient to trigger a substantial EPW (Figure 1D). Only small depolarizations with amplitudes in the range of 2 to 18 mV were observed, and repolarization took place within 60 s (n = 4). No forisome dispersion was observed under these conditions in bright-field images (Figure 1E) or following fluorescent labeling (Figure 1F, n = 8). Likewise, a more gentle burning stimulus in which the leaf tip was not in contact with the flame only gave a brief depolarization with no plateau phase (n = 5). In this case, the forisome dispersed at both ends, while the mid-section stayed condensed.
Figure 1.
The Effect of Ca2+ Channel Blockers on EPWs and Forisome Reactions in Phloem Tissue.
Membrane potentials in SEs were recorded using microelectrodes ([A], [D], and [G]) simultaneously with microscopy observation of forisome reactions during the passage of an EPW in response to a remote burning stimulus ([A] to [C]), remote depolarization induced by 100 mM KCl ([D] to [F]), and burning in the presence of 2 mM La3+ ([G] and [H]). Selected bright-field images ([B], [E], and [H]) are shown at the time points indicated by arrows. In case of burning in the presence of 250 μM nifedipine, recording of EPW (I) and microscopy observation of the forisome (J) were performed in a separate approach. For better visualization of forisome dynamics in response to distant burning (C) and KCl stimulus (F), forisomes were also labeled with CMEDA/CMFDA. The arrowhead ([B], [E], and [H]) indicates the tip of the microelectrode for membrane voltage recording. Asterisks mark the condensed forisomes. CC, companion cells; PPC, phloem parenchyma cell; SP, sieve plate. Verapamil, representing a different class of animal L-type Ca2+ channel blocker, caused hyperpolarization of the resting potential (RPse ± sd; [K]) and limited inhibition of the early phase of the EPW ([L], black trace, 500 μM verapamil) in comparison to the absence of the inhibitor (L, gray trace). Bars = 10 μm.
To determine whether apoplastic Ca2+ influx was required for propagation of EPWs and for forisome dispersion, phloem tissue was preincubated with 2 mM La3+, which is thought to act as a cell-impermeant Ca2+ channel blocker as described for animal systems (Hille, 1992). The electrical signal showed a pronounced reduction in amplitude of the initial depolarization by 50 to 70% for La3+ (Figure 1G, n = 3). La3+, as well as Gd3+ (see Supplemental Figure 1 online), was also sufficient to inhibit forisome dispersion in response to remote burning stimuli (Figure 1H, n = 10) or osmotic shocks (500 mM mannitol, n = 3) in intact phloem tissue.
The effect of the animal L-type Ca2+ channel blockers nifedipine and verapamil were also investigated. Nifedipine at 250 μM was sufficient to inhibit depolarization in response to burning by 30 to 50% at the peak and reduce the plateau phase (Figure 1I, n = 3). Furthermore, nifedipine inhibits the forisome dispersion (Figure 1J). Responses to verapamil (n = 4) were more complex. Verapamil hyperpolarized the resting membrane potential in a concentration-dependent manner (Figure 1K) and inhibited forisome dispersion (see Supplemental Figure 2 online). The initial transient depolarization in response to burning was reduced. However, the plateau phase was prolonged and in some cases showed a subsequent increase accompanied by breakdown of the membrane potential (Figure 1L, 500 μM verapamil, black trace).
Confocal Imaging of Ca2+ Dynamics in Intact SEs
The effect of different Ca2+ channel inhibitors was consistent with a role for Ca2+ influx in response to remote stimuli for both EPW propagation and forisome dispersion. To test whether Ca2+ increases in the sieve element/companion cell (SE/CC) complex could be observed directly, we measured changes in fluorescence from the single-wavelength fluorescent Ca2+ indicators Oregon Green 488-BAPTA-1 (OGB-1) or Fluo-3, loaded as either acetoxymethyl (AM) esters in the presence of Pluronic F127 or at low pH (pH 4.3) in their free acid form (Fricker et al., 1999; Camacho et al., 2000). Both methods were effective in loading OGB-1 or Fluo-3 into intact phloem tissue, as judged by diffuse fluorescence in the densely cytoplasmic CCs, a good marker for cytosolic distribution. Labeling of the SEs showed fluorescence around the periphery of the cell in the unstirred mictoplasmic layer along the SE-PM and at the sieve plate, which is protected from the phloem stream by the overlying SER. The tissue remained viable, as judged from the continuation of rapid cytoplasmic streaming of plastids. Nevertheless, there were some differences in probe distribution. Tissue loaded with AM esters showed additional fluorescence accumulation over time in other cytoplasmic compartments, probably through continued uptake and internal cleavage of the AM ester groups, leading to punctuate staining, and some labeling of vacuoles in cells other than the SEs. By contrast, tissue loaded at low pH showed good intracellular fluorescence distribution in CCs but required more extensive washing to remove the free acid form of the dyes from the cell walls. The removal of apoplastic dye was determined by the absence of fluorescence from the lumen of cut cells adjacent to the phloem tissue. Nevertheless, it is possible that some free dye remained bound to the cell wall.
While it would be desirable to use ratiometric Ca2+ indicators for such measurements, it was not possible to load phloem tissue with Fura-2 or Fura-Red either as AM esters, at low pH, or following microinjection. Without the benefit of ratiometric Ca2+ measurements, it was important to have tight control over changes in the amount of dye, its subcellular location, the optical path length, and the amount of photobleaching, which may all result in changes in fluorescence intensity independently of any changes in cytosolic-free Ca2+ (Fricker et al., 1999; Camacho et al., 2000). We therefore developed a set of imaging protocols designed to minimize the effects of tissue movement and photobleaching on single wavelength measurements.
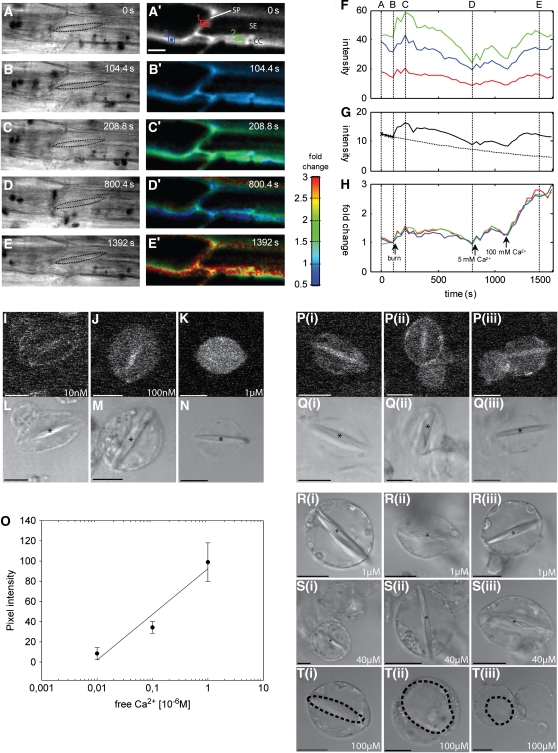
Tissue movements are an inevitable consequence of pressure waves arising from burning. To ensure that the fluorescence signals were consistently drawn from the same part of the cell, images were initially collected as three-dimensional (x,y,z) stacks of optical sections at 20- to 40-s time intervals to give four-dimensional (4-D) (x,y,z,t) image series. Specific image planes were then manually selected, typically to include the forisome in the corresponding transmission images (Figures 2A to 2E), averaged in z with the two adjacent optical sections and then automatically aligned over time by cross-correlation to yield a completely registered (x,y,t) time series (Figure 2A′). The decay in the total fluorescence intensity prior to addition of a stimulus was fit with a monoexponential curve to determine the intrinsic bleaching rate for each time series (Figure 2G) and used to correct the intensity values to compensate for photobleaching. In general, bleaching rates varied from 0.05 to 0.2% s−1 for OGB-1 depending on the pixel dwell time, number of optical sections per stack, and sampling frequency. Bleaching rates for Fluo-3 were significantly greater than for OGB-1, so most experiments were conducted with OGB-1. Finally, images (Figures 2B′ to 2E′) and measurements from specific regions of interest (ROIs; Figure 2F) were expressed as the fold change (see Methods) from the fluorescence intensity immediately prior to the stimulus (Figure 2H). In some experiments, high levels of external Ca2+ were added at the end of the experiment to drive the intracellular indicator to its maximum fluorescence value (e.g., Figures 2F and 2H). This indicated a maximum fold change in vivo of ∼2.6-fold above resting levels for OGB-1 (Figure 2H). This is consistent with the theoretical expectations from the in vitro spectra with a resting cytosolic Ca2+ of ∼100 nM.
Figure 2.
In Vivo Estimation of Resting Ca2+ Levels in Intact Phloem and SEPs Using OGB-1 and CLSM.
Transmission bright-field images with stippled contours of the forisome ([A] to [E]) and confocal optical sections of OGB-1 fluorescence ([A′] to [E′]) from 4-D (x,y,z,t) images were aligned in x,y and z to give a completely registered time series and converted to floating-point format for all subsequent operations. Following subtraction of background signals, a bleaching correction was determined from a monoexponential fit (dotted line) to the trend in the total intensity before the stimulus (solid line) (G). Corrected images were normalized to a reference image, taken immediately prior to the burning stimulus to determine the fold change in intensity and visualized using a pseudo-color scale ([B′] to [E′]) in which hue represents the fold change and the intensity represents the signal strength. Measurements for specific ROIs (green, blue and red boxes in [A′], represented by the same colors in [F] and [H]) are shown for the original averaged intensity (F) and as the fold change after correction for photobleaching (H). A short transient was observed following a remote burning stimulus ([C′] and [H]), followed by a prolonged plateau that returned to resting levels over the next 8 min ([D′] and [H]). Addition of increasing levels of external Ca2+ was used to drive the internal Ca2+ to saturation (H). This gave a maximum fold change of ∼2.6 in vivo. More precise calibration of the OGB-1 response was achieved in SEPs, following loading with OGB-1-AM, in which internal Ca2+ was clamped using ionomycin to calibration solutions with increasing Ca2+ and fluorescence imaged using CLSM ([I] to [K]). Average intensity measurements from the cytoplasm, excluding the labeled forisome, were used to construct a calibration curve ([O]; n = 7). No forisome dispersion was observed during calibration, even at 1 μM ([L] to [N]). The in vivo calibration was used to determine the resting Ca2+ in the absence of ionophore (three examples of each treatment are shown in [P]) as 35 ± 15 nM (n = 7). To determine the threshold internal Ca2+ needed to trigger forisome dispersion in SEPs, the level of Ca2+ was clamped at 1, 40, and 100 μM using ionomycin (three examples are shown in [R] to [T]). Forisome dispersion (dashed lines) only occurred when the Ca2+ concentration was >40 μM (T). Asterisks mark forisomes in the condensed state. Bars = 10 μm.
While it was not possible to achieve a full Ca2+ calibration in intact tissues, to confirm that internal OGB-1 was fully Ca2+ responsive with similar sensitivity to in vitro titration conditions, the Ca2+ sensitivity for OGB-1 was measured in sieve element protoplasts (SEPs), as these were easier to manipulate using ionophores compared with SEs and CCs in intact tissue (Figures 2I to 2K). SEPs were isolated from V. faba transport phloem (Hafke et al., 2007) and loaded with OGB-1AM. Ca2+-dependent fluorescence was recorded in number of SEPs using low-intensity laser scans to minimize photobleaching of OGB-1AM as described before for Fluo-3 (Plank and Sussman, 2003). For calibration, SEPs loaded with OGB-1AM taken from the same protoplast batch used for measurement were permeabilized by the Ca2+ ionophore ionomycin to allow intracellular/extracellular Ca2+ equilibration. Protoplasts were calibrated using standards with defined free Ca2+ concentrations according to a protocol designed for one-wavelength Ca2+ indicators (Plank and Sussman, 2003).
The fluorescence intensity from optical sections of SEPs loaded with OGB-1AM increased when equilibrated with different external Ca2+ concentrations using 4 μM ionomycin to permeabilize the PM to Ca2+ (Figures 2I to 2K). Forisome staining or intensive fluorescence near the PM from adherent compartments were excluded from the calibration procedure. The averaged pixel intensity values, corrected for background, exhibited an ∼10-fold change in intensity when Ca2+ was clamped over the range 0.01 to 1 μM (Figure 2O, n = 7). This compares well with the theoretical maximum of ∼14-fold change expected between zero and saturating Ca2+. Using this calibration, the resting Ca2+ concentration in SEPs was estimated as 35 ± 15 nM (Figures 2P and 2Q, n = 7). It is noteworthy that the forisomes did not disperse during the calibration at the highest Ca2+ concentration (1 μM) used (Figures 2L to 2N). As an independent approach to confirm that resting Ca2+ was indeed low and comparable to resting levels in other cell types, we also measured free Ca2+ using Ca2+-selective electrodes in picoliter droplets of pure phloem sap following aphid stylectomy. With this approach, free Ca2+ concentrations in the range of 40 to 70 nM (n = 4) were found for sieve tube sap.
We infer that OGB-1 provides a useful Ca2+ indicator with a reasonable dynamic range for phloem tissues in vivo. The calibrated measurements in SEPs and fold change observed following artificial elevation of internal Ca2+ are consistent with a low resting level of Ca2+ in these tissues. We also infer from the full dynamic range observed that the contribution of any remaining wall-bound dye was negligible. Any external dye would be saturated with Ca2+ at apoplastic Ca2+ resting levels of ∼60 μM in V. faba (Felle et al., 2000) and would not respond further to elevated external Ca2+ (although fluorescence would also be quenched in the buffered medium at pH 5.7). This ought to provide a constant offset that would reduce the dynamic range of the response. However, this was not observed. The data also indicate that relatively high Ca2+ concentrations may be required in vivo to trigger forisome dispersion, as already observed in vitro (Knoblauch et al., 2005).
We therefore took advantage of the SEP system to facilitate manipulation of internal Ca2+ concentrations in the presence of ionomycin (Figures 2R to 2T) to confirm that substantial increases in Ca2+ were required for forisome dispersion in vivo. Up to an external Ca2+ concentration of 40 μM, forisomes stayed in the condensed state (Figure 2S), but forisomes did disperse when the external Ca2+ concentration was raised further to 100 μM (Figure 2T).
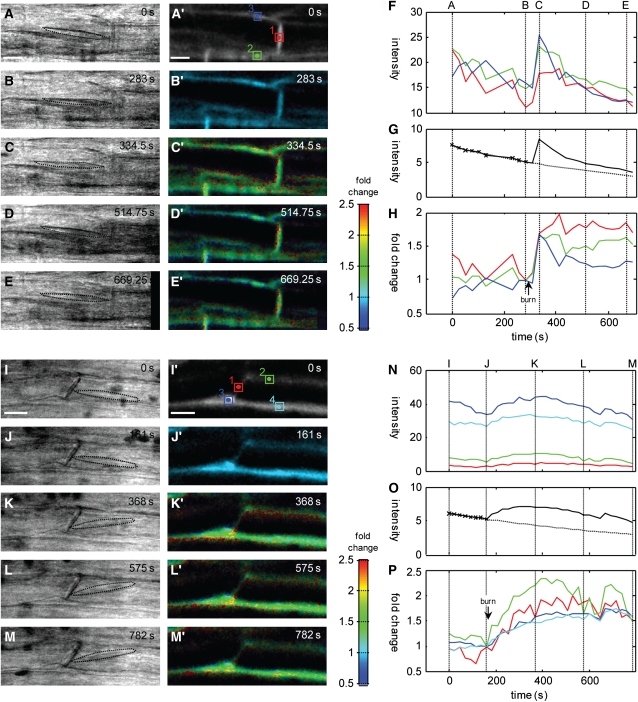
Burning Stimuli Trigger Transient Increases in Ca2+
An increase in free Ca2+ was detected in phloem tissue after burning the leaf tip using OGB-1 and CLSM (n = 14). In some experiments, peak responses were observed within 60 s and were followed by an extended plateau over a period of 10 min (Figuress 2H and 3A to 3H), strongly reminiscent of the kinetics of EPWs in response to burning (Figure 1A). In other cases, the increase in Ca2+ was more gradual, peaking several minutes after the original stimulus was applied (Figures 3I to 3P). There was relatively little difference in the kinetics of the responses between SEs, CCs, or phloem parenchyma cells in each experiment, although the response from the SEs and CCs was marginally greater in amplitude. Across all experiments, the amplitude was quite variable, ranging from ∼1.5-fold (see Figures 2C′, 2H, 3C′, and 3H) to >2-fold (Figures 3I′ to 3M′ and 3P). Assuming resting Ca2+ levels were in the range of 50 to 100 nM, these changes represented increases in Ca2+ to 200 to 500 nM.
Figure 3.
In Vivo Ca2+ Dynamics in Response to Burning Stimuli.
Different profiles of Ca2+ were observed in response to remote burning stimuli. In some cases ([A] to [H]; n = 5), there was a rapid initial response and a prolonged plateau phase. In other cases ([I] to [P]; n = 7), increases occurred more gradually. In some others (n = 3), changes were minimal. Transmission bright-field images, shown with a stippled outline around the forisome ([A] to [E], [I], and [J]), and confocal optical sections of OGB-1 fluorescence ([A′] to [E′], [I′], and [J′]) from 4-D (x,y,z,t) images were aligned in x,y and z to give a completely registered time series and converted to floating-point format for all subsequent operations. Following subtraction of background signals, a bleaching correction was determined from a monoexponential fit (dotted line) to the trend in the total intensity before the stimulus (solid line) ([G] and [O]). Corrected images were normalized to a reference image, taken immediately prior to the stimulus to determine the fold change in intensity and visualized using a pseudo-color scale ([B′] to [E′] and [I′] to [M′]), in which hue represents the fold change and the intensity represents the signal strength. Measurements for specific ROIs (boxed areas in [A′] and [I′], represented by the same colors in [F], [H], [N], and [P], respectively) are shown for the original averaged intensity ([F] and [N]) and as the fold change after correction for photobleaching ([H] and [P]). In the first experiment, a pronounced transient was observed within 20 s of a remote burning stimulus ([C′] and [H]). Measurements including the sieve plate or associated CC showed a sustained increase, while signal from an overlying phloem parenchyma cell (PPC) decayed more quickly ([F] and [H]). The other class of responses observed included a more gradual rise to an extended plateau ([I] to [P]). In this case, responses including the SE and CC and PPC showed similar kinetics, but the amplitude was marginally greater from the SE/CCs (P). The maximum fold change observed in the experiments shown here was ∼2- to 2.5-fold, which corresponds to an increase in Ca2+ up to ∼500 nM.
In comparable measurements using a remote KCl application to trigger membrane depolarization, no long-term changes in Ca2+ were detectable in confocal time series (n = 3). As EPWs in response to KCl only show a rapid AP-like response, we also used rapid line scanning to achieve better temporal resolution over 4-D image collection. Following a shift from 2 to 100 mM KCl, there was a gradual rise in intensity over ∼20 s followed by a return to resting levels by 40 s (see Supplemental Figure 3 and Supplemental Methods 1 online). The relative magnitude of the change was small, in the order of a 20% increase. This would equate to an increase in free Ca2+ of <50 nM.
Despite the transient increases in Ca2+ observed, particularly with the burning stimuli, no forisome dispersion was observed in any of the experiments in which tissue was loaded with OGB-1 (n = 14), consistent with the much higher threshold identified in the SEPs (Figure 2T). In stimuli with the lowest amplitude, the forisomes remained associated with the sieve plate and barely altered position during the Ca2+ response (e.g., Figures 2A to 2E). This contrasts with dispersion of 84% of forisomes in this location in unlabeled tissues (n = 45; see Figure 4B). In SEs with larger Ca2+ changes, forisomes shifted position with the onset of the Ca2+ transient (Figures 3A to 3E and 3I to 3M) but still did not disperse. These data suggest that the normal coupling between EPW and forisome dispersion was specifically disrupted by the presence of the cytosolic Ca2+ indicator. We estimate the concentration of OGB-1 in these experiments was in the range 50 to 100 μM. As OGB-1 is not known to have any other intracellular site of action, the most parsimonious explanation is that such concentrations of a mobile Ca2+ buffer were sufficient to breakdown local Ca2+ hotspots that might be needed for forisome dispersion. This explanation is consistent with the observed disruption of Ca2+ gradients by mobile buffers widely reported for animal systems (Neher, 1998; Demuro and Parker, 2006).
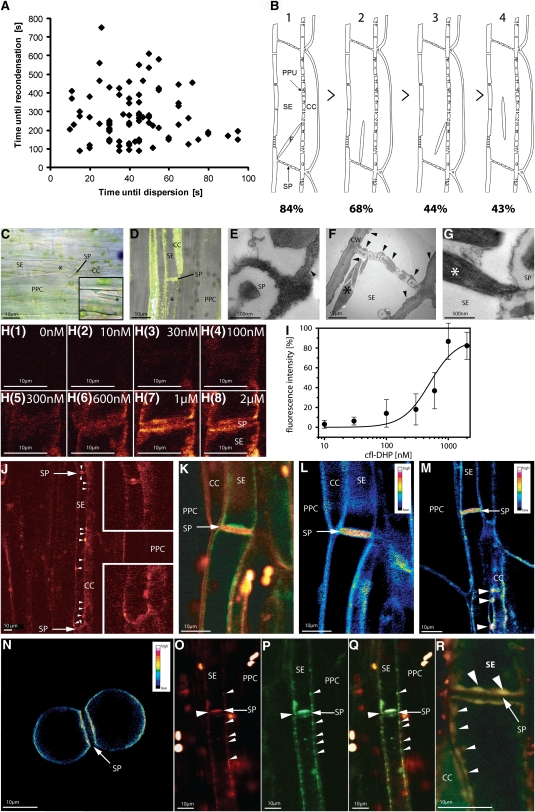
Figure 4.
Relationship between Forisome Position and Responsiveness to Stimuli and Localization of Ca2+ Channels in SEs of V. faba Main Veins.
(A) Time correlation between forisome dispersion in response to distant burning of the leaf tip of intact V. faba plants and forisome recondensation. The time points on the x axis indicate the lag before forisome dispersion after the remote burning stimulus. Those on the y axis represent the recondensation time after burning stimulus was applied.
(B) The relationship between forisome reactivity and forisome position inside SEs as identified by microscopy. Different positions are coded by numbers: (1) forisome with one end attached to the sieve plate (SP) region and the other end to the SE-PM), (2) forisome with one end attached to the SE-PM in the SP region, (3) forisome with one end attached to the SE-PM at the CC side, and (4) forisome positioned remote from the SP with no apparent attachment to the SE-PM. The percentage of forisomes dispersing in response to distant burning is given for each category.
(C) Attachment of forisomes in intact phloem tissue in the V. faba main vein visualized using light microscopy. The forisome (asterisk) has one forked end attached to SER and the other to the SP. The inset shows a higher magnification of the attachment of forisome forks to the SER.
(D) Overlay of phloem tissue stained with ER-Tracker green (green) and the transmission picture of SEs as recorded by CLSM. The forisome (asterisk) is attached to the SP.
(E) to (G) High-magnification electron micrograph (×8600) of well-preserved V. faba SEs. Deposits of stacked ER cisternae ([E] and [F]; arrowheads) occur near the SP and are attached to the PM. The large forisome (F; asterisk) in the SE is in the condensed state. The sieve pores possess callose collars due to the section plane. CW, cell wall.
(H) Binding of the fluorescent Ca2+ channel inhibitor DM-BODIPY-DHP (fl-DHP) to the SP region of intact V. faba tissue. Representative titration (one out of eight) with various concentrations (0 to 2 μM) of fl-DHP as indicated in the micrographs.
(I) Titration curve. Plot of the measured fl-DHP fluorescence intensity (averaged pixel density) recorded from the SP region (see [H]) versus different fl-DHP concentrations (n = 8). Pixel density values were corrected for background. The titration curve was fitted by a Hill equation revealing a high-affinity fl-DHP binding system with Kd values of 600 nM.
(J) CLSM micrograph of V. faba phloem tissue, stained with the fluorescent Ca2+ channel inhibitor (red color) DM-BODIPY-DHP (fl-DHP). Insets show a higher magnification of both SP regions.
(K) Subcellular distribution of Ca2+ channels in double-stained SEs (RH-414, red; BODIPY-DHP, green) observed by CLSM. Ca2+ channels are localized in clusters bearing similarity to SER distribution.
(L) to (N) Ratio images of intact phloem tissue ([L] and [M]) and isolated SE protoplasts (N) are presented as pseudo-colors, obtained by division of the measured fluorescence intensities of BODIPY-DHP by RH-414. (L) represents the ratio image of (K). Highest Ca2+ channel frequency was observed at SPs and at pore plasmodesma units ([M]; arrowheads).
(O) to (R) Localization of BODIPY-DHP (red, Ca2+ channels) (O) and ER-Tracker Red (green, ER) (P). An overlay results in a yellowish color (Q). Colocalization of fl-DHP and SER in an independent micrograph in the SP area (R). Large arrowheads mark the colocalization of ER and Ca2+ channels at the SP and small arrowheads the colocalization of ER and Ca2+ channels near the PM. The direction of translocation is from top to bottom in all pictures. PPC, phloem parenchyma cell.
Forisome Dispersion in Response to a Heat Stimulus Correlates with the Location inside the SE
As noted above, the majority of forisomes adjacent to the sieve plate were observed to respond to a burning stimulus in unlabeled SEs. However, across >150 experiments, the reactivity of forisomes was variable, depending on their location in the SE, and some forisomes did not react at all. For each individual forisome that did respond, there was no correlation between the lag time following the stimulus until dispersion occurred and the subsequent time until recondensation (Figure 4A).
The propensity to disperse appeared to be related to the location of the forisome inside the SE. Forisomes were divided in four categories (Figure 4B): (1) forisomes near the sieve plate, with one end against the sieve plate and the other associated with the SE-PM; (2) forisomes with one end near the sieve plate; (3) forisomes distant from the sieve plate but with one end in the vicinity of the SE-PM; and (4) forisomes in the middle of the SE with no apparent association with the SE-PM. Eighty-four percent of the forisomes of category 1 (n = 45) and 70% of forisomes of category 2 (n = 44) dispersed in response to a distant heat stimulus (Figure 4B). By contrast, <50% of the forisomes of categories 3 (n = 40) and 4 (n = 41) reacted to burning.
Taken together with the proposal that highly localized Ca2+ hotspots might be required for forisome dispersion, this response profile might point to clustered Ca2+ stores or Ca2+ release channels leading to varying probabilities for forisome dispersion depending on forisome location. At the light microscopy level, attachment sites were sometimes observed between forisomes and the PM, effectively tethering the forisome in place (Figure 4C). Labeling with the ER dye, ER-Tracker Green, suggested that there was plentiful SER associated with these attachment sites (Figure 4D). At higher resolution, TEM images also highlighted close association between the forisome and clusters of stacked SER, particularly near the sieve plates (Figures 4E to 4G).
Cellular and Subcellular Localization of Ca2+ Channels in SEs
As a working hypothesis, we proposed that the configuration of the SER and apparent need for highly localized Ca2+ elevation to trigger forisome dispersion might be functionally linked if these SER stores were also endowed with a high density of Ca2+ channels. There are relatively few well-characterized tools available to quantify Ca2+ channel distributions in vivo, particularly given that it is extremely difficult to make electrophysiological measurements in phloem tissues. Nevertheless, there is evidence that the fluorescently tagged dihydropyridine, BODIPY-DHP, inhibits Ca2+ channels in plants through binding to voltage-sensitive Ca2+ channels (cf. White, 2000) and can be used to visualize Ca2+ channel distributions (Vallée et al., 1997). Given that nifedipine was observed to reduce EPWs (Figure 1A), we therefore tried to map DHP-sensitive channels in intact phloem tissue using fl-DHP.
Increasing concentrations of fl-DHP gave increased labeling within the SEs, particularly at the sieve plates (Figure 4H). Moreover, preincubation of intact phloem tissue in 500 μM of unlabeled nifedipine strongly reduced the fluorescence of fl-DHP, suggesting that nifedipine and the fl-DHP compete for the same binding site and does not simply reflect partitioning into a lipid environment. This has been taken to indicate that DHP is specifically binding to Ca2+ channels in other systems (Vallée et al., 1997). Nevertheless, nifedipine also inhibits outward K+ channels in patch-clamp studies at higher micromolar concentrations (e.g., Terry et al., 1992; Thomine et al., 1994). Thus, to determine the specificity of binding, intact phloem tissue was titrated with increasing concentrations of fl-DHP (n = 8). A binding curve was obtained by plotting the fluorescence intensity of fl-DHP recorded by CLSM at each fl-DHP concentration (Figure 4I). The titration curve reveals fl-DHP binding to a high affinity system with a Kd of 600 nM. This is 10- to 100-fold lower than the Kd (5 to 90 μM) reported for nonspecific binding to K+ channels (Terry et al., 1992; Thomine et al., 1994). There was also a suggestion of a second, even higher affinity binding system with a Kd of 40 nM. Thus, to localize putative Ca2+ channels in intact phloem, we used a concentration of 40 nM to reduce potential artifacts from nonspecific binding.
CLSM revealed patches of fl-DHP fluorescence at the highest density near the sieve plates (Figure 4J, insets, magnification of the sieve plate region) and along the PM of SEs. There was insufficient spatial resolution to unequivocally attribute labeling to the PM or internal membranes. Nevertheless, the irregular shape of the fl-DHP patches projecting into the luminal side of the SE suggested that fl-DHP labeling included internal membranes, such as the ER. Equally, the apparent high density of labeling at the sieve plates could include PM localization and localization to the ER within the numerous pore plasmodesma units. We therefore used colocalization with PM and ER-specific fluorophores to characterize further the predicted channel distribution.
The PM was labeled with the potential-sensitive dye RH-414 to give an independent marker for the cell boundaries (Figure 4K). The merged image clearly shows that fl-DHP labeling channels occur inside the SE lumen, most likely associated with the SER (Sjolund and Shih, 1983), as well as the SE-PM. RH-414 is known to stain solely the PM and would be expected to be distributed evenly in the PM. This provides a convenient reference to quantify the relative distribution of fl-DHP signal in or near the PM. Thus, the Ca2+ channel density was approximated by division of the fluorescence intensities of fl-DHP and RH-414 (Schild et al., 1995). The ratio fl-DHP/RH-414 image is presented in pseudocolor for intact phloem tissue (Figures 4L and 4M) as well as for an isolated twin SEP (Figure 4N; cf. Hafke et al., 2007). In all cases, a high ratio was specifically associated with the sieve plate and the pit fields connecting the SEs with the CCs, suggesting the absolute numbers of DHP-sensitive channels are higher in these regions.
Using a similar approach, we colocalized fl-DHP fluorescence with ER membrane labeled with ER-Tracker Red (Figures 4O to 4Q). Figure 4O (red channel) shows fl-DHP staining and Figure 4P (green channel) ER-Tracker Red staining of intact phloem tissue. The merged image (Figure 4Q) shows a high degree of overlap in the two signals, suggesting that DHP-sensitive Ca2+ channels and SER are colocalized in clusters at the sieve plate region and along the SE, mostly at the junction with the CCs (Figure 4R).
DISCUSSION
Changes in Cytosolic-Free Ca2+ Accompany EPWs in SEs and CCs
We showed that the free Ca2+ concentrations in SEs and CCs are ≤100 nM using two independent techniques. These values are significantly lower than previous estimates in the micromolar range (Brauer et al., 1998) but match well with cytoplasmic free Ca2+ concentrations of 10 to 200 nM reported for other plant systems (Plieth et al., 1999; Trewavas, 1999; Mithöfer and Mazars, 2002; Logan and Knight, 2003).
Such low resting Ca2+ levels are a prerequisite for Ca2+ to act in conventional signaling cascades, and we have demonstrated that Ca2+ increases occur in the SE/CC complex and adjacent cells following burning stimuli, with similar profiles and kinetics to EPWs measured in parallel experiments. The average Ca2+ increases in the SE/CC complex observed during burning responses reached only a few hundred nanomolar (200 to 500 nM), similar in magnitude to Ca2+ changes in response to hypo- and hyperosmotic treatments in Arabidopsis thaliana (Plieth, 2001) and duration to heat responses in tobacco (Nicotiana tabacum; summarized in Malho et al., 1998).
By contrast, only a brief AP, small transient change in Ca2+cyt (< 50 nM) and no forisome dispersion was detected in SE/CC after PM depolarization by remote application of KCl. Taken together (Figures 2H and 3P; see Supplemental Figure 3 online), these data provide the evidence that the stimulus strength is reflected in the form of specific spatio-temporal Ca2+cyt elevations (for review, see Ng and McAinsh, 2003; McAinsh and Pittman, 2009).
Ca2+ Channels Act as Relay Stations between Remote Stimuli and Occlusion Response
The inhibitory effect of a range of Ca2+ channel blockers, particularly membrane-impermeant La3+ (Figure 1G), on the amplitude of EPWs recorded from V. faba SE/CC complex was consistent with a requirement for external Ca2+ influx during both the early transient AP-like phase of the phloem EPW and the prolonged VP-like depolarization phase. Besides La3+ (Figure 1H), Gd3+ (see Supplemental Figure 1 online) acts as a potent inhibitor for Ca2+-dependent forisome dispersion in intact V. faba tissue, as previously shown for isolated SEPs (Hafke et al., 2007).
While the propagation of APs involves recruitment of PM voltage-dependent Ca2+ channels (cf. White, 2000; Demidchik and Maathuis, 2007), osmo-sensitive PM Ca2+-conducting channels (Knoblauch et al., 2001; Hafke et al., 2007; Zhang et al., 2007) may also operate during VP in response to the hydraulic pressure wave (Stahlberg and Cosgrove, 1997). Despite the importance of PM Ca2+ channels in AP-like responses, there is also good evidence that propagation of APs in other systems is strongly dependent on additional release from internal stores (Plieth et al., 1999). As SEs lack vacuoles, the SER is the most obvious candidate to act as a major internal Ca2+ store in SEs (Sjolund and Shih, 1983; Arsanto, 1986). We showed that EPWs were inhibited by the membrane-permeant Ca2+ channel blocker nifedipine and that a fluorescent derivative of nifedipine (fl-DHP), known to bind to voltage-sensitive Ca2+ channels in animals (e.g., Knaus et al., 1992), specifically labeled ER membranes when applied at extremely low concentrations.
In vivo binding of fl-DHP was abolished by excess unlabeled nifedipine and showed high affinity binding (Kd 600 nM; Figure 4I). The Kd is significantly lower than binding of nifedipine to outward K+ channels that are present on the PM of SE (Hafke et al., 2007), which show Kd values in the range 5 to 90 μM (Terry et al., 1992; Thomine et al., 1994). We infer that binding of fl-DHP is specific to Ca2+ channels in Vicia SE/CCs, as suggested for other plant tissues (Vallée et al., 1997; Volk and Franceschi, 2000). Maps of fl-DHP binding showed substantial concentration of putative Ca2+ channels at pore plasmodesma units and SE-CC junctions (Figures 4L and 4M) and colocalized with fluorescent ER markers. This latter observation is consistent with reports of fl-DHP binding to voltage-gated ER Ca2+ channels in crystal idioblasts of Pistia stratiotes (Volk et al., 2004).
DHP-sensitive Ca2+ channels reside on both the PM and SER in the SE-CC complex. Although the functional significance of the DHP-sensitive channels in the overall response is not yet clear, the similarity between Ca2+ kinetics and the long-lasting plateau phase of the EPW (Figures 1A and 1L) suggests a temporal correlation between Ca2+ and prolonged depolarization. While cell-impermeant La3+ appeared to preferentially inhibit the initial AP-like phase and had relatively little effect on the plateau, nifedipine gave a pronounced reduction in plateau phase of the EPW. We infer that nifedipine may enter the cell and bind to SER Ca2+ channels, as suggested from fl-DHP binding studies (Figure 4). This may result in suppression of Ca2+ release from SER and a reduction in internal signal.
Forisomes Must Be Tethered in the Vicinity of High Ca2+ Channel Concentration for Full Responses
In the absence of a cytosolic Ca2+ indicator, the majority (84%) of forisomes near the sieve plate dispersed in response to a burning stimulus (Figure 4B). Conversely, in the presence of OGB-1, no forisome dispersion was ever observed, and the most marked response was limited to rapid lateral movement or translocation of the forisome. Inhibition of dispersion was specifically associated with Ca2+ dyes, rather than arising as a general consequence from laser illumination during confocal imaging or dye toxicity (e.g., Camacho et al., 2000), as forisome dispersion occurred normally in a wide range of other imaging and labeling regimes.
One plausible explanation for both the low Ca2+ elevations observed and the inhibition of forisome response is that OGB-1 acts as a mobile Ca2+ buffer and collapses any locally high Ca2+ gradients that are needed to trigger forisome dispersion. Consistent with this view, we confirm here that high (> 50 μM) Ca2+ concentrations (Figures 2R to 2T) are needed for forisome dispersion in vivo as observed in vitro (Knoblauch et al., 2005).
Dispersion of local Ca2+ hot spots is well established in animal systems (Bolsover and Silver, 1991; Neher, 1998; Demuro and Parker, 2006) and can be observed with dye concentrations as low as 10 μM. There is less data specifically for plants, but loading with BAPTA buffers at comparable concentrations to OGB-1 used here is known to dissipate standing Ca2+ gradients in pollen tubes (Miller et al., 1992; Pierson et al., 1994) and Fucus rhizoids (Speksnijder et al., 1989; Taylor et al., 1996).
Current models of Ca2+ channel behavior suggest that very high (100 μM) localized Ca2+ increases can occur in the vicinity of the Ca2+ channel pore (Trewavas, 1999). If no other signals than Ca2+ alone are needed for full response, this concentration might normally be sufficient to trigger forisome dispersion. However, as OGB-1 is mobile and has high affinity and fast binding kinetics, it might act to dissipate these local gradients. Diffusion and dissociation of the dye Ca2+ complex would be measured as a lower and more gradual increase in Ca2+ throughout the cytoplasm.
A corollary of this proposal is that the forisomes might be expected to reside in close physical proximity to Ca2+ channel pores to experience a sufficiently high Ca2+ concentration. Several observations suggest the precise location of the forisome on both a macro and micro scale was critical to its responsiveness. The chance of dispersion in response to burning depended on the forisome position within the SEs (Figure 4B) and increased with proximity to the sieve plate where the highest frequencies of Ca2+ channels were observed (Figures 4J to 4R). Furthermore, the more intimately forisomes were associated with SER and/or the PM, the greater the probability of dispersion. The forisome ends, which are frequently forked, were sometimes observed at the TEM level to be tethered to the PM or to the SER stacks or often inserted into the ER interstices (Figures 4C to 4G). This configuration provides narrow spaces between SER stacks (Sjolund and Shih, 1983; Ehlers et al., 2000) that form an unstirred layer, in which Ca2+ levels may increase rapidly to high levels. The close physical proximity between PM and SER in these regions may also contribute to coupling between the initial PM depolarization and Ca2+ release from the ER via some form of mechanical or electrical coupling (Hepler et al., 1990). In SEs, SE-PM and SER are linked by protein anchors of unknown composition with a length of ∼7 nm (Ehlers et al., 2000). Thus, voltage changes in the one membrane may be transmitted to the other membrane and influence voltage-activated Ca2+ channels there (Klüsener et al., 1995; Klüsener and Weiler, 1999). Alternatively, Ca2+ influx through the SE-PM may trigger Ca2+-induced Ca2+ release from putative Ca2+-dependent Ca2+ channels, analogous to the situation reported so far for PM to tonoplast coupling (Bewell et al., 1999; Sanders et al., 2002).
METHODS
Plant Material
Plants of Vicia faba cv Witkiem major (Nunhems Zaden) were cultivated in pots in a greenhouse at 20 to 30°C, 60 to 70% relative humidity, and with a 14/10-h light/dark regime. Supplementary lighting (model SONT Agro 400 W; Phillips) was used to give an irradiance level of 200 to 250 μmol−2 s−1 at the plant apex. Plants were used 17 to 21 d after germination in the vegetative phase just before flowering.
Preparation of Intact Plants for Electrophysiology and Observation by CLSM
For in vivo observation of sieve tubes, cortical cell layers were removed to create an observation window on the phloem from the abaxial side of the main vein of a mature leaf that was still attached to the intact plant. The cortical tissue was locally removed by manual paradermal slicing with a fresh razor blade, while avoiding damage to the phloem (Knoblauch and van Bel, 1998). The distance between the observation window and the leaf tip where stimuli were applied was 3 to 4 cm. In experiments with a KCl stimulus (100 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 5 mM MES/NaOH, pH 5.7) two paradermal windows 3 cm apart were cut according to Knoblauch and van Bel (1998). The KCl stimulus was applied to the apical application window and observed through the more basal observation window. The cut tissue was covered with bathing medium containing 2 mol m−3 KCl, 1 mol m−3 CaCl2, 1 mol m−3 MgCl2, 50 mol m−3 mannitol, and 2.5 mol m−3 MES/NaOH buffer, pH 5.7. In the case of the KCl stimulus, the mannitol was increased to 200 mol m−3 to maintain the osmotic balance. The leaf was fixed to a microscope slide with two strips of double-sided adhesive tape and mounted on the stage of a CLSM. The intactness of the phloem tissue was checked microscopically using a water immersion objective (HCX APO L40x0.80 W U-V-l objective; Leica).
Electrophysiology on Intact Phloem Tissue
Electrophysiology on intact phloem tissue was described in detail previously (Hafke et al., 2005). After submersion of the exposed phloem tissue in bathing medium for 1 h, SEs were impaled with the microelectrode under continuous microscopic surveillance using a LN SM-1-micromanipulator (Luigs and Neumann). All measurements were performed at room temperature (23 to 25°C). After stabilization of the SE resting potential, a heat shock or a KCl stimulus was applied; leaf tip burning occurred at 3 to 4 cm distance from the observation window, and the KCl stimulus was applied at the application window in the two chambered system. Changes in SE membrane potential in response to the heat pulse or KCl stimulus were recorded.
Ca2+ channel blockers were prepared as stock solutions (50 to 100 mM in bathing medium with the exception of the stock solution of nifedipine, which was dissolved in DMSO) and diluted prior to use to the final concentration using standard bathing medium to give working concentrations of 2 mM for La3+ and Gd3+ and 100 to 500 μM for verapamil and nifedipine. Incubation times were between 2 and 3 h.
Bright-field images were taken at different times after applying the stimulus to correlate the forisome response with the passage of the EPW using a digital camera (Canon Power Shot S40) connected to a computer (Canon Digital Camera solutions disk version 8.0 software package).
Fluorescent Probes
Fluorochrome solutions were freshly prepared in bathing medium shortly before the experiments. Preparation of the solutions is described in detail in the online version of the article (see Supplemental Methods 2 online).
Confocal Microscopy of Fluorophore Distribution
Fluorochromes were imaged by CLSM using a Leica TCS 4D (Leica Microsystems) equipped with a 75-mW argon/krypton laser (Omnichrome). Tissue was observed with a water immersion objective (HCX APO L40x0.80 W U-V-l objective; Leica) in dipping mode. The exposed phloem tissue was stained with the fluorochromes for 1 to 2 h after preincubation of at least 1 h within the bathing medium.
CellTracker Yellow-Green CMEDA and CellTracker Green CMFDA (Molecular Probes), OGB-1 (Molecular Probes), DM-BODIPY dihydropyridine (fl-DHP; Molecular Probes), and ER-Tracker Green (Molecular Probes) were excited with the 488-nm line of the argon-ion laser with emission at >510 nm, while RH-414 and ER-Tracker Red were excited by the 564-nm line of the argon/krypton laser with emission at >590 nm.
Digital images were processed with Adobe Photoshop to optimize brightness, contrast, and color and to enable an overlay of the photomicrographs. For the ratio images, the fluorescence intensities of fl-DHP and RH-414 were divided using Adobe Photoshop. The obtained ratio images fl-DHP/RH-414 are presented as a 16-color scale using Image J (Image Processing and Analysis in Java; National Institute of Health).
Ca2+ Dynamics in Intact Phloem Tissue
Exposed phloem tissue in intact leaves was labeled by flooding the observation window with 5 μM OGB-1 in a buffer containing 2.5 to 10 mM dimethylglutaric acid at pH 4.3 for 20 to 30 min at room temperature (21°C). Tissue was washed extensively in bathing medium and then allowed to recover for 30 to 60 min until condensed forisomes were observed before a heat stimulus at 3 to 4 cm or application of 100 mM KCl 3 to 4 cm upstream from the observation window took place.
Tissue was imaged using a Zeiss LSM510 META CLSM (Carl Zeiss MicroImaging) equipped with a ×25 lens (Zeiss ×25 0.8 numerical aperture Plan-NEOFLUAR multi-immersion lens) in dipping mode. Excitation of OGB-1 was achieved with the 488-nm line of a 30 mW argon-ion laser operating with a tube current of 6.1 A and attenuated to 0.5 to 2% of full power. OGB-1 fluorescence was collected with emission at 505 to 550 nm and chloroplast autofluorescence at 690 to 711 nm. The pinhole was adjusted to give a notional optical section thickness (axial full-width at half-maximum) of 4 μm as a compromise between axial resolution and signal strength. Z-stacks of 4 to 13 optical sections were collected with a pixel dwell time of 1.6 μs and 2× line averaging and an (x,y) pixel spacing of 0.18 to 0.27 μm and sampling repeated at 20- to 40-s intervals over ∼10 min. Simultaneous nonconfocal bright-field images were collected with a transmission detector.
To compensate for specimen movement in z, particularly during passage of the pressure wave following the burning stimulus, specific z-planes were extracted from each z-stack to maintain the same cellular structures in focus. In some experiments, the signal from the adjacent z-planes was averaged to reduce noise. The extracted image series was automatically aligned in (x,y) by cross-correlation with the first image in the series. Images were converted from 8 or 16 bit to floating point to avoid rounding errors during processing. All subsequent manipulations were conducted at single precision. Images were smoothed using a 5 × 5 averaging filter to reduce noise. The background signal was measured from the vacuolar regions of a phloem parenchyma cell and subtracted from each time point. The amount of photobleaching in each experiment was estimated by fitting a monoexponential decay to the total signal from each image prior to stimulus application. The inverse of this decay function was then used to correct images throughout the time course. To determine the relative fluorescence change following a stimulus, images were ratioed against a reference image taken immediately prior to the stimulus. Ratios were calculated on a pixel-by-pixel basis and pseudo-color-coded on a rainbow hue scale, with the image intensity and saturation reflecting the fluorescence intensity.
Regions <2 sd units above background or within 10% of saturation were excluded from the ratio images and further analysis. Changes in fluorescence intensity were averaged from user-defined ROIs and used to calculate the fold change in fluorescence for specific cell types. All image processing routines were implemented in a custom MatLab (The Mathworks) available from M.D.F. on request.
Isolation of SEPs
SEPs were isolated according to Hafke et al. (2007) (see Supplemental Methods 3 online). Isolated SEPs were washed with the bathing medium (including 400 mM) and transferred into a bathing chamber before application of the fluorescent dyes and microscopy examination of fluorochrome treatments.
Ca2+ Concentration Measurements in Isolated SEPs
SEPs were stained with 13.25 μM of OGB-1AM for 2 h. After washing with bathing medium, SEPs were incubated in 4 μM Ca2+ ionophore ionomycin to permeabilize the PM for Ca2+ at least for 1 h. Aliquots of permeabilized SEPs were transferred into Ca2+ buffer solutions (CALBUF-2; WPI) containing 10 nM, 100 nM, and 1 μM free Ca2+ ions. Ca2+-dependent fluorescence was recorded via CLSM with low-intensity laser scans to minimize photobleaching of OGB-1AM. A calibration curve was obtained by measuring the averaged pixel density from the recorded calcium images using Image J software. The resting free Ca2+ level in SEP lumen was determined from the calibration curve in the absence of ionomycin.
Ca2+ Dependence of Forisome Dispersion
SEPs were equilibrated for 30 min with Ca2+ buffer solutions (CALBUF-2) containing 1, 40, or 100 μM free Ca2+ following ionomycin treatment and imaged using light microscopy to detect forisome responses.
Stylet Droplets and Ca2+ Determination by Ion-Selective Electrodes
Ca2+-selective microelectrodes were fabricated as described previously (Felle and Bertl, 1986; Felle, 1988, 1989). Calibration solutions in the range of 10−8 to 10−3 M free Ca2+ were prepared according to Tsien and Rink (1980). Calibration curves of the microelectrode yielded a linear slope of 27 to 28 mV/pCa down to pCa = 8. Measurement of Ca2+ from droplets of phloem sap, obtained from V. faba sieve tubes using microcauterized aphid stylets, was performed as described previously (Hafke et al., 2005).
Electron Microscopy
Tissue was prepared and sectioned for TEM according to Ehlers et al. (2000) (see Supplemental Methods 4 online). The region containing the phloem window (∼5 mm in length) was excised immediately before embedding in Spurr's resin and polymerization for 24 h at 68°C. Ultrathin sections (90 nm) were examined at 120 kV in an EM 912 Omega electron microscope (Carl-Zeiss). Electron micrographs were scanned as grayscale images with a Proscan slow-scan CCD camera with Esi vision 3.2 analysis (Soft Imaging Systems, Olympus).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. The effect of the Ca2+ Channel Blocker Gd3+ on the Forisome Reaction in Phloem Tissue.
Supplemental Figure 2. The Effect of the Ca2+ Channel Blocker Verapamil on the Forisome Reaction in Phloem Tissue.
Supplemental Figure 3. Rapid Line Scan Measurements of Ca2+ Transients.
Supplemental Methods 1. Rapid Line Scan Measurements of Ca2+ Transients.
Supplemental Methods 2. Fluorescent Probes.
Supplemental Methods 3. Isolation of SEPs.
Supplemental Methods 4. Electron Microscopical Preparation of Phloem Tissue.
Supplementary Material
Acknowledgments
We thank Rob Roelfsema, Dirk Becker (Julius-von-Sachs-Institut für Biowissenschaften, University of Würzburg, Germany), and Anne Holz (Justus-Liebig-University) for helpful discussions, Marco Reitz, Tina Henrich, and Christian Michalski (Justus-Liebig-University) for technical assistance, and Martin Hardt (Zentrale Biotechnische Betriebseinheit, Justus-Liebig-University) for introducing the electron microscopy facilities. This work was supported by grants from the Deutsche Forschungsgemeinschaft in the frame of the Schwerpunktprogramm 1108 (BE1925/8-2, 8-3, and 15-1).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Jens B. Hafke (jens.hafke@bot1.bio.uni-giessen.de).
Online version contains Web-only data.
References
- Arsanto, J.P. (1986). Calcium-binding sites and phosphatase activities in sieve element reticulum and P-protein of chick-pea phloem. A cytochemical and X-ray microanalysis survey. Protoplasma 132 160–171. [Google Scholar]
- Bewell, M.A., Maathuis, F.J.M., Allen, G.J., and Sanders, D. (1999). Calcium-induced calcium release mediated by a voltage, activated cation channel in vacuolar vesicles from red beet. FEBS Lett. 458 41–44. [DOI] [PubMed] [Google Scholar]
- Bischofberger, J., and Schild, D. (1995). Different spatial patterns of [Ca2+] increase caused by N- and L-type Ca2+ channel activation in frog olfactory bulb neurons. J. Physiol. 487 305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolsover, S., and Silver, R.A. (1991). Artifacts in calcium measurements: Recognition and remedies. Trends Cell Biol. 1 71–74. [DOI] [PubMed] [Google Scholar]
- Brauer, M., Zhong, W.-Z., Jelitto, T.J., Schobert, C., Sanders, D., and Komor, E. (1998). Free calcium ion concentration in sieve-tube sap of Ricinus communis L. seedlings. Planta 206 103–107. [Google Scholar]
- Camacho, L., Parton, R., Trewavas, A.J., and Malho, R. (2000). Imaging cytosolic free-calcium distribution and oscillations in pollen tubes with confocal microscopy: A comparison of different dyes and loading methods. Protoplasma 212 162–173. [Google Scholar]
- Colombani, A., Djerbi, S., Bessueille, L., Blomqvist, K., Ohlsson, A., Berglund, T., Teeri, T.T., and Bulone, V. (2004). In vitro synthesis of (1→3)-β-D-glucan (callose) and cellulose by detergent extracts of membranes from cell suspension cultures of hybrid aspen. Cellulose 11 313–327. [Google Scholar]
- Demidchik, V., and Maathuis, F.J.M. (2007). Physiological roles of nonselective cation channels in plants: From salt stress to signalling and development. New Phytol. 175 387–404. [DOI] [PubMed] [Google Scholar]
- Demuro, A., and Parker, I. (2006). Imaging single-channel calcium microdomains. Cell Calcium 40 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers, K., Knoblauch, M., and van Bel, A.J.E. (2000). Ultrastructural features of well- preserved and injured sieve elements: Minute clamps keep the phloem transport conduits free for mass flow. Protoplasma 214 80–92. [Google Scholar]
- Felle, H.H. (1988). Cytoplasmatic free calcium in Ricca fluitans L. and Zea mays L.: Interaction of calcium and pH? Planta 176 248–255. [DOI] [PubMed] [Google Scholar]
- Felle, H.H. (1989). PH as a second messenger in plants. In Second Messengers in Plant Growth and Development, W.F. Boss, and D.J. Morre, eds (New York: Alan R. Liss), pp. 145–166.
- Felle, H., and Bertl, A. (1986). The fabrication of H+-selective liquid membrane microelectrodes for use in plant cells. J. Exp. Bot. 37 1416–1428. [Google Scholar]
- Felle, H.H., Hanstein, S., Steinmeyer, R., and Hedrich, R. (2000). Dynamics of ionic activities in the apoplast of the sub-stomatal cavity of intact Vicia faba leaves during stomatal closure evoked by ABA and darkness. Plant J. 24 297–304. [DOI] [PubMed] [Google Scholar]
- Felle, H.H., and Zimmermann, M.R. (2007). Systemic signalling in barley through action potentials. Planta 226 203–214. [DOI] [PubMed] [Google Scholar]
- Fricker, M.D., Plieth, C., Knight, H., Blancaflor, E., Knight, M.R., White, N.S., and Gilroy, S. (1999). Fluorescent and luminescent techniques to probe ion activities in living plant cells. In Fluorescent and Luminescent Probes, 2nd ed., W.T. Mason, ed (London, San Diego: Academic Press), pp. 569–596.
- Fromm, J. (1991). Control of phloem unloading by action potentials in Mimosa. Physiol. Plant. 83 529–533. [Google Scholar]
- Fromm, J., and Bauer, T. (1994). Action potentials in maize sieve tubes change phloem translocation. J. Exp. Bot. 45 463–469. [Google Scholar]
- Fromm, J., and Lautner, S. (2007). Electrical signals and their physiological significance in plants. Plant Cell Environ. 30 249–257. [DOI] [PubMed] [Google Scholar]
- Fromm, J., and Spanswick, R. (1993). Characteristics of action potential in willow (Salix viminalis L.). J. Exp. Bot. 44 1119–1125. [Google Scholar]
- Furch, A.C.U., Hafke, J.B., Schulz, A., and van Bel, A.J.E. (2007). Ca2+-mediated remote control of reversible sieve-tube occlusion in Vicia faba. J. Exp. Bot. 58 2827–2838. [DOI] [PubMed] [Google Scholar]
- Gilroy, S., Bethke, P.C., and Jones, R.L. (1993). Calcium homeostasis in plants. J. Cell Sci. 106 453–462. [DOI] [PubMed] [Google Scholar]
- Grams, T.E.E., Lautner, S., Felle, H.H., Matyssek, R., and Fromm, J. (2009). Heat-induced electrical signals affect cytoplasmic and apoplasmic pH as well as photosynthesis during propagation through the maize leaf. Plant Cell Environ. 32 319–326. [DOI] [PubMed] [Google Scholar]
- Hafke, J.B., Furch, A.C.U., Reitz, M.U., and van Bel, A.J.E. (2007). Functional sieve element protoplasts. Plant Physiol. 145 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafke, J.B., van Amerongen, J.-K., Kelling, F., Furch, A.C.U., Gaupels, F., and van Bel, A.J.E. (2005). Thermodynamic battle for photosynthate acquisition between sieve tubes and adjoining parenchyma in transport phloem. Plant Physiol. 138 1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler, P.K., Palevitz, B.A., Lancelle, S.A., McCauley, M.M., and Lichtscheidl, I. (1990). Cortical endoplasmic reticulum in plants. J. Cell Sci. 96 355–373. [Google Scholar]
- Hille, B. (1992). Ionic Channels of Excitable Membranes, 2nd ed. (Sunderland, MA: Sinauer Associates).
- Hong, B., Ichida, A., Wang, Y., Gens, J.S., Pickard, B.G., and Harper, J.F. (1999). Identification of a calmodulin-regulated Ca2+-ATPase in the endoplasmic reticulum. Plant Physiol. 119 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss, H. (1987). Some aspects of calcium-dependent regulation in plant metabolism. Annu. Rev. Plant Physiol. 38 47–72. [Google Scholar]
- Klüsener, B., Boheim, G., Liß, H., Engelberth, J., and Weiler, E.W. (1995). Gadolinium-sensitive, voltage-dependent calcium release channels in the endoplasmic reticulum of a higher plant mechanoreceptor organ. EMBO J. 14 2708–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüsener, B., and Weiler, E.W. (1999). A calcium-selective channel from root-tip endomembranes of garden cress. Plant Physiol. 119 1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus, H.G., Moshammer, T., Friedrich, K., Kang, H.C., Haugland, R.P., and Glossman, H. (1992). In vivo labeling of L-type Ca2+ channels by fluorescent dihydropyridines: Evidence for a functional, extracellular heparin-binding site. Proc. Natl. Acad. Sci. USA 89 3586–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch, M., Peters, W.S., Ehlers, K., and van Bel, A.J.E. (2001). Reversible calcium-regulated stopcocks in legume sieve tubes. Plant Cell 13 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch, M., Noll, G., Müller, T., Prüfer, D., Schneider-Hüther, I., Scharner, D., van Bel, A.J.E., and Peters, W.S. (2005). ATP-independent contractile proteins from plants. Nat. Mater. 4 353. [DOI] [PubMed] [Google Scholar]
- Knoblauch, M., and van Bel, A.J.E. (1998). Sieve tubes in action. Plant Cell 10 35–50. [Google Scholar]
- Logan, D.C., and Knight, M.R. (2003). Mitochondrial and cytosolic calcium dynamics are differentially regulated in plants. Plant Physiol. 133 21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunevsky, V.Z., Zherelova, O.M., Vostrikov, I.Y., and Berestovsky, G.N. (1983). Excitation of Characeae cell membranes as a result of activation of calcium and chloride channels. J. Membr. Biol. 72 43–58. [Google Scholar]
- Malho, R., Moutinho, A., Van der Luit, A., and Trewavas, A.J. (1998). Spatial characteristics of calcium signalling: the calcium wave as a basic unit in plant cell calcium signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353 1463–1473. [Google Scholar]
- Mancuso, S. (1999). Hydraulic and electrical transmission of wound-induced signals in Vitis vinifera. Aust. J. Plant Physiol. 26 55–61. [Google Scholar]
- McAinsh, M.R., and Pittman, J.K. (2009). Shaping the calcium signature. New Phytol. 181 275–294. [DOI] [PubMed] [Google Scholar]
- Miller, D.D., Callaham, D.A., Gross, D.J., and Hepler, P.K. (1992). Free Ca2+ gradient in growing pollen tubes of Lilium. J. Cell Sci. 101 7–12. [Google Scholar]
- Mithöfer, A., and Mazars, C. (2002). Aequorin-based measurements of intracellular Ca2+-signatures in plant cells. Biol. Proced. Online 4 105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero, M., Brini, M., Marsault, R., Alvarez, J., Sitia, R., Pozzan, T., and Rizzuto, R. (1995). Monitoring dynamic changes in free Ca2+ concentration in the ER of intact cells. EMBO J. 14 5467–5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher, E. (1998). Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron 20 389–399. [DOI] [PubMed] [Google Scholar]
- Ng, C.K.-Y., and McAinsh, M.R. (2003). Encoding specificity in plant calcium signalling: hot-spotting the ups and downs and waves. Ann. Bot. (Lond.) 92 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson, E.S., Miller, D.D., Callaham, D.A., Shipley, A.M., Rivers, B.A., Cresti, M., and Hepler, P.K. (1994). Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: Effect of BAPTA-type buffers and hypertonic media. Plant Cell 6 1815–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plank, D.M., and Sussman, M.A. (2003). Intracellular Ca2+ measurements in live cells by rapid line scan confocal microscopy: Simplified calibration methodology. Methods Cell Sci. 25 123–133. [DOI] [PubMed] [Google Scholar]
- Plieth, C. (2001). Plant calcium signaling and monitoring: Pros and cons and recent experimental approaches. Protoplasma 218 1–23. [DOI] [PubMed] [Google Scholar]
- Plieth, C., Hansen, U.-P., Knight, H., and Knight, M.R. (1999). Temperature sensing by plants: The primary characteristics of signal perception and calcium response. Plant J. 18 491–497. [DOI] [PubMed] [Google Scholar]
- Reynolds, E.S. (1963). The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes, J.D., Thain, J.F., and Wilson, D.C. (1996). The pathway for systemic electrical signal conduction in the wounded tomato plant. Planta 200 50–57. [Google Scholar]
- Samejima, M., and Sibaoka, T. (1983). Identification of the excitable cells in the petiole of Mimosa pudica by intracellular injection of procion yellow. Plant Cell Physiol. 24 33–39. [Google Scholar]
- Sanders, D., Pelloux, J., Brownlee, C., and Harper, J.F. (2002). Calcium at the crossroads of signaling. Plant Cell 14 401–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild, D., Geiling, H., and Bischoffberger, J. (1995). Imaging of L-type Ca2+ channels in olfactory bulb neurones using fluorescent dihydropyridine and a styryl dye. J. Neurosci. Methods 59 183–190. [DOI] [PubMed] [Google Scholar]
- Sjolund, R.D., and Shih, C.Y. (1983). Freeze fracture analysis of phloem structure in plant tissue cultures. I. The sieve element reticulum. J. Ultrastruct. Res. 82 111–121. [DOI] [PubMed] [Google Scholar]
- Speksnijder, J.E., Miller, A.L., Weisenseel, M.H., Chen, T.-H., and Jaffe, L.F. (1989). Calcium buffer injections block fucoid egg development by facilitating calcium diffusion. Proc. Natl. Acad. Sci. USA 86 6607–6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlberg, R., and Cosgrove, D.J. (1997). The propagation of slow wave potentials in pea epicotyls. Plant Physiol. 113 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlberg, R., Stephens, N.R., Cleland, R.E., and Van Volkenburgh, E. (2006). Shade-induced action potentials in Helianthus annuus L. originate primarily from the epicotyl. Plant Signal. Behav. 1 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic, B., Witters, D.L., Zawadzki, T., and Davies, E. (1998). Action potentials and variation potentials in sunflower: An analysis of their relationships and distinguishing charateristics. Physiol. Plant. 103 51–58. [Google Scholar]
- Taylor, A.R., Manison, N.F.H., Fernandez, C., Wood, J., and Brownlee, C. (1996). Spatial organization of calcium signalling involved in cell volume control of the Fucus rhizoid. Plant Cell 8 2015–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry, B.R., Findlay, G.P., and Tyerman, S.D. (1992). Direct effects of Ca2+-channel blockers on plasma membrane cation channels of Amaranthus tricolor protoplasts. J. Exp. Bot. 256 1457–1473. [Google Scholar]
- Thomine, S., Zimmermann, S., van Duijn, B., Barbier-Bryboo, H., and Guern, J. (1994). Calcium channel antagonists induce direct inhibition of the outward rectifying potassium channel in tobacco protoplasts. FEBS Lett. 340 45–50. [DOI] [PubMed] [Google Scholar]
- Trewavas, A. (1999). Le calcium, c'est la vie: Calcium makes waves. Plant Physiol. 120 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien, R.Y., and Rink, T.J. (1980). Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim. Biophys. Acta 599 623–638. [DOI] [PubMed] [Google Scholar]
- Vallée, N., Briere, C., Petitprez, M., Barthou, H., Souvre, A., and Alibert, G. (1997). Studies on ion channel antagonist-binding sites in sunflower protoplasts. FEBS Lett. 411 115–118. [DOI] [PubMed] [Google Scholar]
- van Bel, A.J.E. (2003). The phloem, a miracle of ingenuity. Plant Cell Environ. 26 125–149. [Google Scholar]
- Volk, G.M., and Franceschi, V.R. (2000). Localization of a calcium channel-like protein in the sieve element plasma membrane. Aust. J. Plant Physiol. 27 779–786. [Google Scholar]
- Volk, G.M., Goss, L.J., and Franceschi, V.R. (2004). Calcium channels are involved in calcium oxalate crystal formation in specialized cells of Pistia stratiotes L. Ann. Bot. (Lond.) 93 741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, P.J. (2000). Calcium channels in higher plants. Biochim. Biophys. Acta 1465 171–189. [DOI] [PubMed] [Google Scholar]
- Zawadzki, T., and Trebacz, K. (1982). Action potentials in Lupinus angustifolius L. shoots. J. Exp. Bot. 33 100–110. [Google Scholar]
- Zhang, W., Fan, L.-M., and Wu, W.-H. (2007). Osmo-sensitive and stretch-activated calcium-permeable channels in Vicia faba guard cells are regulated by actin dynamics. Plant Physiol. 143 1140–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.