Abstract
Plant disease resistance is commonly triggered by early pathogen recognition and activation of immunity. An alternative form of resistance is mediated by recessive downy mildew resistant 1 (dmr1) alleles in Arabidopsis thaliana. Map-based cloning revealed that DMR1 encodes homoserine kinase (HSK). Six independent dmr1 mutants each carry a different amino acid substitution in the HSK protein. Amino acid analysis revealed that dmr1 mutants contain high levels of homoserine that is undetectable in wild-type plants. Surprisingly, the level of amino acids downstream in the aspartate (Asp) pathway was not reduced in dmr1 mutants. Exogenous homoserine does not directly affect pathogen growth but induces resistance when infiltrated in Arabidopsis. We provide evidence that homoserine accumulation in the chloroplast triggers a novel form of downy mildew resistance that is independent of known immune responses.
INTRODUCTION
Major plant diseases, such as powdery mildews, rusts, and downy mildews, are caused by obligate biotrophic fungi and oomycetes (fungal-like members of the kingdom Stramenopila). The obligate biotrophs are often highly specialized, and for their growth and reproduction they fully depend on particular host plants. Obligate biotrophs do not cause disease on nonhost plant species, as infection is obstructed by the plant-controlled process of nonhost resistance that involves both pre- and postinvasion defenses (Lipka et al., 2005). In host plants, pathogens can circumvent or actively suppress nonhost resistance (Chisholm et al., 2006). However, a second layer of defense has evolved that is mediated by single dominant resistance (R) genes. The encoded R proteins mediate direct or indirect detection of pathogen determinants followed by the activation of plant immunity (Jones and Dangl, 2006). Despite these effective intrinsic defense mechanisms, plants are still vulnerable to pathogen attack. The molecular mechanisms underlying disease susceptibility, and more specifically the role of host-specific processes that sustain pathogen development and growth, are still largely unknown. In recent years, genetic studies on the model plant Arabidopsis thaliana have resulted in the identification of a number of genes that are involved in susceptibility to biotrophic pathogens (Vogel and Somerville, 2000; O'Connell and Panstruga, 2006). Mutations in the POWDERY MILDEW RESISTANT genes PMR6 and PMR5, encoding a pectate-lyase and a protein of unknown function, respectively, result in plants with an altered cell wall that is thought to underlie their phenotype (Vogel et al., 2002, 2004). The pmr4 mutant has a lesion in a callose synthase gene that leads to enhanced defense responses upon pathogen infection (Nishimura et al., 2003). A fourth example of powdery mildew resistance is the loss-of-function of the Arabidopsis PMR2/MLO2 gene (Consonni et al., 2006), a co-ortholog of the barley (Hordeum vulgare) Mildew resistance locus O (Mlo) gene (Panstruga, 2005). Most of the pmr mutants are pathogen specific, reducing susceptibility to powdery mildew, but not to the bacterial pathogen Pseudomonas syringae and the downy mildew pathogen Hyaloperonospora arabidopsidis (Vogel and Somerville, 2000). Pathogen-specific resistance was also found for the Arabidopsis downy mildew resistant 1 (dmr1) mutant, which is resistant to H. arabidopsidis but susceptible to infection by other pathogens, for example, the powdery mildew fungus Golovinomyces orontii and P. syringae bacteria (Van Damme et al., 2005). The dmr1-mediated resistance to H. arabidopsidis could be due to the absence of a specific host protein required for infection or to a hitherto unknown defense mechanism. Here, we demonstrate that accumulation of the amino acid homoserine in dmr1 mutant plants is sufficient to provide resistance to H. arabidopsidis. The basis of this discovery was the map-based cloning of the DMR1 gene, which was found to encode homoserine kinase (HSK; Arabidopsis gene At2g17265), a key enzyme in primary amino acid metabolism. Surprisingly, biosynthesis of the amino acids Thr, Met, and Ile is not reduced in the dmr1 mutants, whereas homoserine accumulates to high levels. Homoserine-induced resistance is independent of known defense signaling pathways and could provide a novel method for protecting crops against downy mildew disease.
RESULTS
dmr1-Mediated Resistance to H. arabidopsidis
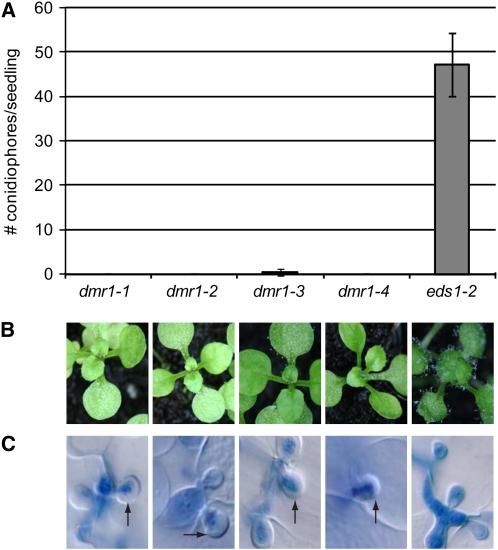
Multiple independent alleles of dmr1, dmr1-1, dmr1-2, dmr1-3, and dmr1-4, were initially identified from a genetic screen for loss of susceptibility to H. arabidopsidis (Van Damme et al., 2005). These mutants were each backcrossed twice (BC2) to the parental line Landsberg erecta (Ler) eds1-2 to reduce the number of unlinked ethyl methanesulfonate (EMS)-induced mutations. The dmr1 BC2 mutants retained strong resistance to H. arabidopsidis compared with the parental line Ler eds1-2 (Figures 1A and 1B). Of the mutants, dmr1-3 still supports a low level of sporulation, suggesting that it carries a weak dmr1 allele. Microscopy analysis of the infection process in the different dmr1 BC2 mutants showed that most H. arabidopsidis hyphae were arrested after formation of the first haustoria. The inhibition of growth was associated with the encasement of haustoria by papillae, translucent structures surrounding the feeding structures that are absent in the susceptible parental line (Figure 1C, papillae are indicated by the arrows). Aniline blue staining confirmed that the papillae contain callose, a polysaccharide that is commonly present in these pathogen-induced physical barriers (Aist, 1976), which are thought to prevent further penetration and nutrient uptake by the pathogen. Besides papillae formation, no other detectable defense responses, such as production of reactive oxygen species or activation of defense gene expression (Van Damme et al., 2005), were observed in the dmr1 mutants.
Figure 1.
Quantification and Visualization of H. arabidopsidis Growth on the dmr1 Mutants (BC2).
The dmr1 mutants confer nearly complete resistance to the downy mildew pathogen H. arabidopsidis.
(A) Conidiophore formation 5 d after inoculation on 10-d-old Arabidopsis seedlings. The average number (with standard deviation, n = 40) of conidiophores/seedling is displayed.
(B) Macroscopy images of conidiophores on Arabidopsis seedlings 8 d after inoculation with H. arabidopsidis.
(C) Microscopy examination of trypan blue–stained hyphae and haustoria in leaves of Arabidopsis seedlings 5 d after inoculation with H. arabidopsidis. The arrest of H. arabidopsidis growth and appearance of papillae (indicated by the arrows) in the dmr1 mutants was observed in multiple independent experiments.
DMR1 Encodes HSK
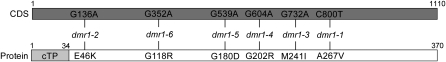
The recessive dmr1 locus was previously mapped on the long arm of chromosome 2 (Van Damme et al., 2005). More precisely, dmr1 could be assigned to a chromosomal region of ∼130 kb that is covered by the BACs F6P23, T23A1, and F5J6. Five recombinants (from 650 F2 plants) between markers designed on BACs F6P23 to F5J6 were instrumental in the fine mapping of dmr1 to a region encompassing eight genes: At2g17230 to At2g17290. Single point mutations in the At2g17265 gene were identified by nucleotide sequencing of the dmr1-1, dmr1-2, dmr1-3, and dmr1-4 mutants. In addition, two resistant mutants, dmr1-5 and dmr1-6, that were isolated later, also contained single point mutations in the At2g17265 coding sequence (Figure 2). At2g17265 encodes HSK, a key enzyme in the Asp pathway for the biosynthesis of the essential amino acids Met, Thr, and Ile. All six mutations result in amino acid substitutions in the HSK protein that could reduce or abolish the activity of the enzyme. Confirmation that mutations in the HSK gene are responsible for the dmr1 resistance phenotype was obtained by Agrobacterium tumefaciens–mediated transformation of the dmr1-1 and dmr1-3 mutants with the wild-type Arabidopsis HSK coding sequence, which reestablished susceptibility to H. arabidopsidis (see Supplemental Figure 1 online).
Figure 2.
dmr1 Mutations Affect the HSK Protein.
The position of the nucleotide changes (GC-to-AT transitions, typical of EMS-induced mutations) in the HSK coding sequence (CDS) and corresponding amino acid substitutions in the HSK protein are indicated for the dmr1-1, dmr1-2, dmr1-3, dmr1-4, dmr1-5, and dmr1-6 mutants. The amino acid substitutions are in the mature protein, which has recently been confirmed to be localized in the chloroplast (Zybailov et al., 2008). Based on prediction and proteomics data, the chloroplast transit peptide (cTP) is cleaved between positions 34 and 35.
The nuclear-encoded Arabidopsis HSK protein carries a predicted N-terminal transit sequence for chloroplast targeting. Using the ChloroP algorithm (Emanuelsson et al., 1999), the HSK protein of Arabidopsis is predicted to be cleaved after amino acid 62 (cleavage site [CS] score 3.97). As this position falls within motif 1, involved in substrate and cofactor binding (see Supplemental Figure 2 online), this predicted site is highly unlikely to be the cleavage site. Motif 1 is highly conserved and is also present in HSK proteins from bacteria (e.g., Escherichia coli THRB) and fungi (e.g., Saccharomyces cerevisiae THR1) that are cytoplasmic and lack a chloroplast transit peptide. The transit peptide of the homologous HSK protein of Brassica oleracea (of which the first 171 amino acids that were predicted from EST sequences are 92% identical to HSK of Arabidopsis) is predicted to be cleaved after amino acid 34 with a CS score of 5.324. For the Arabidopsis HSK protein, the second best transit peptide cleavage site score is also after amino acid 34 (CS score 2.787). Experimental support for this cleavage site is provided by a high-throughput proteomic study using mass spectrometry (Baerenfaller et al., 2008). The HSK-derived tryptic peptide ASVQTLVAVEPEPVFVSVK (position 37 to 55) was identified multiple independent times from different Arabidopsis tissues, indicating that the mature protein contains this peptide and supporting a cleavage site before position 37.
Amino Acid Substitutions in DMR1 Are at Conserved Positions in Plant HSKs
HSK is a single-copy gene in Arabidopsis, rice (Oryza sativa), grapevine (Vitis vinifera), and the moss Physcomitrella patens, based on their annotated genome sequences. In addition, for many other plant species, ESTs were identified that correspond to single orthologous HSK genes. However, in poplar (Populus trichocarpa) and potato (Solanum tuberosum), two putatively orthologous genes appear to be present that give a reciprocal best hit to Arabidopsis HSK. Twelve plant HSK protein sequences, including the best orthologs of potato and poplar, are depicted in a multiple alignment generated by ClustalW (see Supplemental Figure 2 online). Except for the N-terminal part, which is the chloroplast transit peptide sequence, the plant HSKs show high amino acid similarity. The first conserved position in these 12 plant HSK proteins is a negatively charged residue (E or D) at position 46 in the Arabidopsis HSK protein. In the dmr1-2 mutant, this acidic residue is substituted with Lys and is predicted to be located in the mature chloroplast-localized enzyme. Similarly, the other five dmr1 amino acid substitutions are all at positions that are identical or similar in all 12 plant HSK proteins, suggesting that they are functionally important residues. However, the substitutions do not overlap with the three motifs (indicated by the dashed lines in Supplemental Figure 2 online) that are highly conserved in all members of the GHMP kinase superfamily, which includes galacto kinase, HSK, mevalonate kinase, and phosphomevalonate kinase (Zhou et al., 2000). In addition, the dmr1 mutations do not overlap with the conserved amino acids that bind ATP or are in the active site (indicated by the diamonds in Supplemental Figure 2 online), based on alignment of the Arabidopsis HSK amino acid sequence with that of the HSK protein of Methanococcus jannashii, of which the crystal structure has been described (Zhou et al., 2000). Therefore, we investigated if the dmr1 mutations cause a reduction or loss of HSK enzyme activity.
dmr1 Mutations Strongly Affect HSK Activity
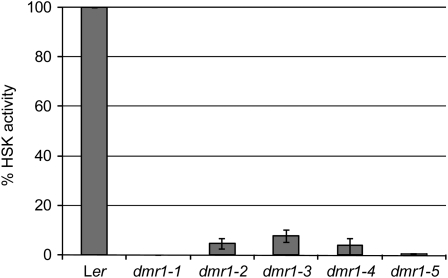
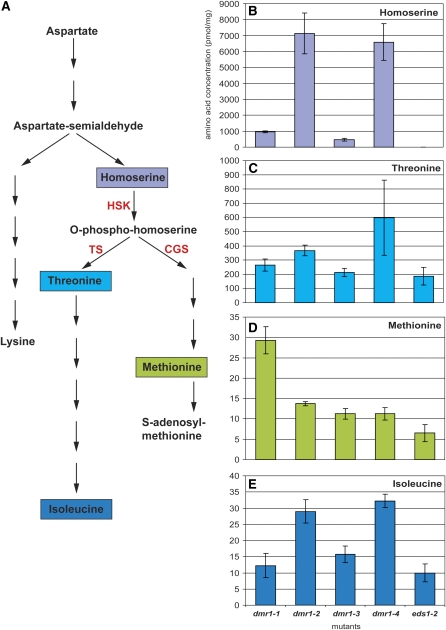
The dmr1 mutations all lead to amino acid substitutions in residues that are conserved in plant HSK proteins, suggesting that they correspond to important positions in the enzyme and possibly affect its activity. We tested this in vitro by measuring the activity of recombinant HSK enzyme from the wild type and four dmr1 mutants, produced and purified from E. coli. In this assay, the consumption of ATP, which is required for the phosphorylation of homoserine, is an indirect measure of HSK activity. As shown in Figure 3, the relative activity of the DMR1-2, 1-3, and 1-4 mutant enzymes was strongly reduced (∼5 to 10% of wild-type activity), whereas DMR1-1 enzyme activity was undetectable. This strong reduction or complete loss of in vitro activity is expected to have a profound effect on the level of soluble amino acids in the dmr1 mutants in vivo, as HSK is a key enzyme in the Asp pathway (Figure 4A). This could directly affect H. arabidopsidis as it is an obligate biotroph that could rely on host-derived amino acids for its growth and development. To test if the dmr1 mutations in HSK affect the levels of amino acids in the Asp pathway, soluble amino acids were quantified in the aboveground parts of mutant and wild-type seedlings. Surprisingly, the levels of the amino acids Thr, Met, and Ile (downstream of HSK in the Asp pathway; Figure 4A) were not reduced in the dmr1 mutants (Figures 4C to 4E), indicating that dmr1-resistance is not caused by a depletion of these amino acids within the host. By contrast, the levels in the dmr1 mutants were higher than in the parental line Ler eds1-2, with Met (Figure 4D) levels being highest in dmr1-1 and Thr (Figure 4C) and Ile (Figure 4E) levels being highest in dmr1-2 and dmr1-4, respectively. The dmr1 mutants were found to affect free homoserine levels profoundly (Figure 4B). This amino acid is not detectable in seedlings of the parental line Ler eds1-2. High levels of homoserine were detected in the dmr1-2 and dmr1-4 mutants, and lower, but still significant, levels were found in the dmr1-1 and dmr1-3 mutants. The high homoserine levels in the dmr1 mutants confirm that the substitutions in HSK lead to reduced enzyme activity.
Figure 3.
HSK Activity of Recombinant Enzyme from Wild-Type Arabidopsis (Accession Ler) and Five dmr1 Mutants Produced and Purified from E. coli.
The dmr1 mutations strongly reduce HSK activity relative to that of the wild type (set at 100% and corrected for recombinant protein input). The average activity (with se) from three independent recombinant enzyme isolations and assays is displayed.
Figure 4.
Mutations in the HSK Gene in the dmr1 Mutants Result in Homoserine Accumulation but Not in Depletion of the Downstream Amino Acids Thr, Met, and Ile.
(A) Position of HSK in the aspartate metabolic pathway in Arabidopsis (adapted from www.biocyc.org). Homoserine is the common precursor for the essential amino acids Thr, Ile, and Met. In the chloroplast stroma, HSK produces O-phospho-homoserine, which is the direct substrate for Thr synthase (TS) and cystathionine γ-synthase (CGS).
(B) to (E) The homoserine (B), Thr (C), Met (D), and Ile (E) content (in pmol/mg fresh weight) of four 14-d-old dmr1 mutants was determined and compared with that of the parental line Ler eds1-2. It is striking to see that Thr and Ile levels are high in the dmr1-2 and dmr1-4 mutants, whereas Met is particularly high in dmr1-1, suggesting that the different dmr1 mutations affect interactions between HSK and enzymes further downstream in the pathway. Amino acid analysis was performed on three biological replicates per mutant. Average amino acid concentrations (with sd) are displayed. Similar relative amino acid levels were confirmed in two independent experiments.
Homoserine Induces Resistance to Downy Mildew
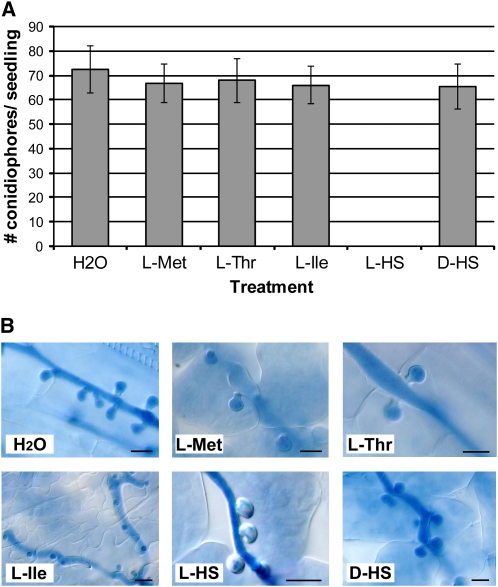
High homoserine levels in the dmr1 mutants could provide direct resistance to downy mildew by inhibiting pathogen growth. However, no inhibitory effect of homoserine was found in vitro. As the obligate biotroph H. arabidopsidis cannot be cultured in vitro, we tested the effect of homoserine on radial growth of the related oomycete pathogen Phytophthora capsici. l-homoserine did not inhibit radial growth when compared with d-homoserine at concentrations of up to 10 mM (see Supplemental Figure 3A online). When tested on H. arabidopsidis conidiospores, l-homoserine concentrations of up to 50 mM did not negatively affect spore germination (see Supplemental Figure 3B online). It is likely that oomycetes can metabolize homoserine because putative HSK ortholog sequences are present in the genome sequence of H. arabidopsidis and other oomycete pathogens (see Supplemental Figure 4 online). Our data indicate that homoserine, at the concentrations used, is not toxic to the pathogen, suggesting that processes in the plant mediate homoserine-induced resistance. Indeed, when l-homoserine (5 mM) was applied exogenously to wild-type Arabidopsis seedlings, by infiltration into the leaf intercellular space, resistance to H. arabidopsidis was observed (Figure 5A). Resistance was not induced upon infiltration with water, Met (5 mM), Thr (5 mM), Ile (5 mM), or the stereo-isomer d-homoserine (5 mM), indicating that the effect is l-homoserine specific. Microscopy analysis of the homoserine-treated leaves showed arrested H. arabidopsidis growth and absence of sporulation. Homoserine-induced resistance was also associated with the occurrence of encased haustoria (Figure 5B). Very similar host responses were observed in the dmr1 mutants (Figure 1), suggesting that high homoserine levels in planta induce the responses.
Figure 5.
Exogenous Application of l-Homoserine Leads to H. arabidopsidis Resistance in Arabidopsis.
(A) Infiltration of H. arabidopsidis–infected seedlings with l-homoserine (5 mM) leads to resistance to H. arabidopsidis, whereas treatment with 5 mM of l-Met, l-Thr, l-Ile, or d-homoserine does not reduce the level of H. arabidopsidis sporulation. The average number (with sd, n = 30) of conidiophores/seedling is displayed. The results were confirmed in two additional independent experiments.
(B) A high frequency of papillae formation, visible as translucent encasements surrounding the trypan blue–stained haustoria, is observed in l-homoserine (L-HS)–treated seedlings and not after treatment with the other amino acids or the d-stereo-isomer of homoserine. Bars = 10 μm.
Homoserine-Induced Resistance Is Independent of Known Defense Pathways
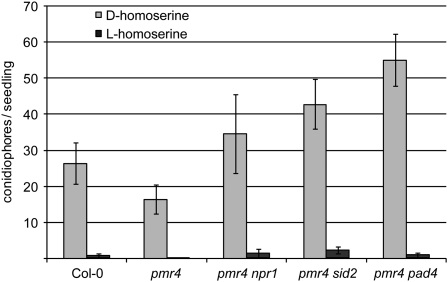
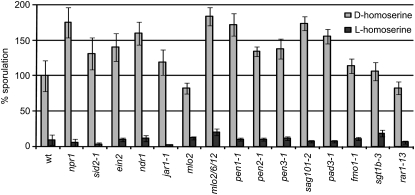
The high prevalence of callose-containing papillae in the dmr1 mutants and homoserine-treated seedlings in response to infection raised the question whether papillae formation is the primary cause of H. arabidopsidis resistance. To test this, we exogenously applied homoserine to seedlings of the Arabidopsis pmr4-1 mutant, which is strongly impaired in the production of pathogen-induced callose because of a lesion in a callose synthase gene (Nishimura et al., 2003). As pmr4 plants show enhanced activation of plant defense responses upon pathogen infection, double mutants impaired in defense signaling, pmr4-1 npr1-1 (for nonexpressor of PR genes), pmr4-1 sid2-1 (for salicylic acid induction deficient), and pmr4-1 pad4-1 (for phytoalexin deficient), were analyzed. In all pmr4-1 single and double mutants tested, resistance to H. arabidopsidis could still be induced by exogenous application of l-homoserine and not by d-homoserine (Figure 6). This indicates that homoserine-induced resistance does not require the pathogen-induced callose synthase PMR4 nor the defense signaling genes NPR1, PAD4, SID2, and EDS1 (the dmr1 mutants were generated in the eds1-2 background). A larger collection of Arabidopsis mutants impaired in immune responses (ethylene insensitive2 [ein2], non-race specific disease resistance1 [ndr1], jasmonate resistant [jar1-1], mlo2, mlo2 mlo6 mlo12, penetration [pen1-1], pen2-1, pen3-1, senescence-associated gene [sag101-2], pad3-1, flavin dependent monooxygenase [fmo1-1], suppressor of G2 allele of skp1 [sgt1b-3], and required for ML-a12 conditioned resistance [rar1-13]) was tested to analyze if homoserine-induced resistance required any of the corresponding genes. In all mutants tested l-homoserine was able to induce resistance to H. arabidopsidis (Figure 7), indicating that it does not rely on known immune responses. This was further supported by the fact that we did not observe activation of expression of the defense-associated genes PR-1, PR-2, and DMR6 (van Damme et al., 2008) in uninoculated dmr1 mutants (see Supplemental Figure 5A online). As resistance in the dmr1 mutants is already visible microscopically at 1 and 2 d postinoculation, we analyzed the expression of PR-1 and HSK after inoculation with H. arabidopsidis Cala2. As shown in Supplemental Figure 5B online, there is no strong activation of DMR1/HSK and PR-1 in the dmr1 mutants in response to infection with the compatible isolate Cala2. dmr1-mediated resistance is clearly not based on well-known defense responses.
Figure 6.
Sporulation of H. arabidopsidis on Arabidopsis pmr4-1 Single and Double Mutants after d- and l-Homoserine (10 mM) Application.
l-homoserine-induced resistance is unaffected in the pmr4 mutants (compared with wild-type Col-0), indicating that the pathogen-induced callose synthase PMR4 is not required for homoserine-induced resistance. The average number (with se, n = 20) of conidiophores/seedling is displayed. Similar results were obtained in a separate independent experiment.
Figure 7.
L-Homoserine-Induced Resistance Is Not Affected in a Large Set of Arabidopsis Immune Response Mutants.
The level of sporulation of d-homoserine- and l-homoserine-treated (10 mM) seedlings is displayed compared with the wild-type control plant Col-0 (set to 100% sporulation). Several mutants demonstrated enhanced susceptibility to H. arabidopsidis, but in all cases, infiltration with l-homoserine, but not d-homoserine, strongly reduced the H. arabidopsidis sporulation level. The relative level (with se, n = 20) of conidiophores/seedling is displayed. Similar results were obtained in a separate independent experiment.
Homoserine Acts in the Chloroplast to Induce Resistance
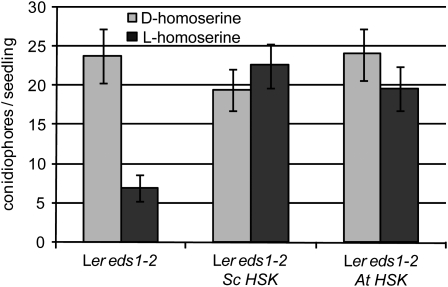
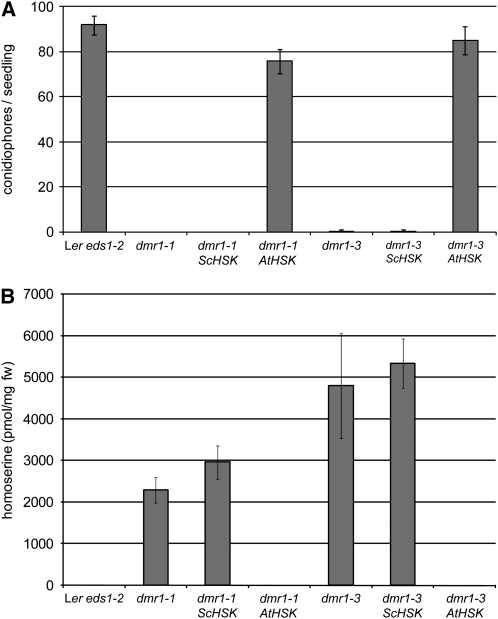
In plants, most enzymatic reactions in the Asp pathway, including the phosphorylation of homoserine, are known to take place in the chloroplast (Azevedo, 2002). The nuclear-encoded HSK protein indeed carries a predicted N-terminal transit sequence for chloroplast targeting (Lee and Leustek, 1999) and has been confirmed by proteomic analysis of purified chloroplasts of wild-type Arabidopsis to be present in this organelle (Zybailov et al., 2008). To test whether dmr1-mediated resistance is induced by homoserine accumulation in the chloroplast or cytosol, we expressed the cytosolic HSK protein of the yeast S. cerevisiae (HSK or THR1 [for threonine requiring]), which lacks a chloroplast transit peptide (Mannhaupt et al., 1990), in dmr1 plants. Transgenic plants expressing S. cerevisiae HSK did not gain resistance to H. arabidopsidis after exogenous homoserine application (Figure 8). This suggests that exogenously applied homoserine is metabolized in the cytoplasm by S. cerevisiae HSK before it can induce resistance, demonstrating that the cytosolic enzyme is biologically active in the transgenic Arabidopsis lines. However, transgenic dmr1 mutants expressing S. cerevisiae HSK remained as resistant as the dmr1 mutants, whereas transgenic dmr1 plants expressing the chloroplastic Arabidopsis HSK regained full susceptibility to H. arabidopsidis (Figure 9A). In addition, amino acid analysis showed that S. cerevisiae HSK-expressing dmr1 plants retained a high level of homoserine, whereas in Arabidopsis HSK-expressing lines, homoserine was completely absent (Figure 9B). To rule out the possibility that the S. cerevisiae HSK gene was corrupted, we PCR amplified the gene from genomic DNA of the S. cerevisiae HSK transgenic plants. Sequence analysis confirmed that the coding sequence was unaltered. HSK enzyme assays on recombinant S. cerevisiae HSK protein, produced in E. coli from the cloned PCR product, showed that the recombinant yeast protein had an activity that was comparable to that of recombinant Arabidopsis HSK protein (which is shown in Figure 4). We conclude that dmr1-mediated resistance is a consequence of homoserine accumulation in the chloroplast.
Figure 8.
Exogenous Application of Homoserine (5 mM) Does Not Induce Resistance in Transgenic Lines Overexpressing a Cytoplasmic or Chloroplastic HSK Enzyme.
H. arabidopsidis sporulation (average number [with se, n = 35] of conidiophores/seedling at 6 d after Cala2 inoculation) is strongly reduced as a result of l-homoserine in the Arabidopsis control line Ler eds1-2. By contrast, transgenic lines overexpressing the cytoplasmic S. cerevisiae HSK (Sc HSK) or the chloroplastic Arabidopsis HSK (At HSK) no longer show l-homoserine-induced resistance. This shows that the transformed plants have an enhanced HSK activity, indicating that S. cerevisiae HSK is functional in planta. Similar results were obtained in a separate independent experiment.
Figure 9.
Downy Mildew Resistance and Homoserine Levels of dmr1 Mutants Are Not Reduced by Transgenic Expression of S. cerevisiae HSK Encoding a Cytoplasmic HSK.
Transgenic dmr1-1 and dmr1-3 Arabidopsis lines were transformed with the wild-type Arabidopsis Col-0 HSK or the S. cerevisiae HSK coding sequence under control of a P35S promoter, and homozygous T3 plants were selected for the analyses.
(A) Susceptibility of dmr1 is only restored to parental levels (Ler eds1-2) by expression of the Arabidopsis HSK gene encoding a chloroplastic HSK and not by S. cerevisiae HSK that is active in the cytoplasm. Conidiophores were counted at 6 d after H. arabidopsidis Cala2 inoculation. The average number (with sd, n = 20) of conidiophores/seedling is displayed. This result was confirmed in four independent T3 lines per construct. Similar results were obtained in a separate independent experiment.
(B) In an independent experiment, homoserine levels were measured, showing that homoserine is completely metabolized in the At HSK complementation lines, which express a chloroplast-targeted HSK, but not in lines expressing the cytoplasmic Sc HSK. The average concentration (with sd, n = 3) of homoserine is displayed. These data suggest that, in the dmr1 mutants, homoserine accumulates predominantly in the chloroplast.
DISCUSSION
The DMR1 gene was map base cloned and identified as At2g17265 encoding HSK. dmr1 alleles from six independent mutants all carried GC-to-AT transitions in the HSK coding sequence, typical of EMS-induced mutations (Figure 2). All six mutations resulted in amino acid substitutions in the HSK protein. The fact that no null mutations were identified suggests that HSK is an essential protein in Arabidopsis. Indeed, in other organisms, for example, E. coli (Theze and Saint-Girons, 1974) and S. cerevisae (Mannhaupt et al., 1990), mutation or deletion of the HSK gene leads to Thr auxotrophy. For an autotrophic organism, such as Arabidopsis, we expect Thr auxotrophy to be lethal. How can it be that mutation of a gene involved in primary metabolism does not lead to a strong macroscopic phenotype? Amino acid analysis of the dmr1 mutants showed that the levels of Ile, Met, and Thr, downstream of homoserine in the Asp pathway, are not reduced (Figure 4). This suggests there is sufficient HSK activity remaining in the mutants or there is an alternative, so far unknown, biosynthetic route bypassing HSK, allowing the dmr1 mutant to grow and develop normally. However, functional redundancy in Arabidopsis is unlikely as (1) the accumulation of homoserine in the dmr1 mutants indicates that there is a blockage that is not bypassed, and (2) only a single HSK gene is predicted from the Arabidopsis genome sequence. A second gene (At4g35295) is annotated as “putative HSK,” but this gene has many stop codons and the predicted protein is only 111 amino acids, while HSK is 370 amino acids long. At4g35295 is probably a pseudogene that has degenerated from a duplicated HSK gene. The HSK region on chromosome 2 and the At4g35295 region on chromosome 4 are syntenic and are proposed to originate from an ancient segmental duplication (Terryn et al., 1999; Blanc et al., 2000). A single HSK gene was also identified in the sequenced genomes of rice, grape, and Physcomitrella. In poplar, two predicted HSK genes have been identified that could have resulted from a more recent genome duplication (Tuskan et al., 2006).
The observation that dmr1 mutants accumulate homoserine (Figure 4B) confirms that the Asp pathway is blocked at HSK. In addition, our results confirm that homoserine is indeed the substrate of the At2g17265-encoded enzyme, as was previously shown by enzymatic analysis of recombinant HSK (Lee and Leustek, 1999). Homoserine is virtually undetectable in wild-type Arabidopsis as HSK is not rate limiting. Because of this, HSK-overexpressing plants do not produce more Met or Thr, except when additional homoserine is applied exogenously (Lee et al., 2005). The high homoserine content of the dmr1 mutants indicates that HSK has become rate limiting. It was expected that the reduced HSK activity would lead to lower levels of downstream amino acids. However, the levels of Met, Thr, and Ile were increased rather than decreased in the dmr1 mutants (Figure 4). We postulate that a feedback mechanism shuttles more Asp into the pathway so that homoserine accumulates to high levels. In the presence of high substrate concentrations, the residual HSK activity may be sufficient to produce equal amounts or even more of Met, Thr, and Ile. When tested in vitro, recombinant HSK from most dmr1 mutants indeed retained some residual enzyme activity (Figure 3). However, mutant HSK from dmr1-1 appeared to be completely inactive when produced in E. coli and tested at 37°C. The mutant enzyme could be inactive when produced at 37°C in E. coli, while in vivo (in Arabidopsis), it could retain some residual activity. Since no T-DNA insertion lines are available for HSK, we are currently investigating a large collection of TILLING mutants to determine if HSK is an essential gene.
Exogenous application of amino acids to Arabidopsis showed that only homoserine accumulation causes resistance to downy mildew (Figure 5). Resistance was not induced by exogenous application of any of the other amino acids that were increased in levels in the dmr1 mutants. The fact that H. arabidopsidis spore germination or P. capsici mycelium growth was not inhibited by direct treatment with l-homoserine implies that the plant plays an active role in mediating homoserine-induced resistance. Homoserine synthesis and the phosphorylation of homoserine by HSK, in the Asp pathway, has been shown to take place in the chloroplast (Azevedo, 2002). However, H. arabidopsidis is not in direct contact with chloroplasts as it is physically separated from them by the plant cell membrane and cytoplasm. It is currently unknown if homoserine is transported out of the chloroplast in Arabidopsis.
Expression of the HSK gene from the yeast S. cerevisiae (Sc HSK) in the cytoplasm of dmr1 mutants did not lead to a reduction in homoserine level (Figure 9). This indicates that the amino acid is not transported from the chloroplast to the cytoplasm and that it thereby remains inaccessible to the cytoplasmic S. cerevisiae HSK. This observation is supported by the fact that exogenous application of homoserine to S. cerevisiae HSK-expressing plants does not effectively induce resistance. We postulate that in wild-type plants, which lack cytoplasmic HSK activity, exogenous homoserine is taken up by the cell and transported to the chloroplast where it induces resistance. In plants expressing a cytoplasmic yeast HSK, exogenously applied homoserine is metabolized in the cytoplasm and is thus unable to reach the chloroplast and therefore does not induce resistance.
Resistance of the dmr1 mutants is specific to H. arabidopsidis, as these plants are still susceptible to other pathogens. Previously, we reported that the dmr1 mutants are susceptible to P. syringae pv tomato and Golovinomyces orontii (Van Damme et al., 2005). Preliminary data indicate that dmr1 plants are also susceptible to the anthracnose fungus Colletotrichum higginsianum (R. O'Connell, personal communication) and the white rust pathogen Albugo candida (E. Holub, personal communication). The specific resistance is directly linked to high homoserine levels and could be caused by (1) the activation of a highly specific and so far unknown defense response or (2) the sensitivity of H. arabidopsidis to high homoserine levels in planta. This latter explanation is unlikely because homoserine must accumulate in the chloroplast to induce resistance. As tested by homoserine infiltration in a large collection of Arabidopsis mutants, resistance was found to be independent of known defense signaling genes (Figures 6 and 7). To identify Arabidopsis genes required for homoserine-induced resistance, we have identified suppressors of dmr1 (so-called loss of downy mildew resistant one resistance or ldo mutants).
Homoserine was not detectable in wild-type Arabidopsis seedlings. However, in other plant species, such as pea (Pisum sativum) and other members of the legume subfamily Vicieae, high levels of homoserine are present. As homoserine is abundant in the phloem sap of these plants, it is thought to be a transport molecule for nitrogen and carbon allocation. Interestingly, in pea, an alternative biosynthetic route exists, by transamination of a keto acid precursor, to produce high amounts of homoserine (Joy and Prabha, 1986). Aminooxyacetate, a transamination inhibitor, can inhibit this reaction, whereas homoserine synthesis through the Asp pathway remains unaffected by this drug. This alternative pathway could explain why relatively more homoserine was found in the cytoplasm than in the chloroplasts of pea plants (Mills, 1980). The high endogenous homoserine level in pea was found to act as an inducer of the fungal pathogen Nectria hematococca. It was hypothesized that N. hematococca has evolved the ability to sense homoserine, but also Asn, as a signal to induce expression of virulence genes in planta (Yang et al., 2005).
We conclude that, with the isolation of the DMR1 gene encoding HSK, we have identified an alternative form of plant disease resistance caused by the accumulation of homoserine. The molecular mechanism by which homoserine triggers resistance in the chloroplast is still an enigma. The fact that putative DMR1/HSK orthologs are present in all higher plants allows us to address the agricultural application of dmr1 technology in breeding downy mildew resistant crops that is based on a novel mechanism of resistance caused by modulation of host amino acid metabolism.
METHODS
Plant and Pathogen Growth Conditions
All Arabidopsis thaliana accessions used in this study were grown on potting soil in a growth chamber (Snijders) at 22°C with 16 h of light (100 μE/m2/s) and a relative humidity of 75%. Hyaloperonospora arabidopsidis isolate Cala2 was maintained on Arabidopsis Ler by weekly transfer to healthy 10- to 14-d-old seedlings (Holub et al., 1994). To obtain large amounts of conidiospores for bioassays, inoculum was collected from Ler eds1-2 seedlings that support abundant Cala2 growth and sporulation (Parker et al., 1996). Inoculum (4 × 104 spores·mL−1) was applied on 14-d-old seedlings using a spray gun. After inoculation, plants were allowed to dry for ∼30 min and were subsequently incubated under a sealed lid (100% relative humidity) in a growth chamber at 16°C with 9 h light/day (100 μE/m2/s). The amount of sporulation was quantified at 5 to 6 d after inoculation by counting the number of conidiophores on the cotyledons and leaves.
Microscopy
Infections of H. arabidopsidis in Arabidopsis leaves were visualized by trypan blue staining. Infected seedlings or leaves were stained in lactophenol (1:1:1:1 volume of lactic acid/glycerol/phenol/water) containing 1 mg/mL trypan blue, by boiling for 1 to 2 min and destaining overnight in choral hydrate. Trapped air bubbles were removed by 1 min speed vacuum infiltration. H. arabidopsidis growth was visualized by differential interference contrast microscopy.
Cloning of DMR1
The isolation of the dmr1 mutants, which have been generated by EMS mutagenesis, has been described previously (Van Damme et al., 2005). The dmr1 mutants were backcrossed twice (BC2), to reduce the number of unlinked EMS-induced mutations, to the parental line Ler eds1-2 and to Ler containing the wild-type EDS1 gene. A mapping population was generated by crossing the mutant to Columbia-0 (Col-0) FN2 (Sinapidou et al., 2004), and the resistant F2 plants were selected, genotyped, and rescreened for resistance in the F3. The dmr1 phenotype was found to be linked to the ciw3 and nga1126 markers (www.arabidopsis.org) on chromosome 2. The dmr1 mutation was fine-mapped to an ∼130-kb region covered by three BACs between two IND-based markers (marker designed by use of http://www.arabidopsis.org/browse/Cereon/index.jsp site for polymorphisms), located on BAC F6P23, at 7.43 Mb, and F5J6, at 7.56 Mb, resulting in an area of 30 candidate DMR1 genes. Additional cleaved amplified polymorphic markers in six genes, At2g17190, At2g17200, At2g17270, At2g17300, At2g17310, and At2g17360, allowed the further reduction of the dmr1 region to eight candidate genes At2g17230 to At2g17290. DNA sequencing of the genes in the dmr1 mutants resulted in the finding of mutations in At2g17265, which was thereby identified as DMR1.
Identification of Putative HSK Orthologs
Putative HSK orthologs were identified from the annotated genome sequences of grape (Vitis vinifera), poplar (Populus trichocarpa), rice (Oryza sativa), and the moss Physcomitrella patens by BLASTP searches on RefSeq proteins using the Arabidopsis HSK protein as a query. The identified putative HSK orthologs were used as query in reciprocal BLASTP searches on all Arabidopsis proteins. Identification of Arabidopsis HSK as the highest scoring protein confirmed the finding of a reciprocal best hit; therefore, the identified protein was considered a putative ortholog. For the identification of putative orthologs from other plant species, EST databases were employed. The Arabidopsis HSK protein sequence was used as query in TBLASTN searches against the EST database. For each plant species analyzed, the high-scoring ESTs were collected and assembled into contigs using the CAP3 program (http://pbil.univ-lyon1.fr/cap3.php). Reciprocal BLASTX analysis using the contig nucleotide sequences against all Arabidopsis proteins was used to select those contigs that had the best hit with Arabidopsis HSK. All collected HSK putative orthologs formed a single clade in a phylogenetic tree generated using ClustalW (standard settings).
Amino Acid Analysis
Amino acids were extracted from 100 mg of aboveground parts of 14-d-old seedlings that were ground in liquid nitrogen. Amino acids were extracted with 80% methanol (twice), and the supernatant was collected in a fresh tube. The remaining pellet was extracted with 20% methanol (twice), and the extract was added to the previously collected 80% methanol extracted supernatant. Samples were lyophilized and dissolved in 150 μL of deionized water. An equal volume (150 μL) of internal standard, 750 μmol/L, S-amino-ethyl-cysteine was added. Prior to amino acid detection, the remaining proteins were removed by treatment with 10% sulfosalicylic acid. Plant amino acids were detected and quantified by automated ion-exchange chromatography with post column ninhydrin derivatization on a JEOL AminoTac JLC-500/V.
Homoserine Treatment of Arabidopsis Seedlings
The homoserine treatment was combined with an H. arabidopsidis pathogenicity assay. Ten-day-old Arabidopsis seedlings were spray inoculated with H. arabidopsidis Cala2 (4 × 104 spores·mL−1) and 3 d later the infected seedlings were removed from the soil and submerged in l- or d-homoserine solution (or another amino acid) at the indicated concentration in an Eppendorf tube and vacuum infiltrated. After infiltration, the seedlings were transplanted back into the soil. Pathogen growth and sporulation were monitored 5 to 6 d after infection and 2 to 3 d after infiltration.
Construction of dmr1 Transgenic Lines Overexpressing HSK
Complementation lines were generated by transforming dmr1 plants by the floral dip method (Clough and Bent, 1998) with Agrobacterium tumefaciens containing the HSK gene from Col-0 (At HSK) or the HSK gene THR1 from Saccharomyces cerevisiae (Sc HSK) under control of the 35S promoter. The Arabidopsis HSK construct was generated by PCR amplification of the full-length coding sequence from Col-0 cDNA with primers For.HSKclonAT._BamHI, 5′-CTCATTACTGGATCCTCAATGGCAAGTCT-3′, containing a BamHI restriction site near the start codon (ATG), and Rev.HSKclonAT._EcoRI, 5′-GTTCCAATCTTAACGAATTCAAACAGCACAC-3′, containing an EcoRI site after the stop codon. The fragment was cloned directionally between the P35S promoter and the Nos terminator and inserted into pGreenII0229 (http://www.pgreen.ac.uk; Hellens et al., 2000). The S. cerevisiae HSK gene was PCR-amplified using primers For.HSKclonYeast._BamHI, 5′-TAGTGGGATCCGCAGATGGTTCGTGCCTTC-3′, and Rev.HSKclonYeast._EcoRI, 5′-CTGCAGAATTCCTATTCATTGCTGTTCGACGC-3′, and cloned as described for Arabidopsis HSK. Transformed seedlings containing the P35S-HSK constructs were selected for BASTA resistance. dl-Phosphinothricin (300 μM; BASTA) was sprayed on 10-d-old seedlings, and resistant seedlings (T1) were transplanted for seed set. The T2 and T3 generations were analyzed for H. arabidopsidis susceptibility.
HSK Recombinant Protein Production and Enzyme Assay
The coding sequences of the wild-type HSK gene (Arabidopsis accession Ler) and that of the dmr1 mutants were PCR amplified from genomic DNA using primers HSK_pET_F, 5′-CCCCCATGGCAAGTCTTTGTTTCCAA-3′, and HSK_pET_R, 5′-CCCCTCGAGTCATCTGGAGACGCTGTTGA-3′. PCR fragments were digested with NcoI and XhoI and ligated in pET-30(a)+ (Novagen). The coding sequence of the yeast HSK was PCR amplified using primers FwScHSK, 5′-CCGGATCCATGGTTCGTGCCTTCAA-3′, and RvScHSK, 5′-CCCCTCGAGCTATTCATTGCTGTTCGACGC-3′. PCR fragments were digested with BamHI and XhoI and ligated in pET-30(a)+. Production of recombinant enzyme and the HSK enzyme assay was performed as previously described (Lee and Leustek, 1999), with several modifications, as described below. Bacterial pellets were resuspended in lysis buffer (50 mM NaH2PO4, pH 8.0, 300 mM NaCl, and 10 mM imidazole) and sonicated six times for 10 s. Cleared lysates were filtered (0.2 μm) and incubated with Ni-NTA beads (Qiagen) for 1 h at 4°C. The beads were washed three times with washing buffer (50 mM NaH2PO4, pH 8.0, 300 mM NaCl, and 20 mM imidazole) and finally eluted in elution buffer (50 mM NaH2PO4, pH 8.0, 300 mM NaCl, and 250 mM imidazole). HSK activity was assayed indirectly by measuring ADP levels spectrophotometrically in 96-well plates at 340 nm.
Input protein levels were quantified by protein gel blot analysis using anti-His antibodies (Amersham). Specific activities were calculated using the activity assay data and protein input levels that were plotted relative to the activity of wild-type HSK protein.
Q-PCR Analysis
Total RNA was extracted using an RNeasy kit and treated with the RNase-free DNase set (Qiagen). Total RNA was quantified using a Biowave II UV/visible spectrophotometer (WPA). cDNA was synthesized with SuperScript-III reverse transcriptase (Invitrogen) and oligo(dT)15 (Promega). Cycle tresholds were determined in triplicate per transcript in three biological replicas using the ABI PRISM 7700 sequence detection system (Applied Biosystems) using SYBR Green I as the reporter dye. The data were normalized using Arabidopsis Actin2 levels. Primers used for detecting transcripts analyzed in this study are Actin2 (QACT2F, 5′-AATCACAGCACTTGCACCA-3′, and QACT2R, 5′-GAGGGAAGCAAGAATGGAAC-3′), PR-1 (QPR-1F, 5′-GAACACGTGCAATGGAGTTT-3′, and QPR-1R, 5′-GGTTCCACCATTGTTACACCT-3′), PR-2 (QPR-2F, 5′-CCCGTAGCATACTCCGATTT-3′, and QPR-2R, 5′-AAGGAGCTTAGCCTCACCAC-3′), DMR6 (QDMR6F, 5′-TGTCATCAACATAGGTGACCA-3′, and QDMR6R, 5′-CGATAGTCACGGATTTTCTGTG-3′), and HSK (QHSKF, 5′-CTGCTTTAGTCGCTGCTGTG-3′, and QHSKR, 5′-GAATCAACGGCGCTCTAGTC-3′). The size of amplicons was between 99 and 101 bp.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: DMR1/HSK (At2g17265), PMR4 (At4g03550), NPR1 (At1g64280), Sid2-1 (At1g74710), PAD4 (At3g52430), EIN2 (At5g03280), NDR1 (At3g20600), JAR1 (At2g46370), PMR2/MLO2 (At1g11310), MLO6 (At1g61560), MLO12 (At2g39200), PEN1 (At3g11820), PEN2 (At2g44490), PEN3 (At1g59870), SAG101 (At5g14930), PAD3 (At3g26830), FMO1 (At1g19250), SGT1b (At4g11260), RAR1 (At5g51700), DMR6 (At5g24530), S. cerevisiae THR1 (NP_011890), P. trichocarpa HSK (XP_002328135), V. vinífera HSK (XP_002277887), and O. sativa HSK (NP_001048623).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Complementation of dmr1 Mutants with the HSK Gene.
Supplemental Figure 2. Multiple Sequence Alignment of Plant Homoserine Kinase Amino Acid Sequences.
Supplemental Figure 3. Oomycete Hyphal Growth and Spore Germination Are Not Affected by l-Homoserine Compared with d-Homoserine Treatment.
Supplemental Figure 4. Multiple Alignment of Three Oomycete and Two Plant HSK Protein Sequences.
Supplemental Figure 5. Defense-Associated Genes and HSK Are Not Strongly Activated in the Mutants dmr1-1, dmr1-2, and dmr1-3.
Supplementary Material
Acknowledgments
We thank J. Stassen and M. Roeleveld for their excellent technical assistance, P. Weisbeek and C. Pieterse for critical reading of the manuscript, and F. Kindt and R. Leito for the artwork. We thank S. Somerville, M. Nishimura, R. Panstruga, and J. Parker for generously providing seeds of Arabidopsis immune response mutants and R. O'Connell and E. Holub for testing the dmr1 mutants for resistance to C. higginsianum and A. candida, respectively.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Guido van den Ackerveken (g.vandenackerveken@uu.nl).
Online version contains Web-only data.
References
- Aist, J.R. (1976). Papillae and related wound plugs of plant cells. Annu. Rev. Phytopathol. 14 145–163. [Google Scholar]
- Azevedo, R.A. (2002). Analysis of the aspartic acid metabolic pathway using mutant genes. Amino Acids 22 217–230. [DOI] [PubMed] [Google Scholar]
- Baerenfaller, K., Grossmann, J., Grobei, M.A., Hull, R., Hirsch-Hoffmann, M., Yalovsky, S., Zimmermann, P., Grossniklaus, U., Gruissem, W., and Baginsky, S. (2008). Genome-scale proteomics reveals Arabidopsis thaliana gene models and proteome dynamics. Science 320 938–941. [DOI] [PubMed] [Google Scholar]
- Blanc, G., Barakat, A., Guyot, R., Cooke, R., and Delseny, M. (2000). Extensive duplication and reshuffling in the Arabidopsis genome. Plant Cell 12 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm, S.T., Coaker, G., Day, B., and Staskawicz, B.J. (2006). Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 124 803–814. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Consonni, C., Humphry, M.E., Hartmann, H.A., Livaja, M., Durner, J., Westphal, L., Vogel, J., Lipka, V., Kemmerling, B., Schulze-Lefert, P., Somerville, S.C., and Panstruga, R. (2006). Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nat. Genet. 38 716–720. [DOI] [PubMed] [Google Scholar]
- Emanuelsson, O., Nielsen, H., and von Heijne, G. (1999). ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci. 8 978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens, R.P., Edwards, E.A., Leyland, N.R., Bean, S., and Mullineaux, P.M. (2000). pGreen: A versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 42 819–832. [DOI] [PubMed] [Google Scholar]
- Holub, E.B., Beynon, J.L., and Crute, I.R. (1994). Phenotypic and genotypic characterization of interactions between isolates of Peronospora parasitica and accessions of Arabidopsis thaliana. Mol. Plant Microbe Interact. 7 223–239. [Google Scholar]
- Jones, J.D., and Dangl, J.L. (2006). The plant immune system. Nature 444 323–329. [DOI] [PubMed] [Google Scholar]
- Joy, K.W., and Prabha, C. (1986). The role of transamination in the synthesis of homoserine in peas. Plant Physiol. 82 99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M., and Leustek, T. (1999). Identification of the gene encoding homoserine kinase from Arabidopsis thaliana and characterization of the recombinant enzyme derived from the gene. Arch. Biochem. Biophys. 372 135–142. [DOI] [PubMed] [Google Scholar]
- Lee, M., Martin, M.N., Hudson, A.O., Lee, J., Muhitch, M.J., and Leustek, T. (2005). Methionine and threonine synthesis are limited by homoserine availability and not the activity of homoserine kinase in Arabidopsis thaliana. Plant J. 41 685–696. [DOI] [PubMed] [Google Scholar]
- Lipka, V., et al. (2005). Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310 1180–1183. [DOI] [PubMed] [Google Scholar]
- Mannhaupt, G., Pohlenz, H.D., Seefluth, A.K., Pilz, U., and Feldmann, H. (1990). Yeast homoserine kinase. Characteristics of the corresponding gene, THR1, and the purified enzyme, and evolutionary relationships with other enzymes of threonine metabolism. Eur. J. Biochem. 191 115–122. [DOI] [PubMed] [Google Scholar]
- Mills, W.R. (1980). Photosynthetic formation of the aspartate family of amino acids in isolated chloroplasts. Plant Physiol. 65 1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, M.T., Stein, M., Hou, B.H., Vogel, J.P., Edwards, H., and Somerville, S.C. (2003). Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301 969–972. [DOI] [PubMed] [Google Scholar]
- O'Connell, R.J., and Panstruga, R. (2006). Tete a tete inside a plant cell: Establishing compatibility between plants and biotrophic fungi and oomycetes. New Phytol. 171 699–718. [DOI] [PubMed] [Google Scholar]
- Panstruga, R. (2005). Serpentine plant MLO proteins as entry portals for powdery mildew fungi. Biochem. Soc. Trans. 33 389–392. [DOI] [PubMed] [Google Scholar]
- Parker, J.E., Holub, E.B., Frost, L.N., Falk, A., Gunn, N.D., and Daniels, M.J. (1996). Characterization of eds1, a mutation in Arabidopsis suppressing resistance to Peronospora parasitica specified by several different RPP genes. Plant Cell 8 2033–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinapidou, E., Williams, K., Nott, L., Bahkt, S., Tor, M., Crute, I., Bittner-Eddy, P., and Beynon, J. (2004). Two TIR:NB:LRR genes are required to specify resistance to Peronospora parasitica isolate Cala2 in Arabidopsis. Plant J. 38 898–909. [DOI] [PubMed] [Google Scholar]
- Terryn, N., et al. (1999). Evidence for an ancient chromosomal duplication in Arabidopsis thaliana by sequencing and analyzing a 400-kb contig at the APETALA2 locus on chromosome 4. FEBS Lett. 445 237–245. [DOI] [PubMed] [Google Scholar]
- Theze, J., and Saint-Girons, I. (1974). Threonine locus of Escherichia coli K-12: Genetic structure and evidence for an operon. J. Bacteriol. 118 990–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuskan, G.A., et al. (2006). The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313 1596–1604. [DOI] [PubMed] [Google Scholar]
- Van Damme, M., Andel, A., Huibers, R.P., Panstruga, R., Weisbeek, P.J., and Van den Ackerveken, G. (2005). Identification of Arabidopsis loci required for susceptibility to the downy mildew pathogen Hyaloperonospora parasitica. Mol. Plant Microbe Interact. 18 583–592. [DOI] [PubMed] [Google Scholar]
- van Damme, M., Huibers, R.P., Elberse, J., and Van den Ackerveken, G. (2008). Arabidopsis DMR6 encodes a putative 2OG-Fe(II) oxygenase that is defense-associated but required for susceptibility to downy mildew. Plant J. 54 785–793. [DOI] [PubMed] [Google Scholar]
- Vogel, J., and Somerville, S. (2000). Isolation and characterization of powdery mildew-resistant Arabidopsis mutants. Proc. Natl. Acad. Sci. USA 97 1897–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, J.P., Raab, T.K., Schiff, C., and Somerville, S.C. (2002). PMR6, a pectate lyase-like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell 14 2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, J.P., Raab, T.K., Somerville, C.R., and Somerville, S.C. (2004). Mutations in PMR5 result in powdery mildew resistance and altered cell wall composition. Plant J. 40 968–978. [DOI] [PubMed] [Google Scholar]
- Yang, Z., Rogers, L.M., Song, Y., Guo, W., and Kolattukudy, P.E. (2005). Homoserine and asparagine are host signals that trigger in planta expression of a pathogenesis gene in Nectria haematococca. Proc. Natl. Acad. Sci. USA 102 4197–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, T., Daugherty, M., Grishin, N.V., Osterman, A.L., and Zhang, H. (2000). Structure and mechanism of homoserine kinase: Prototype for the GHMP kinase superfamily. Structure 8 1247–1257. [DOI] [PubMed] [Google Scholar]
- Zybailov, B., Rutschow, H., Friso, G., Rudella, A., Emanuelsson, O., Sun, Q., and van Wijk, K.J. (2008). Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS One 3 e1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.