Abstract
The development and activity of the procambium and cambium, which ensure vascular tissue formation, is critical for overall plant architecture and growth. However, little is known about the molecular factors affecting the activity of vascular meristems and vascular tissue formation. Here, we show that the His kinase CYTOKININ-INDEPENDENT1 (CKI1) and the cytokinin receptors ARABIOPSIS HISTIDINE KINASE2 (AHK2) and AHK3 are important regulators of vascular tissue development in Arabidopsis thaliana shoots. Genetic modifications of CKI1 activity in Arabidopsis cause dysfunction of the two-component signaling pathway and defects in procambial cell maintenance. CKI1 overexpression in protoplasts leads to cytokinin-independent activation of the two-component phosphorelay, and intracellular domains are responsible for the cytokinin-independent activity of CKI1. CKI1 expression is observed in vascular tissues of inflorescence stems, and CKI1 forms homodimers both in vitro and in planta. Loss-of-function ahk2 and ahk3 mutants and plants with reduced levels of endogenous cytokinins show defects in procambium proliferation and an absence of secondary growth. CKI1 overexpression partially rescues ahk2 ahk3 phenotypes in vascular tissue, while the negative mutation CKI1H405Q further accentuates mutant phenotypes. These results indicate that the cytokinin-independent activity of CKI1 and cytokinin-induced AHK2 and AHK3 are important for vascular bundle formation in Arabidopsis.
INTRODUCTION
Vascular tissue formation in plants is a process with broad developmental and physiological consequences. Factors regulating the proper formation of vascular tissue affect important developmental processes in plants, including the establishment of apical/basal symmetry during embryogenesis (Friml et al., 2003), organogenesis (Scheres et al., 1995; Mähönen et al., 2000), adaxial/abaxial cell fate determination (Emery et al., 2003; Prigge et al., 2005), and cell elongation and differentiation (Szekeres et al., 1996; Cano-Delgado et al., 2004).
Development of vascular tissue entails the differentiation of primary phloem and xylem from procambium, which contains vascular stem cells. Secondary vascular growth is characterized by vascular cambium originating from procambium and interfascicular cambium differentiating from phloem parenchyma and starch sheath cells (Altamura et al., 2001). The mitotic activity and differentiation of vascular and interfascicular cambial cells leads to the formation of secondary xylem and secondary phloem (Altamura et al., 2001; Ye et al., 2002).
Phytohormones appear to be regulatory factors of both primary and secondary vascular growth. Polar auxin transport is presumed to be necessary for the continuity of procambium (Jacobs, 1952; Sachs, 2000), and gibberellins are positive regulators of biomass production in hybrid aspen (Populus tremula × Populus tremuloides; Eriksson et al., 2000). Cytokinins have been suggested to be important regulators of primary vascular growth (Aloni, 1987; Medford et al., 1989), but their role in the regulation of procambium is just starting to emerge. Two-component signaling, wherein a His kinase receptor transfers a phosphate to downstream response regulators, is key for the cytokinin response (Hwang and Sheen, 2001; Kim et al., 2006). Cytokinin-induced signaling via its receptor ARABIOPSIS HISTIDINE KINASE4 (AHK4) and the type-B response regulators ARR1, ARR10, and ARR12 is necessary for procambium formation in Arabidopsis thaliana roots (Scheres et al., 1995; Mähönen et al., 2000; Yokoyama et al., 2007). Reduction of endogenous cytokinins by ectopic overexpression of CYTOKININ OXIDASE/DEHYDROGENASE1 (CKX1) or CKX2 results in the exclusive formation of protoxylem in root vascular bundles (VBs) (Mähönen et al., 2000, 2006a). The role of cytokinin in vascular tissue formation is further suggested by the vascular tissue-specific expression of genes involved in cytokinin biosynthesis (Miyawaki et al., 2004; Zhao et al., 2005) and transport (Hirose et al., 2005, 2008). Factors involved in cytokinin signaling in poplar (Populus spp; Nieminen et al., 2008) and cytokinin biosynthesis in Arabidopsis (Matsumoto-Kitano et al., 2008) were shown to be principal regulators of the cambium activity and positive regulators of the radial growth via secondary thickening. Nonetheless, the nature of cytokinin action in the primary vascular meristems of shoots, which supply the majority of economically useful plant biomass, is still largely unknown. In addition to hormonal regulations, recent studies of dodeca-peptides, CLV3/ESR-related41 (CLE41) and CLE44, and their receptor, PHLOEM INTERCALTED WITH XYLEM, showed that non-cell-autonomous communication between phloem and procambium is essential for procambium proliferation and polarity as well as xylem differentiation in the VB development (Fisher and Turner, 2007; Hirakawa et al., 2008). However, although few molecular factors regulating individual processes during vascular tissue formation and differentiation have been identified (Fukuda, 2004; Carlsbecker and Helariutta, 2005; Baucher et al., 2007), our knowledge of the molecular regulators of procambium and vascular cambium is still fragmentary.
Here, we report that the His kinase CYTOKININ-INDEPENDENT1 (CKI1) is important for vascular development via the regulation of procambium proliferation and/or the maintenance of its identity. Genetic manipulation of CKI1 activity leads to abnormal two-component signaling and defects in vascular tissue formation in Arabidopsis shoots. Cytokinin depletion and mutations in the cytokinin receptors AHK2 and AHK3 result in defects in vascular tissue formation in the inflorescence stem. Collectively, these results suggest that the two-component phosphorelay system is a key regulatory pathway for VB development in Arabidopsis shoots.
RESULTS
CKI1 Is Expressed in Specific Cell Types of VBs in Arabidopsis Inflorescences
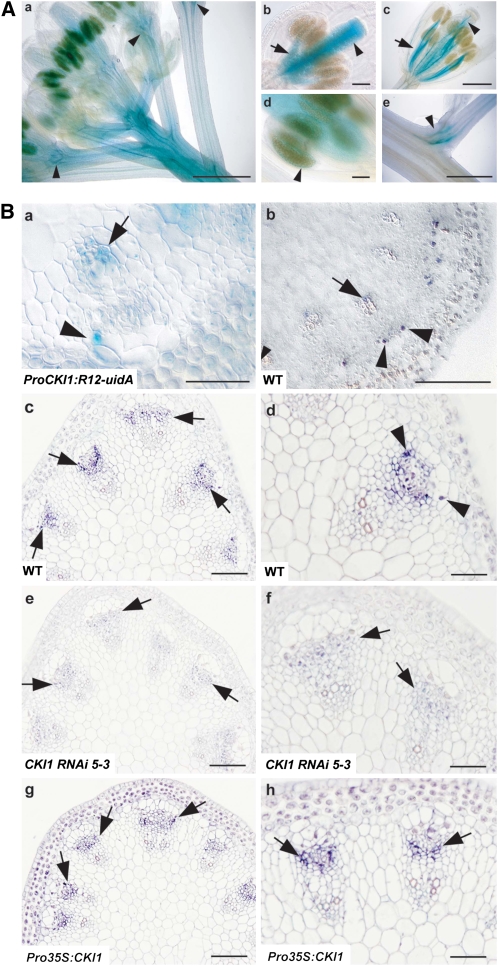
To investigate the physiological function of the putative sensor His kinase CKI1 in Arabidopsis sporophyte development, we first determined the transcriptional activity of CKI1 in ProCKI1:uidA and ProCKI1:R12-uidA transgenic lines that carry the uidA marker gene under the control of the CKI1 promoter (Hejátko et al., 2003; Figure 1A; see Supplemental Figure 1 online). In ProCKI1:uidA and ProCKI1:R12-uidA plants (see Methods), β-glucuronidase (GUS) activity was mostly detected in the vascular tissue of all floral organs, the top of the inflorescence stem and flower pedicels, and in the branching points adjacent to axillary meristems (Figures 1Aa to 1Ac and 1Ae). Weak but distinct GUS activity was also detectable in male sporogenous tissue (Figure 1Ad). In transverse sections of inflorescence stems, GUS activity was limited to specific cell types of VBs (Figure 1Ba).
Figure 1.
Expression of CKI1 in VBs.
(A) GUS activity in flowering transgenic plants harboring ProCKI1:R12-uidA ([a] and [c] to [e]) or ProCKI1:uidA (b). (a) Top of the Arabidopsis inflorescence. Note the intensity of the signal in the subapical region of the inflorescence stem, vascular tissues of floral organs, and floral pedicels (arrowheads). (b) and (c) Floral organs before (b) and at/just after anthesis (c). Note the predominant GUS staining in the pistil in the flowers before anthesis ([b]; arowhead); conversely, the signal in the vascular tissue of stamens is stronger in flowers at/just after anthesis ([c]; arrow). (d) Male sporophytic tissue (arrowhead). (e) Axillary meristem. Bars = 500 μm in (a), (c), and (e) and 100 μm in (b) and (d).
(B) CKI1 expression in VBs of the inflorescence stem. (a) GUS staining in a cross section of the inflorescence stem of a ProCKI1:R12-uidA plant. GUS activity is seen in cells of the VB sheath located at the lateral (outer) borders of the VB (arrowhead) and xylem (arrows; see also [b]). (b) In situ localization of CKI1 mRNA. (c) to (h) In situ immunolocalization of CKI1 using αCKI1ED polyclonal antibodies in the cambium of VBs (deep-purple signal, arrows) on cross sections of inflorescence stems of wild-type ([c] and [d]), CKI1RNAi ([e] and [f]), and Pro35S:CKI1 plants ([g] and [h]). px, protoxylem; mx, metaxylem; arrowheads point to the strongest signal, located in cells on the outer border of the VB (cf. with [a] and [b]; arrowheads). Note the procambial localization of CKI1 even in the Pro35S:CKI1 line. Bars = 100 μm in (b), (c), (e), and (g) and 50 μm in (a), (d), (f), and (h).
To confirm the relevance of the GUS data with CKI1 expression, the localization of CKI1 mRNA and CKI1 protein was determined in situ on cross sections of inflorescence stems (Figure 1B). Similar to what was seen for GUS activity, CKI1 mRNA was detected in differentiating xylem cells and in VB sheath cells (Figure 1Bb). Antibodies raised against the extracellular domain of CKI1 (αCKI1ED) identified the protein in the procambium of VBs, with the most intense signals in the VB sheath cells located at the lateral procambium borders (Figures 1Bc and 1Bd). Weak CKI1 signals were also distinguishable in the xylem (Figure 1B; for the specificity of αCKIED, see Supplemental Figure 2 online). The absence of CKI1 promoter activity and CKI1 mRNA in the procambium suggests the presence of a signal for procambial CKI1 localization. This was confirmed by immunolocalization of CKI1 in CKI1-overexpressing (Pro35S:CKI1) lines. Similar to wild-type plants, in Pro35S:CKI1 lines, the CKI1 protein localized predominantly to procambial cells (Figures 1Bg and 1Bh). Collectively, these data suggest that CKI1 may be involved in growth and development of VB, particularly in procambium development. Weak expression of CKI1 in the cortex (Figure 1B) might account for an additional role of CKI1 in other aspects of inflorescence stem growth.
CKI1 Is Involved in Controlling Meristematic Activity and Vascular Tissue Formation
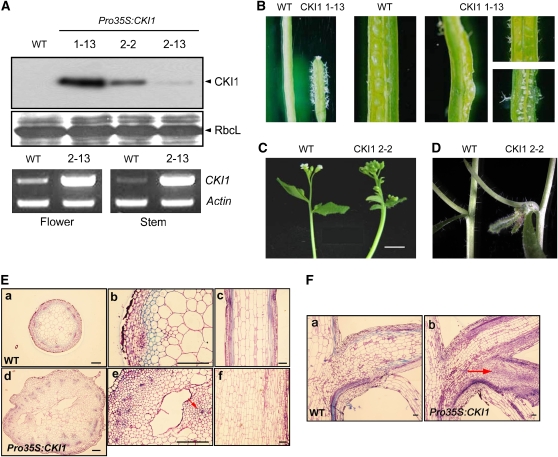
To further assess the role of CKI1 in the sporophyte development of Arabidopsis, we employed a gain-of-function approach, as mutants completely lacking CKI1 cannot be obtained due to the infertility of female gametes carrying cki1 insertion alleles (Pischke et al., 2002; Hejátko et al., 2003). Ectopic overexpression of CKI1 caused pleiotropic developmental changes (Figures 2A and 2B). Pro35S:CKI1 transgenic lines were found to be partially or almost completely sterile and to have dramatically shorter siliques. Immunoblot analysis showed that sterility correlated well with CKI1 expression levels (Figures 2A and 2B). Pro35S:CKI1 lines also had unusually thick fasciated inflorescence stems, along with changes in overall VB architecture (Figure 2C). Ectopic formation of increased numbers of VBs was observed in transverse sections of inflorescence stems, which suggests higher mitotic activity and abnormal differentiation (Figure 2E). The overall number of cells in transgenic stems was dramatically increased compared with wild-type stems, as seen in transverse sections of inflorescence stems (Figures 2Ea and 2Ed). CKI1-overexpressing plants also developed additional inflorescence branches that were initiated from axillary meristems (Figures 2D and 2F). Longitudinal sections of axillary buds revealed additional meristematic tissues bearing many smaller cells (Figure 2Fb, arrow). These findings further suggest that CKI1 might be involved in the regulation of cell division in vascular and meristematic tissues and in their development.
Figure 2.
Phenotype Analysis of CKI1-Overexpressing Plants.
(A) Expression analysis of Pro35S:CKI1-HA transgenic lines. Total protein and RNA from 2-week-old wild-type and transgenic plants were subjected to an immunoblot assay (top) and RT-PCR assay (bottom). RbcL and actin serve as input controls in the two assays.
(B) and (C) Ectopic expression of CKI1 leads to sterility, many trichomes (B), and thick fasciated inflorescence stems (C).
(D) Ectopic expression of CKI1 leads to additional vegetative tissues initiated from lateral meristems.
(E) The architecture of VBs in Pro35S:CKI1 transgenic plants. Transverse sections ([a], [b], [d], and [e]) and longitudinal sections ([c] and [f]) of the inflorescence stems of wild-type (top) and Pro35S:CKI1 transgenic plants (bottom). The arrows indicate ectopically formed VBs.
(F) The node structures of wild-type and Pro35S:CKI1 transgenic plants. Longitudinal sections of wild-type (a) and Pro35S:CKI1 transgenic nodes (b). The arrow indicates an ectopic axillary bud in a Pro35S:CKI1 transgenic plant.
Bars = 100 μm.
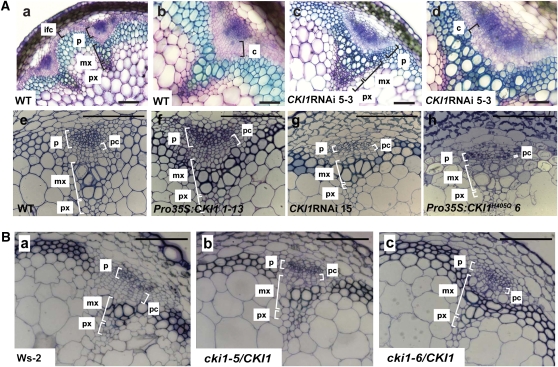
To examine CKI1 action in vascular development and to avoid possible artifacts due to CKI1 overexpression, we employed RNA interference (RNAi) to knock down the level of CKI1. The relative amounts of CKI1 transcripts and proteins in RNAi transgenic plants were determined by immunostaining and quantitative real-time PCR (Figure 1B; see Supplemental Figure 3A online). Wild-type and transgenic plants were grown under long-day conditions to the stage at which the first silique is formed on the inflorescence. We found that in comparison to wild-type plants, the procambial cell file layers of RNAi lines were decreased (Figures 3Ac, 3Ad, and 3Ag). By contrast, the number of procambial cells in VBs of inflorescence stems in Pro35S:CKI1 plants (122.8 ± 25, n = 6; mean ± se) was increased compared with wild-type plants (77.6 ± 6.1, n = 8) (Figures 3Ae and 3Af; see Supplemental Figure 4B online; for an example of quantification of procambial cells, see Supplemental Figure 4A online). CKI1 expression in analyzed RNAi lines was not completely absent, as shown by both RNA and protein levels (Figure 1B; see Supplemental Figure 3A online), suggesting that even a partial reduction of CKI1 expression might lead to phenotypic changes. We therefore analyzed two independent T-DNA insertion mutants in CKI1, cki1-5/CKI1 and cki1-6/CKI1 (Pischke et al., 2002). CKI1 transcripts in heterozygous plants of both lines were reduced up to 50% of the wild-type level (see Supplemental Figure 3B online). Defects similar to, but not identical, those identified in the CKI1 RNAi plants (i.e., reduction of procambium and abnormal cell shape) were observed in heterozygous cki1-6 plants (Figure 3B; see Supplemental Figures 4A and 4B online). The number of procambial cells in VBs of inflorescence stems in cki1 heterozygotes (51.1 ± 5, n = 14) was significantly lower than in wild-type plants (84.9 ± 5.7, n = 12). These results suggest that quantitative changes in the CKI1 activity result in a mutant phenotype and, furthermore, indicate that CKI1 is important for the maintenance of mitotic activity and/or the identity of procambial cells during VB development in Arabidopsis.
Figure 3.
CKI1 Is Involved in the VB Development of Inflorescence Stems.
(A) Suppression of CKI1 activity in CKI1 RNAi lines ([c], [d], and [g]) results in reduced and disorganized files of cambial cells. Conversely, the overexpression of CKI1 (f) results in an increase in the number of cambium layers. Note the presence of interfascicular cambium in toluidine blue staining of native tissue ([a] to [d]), suggesting the onset of secondary growth and, thus, cambium formation. Fixed material was subjected to phenotypic analysis before the onset of secondary growth, providing evidence for the role of CKI1 in procambium development ([e] to [h]). Overexpression of the negative allele CKI1H405Q leads to a dramatic reduction in procambium formation. Native staining of handmade sections ([a] to [d]) with toluidine blue and thin sections made from fixed and embedded material ([e] to [h]). With native toluidine blue staining, the phloem appears as blue, the undifferentiated cambial zone as pink, metaxylem as blue-green, and protoxylem as purple. c, cambium; ic, interfascicular cambium; pc, procambium; mx, metaxylem; p, phloem. Bars = 100 μm in (a), (c), and (e) to (h) and 50 μm in (b) and (d).
(B) The phenotypes conferred by reducing CKI1 expression by T-DNA insertion resemble those of CKI1RNAi plants. Transverse sections of the inflorescence stems of wild-type plants (Ws-2; [a]) and the heterozygous CKI1 T-DNA insertion lines cki1-5/CKI1 (b) and cki1-6/CKI1 (c). Bars = 100 μm.
CKI1 Acts through the Two-Component Signaling Pathway
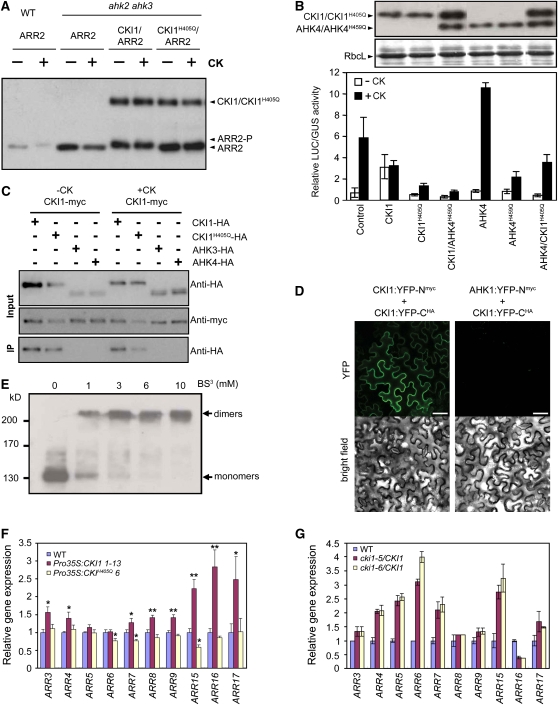
CKI1 shares similarity with members of the His kinase family, and CKI1 His kinase activity has been reported in heterologous and Arabidopsis protoplast systems (Hwang and Sheen, 2001; Yamada et al., 2001; Mähönen et al., 2006a). To understand the mechanism by which CKI1 affects vascular tissue development, we inspected the His kinase activity of CKI1 in a two-component signaling network by measuring both the activity of the cytokinin-responsive ARR6 promoter fused to a luciferase (LUC) reporter gene and measuring cytokinin-dependent ARR2 phosphorylation; both of these approaches have proved to be reliable indicators of two-component signaling outputs (Hwang and Sheen, 2001; Kim et al., 2006). CKI1 induced ARR6-LUC activity in both the presence and absence of cytokinin, as previously shown (Hwang and Sheen, 2001; see Supplemental Figure 5A online). However, CKI1 did not affect expression of the abscisic acid–responsive RD29A or auxin-responsive GH3 promoters, suggesting a specificity of CKI1-mediated responses to the two-component phosphorelay (see Supplemental Figure 5A online). Then we tested whether CKI1 could initiate a phosphorelay to ARR2, a type-B response regulator that is involved in cytokinin-mediated two-component responses (Hwang and Sheen, 2001). As previously demonstrated (Kim et al., 2006), the ARR2 protein was phosphorylated in a cytokinin-dependent manner, resulting in a gel band shift. By contrast, cytokinin-dependent phosphorylation of ARR2 was abolished in protoplasts prepared from the loss-of-function ahk2 ahk3 mutants (Figure 4A). When CKI1 was expressed in ahk2 ahk3 cells, ARR2 phosphorylation was restored regardless of cytokinin treatment. However, overexpression of CKI1H405Q carrying a mutation in the conserved functional His residue could not induce ARR2 phosphorylation in the double mutant (Figure 4A). These results suggest that CKI1 has cytokinin-independent His kinase activity in the two-component phosphorelay system.
Figure 4.
CKI1-Mediated Signaling Is Connected to the Two-Component Signal Transduction Pathway.
(A) CKI1 induces cytokinin-independent ARR2 phosphorylation. Protoplasts from ahk2 ahk3 plants were cotransfected with ARR2-HA along with CKI1-HA or CKI1H405Q-HA, incubated for 6 h, and treated with 100 nM t-zeatin (cytokinin) in the presence of 100 μM cycloheximide for 1 h. Wild-type protoplasts transfected with ARR2 served as a control. The mobility shift of ARR2 induced by phosphorylation was detected with an anti-HA antibody. Equal amounts of protein were loaded on each lane.
(B) A negative form of CKI1 protein represses the AHK4-mediated induction of ARR6. Protoplasts from wild-type plants were transfected with ARR6-LUC alone, wild-type AHK4, wild-type AHK4 plus mutant CKI1, wild-type CKI1, or wild-type CKI1 plus mutant AHK4. Error bars indicate se (n = 2). CKI1H405Q and AHK4H459Q are negative versions of CKI1 and AHK4, respectively. Rubisco large subunit (RbcL) stained by Coomassie blue was used as a protein loading control.
(C) CKI1-HA but none of the tested AHKs-HA proteins c-immunoprecipitate with myc-tagged CKI1. Mesophyll protoplasts from wild-type plants were transfected with CKI1-HA, CKI1H405Q-HA, AHK3-HA, or AHK4-HA, with or without CKI1-myc, incubated for 6 h, and then immunoprecipitated with anti-myc antibodies. CKI1 proteins were detected with an anti-HA antibody.
(D) CKI1 forms homodimers in tobacco leaf cells. Confocal images of abaxial epidermal tobacco leaf cells expressing the indicated YFP-N and YFP-C fusion proteins demonstrate YFP fluorophore reconstitution due to protein–protein interaction of the tested proteins (top row). The bottom row shows the corresponding bright-field images of the transiently transformed cells. Bars = 50 μm.
(E) CKI1 forms dimers. Protoplasts expressing CKI1-HA were solubilized with Triton X-100. Total protein was treated with increasing amounts of the cross-linker BS3 and subjected to SDS-PAGE. Two bands corresponding to the predicted sizes of the CKI1 monomer and dimer were detected with the anti-HA antibody.
(F) and (G) Genetic manipulation of CKI1 activity affects two-component signaling in planta. Transgenic plants overexpressing CKI1 or CKI1H405Q (F) or CKI1 T-DNA insertion lines (G) show changes in the expression of specific type-A ARRs. Quantitative RT-PCR was performed with total RNA extracted from 3-week-old seedlings (F) or inflorescence stems (G) using gene-specific primers for type-A ARRs (see Supplemental Table 1 online for primer sequences). Error bars indicate se (n = 8 [F] and 3 [G]). Asterisks indicate statistically significant differences from wild-type transgenic plants analyzed by Student's t test (*P < 0.05; ** P < 0.01).
The His residue at position 405 of CKI1 amino acid sequence is reported to be a primary target of His kinase activity in the two-component phosphorelay (Hwang and Sheen, 2001). We previously showed that the CKI1H405Q mutation diminishes the cytokinin-dependent activation of the ARR6 promoter in wild-type protoplasts (Hwang and Sheen, 2001), suggesting that this mutation might act in a dominant-negative manner in AHK2-, AHK3-, and AHK4-mediated cytokinin signaling pathway.
To determine the mechanism for this negative regulation, wild-type protoplasts were transfected with CKI1H405Q and ARR6-LUC along with the His kinases AHK2, AHK3, or AHK4 and treated with cytokinin. Interestingly, CKI1H405Q suppressed the AHK2-, AHK3-, and AHK4-mediated ARR6-LUC activation that was induced by exogenous cytokinins (Figure 4B; see Supplemental Figure 5B online). Accordingly, when wild-type protoplasts were transfected with AHK2H597Q, AHK3H460Q, or AHK4H459Q carrying mutation in the conserved His residue along with wild-type CKI1 and the ARR6-LUC reporter gene, the CKI1-mediated activation of the ARR6 promoter was also blocked (Figure 4B; see Supplemental Figure 5B online). These data indicate that CKI1 is connected to the two-component signal transduction pathway via its His kinase activity and that the negative effect of the CKI1H405Q protein is exerted via its interference with signaling mediated by the other His kinases AHK2, AHK3, or AHK4. Moreover, Pro35S:CKI1H405Q transgenic lines displayed defects in VBs (Figure 3Ah). Notably, these lines exhibited abnormal cell morphology with irregularly sized cells in both the xylem and phloem. These results provide additional experimental evidence for the functional importance of two-component mediated signaling in proper VB formation in Arabidopsis.
Dimerization of His kinases in plants and bacteria was previously demonstrated (Schaller et al., 1995; Surette et al., 1996; Tomomori et al., 1999; Gao et al., 2008; Grefen et al., 2008). Thus, we tested whether CKI1 directly interacts with other His kinases in Arabidopsis two-component signaling. To do this, myc-tagged CKI1 was cotransfected with HA-tagged CKI1, AHK3, or AHK4 in Arabidopsis protoplasts. When whole protoplast lysates were immunoprecipitated with an anti-myc antibody, CKI1-HA but not AHK3-HA or AHK4-HA was pulled down together with CKI1-myc, either in the presence or absence of exogenous cytokinins (Figure 4C). Wild-type CKI1 protein still interacted with the CKI1H405Q mutant protein (Figure 4C), suggesting that the His kinase and phosphoryl transfer activities of CKI1 are not required for its dimerization. The self-interaction of CKI1 in planta was confirmed using a bimolecular fluorescence complementation system (Walter et al., 2004). Coexpressed CKI1-cYFP and CKI1-nYFP, but not CKI1-cYFP and AHK1-nYFP, produced strong yellow fluorescent protein (YFP) fluorescence at the plasma membrane in tobacco (Nicotiana tabacum) leaf cells (Figure 4D). To determine whether CKI1 forms a dimer or a higher-order multimer, detergent-solubilized proteins from protoplasts expressing CKI1-HA were treated with the cross-linker bis-sulfosuccinimidyl suberate (BS3) (Figure 4E). The intensity of immunoreactive bands corresponding to the approximate size of the CKI1 monomer was gradually reduced as the BS3 concentration increased, while the intensity of a higher band with the approximate predicted size of a CKI1 dimer was concomitantly increased. Taken together, these results indicate that CKI1 forms homodimers both in vitro and in planta. However, in contrast with sensor His kinases involved in ethylene signaling, which form heterodimers (Gao et al., 2008; Grefen et al., 2008), CKI1 does not form heterodimers with any of the tested His kinases.
To confirm the His kinase activity of CKI1 in planta, we examined the expression of type-A ARR genes, the cytokinin primary response genes (D'Agostino et al., 2000), in Pro35S:CKI1, Pro35S:CKI1H405Q, and CKI1 knockdown lines. The ectopic expression of CKI1 induced the expression of a subset of type-A response regulators, including ARR3, 4, 7, 8, 9, 15, 16, and 17 (Figure 4F). By contrast, overexpression of CKI1H405Q significantly reduced the expression of ARR6, 7, and 15 (Figure 4F), thus confirming the negative regulatory role of CKI1H405Q in the two-component phosphorelay. Moreover, in heterozygous cki1-5 and cki1-6 lines, the expression of most of the inspected ARR genes was upregulated (Figure 4G), further demonstrating that CKI1 exerts its action through the two-component signal transduction pathway in planta. Furthermore, these results imply that changing CKI1 activity via site-directed mutagenesis and/or deregulation of endogenous CKI1 expression leads to differential changes in expression of individual ARRs, suggesting a disturbance of the two-component phosphorelay.
The Cytoplasmic CKI1 Domain Is Necessary for Its His Kinase Activity
Our data suggested that CKI1 can activate the two-component phosphorelay via its His kinase activity, which is independent of exogenously added cytokinins. To unravel the potential importance of extracellular and intracellular CKI1 domains in CKI1-mediated signaling, we constructed chimeric receptors composed of CKI1 and AHK4 (CKI1-AHK4 and AHK4-CKI1) along with truncated forms of CKI1 (see Supplemental Figure 5C online). AHK4-CKI1, which consists of the extracellular and transmembrane domains of AHK4 fused to the kinase and receiver domains of CKI1, could activate the ARR6 promoter as efficiently as wild-type CKI1, either in the presence or absence of cytokinins (see Supplemental Figure 5D online). However, CKI1-AHK4, which consists of the extracellular and transmembrane domains of CKI1 fused to the intracellular domain of AHK4, could not enhance the activity of the ARR6 promoter, regardless of the presence or absence of cytokinins. Moreover, CKI1ΔN, which lacks the extracellular domain of CKI1, still constitutively activated ARR6-LUC, unlike CKI1ΔC, which consists of the extracellular and transmembrane domains of CKI1 (see Supplemental Figure 5D online). Pro35S:AHK4-CKI1 transgenic lines displayed similar CKI1-overexpressing phenotypes with reduced fertility, shorter siliques, and additional inflorescence branches (see Supplemental Figures 6A and 6B online). They also had thick fasciated inflorescence stems with increased mitotic activity (see Supplemental Figure 6C online). Thus, the cytoplasmic kinase domain of CKI1 is sufficient for CKI1 cytokinin-independent His kinase activity in two-component signaling.
Cytokinins Regulate VB Development of Arabidopsis Inflorescence Stems via the AHK2 and AHK3 Signaling Pathway
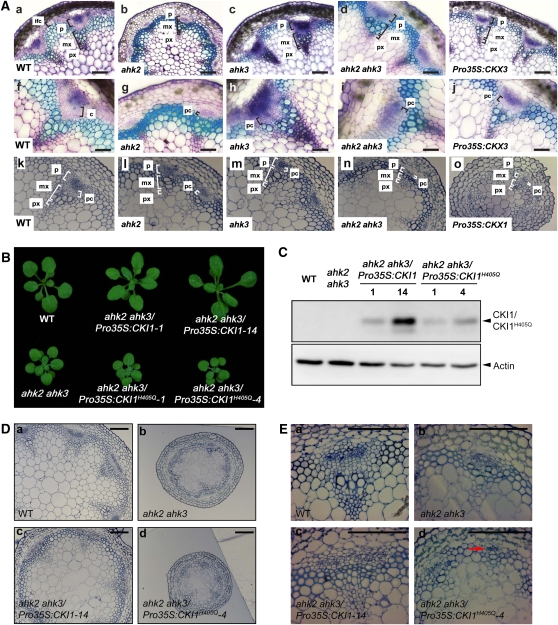
Our data suggest that CKI1 regulates the development of vascular tissue in shoots via its His kinase activity. Proteins involved in the cytokinin-regulated two-component signaling pathway are known to regulate vascular tissue formation in Arabidopsis roots (Mähönen et al., 2000, 2006b; Hutchison et al., 2006). In addition, the role of cytokinins in the cambium growth activity was recently identified (Matsumoto-Kitano et al., 2008; Nieminen et al., 2008). These results raised the possibility that two-component signaling cascades initiated by cytokinins as well as by CKI1 are also involved in the regulation of VB formation in shoots. Thus, we examined vascular tissue morphology in the inflorescence stems of plants with mutations in individual cytokinin receptors and in double mutants. In the ahk2 mutant, the number of cell layers in the procambial region was decreased (Figures 5Ab, 5Ag, and 5Al). A weaker phenotype was identifiable in the ahk3 line (Figures 5Ac, 5Ah, and 5Am). In the ahk2 ahk3 double mutant, reduction of the procambium and in the size of VBs was more pronounced than in either single mutant (Figures 5Ad, 5Ai, and 5An). A similar phenotype was also observed as a result of endogenous cytokinin depletion in Pro35S:CKX3 and Pro35S:CKX1 lines (Figures 5Ae, 5Aj, and 5Ao, respectively). In ahk2 ahk3 plants and in lines with decreased endogenous cytokinin, we further observed that interfascicular cambium failed to form when compared with the wild type in Figure 5Aa, suggesting defects in the onset of secondary growth. Taken together, AHK2 and AHK3 together with CKI1 play important roles in proper VB development, especially in the maintenance of procambial cell identity and/or regulation of procambial cell proliferation.
Figure 5.
Cytokinin Regulates VB Formation via AHK2 and AHK3 Phosphorelay.
(A) Transverse sections of the inflorescence stems of wild-type (Columbia-0 [Col-0]) ([a], [f], and [k]), ahk2 ([b], [g], and [l]), ahk3 ([c], [h], and [m]), ahk2 and ahk3 ([d], [i], and [n]), and Pro35S:CKX ([e], [j], and [o]) lines. Note the reduction of procambial layers in ahk2 and ahk3 plants and particularly in ahk2 ahk3 double mutants. The overall reduction in VB size is apparent in ahk2 ahk3 and in Pro35S:CKX lines, suggesting positive regulation of cytokinin signaling via the AHK2/AHK3 pathway in VB development in Arabidopsis inflorescence stems. Staining of handmade sections ([a] to [j]) with toluidine blue and thin sections made from fixed and embedded material ([k] to [o]). c, cambium; ic, interfascicular cambium; pc, procambium; mx, metaxylem; p, phloem. Bars = 100 μm in (a) to (e) and (k) to (o) and 50 μm in (f) to (j).
(B) The dwarfism resulting from deletion of ahk2 ahk3 is rescued in the presence of CKI1. Three-week-old wild-type (Col-0) and transgenic plants expressing Pro35S:CKI1-HA or Pro35S:CKI1H405Q-HA in the ahk2 ahk3 background were used for phenotypic analysis.
(C) Expression analysis of HA-tagged CKI1 and CKI1H405Q proteins under the control of the Pro35S promoter in transgenic lines. Total proteins from 3-week-old plants of each designated line were subjected to 7.5% SDS-PAGE. Actin proteins detected by immunoblotting serve as input controls.
(D) and (E) Ectopic expression of CKI1 rescues the abnormal vasculature of the ahk2 ahk3 mutant. Microscopy images of transverse sections of the inflorescence stems of wild-type (a), ahk2 ahk3 (b), Pro35S:CKI1-HA/ ahk2 ahk3 (c), and Pro35S:CKI1H405Q-HA/ ahk2 ahk3 (d) plants. The arrow indicates extensively reduced VB. Bars = 100 μm.
To confirm that CKI1 can affect vascular tissue development via the AHK2/3 signaling pathway, we ectopically expressed CKI1 or CKI1H405Q in ahk2 ahk3 mutants. Ectopic expression of CKI1 partially rescued the growth defects of these mutants (Figures 5B and 5C). The rosette leaves and petioles of ahk2 ahk3/Pro35S:CKI1 transgenic plants were similar to those of wild-type plants (Figure 5B). Overexpression of CKI1 in the ahk2 ahk3 background resulted in an increase of cambial layers, with a two- to threefold increase in the diameter of inflorescence stems compared with ahk2 ahk3 (Figure 5D). In addition, a reduced number of cells with the irregular size of ahk2 ahk3 in the xylem, phloem, and cambial layers were partially restored in these transgenic lines (Figure 5E). As a result, the radial growth was rescued, manifested by almost, but still partially, wild-type-like diameter of the inflorescence stem in Pro35S:CKI1/ahk2 ahk3 (Figure 5E). By contrast, ectopic expression of the dominant-negative mutation CKI1H405Q further accentuated the mutant phenotypes of ahk2 ahk3 plants. In comparison to the ahk2 ahk3 mother line, the aerial parts and diameters of inflorescence stems of ahk2 ahk3/Pro35S:CKI1H405Q plants were much smaller (Figures 5B and 5D). The cambial cell layers were unidentifiable, and vascular tissue differentiation was nearly abolished (Figure 5E). Collectively, these results suggest that CKI1 is functionally conserved with AHK2 and AHK3 in VB development but that it still has its own specificity in the regulation of vascular tissue development.
DISCUSSION
Cytokinin-Independent CKI1 Regulates Two-Component Phosphorelay in Arabidopsis
CKI1 was the first His kinase implicated in the perception of cytokinins (Kakimoto, 1996). However, CKI1 does not contain the cytokinin binding CHASE domain and does not bind cytokinins in vitro (Yamada et al., 2001). Defects in megagametogenesis conferred by a cki1 loss-of-function allele, together with observations of CKI1 expression in the ovule and endosperm, show that CKI1 is critical in female gametophyte development (Pischke et al., 2002; Hejátko et al., 2003). However, the mechanisms underlying the involvement and action of CKI1 signaling in specific biological processes during Arabidopsis gametophyte and/or sporophyte development remain largely unknown. Furthermore, when overexpressed in plants, calli, or protoplasts, CKI1 induces typical cytokinin responses, including shoot regeneration, delay of leaf senescence, and activation of the cytokinin-responsive ARR6 promoter in the absence of exogenously applied cytokinins (Kakimoto, 1996; Hwang and Sheen, 2001). It was suggested that ectopic expression of CKI1 allows the expressing cells to sense low concentrations of endogenous cytokinins that are otherwise unable to trigger shoot formation (Kakimoto, 1996). Here, we have shown that CKI1 can mediate cytokinin-independent regulation of the two-component signaling pathway. Thus, rather than recognition of endogenous cytokinin levels as suggested previously (Hwang and Sheen, 2001), the cytokinin-independent His kinase activity of CKI1 probably leads to the cytokinin-like phenotype in calli overexpressing CKI1 (Kakimoto, 1996). However, the possibility that the extracellular domain of CKI1 allows another mode of cytokinin-independent regulation of its His kinase activity cannot be excluded.
We found here that CKI1 shares at least some of the signaling proteins with the two-component phosphorelay system in the cytokinin signaling pathway. Based on our results, and of studies showing dephosphorylation of AHP1 and AHP2 by CKI1 in vitro (Nakamura et al., 1999), functional complementation of bacterial and yeast His kinase mutants by CKI1 (Yamada et al., 2001), and CKI1 interaction with AHP proteins in a yeast two-hybrid system (Dortay et al., 2006), we conclude that CKI1 can activate the two-component phosphorelay in Arabidopsis via proteins involved in the cytokinin signaling pathway. Whether CKI1 activity directly affects cytokinin signaling and/or other adaptive responses mediated by the two-component phosphorelay (e.g., osmosensing or abscisic acid responses), however, remains to be determined. CKI1 overexpression activated ProARR6:LUC, a marker for two-component signaling in protoplasts, but we could not observe similar activation of ARR6 in a late developmental stage of CKI1-overexpressing plants. This result implies that a single cell system may not always reflect different developmental stages at which multiple cells incorporate diverse external and/or internal signals to properly execute growth and development programs (Figure 4; see Supplemental Figure 5 online).
The output of the two-component phosphorelay was proposed to be a result of interactions of multiple His kinases and their kinase and phosphatase activities (Mähönen et al., 2006a). In this model, the final output of the two-component phosphorelay also depends on the expression levels of cytokinin binding and cytokinin nonbinding His kinases, including CKI1. The phosphatase activity of the receiver domain of CKI1 has been demonstrated (Nakamura et al., 1999), suggesting that CKI1 might contribute to two-component phosphorelay regulation via both kinase and phosphatase activities. Here, we have shown that both overexpression and downregulation of CKI1 affects the output of the two-component signaling pathway, as measured by regulation of the expression of ARRs. This observation accords well with the above-described model proposed by Mähönen et al. (2006a) and suggests that an equilibrium of individual inputs into the two-component pathway is critical for proper vascular tissue development in Arabidopsis shoots. Type-A ARR genes have been identified as negative regulators of cytokinin signaling (To et al., 2004). Auxin-induced regulation of ARR7 and ARR15 was recently identified as a mechanism of auxin-dependent spatial-specific attenuation of cytokinin signaling during stem cell niche formation in Arabidopsis roots (Müller and Sheen, 2008). Thus, upregulation of negative type-A ARRs in knockdown CKI1 lines might disrupt the proper regulation of two-component signaling in procambial development; therefore, these lines may partially phenocopy plants deficient in cytokinin signaling. However, whether CKI1-regulated expression of ARR genes represents another cytokinin-independent mechanism for regulation of the cytokinin two-component pathway remains to be determined. Furthermore, it is still uncertain if cytokinin-responsive ARR genes are direct regulators required for cambial development, although it is evident that perturbation of cytokinin homeostasis affects cambial activity in shoots and roots (Matsumoto-Kitano et al., 2008; Nieminen et al., 2008).
CKI1 Together with AHK2/AHK3 Is Involved in the Maintenance of Procambial Activity during VB Development in Arabidopsis Shoots
Vascular development in Arabidopsis can be divided into three major steps: (1) initiation and maintenance of (pro)cambium, (2) asymmetric cell patterning and differentiation into xylem and phloem precursor cells, and (3) their final specification into distinct xylem and phloem cell types. While auxin initiates and maintains continuous vascular pattern formation of procambial cells via polar auxin transport (Fukuda, 2004; Friml et al., 2004), cytokinin signaling mediated by AHK2/3 is unlikely to be involved in the initiation of procambial cell files as knockout lines still contain functional VBs (Figure 5). Rather, our results suggest that CKI1 and AHK2/3 are required for the proliferation and maintenance of procambial cells and vascular stem cells, which give rise to primary vascular tissues and vascular cambium.
Besides the procambium activity, the activity of the shoot apical meristem (SAM) seems to be genetically linked with the regulation of vascular tissue development (Baucher et al., 2007). Cytokinins were shown to be positive regulators of the shoot meristem size (Higuchi et al., 2004; Nishimura et al., 2004; Kurakawa et al., 2007). Thus, the downregulation of the diameter of the inflorescence stem and the size of VBs in ahk2 ahk3 mutants might be at least partially due to the defects in the SAM activity during procambium initiation. Accordingly, formation of enlarged and fasciated inflorescence stems in Pro35S:CKI1 lines could be affected by the increased mitotic activity in the SAM upon CKI1 overexpression. However, we could not observe any quantitatively significant change of the SAM size in the transgenic lines overexpressing CKI1 (see Supplemental Figure 8A online). In addition, we have analyzed the VB phenotype at the very base of the first internodium and, thus, in a position spatially and developmentally well dissected from the shoot apical meristem. The analysis was performed at the stage when the first silique was formed on the inflorescence. This stage corresponds to the end of the primary growth, which is primarily governed by the procambial activity (Altamura et al., 2001). Taken together, although we cannot completely exclude the possibility that some of the observed phenotype changes originate in the SAM, the defects in the procambium activity due to impaired cytokinin signaling seem to be at least one of the substantial contributions to the observed defects in the primary radial growth of ahk2 ahk3 and Pro35S:CKX1(2) lines.
Interestingly, AHK2/3-mediated cytokinin signaling seems to be also involved in secondary VB development. Formation of interfascicular cambium is one of the anatomically well distinguishable markers of the secondary growth initiation in Arabidopsis (Altamura et al., 2001). We observed that the formation of interfascicular cambium was absent and/or substantially reduced in the ahk2 ahk3 double mutant and in Pro35S:CKX1(2) lines, which suggests possible defects in the onset of the secondary thickening. It is possible that CKI1 maintains the basal meristematic activity of procambial cells and that AHK2/3 fine-tunes (pro)cambial activity following environmental and/or developmental cues that regulate endogenous cytokinin levels (Samuelson et al., 1992; Yang et al., 2001; Takei et al., 2004; Werner et al., 2006; Matsumoto-Kitano et al., 2008). Recently, results showing the involvement of cytokinin in the regulation of cambium in Arabidopsis and poplar were published (Matsumoto-Kitano et al., 2008; Nieminen et al., 2008). A reduction of cytokinins in null mutants of the Arabidopsis cytokinin biosynthetic genes ipt1,3,5,7 and in transgenic poplar trees overexpressing Arabidopsis CYTOKININ OXIDASE/DEHYDROGENASE2 resulted in impaired cambial formation, indicating that cytokinins are important regulators of vascular cambium. These results are consistent and complementary with our findings of a role for His kinase–mediated two-component signaling in vascular tissue formation of Arabidopsis shoots.
However, it should be emphasized here that in addition to hormonal regulations, other signals (e.g., weight of the produced biomass of the plant body) (Ko et al., 2004) are also integrated in the regulation of the secondary thickening. Thus, this type of signal might contribute to the observed defects in the onset of secondary thickening in ahk2 ahk3 and cytokinin-deficient plants, both of which are deficient in radial growth, thus revealing lowered production of the shoot biomass.
It has long been known that roots and shoots respond differently to cytokinins in Arabidopsis (Werner et al., 2003). In Arabidopsis roots, cytokinins have been shown to be necessary for the periclinal procambial cell divisions required for the proliferation of vascular cell files (Scheres et al., 1995; Mähönen et al., 2000, 2006b). Similar to what we have found, this suggests a positive role of cytokinins for procambium proliferation and/or maintenance. However, CKX1(2)-mediated depletion of cytokinins in Arabidopsis roots leads to the formation of abnormal vascular tissue that is devoid of phloem but which exhibits abundant protoxylem formation (Mähönen et al., 2006b). This is apparently not the case in the inflorescence stem, where CKX1(3)-mediated cytokinin depletion led to the formation of VBs of reduced size; however, all cell types (i.e., protoxylem, metaxylem, and phloem) still could be detected (Figure 5C). Accordingly, we did not observe specific phenotypic changes in root and hypocotyl vascular development in CKI1 knockdown lines (see Supplemental Figures 8B and 8C online). This could be explained by a lower sensitivity of the inflorescence stem to cytokinin depletion and by the specificity of CKI1 and AHK2/3 signaling. CKI1-driven, cytokinin-independent regulation of VB development could contribute to the lower sensitivity and resulting phenotype in Pro35S:CKX1(3) inflorescence stems. Alternatively, modified developmental pathways might operate in root and shoot vascular tissue development.
Here, we have shown that His kinases in Arabidopsis regulate vascular tissue formation in shoots via the regulation of procambium activity (see Supplemental Figure 9 online). This is of great economic importance as procambium and vascular cambium activities regulate biomass production in plants. Thus, regulation of the activity of individual His kinases by means of genetic engineering might be used to regulate biomass production in plants and might help us to lower our dependence on other, mostly nonrenewable, energy resources.
METHODS
Plant Materials
The Arabidopsis thaliana Col-0 ecotype and the mutant carrying the ahk2-1 and ahk3-1 mutant alleles (Higuchi et al., 2004), both in the Col-0 background, were used. Wild-type and mutant plants were grown in an environmentally controlled room at 23°C under white light with 14-h-light/10-h-dark cycles.
Transient Expression in Arabidopsis Protoplasts
Transient expression in protoplasts was performed as previously described (Hwang and Sheen, 2001). Typically, 2 × 104 protoplasts were transfected with 20 μg total plasmid DNA consisting of different combinations of the reporter, effectors, and internal control. Transfected protoplasts were incubated at 104 cells per mL with or without 100 nM t-zeatin (Sigma-Aldrich) for 6 h. As an internal control, the GUS reporter gene fused to the Arabidopsis ubiquitin promoter (UBI10−GUS) was used. The results shown are the means and error bars of relative LUC activities obtained from duplicate samples. All assays were performed at least three times, and similar results were obtained in all experiments.
Plasmid Constructs and Generation of Transgenic Plants
Full-length and truncated CKI1 were amplified by PCR from genomic Arabidopsis DNA. Full-length AHK4 was obtained by PCR from an Arabidopsis cDNA library. Chimeric AHK4-CKI1 and CKI1-AHK4 constructs were generated by overlap extension PCR using the overlapping primers 5′-CATCTCTCTCCTTGTTGCTTGAGCTGCACCATACAGTATATA-3′ and 5′-CTTCGACTTTTACTATGTGCATCATAAACCACACAAACCATAC-3′, respectively. The coding regions of all proteins were tagged with either two copies of the hemagglutinin epitope (HA), the myc epitope, or green fluorescent protein and inserted into a plant expression vector containing the 35SC4PPDK promoter and the NOS terminator (Hwang and Sheen, 2001). Transgenic Arabidopsis plants expressing CKI1-HA under the control of the 35SC4PPDK promoter were generated by the floral dip method and BASTA selection as described (Clough and Bent, 1998). Pro35S:CKX2 and Pro35S:CKX3 lines were generated as described (Pernisova et al., 2009). The ectopic expression of CKI1 was tested by RT-PCR and immunoblot analysis. Phenotypic analyses of transgenic lines were performed with homozygous T3 plants. All mutants were generated by site-directed mutagenesis using the QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. All constructs were confirmed by sequencing. To analyze the specificity of the CKI1 promoter, two different constructs were made and introduced into Arabidopsis ecotype Col-0. The first construct, ProCKI1:uidA, contains a 2.7-kb fragment of upstream genomic DNA that includes the putative translational start site of the short open reading frame (MKRAF) in the 5′ untranslated region of the CKI1 mRNA. The primers SII-ckpr (5′-GTAACCGCGGGAGGAGGCACAAAATGACGAA-3′) and B-ckpr (5′-GCTGGGATCCTCATATTATCTTCTTCCTCGGAGC-3′) were used for PCR amplification of the putative promoter region of CKI1; this fragment is translationally fused with the uidA coding sequence (Hejátko et al., 2003). The second construct, ProCKI1:R12-uidA, also contains a translational fusion of uidA with the same genomic fragment described above; however, the 3′ end of this fragment was extended to include the CGT codon that encodes the R12 residue of CKI1 (see Supplemental Figure 1 online). Multiple independent transgenic lines were inspected in both cases, and no apparent differences in the resulting distribution of GUS activity were detectable.
Expression Analysis
In situ mRNA and GUS staining were performed as previously described (Hartmann et al., 2000; Hejátko et al., 2003; Brewer et al., 2006). Polyclonal rabbit anti-CKI1 antibody was prepared against the peptide from the CKI1 extracellular domain (GATRIKHQAEKAKYQC, αCKIED; Sigma-Genosys) and used for indirect immunolocalization on Steedman's wax sections as described (Vitha et al., 2000). Two batches of polyclonal sera (anti-CKI1ED120 and anti-CKI1ED121) isolated from two independently immunized rabbits were tested (see Supplemental Figure 2 online). If not otherwise mentioned, anti-CKI1ED121 was used. The alkaline phosphatase–conjugated secondary antibody was visualized by 5-bromo-4-chloro-3-indol phosphate (BCIP)/p-Nitro-Blue tetrazolium chloride (NBT) staining. Antibody specificity was characterized on immunoblots using recombinant proteins expressed in Escherichia coli and on immunoblot using plant protein extracts (see Supplemental Figure 2 online). Preimmune serum was used as a negative control in immunolocalizations of CKI1 on sections, and no signal was obtained.
RT-PCR and Quantitative Real-Time PCR Analysis
Total RNA was isolated using Trizol reagent (Invitrogen) according to the manufacturer's instructions. For RT-PCR, first-strand cDNA was synthesized from 1 μg RNA with oligo(dT) primers and ImProm-II reverse transcriptase (Promega). The expression of CKI1 was verified with 30 cycles using a gene-specific primer set, CKI1fwd (5′-AACAGCTCAAGGACACCAAG-3′) and CKI1rev (5′-GCGTTCTTCATTTTTCAATA-3′), and actin gene as a control using ACTfwd (5′-GTACAACTATGTTCTCAGGT-3′) and ACTrev (5′-GAAGCATTTTCTGTGGACAA-3′) primers. For quantitative real-time RT-PCR, first-strand cDNA was prepared with SuperScript II reverse transcriptase (Invitrogen) and the ACT-L and rCKI1rt primers. The subsequent quantitative PCR was performed in a Light Cycler 2.0 (Roche) with SYBR Premix ExTaq system (Takara) as a fluorescent dye that monitors DNA content. To amplify gene-specific products, the following primers were used: fACTrt (5′-CAGTGTCTGGATCGGAGGAT-3′), rACTrt (5′-TGAACAATCGATGGACCTGA-3′), fCKI1rt (5′-CTATTGGGAACCCAGAGGACG-3′), rCKI1rt (5′- AAGCTTCTTTCCCACTGTCGC-3′), and type-A ARRs (see Supplemental Table 1 online). The steady state levels of the transcripts were determined by standard curve quantitation. All quantitative RT-PCR experiments were performed with biologically independent samples at least three times.
Coimmunoprecipitation and Immunoblot Analysis
Protoplasts were transfected with either HA- or myc-tagged CKI1, AHK3, AHK4, CKI1H405Q, or AHK4 and then incubated for 6 h to allow protein expression. Total protein was extracted from the transfected protoplasts in IP buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, protease inhibitor cocktail [Roche], and 1 mM DTT) and incubated with a monoclonal anti-HA antibody (Roche) or a monoclonal anti-c-myc antibody (Cell Signaling). The protein-antibody complex was precipitated with protein A/G plus-agarose beads (Calbiochem). For cross-linking experiments, protoplasts transfected with CKI1-HA were incubated for 6 h and lysed with protein extraction buffer (50 mM sodium phosphate, pH 7.4, 5 mM EDTA, 1% Triton X-100, 1 mM DTT, and protease inhibitor cocktail). Total protein extracts were incubated with different concentrations of the cross-linker BS3 (Pierce) for 1 h at 4°C before being quenched with 25 mM Tris-HCl, pH 7.5, for 30 min. Immunoprecipitated proteins and total proteins were subjected to 7.5 or 10% SDS-PAGE and blotted onto Immunobilon-P membranes (Millipore). The blots were probed with a peroxidase-conjugated anti-HA antibody (Roche) or a monoclonal anti-myc antibody. Extracellular domains of AHK4 (AHK4ED, D127-P395) and CKI1 (CKI1ED, E28-Q345) were cloned into E. coli expression vector pDEST17 and expressed as a recombinant protein in a translational fusion with His-Tag. One hundred micrograms of the total protein (bacterial lysate) was separated using 15% SDS-PAGE, blotted on polyvinylidene fluoride (PVDF) membrane, and immunodetected using monoclonal anti-polyHistidine (Sigma-Aldrich) or polyclonal anti-CKI1ED 1:10,000 in blocking buffer (5% milk in 10 mM Tris-HCI, pH 7.5, 150 mM NaCl, and 0.1% Tween-20). The detection was performed using alkaline phosphatase–conjugated goat anti-rabbit secondary antibodies (Sigma-Aldrich) diluted 1: 30,000 in blocking buffer and anti-mouse-AP antibodies (Sigma-Aldrich) diluted 1:20,000 in a blocking buffer with BCIP/NBT substrate for 10 min, within the linear range of signal development. All experiments were performed at least three times.
Analysis of CKI1 Dimerization Using Bimolecular Fluorescence Complementation
Entry clones containing CKI1 and AHK1 cDNA were prepared according to the manual for Gateway technology in pDONR207 (Invitrogen), verified by sequencing, and subsequently recombined via the LR reaction into pSPYNE-35S and pSPYCE-35S (Walter et al., 2004). Transient transformation of tobacco (Nicotiana tabacum) leaves and immunodetection of fusion proteins were performed as previously described (Horak et al., 2008). Confocal laser scanning microscopy was performed using an Olympus IX81 microscope equipped with a Fluoview 500 confocal unit at a setup recommended by the manufacturer for YFP fluorescence detection.
Histological Analysis
Tissue samples were fixed for 24 h in 3% glutaraldehyde and 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.2, or in FAA containing 5% acetic acid, 45% ethanol, and 5% formaldehyde. The fixed samples were then rinsed with 0.1 M phosphate buffer, pH 7.2, and dehydrated through a graded ethanol series. The specimens were infiltrated and embedded in Spurr's resin (Ted Pella) or Technovit resin (Kulzer and Co.) for 48 h at 65°C. Sections (0.5 or 4 μm) were made using an MT-X ultramicrotome (RMC), stained in 0.1% toluidine blue, and photographed with a Zeiss Axioplan2 microscope. For native staining, handmade sections were prepared with a razor blade from the base of the inflorescence stems when the first silique appeared. Sections were stained with toluidine blue (0.05% [w/v] solution in water) for 1 min, destained in distilled water for 30 seconds, mounted in 50% glycerol, and observed with a microscope (Olympus BX 61) using differential interference contrast microscopy.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: CKI1 (AT2G47430), AHK1 (AT2G17820), AHK2 (AT5G35750), AHK3 (AT1G27320), AHK4 (AT2G01830), ARR2 (AT4G16110), CKX1 (AT2G41510), CKX2 (AT2G19500), and CKX3 (AT5G56970). Germplasm identification numbers from this article are as follows: cki1-5 (CS6360) and cki1-6 (CS6361).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Schematic Representation of the ProCKI1:uidA (Up) and ProCKI1:R12-uidA (Down) Constructs Used in Analysis of the Transcriptional Specificity of the CKI1 Promoter.
Supplemental Figure 2. Anti-CKI1ED Antibody (αCKI1ED) Specifically Recognizes the Extracellular Domain of CKI1.
Supplemental Figure 3. Transcript Levels of CKI1 in RNAi Lines and T-DNA Insertion Heterozygous Lines.
Supplemental Figure 4. CKI1 Regulates the Number of Procambial Cells.
Supplemental Figure 5. CKI1 Specifically Enhances the Activity of the Two-Component, Cytokinin-Responsive ARR6 Promoter in a Cytokinin-Independent Manner.
Supplemental Figure 6. The Ectopic Expression of CKI1 or AHK4-CKI1 Leads to Sterility and Formation of Short Siliques and to Additional Vegetative Tissues Initiated from Lateral Meristems.
Supplemental Figure 7. Expression of Individual Constructs Used in the BiFC Assay in Figure 5B Was Determined by Immunostaining and Ponceau S Staining to Prove Equal Protein Loading (Red Bands).
Supplemental Figure 8. CKI1 Activity Does Not Affect Either the SAM Activity or the Vascular Bundle Development in Hypocotyl and Root.
Supplemental Figure 9. A Proposed Model for CKI1 and Cytokinin Action Mechanism in the Vascular Bundle Development of Inflorescence Stems.
Supplemental Table 1. Gene-Specific Primers for Type-A ARRs.
Supplementary Material
Acknowledgments
We thank Filip Rolland and Jiří Friml for critically reading the manuscript, Thomas Schumülling for Pro35S:CKX1 and Pro35S:CKX2 seeds, and Chiharu Ueguchi and Yka Helariutta for ahk mutant seeds. This work was supported by grants to I.H. from the Plant Diversity Research Center of MOST, the Plant Signaling Network Research Center, and Technology Development Program for Agriculture and Forestry (309017-5) and, in part, by a grant to G.-T.K. from the Environmental Biotechnology National Core Research Center of MOST. The work was also supported by the Ministry of Education of the Czech Republic (LN00A081, LC06034, and MSM0021622415), the Academy of Sciences of the Czech Republic (IAA600380507), the Bundesministerium für Bildung und Forschung, and Fonds of Chemical Industry. J.H. was supported by the Deutscher Akademischer Austausch Dienst. S.M.C. was a recipient of a Brain Korea 21 fellowship.
The authors responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) are: Jan Hejátko (hejátko@sci.muni.cz) and Ildoo Hwang (ihwang@postech.ac.kr).
Online version contains Web-only data.
References
- Aloni, R. (1987). Differentiation of vascular tissues. Annu. Rev. Plant Physiol. Plant Mol. Biol. 38 179–204. [Google Scholar]
- Altamura, M.M., Possenti, M., Matteucci, A., Baima, S., Ruberti, I., and Morelli, G. (2001). Development of the vascular system in the inflorescence stem of Arabidopsis. New Phytol. 151 381–389. [Google Scholar]
- Baucher, M., El Jaziri, M., and Vandeputte, O. (2007). From primary to secondary growth: Origin and development of the vascular system. J. Exp. Bot. 58 3485–3501. [DOI] [PubMed] [Google Scholar]
- Brewer, P.B., Heisler, M.G., Hejátko, J., Friml, J., and Benkova, E. (2006). In situ hybridization for mRNA detection in Arabidopsis tissue sections. Nat. Protocols 1 1462–1467. [DOI] [PubMed] [Google Scholar]
- Cano-Delgado, A., Yin, Y., Yu, C., Vafeados, D., Mora-Garcia, S., Cheng, J.C., Nam, K.H., Li, J., and Chory, J. (2004). BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development 131 5341–5351. [DOI] [PubMed] [Google Scholar]
- Carlsbecker, A., and Helariutta, Y. (2005). Phloem and xylem specification: pieces of the puzzle emerge. Curr. Opin. Plant Biol. 8 512–517. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- D'Agostino, I.B., Deruere, J., and Kieber, J.J. (2000). Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol. 124 1706–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dortay, H., Mehnert, N., Burkle, L., Schmulling, T., and Heyl, A. (2006). Analysis of protein interactions within the cytokinin-signaling pathway of Arabidopsis thaliana. FEBS J. 273 4631–4644. [DOI] [PubMed] [Google Scholar]
- Emery, J.F., Floyd, S.K., Alvarez, J., Eshed, Y., Hawker, N.P., Izhaki, A., Baum, S.F., and Bowman, J.L. (2003). Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr. Biol. 13 1768–1774. [DOI] [PubMed] [Google Scholar]
- Eriksson, M.E., Israelsson, M., Olsson, O., and Moritz, T. (2000). Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat. Biotechnol. 18 784–788. [DOI] [PubMed] [Google Scholar]
- Fisher, K., and Turner, S. (2007). PXY, a receptor-like kinase essential for maintaining polarity during plant vascular-tissue development. Curr. Biol. 17 1061–1066. [DOI] [PubMed] [Google Scholar]
- Friml, J., Vieten, A., Sauer, M., Weijers, D., Schwarz, H., Hamann, T., Offringa, R., and Jurgens, G. (2003). Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426 147–153. [DOI] [PubMed] [Google Scholar]
- Friml, J., et al. (2004). A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306 862–865. [DOI] [PubMed] [Google Scholar]
- Fukuda, H. (2004). Signals that control plant vascular cell differentiation. Nat. Rev. Mol. Cell Biol. 5 379–391. [DOI] [PubMed] [Google Scholar]
- Gao, Z., Wen, C.K., Binder, B.M., Chen, Y.F., Chang, J., Chiang, Y.H., Kerris III, R.J., Chang, C., and Schaller, G.E. (2008). Heteromeric interactions among ethylene receptors mediate signaling in Arabidopsis. J. Biol. Chem. 283 23801–23810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefen, C., Städele, K., Ruzicka, K., Obrdlik, P., Harter, K., and Horák, J. (2008). Subcellular localization and in vivo interactions of the Arabidopsis thaliana ethylene receptor family members. Mol. Plant 1 308–320. [DOI] [PubMed] [Google Scholar]
- Hartmann, U., Hohmann, S., Nettesheim, K., Wisman, E., Saedler, H., and Huijser, P. (2000). Molecular cloning of SVP: A negative regulator of the floral transition in Arabidopsis. Plant J. 21 351–360. [DOI] [PubMed] [Google Scholar]
- Hejátko, J., Pernisova, M., Eneva, T., Palme, K., and Brzobohaty, B. (2003). The putative sensor histidine kinase CKI1 is involved in female gametophyte development in Arabidopsis. Mol. Genet. Genomics 269 443–453. [DOI] [PubMed] [Google Scholar]
- Higuchi, M., et al. (2004). In planta functions of the Arabidopsis cytokinin receptor family. Proc. Natl. Acad. Sci. USA 101 8821–8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa, Y., Shinohara, H., Kondo, Y., Inoue, A., Nakanomyo, I., Ogawa, M., Sawa, S., Ohashi-Ito, K., Matsubayashi, Y., and Fukuda, H. (2008). Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proc. Natl. Acad. Sci. USA 105 15208–15213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose, N., Makita, N., Yamaya, T., and Sakakibara, H. (2005). Functional characterization and expression analysis of a gene, OsENT2, encoding an equilibrative nucleoside transporter in rice suggest a function in cytokinin transport. Plant Physiol. 138 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose, N., Takei, K., Kuroha, T., Kamada-Nobusada, T., Hayashi, H., and Sakakibara, H. (2008). Regulation of cytokinin biosynthesis, compartmentalization and translocation. J. Exp. Bot. 59 75–83. [DOI] [PubMed] [Google Scholar]
- Horak, J., Grefen, C., Berendzen, K.W., Hahn, A., Stierhof, Y.D., Stadelhofer, B., Stahl, M., Koncz, C., and Harter, K. (2008). The Arabidopsis thaliana response regulator ARR22 is a putative AHP phospho-histidine phosphatase expressed in the chalaza of developing seeds. BMC Plant Biol. 8 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison, C.E., Li, J., Argueso, C., Gonzalez, M., Lee, E., Lewis, M.W., Maxwell, B.B., Perdue, T.D., Schaller, G.E., Alonso, J.M., Ecker, J.R., and Kieber, J.J. (2006). The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell 18 3073–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, I., and Sheen, J. (2001). Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413 383–389. [DOI] [PubMed] [Google Scholar]
- Jacobs, W.P. (1952). The role of auxin in differentiation of xylem around a wound. Am. J. Bot. 39 301–309. [Google Scholar]
- Kakimoto, T. (1996). CKI1, a histidine kinase homolog implicated in cytokinin signal transduction. Science 274 982–985. [DOI] [PubMed] [Google Scholar]
- Kim, H.J., Ryu, H., Hong, S.H., Woo, H.R., Lim, P.O., Lee, I.C., Sheen, J., Nam, H.G., and Hwang, I. (2006). Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis. Proc. Natl. Acad. Sci. USA 103 814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, J.H., Han, K.H., Park, S., and Yang, J. (2004). Plant body weight-induced secondary growth in Arabidopsis and its transcription phenotype revealed by whole-transcriptome profiling. Plant Physiol. 135 1069–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurakawa, T., Ueda, N., Maekawa, M., Kobayashi, K., Kojima, M., Nagato, Y., Sakakibara, H., and Kyozuka, J. (2007). Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445 652–655. [DOI] [PubMed] [Google Scholar]
- Mähönen, A.P., Bishopp, A., Higuchi, M., Nieminen, K.M., Kinoshita, K., Tormakangas, K., Ikeda, Y., Oka, A., Kakimoto, T., and Helariutta, Y. (2006. b). Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science 311 94–98. [DOI] [PubMed] [Google Scholar]
- Mähönen, A.P., Bonke, M., Kauppinen, L., Riikonen, M., Benfey, P.N., and Helariutta, Y. (2000). A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Genes Dev. 14 2938–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mähönen, A.P., Higuchi, M., Tormakangas, K., Miyawaki, K., Pischke, M.S., Sussman, M.R., Helariutta, Y., and Kakimoto, T. (2006. a). Cytokinins regulate a bidirectional phosphorelay network in Arabidopsis. Curr. Biol. 16 1116–1122. [DOI] [PubMed] [Google Scholar]
- Matsumoto-Kitano, M., Kusumoto, T., Tarkowski, P., Kinoshita-Tsujimura, K., Vaclavikova, K., Miyawaki, K., and Kakimoto, T. (2008). Cytokinins are central regulators of cambial activity. Proc. Natl. Acad. Sci. USA 105 20027–20031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford, J.I., Horgan, R., El-Sawi, Z., and Klee, H.J. (1989). Alterations of endogenous cytokinins in transgenic plants using a chimeric isopentenyl transferase gene. Plant Cell 1 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki, K., Matsumoto-Kitano, M., and Kakimoto, T. (2004). Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: Tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J. 37 128–138. [DOI] [PubMed] [Google Scholar]
- Müller, B., and Sheen, J. (2008). Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 453 1094–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, A., Kakimoto, T., Imamura, A., Suzuki, T., Ueguchi, C., and Mizuno, T. (1999). Biochemical characterization of a putative cytokinin-responsive His-kinase, CKI1, from Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 63 1627–1630. [DOI] [PubMed] [Google Scholar]
- Nieminen, K., et al. (2008). Cytokinin signaling regulates cambial development in poplar. Proc. Natl. Acad. Sci. USA 105 20032–20037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, C., Ohashi, Y., Sato, S., Kato, T., Tabata, S., and Ueguchi, C. (2004). Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell 16 1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernisova, M., Klima, P., Horak, J., Valkova, M., Malbeck, J., Soucek, P., Reichman, P., Hoyerova, K., Dubova, J., Friml, J., Zazimalova, E., and Hejatko, J. (2009). Cytokinins modulate auxin-induced organogenesis in plants via regulation of the auxin efflux. Proc. Natl. Acad. Sci. USA 106 3609–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischke, M.S., Jones, L.G., Otsuga, D., Fernandez, D.E., Drews, G.N., and Sussman, M.R. (2002). An Arabidopsis histidine kinase is essential for megagametogenesis. Proc. Natl. Acad. Sci. USA 99 15800–15805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge, M.J., Otsuga, D., Alonso, J.M., Ecker, J.R., Drews, G.N., and Clark, S.E. (2005). Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell 17 61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs, T. (2000). Integrating cellular and organismic aspects of vascular differentiation. Plant Cell Physiol. 41 649–656. [DOI] [PubMed] [Google Scholar]
- Samuelson, M.E., Eliasson, L., and Larsson, C.M. (1992). Nitrate-regulated growth and cytokinin responses in seminal roots of barley. Plant Physiol. 98 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, G.E., Ladd, A.N., Lanahan, M.B., Spanbauer, J.M., and Bleecker, A.B. (1995). The ethylene response mediator ETR1 from Arabidopsis forms a disulfide-linked dimer. J. Biol. Chem. 270 12526–12530. [DOI] [PubMed] [Google Scholar]
- Scheres, B., Dilaurenzio, L., Willemsen, V., Hauser, M.T., Janmaat, K., Weisbeek, P., and Benfey, P.N. (1995). Mutations affecting the radial organization of the Arabidopsis root display specific defects throughout the embryonic axis. Development 121 53–62. [Google Scholar]
- Surette, M.G., Levit, M., Liu, Y., Lukat, G., Ninfa, E.G., Ninfa, A., and Stock, J.B. (1996). Dimerization is required for the activity of the protein histidine kinase CheA that mediates signal transduction in bacterial chemotaxis. J. Biol. Chem. 271 939–945. [DOI] [PubMed] [Google Scholar]
- Szekeres, M., Nemeth, K., Koncz-Kalman, Z., Mathur, J., Kauschmann, A., Altmann, T., Redei, G.P., Nagy, F., Schell, J., and Koncz, C. (1996). Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85 171–182. [DOI] [PubMed] [Google Scholar]
- Takei, K., Ueda, N., Aoki, K., Kuromori, T., Hirayama, T., Shinozaki, K., Yamaya, T., and Sakakibara, H. (2004). AtIPT3 is a key determinant of nitrate-dependent cytokinin biosynthesis in Arabidopsis. Plant Cell Physiol. 45 1053–1062. [DOI] [PubMed] [Google Scholar]
- To, J.P., Haberer, G., Ferreira, F.J., Deruere, J., Mason, M.G., Schaller, G.E., Alonso, J.M., Ecker, J.R., and Kieber, J.J. (2004). Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell 16 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomomori, C., et al. (1999). Solution structure of the homodimeric core domain of Escherichia coli histidine kinase EnvZ. Nat. Struct. Biol. 6 729–734. [DOI] [PubMed] [Google Scholar]
- Vitha, S., Baluska, F., Braun, M., Samaj, J., Volkmann, D., and Barlow, P.W. (2000). Comparison of cryofixation and aldehyde fixation for plant actin immunocytochemistry: Aldehydes do not destroy F-actin. Histochem. J. 32 457–466. [DOI] [PubMed] [Google Scholar]
- Walter, M., Chaban, C., Schutze, K., Batistic, O., Weckermann, K., Nake, C., Blazevic, D., Grefen, C., Schumacher, K., Oecking, C., Harter, K., and Kudla, J. (2004). Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40 428–438. [DOI] [PubMed] [Google Scholar]
- Werner, T., Kollmer, I., Bartrina, I., Holst, K., and Schmulling, T. (2006). New insights into the biology of cytokinin degradation. Plant Biol. 8 371–381. [DOI] [PubMed] [Google Scholar]
- Werner, T., Motyka, V., Laucou, V., Smets, R., Van Onckelen, H., and Schmulling, T. (2003). Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15 2532–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, H., Suzuki, T., Terada, K., Takei, K., Ishikawa, K., Miwa, K., Yamashino, T., and Mizuno, T. (2001). The Arabidopsis AHK4 histidine kinase is a cytokinin-binding receptor that transduces cytokinin signals across the membrane. Plant Cell Physiol. 42 1017–1023. [DOI] [PubMed] [Google Scholar]
- Yang, J., Zhang, J., Wang, Z., Zhu, Q., and Wang, W. (2001). Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol. 127 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, Z.H., Freshour, G., Hahn, M.G., Burk, D.H., and Zhong, R. (2002). Vascular development in Arabidopsis. Int. Rev. Cytol. 220 225–256. [DOI] [PubMed] [Google Scholar]
- Yokoyama, A., Yamashino, T., Amano, Y., Tajima, Y., Imamura, A., Sakakibara, H., and Mizuno, T. (2007). Type-B ARR transcription factors, ARR10 and ARR12, are implicated in cytokinin-mediated regulation of protoxylem differentiation in roots of Arabidopsis thaliana. Plant Cell Physiol. 48 84–96. [DOI] [PubMed] [Google Scholar]
- Zhao, C., Craig, J.C., Petzold, H.E., Dickerman, A.W., and Beers, E.P. (2005). The xylem and phloem transcriptomes from secondary tissues of the Arabidopsis root-hypocotyl. Plant Physiol. 138 803–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.