Abstract
Drug-induced depression has been the focus of intense scrutiny by the US Food and Drug Administration and has serious clinical and medicolegal implications. “Gold standard” studies of drug-induced depression—involving randomized, placebo-controlled design and direct assessment of depressive symptoms—are lacking. Based on the available literature, our review suggests that only a few types of drugs are strongly linked with induction of depression. However, the potential for idiosyncratic reactions—not necessarily detected in large-scale studies—suggests that particular caution and careful monitoring are warranted with several types of drugs, including isotretinoin, rimonabant, and alpha interferons.
Keywords: drug-induced depression, iatrogenic depression, depressogenic, depressogenic drugs, secondary depression
Introduction
In the past two years alone, the US Food and Drug Administration (FDA) has issued alerts concerning suicidal ideation linked to the drug varenicline (Chantix®)1 as well as to numerous antiepileptic drugs.2 Meanwhile, the antiobesity drug rimonabant (Accomplia®)—not yet available in the US—was given a vote of no confidence by an FDA advisory panel,3 owing in part to the drug’s association with suicidality. All this occurs against the backdrop of intense controversy surrounding newer antidepressants and their possible association with increased suicidal ideation in a small percentage of younger patients.4
The notion of a “depressogenic” drug is hardly new to medical practitioners. More than a half century ago, Freis5 first reported on “mental depression” in association with the antihypertensive drug, reserpine. And in his classic, Anatomy of Melancholy (1621), the English scholar Robert Burton identified alcohol as one cause of melancholy. Indeed, if alcohol is considered a drug, the concept of drug-induced depression (DID) may be traced to antiquity: In the Old Testament, for example, we read: “Who has woe? Who has sorrow? …Those who tarry long over wine…” (Proverbs 23:29–30).
In our own time, numerous medications and classes of medications have been implicated in DID, sometimes called substance-induced depression or drug-related depression. DID has important medical, medicolegal, and commercial implications. Any physician who has observed steroid-related mood swings—either mania or depression—knows that DID can drastically affect a patient’s clinical course. For example, one of us (R.P.) reported a case in which a young woman appeared to develop persistent bipolar mood swings after a single course of corticosteroids for treatment of ulcerative colitis.6
The legal implications of alleged or apparent DID are also becoming clear. It is now easy to find online advertisements from law firms with solicitations such as, “If you or a loved one took [antidepressant x] and suffered side effects, please fill out the form at the right for a free case evaluation by a qualified drug side effects attorney.”7 It is obvious that pharmaceutical firms stand to suffer major financial consequences from lawsuits alleging that their product “caused” a patient to become suicidal. As we will see, however, the task of establishing a causal link between a drug and a patient’s subsequent depression or suicide is daunting.
In this paper, we review DID in relation to drugs used mainly in primary care and internal medicine. We do not cover the burgeoning area of depression or “suicidality” in relation to antidepressants or to other agents with FDA labeling for use in psychiatric disorders (e.g., antipsychotics, anxiolytics, and mood-stabilizers, including anticonvulsants). The reader is referred to recent reviews of depression and other putative side effects associated with psychotropic agents.8–10 That said, there is substantial overlap in the pharmacological “territories” now covered by psychiatrists, family practitioners, internists, and neurologists. Most antidepressant prescriptions, for example, are written by primary care physicians (PCP), not psychiatrists.11 And, not uncommonly, psychiatrists nowadays are prescribing agents usually associated with neurologists, such as anticonvulsants or drugs for neuropathic pain.
In this paper, we first review methodological issues in assessing DID; the epidemiology of DID; and hypotheses concerning the pathophysiology of DID. We then review, by general class or specific agent, the medications associated with DID. We attempt to categorize the data according to “strength of evidence,” and conclude with some practical recommendations for PCPs and other physicians.
Methodological Problems, “Causality,” and Levels of Evidence
Let’s say that Dr. Jones prescribes a beta-blocking antihypertensive agent for Mr. Smith. A month later, Mr. Smith complains that he is “feeling really down lately,” lacks energy, and is having some difficulty with sexual performance. It is understandable that Dr. Jones might implicate the recently prescribed beta blocker in the genesis of Mr. Smith’s complaints. But suppose Mr. Smith has a history of recurrent major depression—with eight previous episodes over the past 12 years, all characterized by low mood, anergia, and loss of libido. Now the culprit is not so clear, and the risk of post-hoc fallacy (“after this drug, therefore because of this drug”) is very real. Indeed, as we shall see, epidemiologic studies of beta blockers do not clearly implicate them in DID, notwithstanding many suggestive case reports to the contrary.12 On the other hand, a recent review concluded that, “Evidence from case reports should be carefully considered when relevant, randomized, controlled trials have not been adequately designed to detect adverse effects.”13 Indeed, large epidemiological studies and meta-analyses cannot always reveal the “outlier” who has an idiosyncratic—but quite genuine—depressive reaction to a drug.
The Naranjo causality scale14 for adverse drug reactions (Figure 1) is one instrument that can assist clinicians in assessing the likelihood that a drug is responsible for the onset of depressive or suicidal ideation.
FIGURE 1.
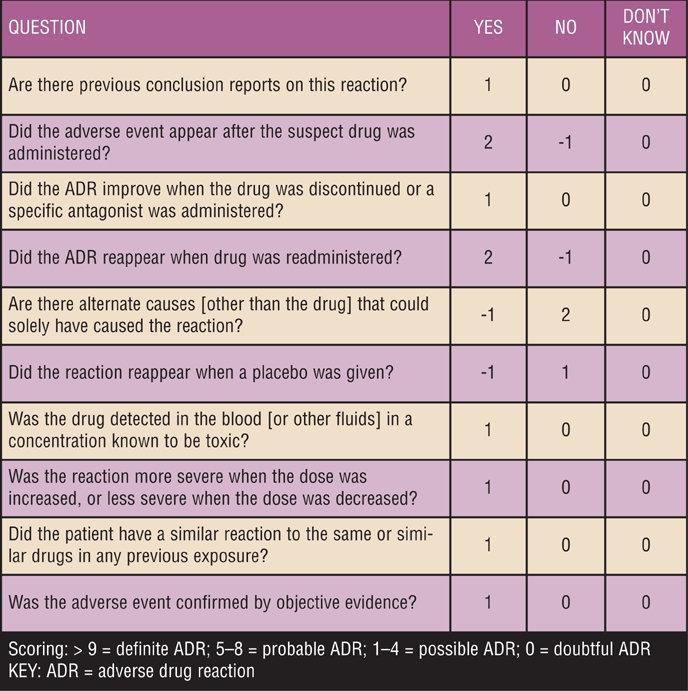
Naranjo causality scale
Another factor in assessing the likelihood of a drug’s depressogenic effect is the type and quality of studies used in the determination. The general principles of “evidence-based medicine” hold that the evidentiary value of clinical and experimental data should be judged hierarchically. Thus, the randomized, prospective, double-blind, placebo-controlled study is usually held up as the “gold standard” of evidence.15 Meta-analyses, uncontrolled studies (including observational epidemiological studies), and case series are considered lower-level evidence, with the lowly, single-case report occupying the bottom rung. In practice, ethical concerns virtually prohibit the use of controlled studies as a means of assessing drug side effects. Dhondt summarizes the predicament as follows:
“The association of medication use with depression is not synonymous with a causal relation. Because of the multifactorial origins of depression, what is needed is a study on the etiology of depression taking into account as many possible other etiological factors. Ideally, the associations found are confirmed in prospective studies. To obtain the strongest evidence for a causal relationship between medication use and depression, randomized, controlled trials would be required. However, as ethical constraints prohibit such studies, observational epidemiological research, including health and disability factors, will probably provide the best possible evidence.15
As Dhondt describes in detail, pharmacoepidemiological research “…merges the concerns of pharmacology with those of epidemiology” by using both internal and external morbidity data. One type of internal morbidity data entails linking the use of drug A with a “rescue” drug B. For example, Thiessen et al16 demonstrated that a greater proportion of patients prescribed propranolol than prescribed other beta blockers received an antidepressant. In this model, antidepressant use is posited as a “marker” for depressive symptoms. External morbidity data entails linkage of medication records and disease records. For example, the incidence of a clinical diagnosis of depression is compared between a cohort of patients who used drug A and those who used drug B (or placebo): If the incidence of depression is higher with drug A, this is considered presumptive evidence of the drug’s depressogenic effect.
In this paper, we consider randomized, controlled trials, pharmacoepidemiological studies, and large-scale meta-analyses to be at the top of the evidence hierarchy. We consider case series data (e.g., three or more patients in the same report) and single-case reports as lower levels of evidence. Such hierarchies, while useful for research purposes, are no substitute for clinical judgment. For example, which of the following “counts” as stronger evidence: two pharmacoepidemiologic studies finding no association between drug A and depression or 85 case reports clearly linking drug A with onset of depression? In the absence of randomized, controlled, prospective studies, the clinician is left to use his or her best judgment. Indeed, our tabular listing of drug classes and their evidentiary link with DID (Table 2) merely reflects our best clinical judgment of the data.
TABLE 2.
Evidence for DID associated with drug groups
| DRUG CLASS/DRUG |
LEVEL OF EVIDENCE |
AVAILABLE LITERATURE |
COMMENTS |
REFERENCES |
|---|---|---|---|---|
| **Reflects authors’ global assessment of evidence; --- little or no convincing evidence; +/- limited evidence; + moderately strong evidence; ++ strong evidence; +++ very strong/unequivocal evidence | ||||
KEY
| ||||
| Beta blockers | +/- | Case reports, RCTs, large scale meta-analyses | Evidence is conflicting—propranolol may have the strongest association with symptoms after starting or increasing dose. | 13, 16, 30–41 |
| Calcium channel blockers | +/- | Case reports, case series, prescription symmetry analysis, cohort study examining suicide rates | Results are conflicting—newer agents have fewer reports. | 42–48 |
| ACE inhibitors | +/- | Prescription symmetry analysis | Some reports have found antidepressant effects. | 46, 49–51 |
| ARBs | +/- | Case reports | Preliminary data suggest some ARBs may have antidepressant effects. | 52, 53 |
| Antiobesity drugs: rimonabant, taranabant | ++ | Case reports, meta-analyses | Neither agent approved in US. Taranabant is no longer being developed due to psychiatric side effects. | 54–56 |
| Alpha interferons | ++ | Uncontrolled and controlled studies | No comment | 57–61 |
| Beta interferons | --- | 4 RCTs and 1 naturalistic study | No comment | 62–67 |
| Finasteride | + | Case series, prospective noncontrolled trial | Evidence of DID only exists for men treated for alopecia; no evidence for BPH, but caution warranted, given high doses | 69, 70 |
| Isotretinoin | +++ | Over 400 case reports, prescription symmetry analysis, case-crossover study | No comment | 71–74 |
| Progesterone inserts (Norplant®) | + | Case reports, case series, large trials | Large trials suggest that women with higher baseline depressive scores may be at risk as well as women with relationship dissatisfaction | 75–81 |
| Leukotriene antagonists (montelukast) | +/- | Collection of single case reports; 3 double-blind RCTs | Conflicting data—cases suggest association; recent analysis of 3 RCTs found no association | 82–84 |
| Corticosteroids | + | Case control study, cross-sectional analysis | Results of trials are suggestive of DID, especially in patients aged >65, but not conclusive | 85–88 |
| Varenicline | + | Case reports, case series | Enhanced FDA warnings—banned by the FAA; increased anxiety reported in 1 placebo RCT. | 89–91, 92 |
Epidemiology of DID
To our knowledge, there are no large-scale, epidemiological data directly answering the question, “What is the incidence and prevalence of DID?” in a specific population.17 To answer this question, we would need not only clear and reliable criteria for DID, but also a very large cohort of subjects and a valid method of assessing them for DID. These requirements have not yet coalesced. It is instructive, however, to note the findings of the 1988 study by Maricle et al,18 in which a community sample of 40 elderly subjects were followed up an average of two and one-half years after a diagnosis of depression. Nine subjects were identified with depressive syndromes associated with depressogenic medication—nearly a quarter of the original sample.
According to Turner from the FDA’s Division of Neuropharmacologic Drug Products, there is a “lack of consensus” on the scope of DID.19 Nonetheless, a cross-sectional, population-based study of 2,646 elderly subjects in the Netherlands yielded some relevant findings.20 Using a combination of structured interviews for depression and careful determination of medication use, the researchers were able to determine the Population Attributable Risk percentage (PAR%) for various drug classes. The PAR% is not a direct measure of incidence or prevalence; rather, it is a quantitative estimate of the proportion of disease in the population that is directly attributable to a particular risk factor.21 The authors concluded that the PAR% for nonselective beta blockers is 2.5 percent, for calcium antagonists five percent; for benzodiazepines 15.42 percent; and for systemic corticosteroids, 2.95 percent. If these figures are at all representative of actual DID prevalence, we are dealing with a significant public health problem in the elderly and perhaps in younger populations as well.
Putative Pathophysiology of DID
Given the plethora of drugs implicated in DID, there is no reason to presume that all drugs produce depression via a common pathophysiological mechanism. And, in as much as the pathophysiology of “ordinary” or idiopathic depression remains uncertain, it would be presumptuous to make confident claims regarding the genesis of DID. Nonetheless, specific depressogenic drugs may induce depression via mechanisms that have been investigated in other contexts. The classic example is that of reserpine, perhaps the first putative depressogenic drug to be the focus of a professional journal report.5 Since in-vitro investigations have shown reserpine to be an “amine-depleting agent,” it was plausible to hypothesize that reserpine induces depression by reducing neuronal stores of biogenic amines. However, this plausible notion has recently been criticized by Baumeister et al22 who have argued that reserpine is not depressogenic and that “the reason for perpetuation of this myth is reluctance to discard the monoamine hypothesis.” Nonetheless, reduction or depletion of biogenic amines continues to be adduced as one possible mechanism for DID and has by no means been ruled out.17 Other putative mechanisms20,23–25 for DID are listed in Table 1, but must be considered highly speculative at this time. This uncertainty is brought home by the observation that one and the same drug—e.g., dexamethasone or varenicline—may be associated with either depressive or manic-like symptoms.25 Finally, the development of DID appears to be more likely in a person who has a predisposition to depression.17
TABLE 1.
Some possible mechanisms of drug-induced depression
| DRUG OR DRUG CLASS |
POSSIBLE MECHANISM FOR DID |
|---|---|
| Nifedipine, other calcium channel blockers | Block slow influx of calcium into the cell, inhibiting calcium-dependent neurotransmitter release and reducing neurotransmitter amplification through the second-messenger system20 |
| Benzodiazepines | Based on rodent studies: decreased release of serotonin in hippocampus (except with alprazolam)23 |
| Exogenous corticosteroids | Based on rodent development studies: dexamethasone administration leads to deficits in the number and size of neural cells; reduced function of G-protein-coupled catecholaminergic or cholinergic receptors24 |
| Varenicline | Displaces nicotine from acetylcholine receptors, produces low-to-moderate levels of dopamine release, and stimulates mesolimbic dopamine system. May upset the balance in cholinergic-adrenergic tone potentially leading to depression or mania25 |
Drugs and DID: Where the Evidence Lies
We have identified more than a dozen classes of medications with putative depressive effects. Some have provoked official alerts from the FDA, whereas others have elicited isolated case-reports of DID. A listing of the agents is presented in Table 2, where we also indicate the level of evidence associated with each class. One should note that the level of evidence may vary within a therapeutic class. This may in part be explained by differences in pharmacokinetic parameters among the drugs within a given class. For example, lipophilic drugs are able to penetrate the blood brain barrier more readily than hydrophilic compounds and as a result may have more profound central nervous system (CNS) effects.
Much of the literature comes by way of case reports, but some is available from large-scale trials in which depression was assessed. The variations in identification of a DID in these trials is worth mentioning. Certain reports extrapolate from patients on both drug X and an antidepressant (prescription symmetry), whereas others actually screen for depressive symptoms at various times during the study. One of the most rudimentary and questionable assessment methods uses the question, “Have you ever felt depressed?”26 Thus, one must carefully examine such screening methods before associating any drug with the development of DID.
Antihypertensives
Beta blockers. One of the most touted yet controversial claims regarding DID implicates beta-1 receptor antagonists, better known as beta blockers. These agents have long been recognized for their ability to reduce morbidity and mortality in patients with hypertension27 and are also used for heart failure and arrhythmias. Unfortunately, this class of medication is often underutilized, possibly owing to concerns about side effects and tolerability.28 Thus, both medical references and patient-education information often include depression as a potential adverse reaction. Interestingly, some agents within the class (e.g., pindolol)29 have also been studied as augmenting agents in the treatment of depression, suggesting that DID may not be associated with all beta blockers.
The oldest citation implicating a beta blocker and DID is from a 1967 letter to the editor of the British Medical Journal.30 It was suggested that a high incidence of depression (30%) was seen in a series of 89 patients receiving propranolol. Two of the 27 patients exhibiting depression completed suicide and two others required antidepressant therapy. These findings were immediately disputed31 and so the controversy began.
Since the original 1967 report, numerous case reports16,32–35 have been published and several randomized trials36–40 have investigated depression as a side effect of many beta blockers. Although the number of studies reporting DID appears convincing, a large-scale meta-analysis conducted by Ko et al41 did not support the association. In this study, seven trials and more than 10,500 patients were evaluated. These authors concluded that the overall incidence of depression was comparable to placebo (20.1% vs. 20.5%).
In a separate multistudy analysis, Steffensmeier et al13 conducted a review of the literature on beta blockers and DID. Using the Naranjo algorithm, they found that DID was most likely to be evident soon after a dose increase; conversely, DID symptoms subsided when the dose was reduced. In addition, the highly lipophilic agent, propranolol, was the beta blocker most frequently implicated in DID, perhaps pointing to CNS penetration as an important variable.13
Large-scale, controlled, and statistically analyzed data suggest that beta blockers may not be as strongly linked to DID as previously thought. Moreover, in evaluating the literature, clinicians need to consider the generation of each beta blocker as well as its lipophilicity and receptor selectivity. Propranolol and timolol are considered first- or early- generation agents, and propranolol, carvedilol, and bucindolol are highly lipophilic. Greater lipophilicity allows greater penetration of the blood brain barrier; in theory, this might predict a greater risk of DID. Propranolol, nadolol, timolol, and pindolol are all nonselective beta blockers, demonstrating equal affinity for both beta1- and beta2-receptors; whereas, metoprolol has greater activity at beta-1 receptors. Compared to selective agents, nonselective beta blockers exert a wider variety of extracardiac manifestations; however, there are insufficient controlled data to determine whether beta selectivity, per se, is associated with greater risk of DID.
It is also important to recognize that propranolol was the first marketed beta blocker. The use of propranolol as a first-line agent has significantly declined with the availability of more cardioselective agents (e.g., metoprolol for the treatment of heart failure).
Given the conflicting data, we believe that the original concern from 1967 is not warranted for the entire beta blocker class. Physicians should not be hesitant to use these medications when appropriate. Nevertheless, individual patients may show idiosyncratic depressive reactions to a given beta blocker, and clinicians concerned about DID should exercise particular caution during initial dosing and after any dosage increase. Dosage reduction should be considered before discontinuation. If discontinuation is necessary, beta blockers should generally be tapered off gradually, to avoid “rebound” tachycardia or hypertension.
Calcium channel blockers. Like beta blockers, calcium channel blockers (CCBs) are an extremely useful class of medication in certain patient populations. CCBs have approved indications for multiple cardiovascular conditions, including hypertension, angina, and arrythmias, as well as for migraine prophylaxis. And, like beta blockers, CCBs have been used in patients with various psychiatric disorders, such as bipolar disorder and panic disorder.
Very few well-designed studies implicate CCBs in DID, though two separate case series (N=6) suggest nifedipine as a potential offender.42,43 Two of the depressed patients, one from each report, were resistant to treatment with nortriptyline; and, in all six patients, resolution of depression occurred when nifedipine was discontinued. Applying the Naranjo algorithm to these case reports, nifedipine would receive a score of 5, suggesting a probable association. Case reports also exist for diltiazem and verapamil.44,45
In 1996, Hallas performed an epidemiologic study examining the depression-provoking effects of various cardiovascular medications.46 The author screened more than 11,000 patients started on various cardiovascular medications and concomitant antidepressants in a predefined period. It was expected that if cardiovascular drugs did not cause DID, then the number of patients starting either class of drugs first would be equal. Of all the cardiovascular medication classes examined, including beta blockers, diuretics, nitrates, and digoxin, only CCB and angiotensin-converting enzyme inhibitors (ACE inhibitors) appeared to have a depression-provoking effect. Although somewhat suggestive, this type of analysis is questionable since it does not directly assess depressive symptoms before and during therapy.
A follow-up investigation was performed two years later and examined suicide rates and the use of various cardiovascular medications including diuretics, beta blockers, ACE inhibitors, nitrates, and calcium channel blockers.47 Approximately 3,400 patients were included in the cohort, of which 18.2 percent were classified as users of CCBs. The only class not found to have a positive correlation was the ACE inhibitors. However, after statistical adjustments for the rates of use among the classes, only CCBs were found to have a statically significant positive correlation (i.e., increased risk of suicide). The authors concluded that the absolute risk of CCB use and suicide was 1.1 suicides per 1,000-person years.
Contrary to the above investigations, Dunn et al48 found no evidence of DID in patients treated with diltiazem and nicardipine, compared with individuals who were not receiving these drugs in general practice settings. This evaluation may be considered stronger than those reviewed above in that it screened for depression based on the general practitioners’ diagnoses over a five-year period. The authors do point out that depression may be under-diagnosed, but this would presumably be true in the control groups as well.
In summary, given the variations in methodology and the conflicting results, the evidence for CCBs causing a DID is limited. As with beta blockers, newer agents are now available, which may be less frequently associated with a DID.
ACE inhibitors. The Hallas study46 did find a significant positive correlation between ACE inhibitor use and concomitant antidepressant prescribing. However, this study did not directly assess for depression, and the method of prescription sequence symmetry may not truly assess DID. Furthermore, case reports, case series, and an open-label trial have actually found certain ACE inhibitors effective in the treatment of major depression.49–51 Accordingly, we would conclude that, based on such limitations in study design, there is only limited evidence linking ACE inhibitors with DID.
Angiotensin II blockers (ARBs). The angiotensin II blockers valsartan and losartan are generally reserved for patients who cannot tolerate or are resistant to ACE inhibitor therapy. As with the ACE inhibitors, the link between ARBs and DID is weak. A single case report of valsartan-induced depression and attempted suicide was reported in a 43-year-old female patient.52 The patient was also taking atenolol and the diuretic hydrochlorthiazide at the time of evaluation; however, the regimen of valsartan and hydrochlorothiazide was implicated in DID, since it was initiated four weeks prior to the suicide attempt. Upon discontinuation of the valsartan and hydrochlorthiazide, the symptoms resolved. Although a weak association may exist, others have found losartan to possess antidepressant-like effects in mice.53 In short, the evidence implicating ARBs in DID is based on a single case report. Future research may be geared to examining these agents as antidepressants, not depressogenics.
Antiobesity Agents
Rimonabant, a cannabinoid antagonist and antiobesity agent, is available in numerous countries, but did not receive approval from the FDA. Rimonabant’s utility as an antiobesity agent may be limited by its high incidence of psychiatric adverse events. In a meta-analysis, Christensen et al54 found that, compared to those taking placebo, patients treated with rimonabant were 2.5 times more likely to discontinue therapy secondary to depression. The FDA also reviewed the same studies included in this meta-analysis and concluded that 26 percent of rimonabant-treated patients were more likely to have an adverse psychiatric event. Suicidal ideation and/or attempt were almost twice as likely.55 Further strengthening the concern for these agents was the decision by Merck to halt the development of the related agent, taranabant.56 They did so based on psychiatric side effects, including depression, experienced by patients. Thus, the evidence linking cannabinoid antagonists to DID appears strong.
Antivirals
The clinically used interferons are classified as alpha or beta. Alpha interferons are used in the treatment of hepatitis C as well as various forms of cancer; whereas, beta interferons are used in the treatment of multiple sclerosis. Surprisingly, the literature suggests different levels of DID risk associated with the two types of interferon.
Alpha interferons. The alpha interferons (2a and 2b) have been associated with depression in many uncontrolled and controlled investigations.57–61 The incidence of depression in interferon-treated hepatitis C patients ranges widely from 3 to 50 percent. Much of this discrepancy is due to the variations in screening and to the individual evaluator (i.e., psychiatrist vs. gastroenterologist). The most recent prospective, open-label investigation60 found that approximately 33 percent of interferon-treated patients developed new-onset depression after 12 weeks. Similar studies have reported essentially the same findings. It is hypothesized that interferon alpha therapy causes neurotransmission abnormalities in the basal ganglia and limbic system. Screening for depression before and during interferon alpha therapy is recommended and should be performed by a psychiatric practitioner if possible. Based on our overall assessment of the evidence, we conclude that the link between alpha interferon and DID is strong.
Beta interferons. The literature is less convincing in demonstrating an association between DID and beta interferons, and clinicians should avoid extrapolating from data on alpha interferons. Although one randomized, controlled trial suggested depression as a side effect of beta interferons,62 three randomized, controlled trials and the work from the European Study Group have failed to validate such findings.63–66 Antidepressant use with interferons and glatiramer, a non-interferon used for multiple sclerosis, was also assessed.67 This study was designed to mimic real-world practice rather than to use a tightly controlled design. Cohorts of patients choosing beta interferons or glatiramer were followed and monitored for depression. Depression scores at baseline and throughout the trial did not differ between groups. Thus, this naturalistic study further suggests that DID is not a significant concern with beta-interferon therapy.
Dermatologics
Finasteride. Finasteride is currently used in the treatment of benign prostatic hyperplasia (BPH) as well as androgenic (androgenetic) alopecia. All reports of finasteride-induced depression have involved its use in alopecia. Finasteride works primarily by blocking conversion of testosterone to dihydrotestosterone via inhibition of the 5-alpha reductase enzyme. Finasteride also inhibits the conversion of progesterone to dihydroprogesterone, which is subsequently converted to allopregnanolone—a neurosteroid with antiepileptic and anxiolytic properties. Changes in the levels of allopregnanolone have also been linked to depression.68
A case series of 19 (14 male and 5 female) out of 23 patients who developed moderate-to-severe depression during finasteride treatment for alopecia was published in 2002.69 In a confirmatory, prospective, noncontrolled trial, 128 men with androgenic alopecia were prescribed 1mg of finasteride daily and monitored for depressive and anxiety symptoms.70 After two months of therapy, finasteride was found to increase the depressive symptom scores for both the Beck Depression Inventory (BDI) and the Hospital Anxiety Depression Scale (HADS). Changes in anxiety scores were not significant. In the case series and prospective trial, the mean ages were 28.16 and 25.8 years, respectively, and the doses of finasteride were lower than that used for the treatment of BPH.
In our view, the available literature provides moderately strong evidence for a DID-finasteride association, especially in the treatment of alopecia. Although there is no literature for DID in men being treated for BPH, caution is still warranted, in that the doses for BPH are typically five times higher for this indication and are often used in an older population. On this basis, we would hypothesize that finasteride-induced depression may be more prevalent than currently reported.
Isotretinoin. Between 1982 and 2000, the FDA received approximately 400 reports of depression and 37 of suicide for patients who had received the acne medication isotretinoin.71,72 The FDA issued enhanced warnings in 2005 based upon these postmarketing reports. In April of 2008, Azoulay et al73 published their findings from a case-crossover study, which examined 19 years worth of prescription data from Quebec. Patients who received isotretinoin during this period were identified, as were cases of patients who received new diagnoses for depression, underwent hospitalization for depression, or filled a prescription for an antidepressant. The authors found that the adjusted relative risk for DID with isotretinoin was 2.68. Although a previously conducted study using prescription sequence symmetry alone reported no increase in antidepressant use with isotretinoin versus minocycline,74 the Azoulay et al73 investigation is the first and only controlled (crossover design) study of this question. Given that this study did find a statistical association between isotretinoin and DID and considering the data from more than 400 case reports of the FDA, we conclude that the evidence linking isotretinoin and DID is very strong.
Hormonal (Contraceptives)
In 1999, the manufacturer of the birth-control agent, Norplant® (a formulation of levonorgestrel) agreed to pay a settlement of $50 million in a claim filed by more than 36,000 women.75 The suit claimed that the company had downplayed side effects, such as irregular menstrual bleeding, headaches, nausea, and depression. Prior to this settlement, a 1988 study randomizing women to Norplant or Norplant-2 reported the second most common reason for discontinuation as depression.76 In 1994 and 1996, Wagner cited two separate reports of Norplant-induced major depression.77,78 The first was a case report of two women with no prior psychiatric history who developed major depression and panic disorder within two months after Norplant was inserted.77 Upon removal of the implants, the symptoms resolved within one month. The second was a case series78 in which Wagner presented the cases of five women with no psychiatric history who developed major depression 1 to 3 months after Norplant insertion. Again, the depressive symptoms resolved 1 to 2 months after removal.
Contrary to the above reports, Westhoff et al79 described the results of a multicenter, prospective investigation evaluating depression in association with Norplant. Unlike the previous studies, this study evaluated depressive symptom scores at baseline and at six and 24 months. Of 910 women evaluated, 138 were lost to follow up and 295 had discontinued use. However, only 4.4 percent of the 295 had done so because of mood changes. The remaining women still using the implant were reported to have had lower depressive scores at baseline than those who discontinued the medication. In addition, for those users that experienced an increase in depressive score at the end of two years, it was found that relationship dissatisfaction was a strong predictor of higher scores. The authors concluded that Norplant use does not exacerbate pre-existing depression.
In a study of 212 postpartum teenage mothers, depressive symptoms were monitored during the first 12 months after Norplant insertion.80 Three independent factors were found in those women who experienced increased depression scores: depression prior to Norplant; a new boyfriend at time of delivery; and late Norplant insertion (>4 weeks after giving birth). These researchers concluded that insertion within the first four weeks after birth did not exacerbate depression, and delaying insertion might actually increase the risk of postpartum depression. It is interesting to note that 2 of the 3 factors are similar to those suggested by Westhoff (higher depressive scores and potential for reduced relationship satisfaction).
In our view, these two large trials suggest that clinicians should screen for depressive symptoms prior to Norplant implantation. If depression scores are high in a given patient, this would not necessarily be a contraindication to use Norplant; rather, it would point to the need for effective treatment of the depression. Indeed, such treatment might avert unsupervised discontinuation of Norplant. The most recent piece of literature supports this approach.81 This study examined discontinuation and depression scores in women who received Norplant for treatment of menorrhagia. The authors found that depressive symptoms, based on Beck’s Depression Inventory measured six months after the beginning of the treatment, were related to discontinuation of Norplant [Odds ratio (OR)=3.70]. The authors opined that diagnosing and treating depression among patients having menstrual problems may improve adherence to Norplant treatment.
Considering all the (sometimes conflicting) data, we conclude that the association of Norplant and DID is only moderately strong. Clinicians should screen all patients prior to initiating therapy and again some months after implantation. Women with lower depression symptom ratings and those who are in stable relationships may be less likely to discontinue Norplant, secondary to depression. Some women may benefit from either antidepressant medication or psychotherapy, if screening points to clinically significant depressive symptoms. In any case, the evidence does not demonstrate a strong association between Norplant and DID.
Respiratory Agents (Leukotriene Inhibitors)
Leukotriene inhibitors comprise a new class of medications for the treatment of persistent asthma. In September of 2006, four case reports of depression were cited in the Netherlands Pharmacovigilance Centre Lareb for the leukotriene inhibitor montelukast.82 At that time, 43 cases were contained in the World Health Organizations database. Seven months later, depression was added as a side effect in the manufacturer’s labeling. The FDA has since issued an early communication letter noting that it was investigating reports of depression associated with this medication.83 Although the number of cases may suggest a relationship to DID, concomitant therapy with other treatments for asthma or seasonal allergic rhinitis (i.e., corticosteroids) cannot be dismissed.
Holbrook and Harik-Khan recently analyzed three randomized, controlled trials examining “emotional well being” in 1,356 asthma patients, 536 of whom were treated with montelukast.84 In the three trials, measures of “quality of life” were assessed using the Juniper Mini Asthma Quality of Life Questionnaire and the Juniper Paediatric Asthma Quality of life Questionnaire. The authors attempted to correlate these measures with recognized measures of depression, such as the Center for Epidemiological Studies Depression Scale (CES-D). The authors’ findings did not support an association between montelukast and adverse effects on emotional well being. They acknowledged that their quality-of-life instruments were not designed to evaluate depression directly, but noted that they found “…good correlation between our measures of well being and recognized measures of depression.”
Our overall assessment of the admittedly conflicting data is that the association between leukotriene inhibitors and DID is limited at this time.
Corticosteroids
The polymorphous psychiatric complications of corticosteroids have long been recognized. Symptoms of euphoria, depression, agitation, irritability, and psychosis are all potential side effects. Surprisingly, data confirming a direct causal link between corticosteroids and DID are limited and conflicting. When carefully evaluated, the three most frequently cited referenced articles in the literature85–87 do not clearly establish a causal link between corticosteroids and DID, in our judgment. Although one may agree that depression is evident in corticosteroid-treated patients, the ultimate question is whether the medication actually caused the depressive symptoms.
Two cohort studies involving medical inpatients have suggested an association between corticosteroids and depression.85,86 Both investigations identified the cases through self reporting of depressive symptoms. Gift et al85 evaluated 40 inpatients, 20 of whom were receiving corticosteroids and 20 of whom were not. Although the study found that depression was more frequent in the corticosteroid group, the small sample size and use of self reports are significant limitations of the study. The second investigation by Patten et al86 (which also used self reporting as identification of depression) also found more frequent depression in the corticosteroid group. However, the results did not achieve statistical significance (p=0.07). Interestingly, the same authors presented a study one year earlier evaluating the association between corticosteroid use and the development of a depressive disorder.87 They concluded that although depressive symptoms may be a side effect of corticosteroids, their use is not associated with an elevated risk of a depressive disorder diagnosis in hospitalized patients.
In contrast, in a cross-sectional analysis of 2,804 adults older than 55, Feng et al88 did find that corticosteroid use was more common among patients with depressive symptoms than among those without depressive symptoms (1.9% vs. 0.7%). Out of the total population included, 368 (13%) patients met the criteria for depressive symptoms based on the 15-item Geriatric Depression Scale (GDS). Of these 368 patients with depressive symptoms, only seven (1.9%) were receiving corticosteroids. Of the remaining 2,436 patients who did not have depressive symptoms, 16 were receiving corticosteroids (0.7%). The authors found that corticosteroid use was associated with depressive symptoms in the entire sample, although this association was of only marginal statistical significance. However, in participants aged 65 or older, systemic corticosteroid use was significantly associated with depressive symptoms (OR 4.02, 95% CI 1.12,14.42).
There is certainly abundant observational evidence that depressive symptoms are present in some patients treated with corticosteroids, perhaps especially those in older age groups. However, the evidence that corticosteroids are causally related to depression is no more than moderately strong, in our judgment.
Smoking Cessation Agents (Varenicline)
Two months after an early communication letter in November of 2007, the FDA issued an alert to the Warnings and Precautions sections for the smoking cessation agent varenicline (Chantix®), a partial agonist at the nicotinic receptor.89 Three months later, the Federal Aviation Administration banned this medication for pilots and air traffic controllers, suggesting that its neuropsychiatric effects could jeopardize public safety.90 These warnings came only two years after this agent received a “priority review” by the FDA.
The FDA and the manufacturer agreed to these warnings based on post-marketing case reports received by the FDA (the actual number of reports is not provided on the FDAs webpage89). These case reports—which are subject to a variety of reporting errors—described new-onset suicidal ideation and behavior in association with varenicline. Many of the symptoms developed within days to weeks after initiation. However, a recent case report describes a patient with a history of depression developing worsening symptoms six weeks after the initiation of varenicline.91 Given this report, it seems premature to assume that depressive symptoms will always occur within the first few weeks of therapy. One may argue that smoking cessation in itself may cause signs of depression; however, in the case report above, the patient was still smoking during his onset of symptoms. In addition, not all of the patients in the postmarketing data had discontinued smoking either.
To our knowledge, randomized, placebo-controlled studies of varenicline have generally not assessed depression in a systematic fashion. However, one such study by Tsai et al92 found that, compared with placebo, anxiety and abnormal dreams were significantly more common in the varenicline group [anxiety (5.6% vs 2.4%), abnormal dreams (5.6% vs 0.8%)], based solely on self reports.
Clearly, there is a pressing need, among varenicline-treated patients, for randomized, placebo-controlled studies that directly assess baseline and follow-up depression scores. In the absence of such studies or of large-scale meta-analyses implicating varenicline in DID—and without knowing the number of case reports in the FDA database—we would rate the evidence of a varenicline/DID link as only moderately strong. However, given current FDA warnings, clinicians should be cautious when prescribing this medication and seriously weigh the risks of precipitating or exacerbating depression in predisposed individuals.
Conclusion
Drug-induced depression is a significant clinical, medicolegal, and public health problem. However, our review suggests that high-quality studies of DID are generally lacking, and that causal relationships are difficult to ascertain. Moreover, ethical constraints may always impose limits on the kind of studies that can be conducted. Nonetheless, a review of the available evidence finds that some drugs or drug classes commonly used in general medicine probably do pose a relatively high risk of DID. While our findings should be considered provisional, isotretinoin, rimonabant, and alpha-interferons appear to pose the highest risk of DID. Corticosteroids, varenicline, progesterone inserts, and finasteride may pose a moderately high risk of DID. Since all these agents have legitimate medical indications, the risks and benefits of each drug must be carefully weighed on a case-by-case basis.
Contributor Information
Donald Rogers, Dr. Rogers is from the State Office of Pharmacy, Tewksbury, Massachusetts.
Ronald Pies, Dr. Pies is Professor of Psychiatry, SUNY Upstate Medical University, Syracuse, New York, and Clinical Professor of Psychiatry, Tufts University School of Medicine, Boston, Massachusetts.
References
- 1.US Food and Drug Administration. FDA Issues Public Health Advisory on Chantix. [November 6, 2008]. http://www.fda.gov/bbs/topics/NEWS/2008/NEW01788.html.
- 2.US Food and Drug Administration. Information for Healthcare Professionals Suicidality and Antiepileptic Drugs. [November 6, 2008]. http://www.fda.gov/Cder/Drug/Info Sheets/HCP/antiepilepticsHCP.htm.
- 3.Summary Minutes. FDA Advisory Committee Meeting; 13 June 2007; FDA. [Google Scholar]
- 4.US Food and Drug Administration. Antidepressant Use in Children, Adolescents, and Adults. [November 6, 2008]. http://www.fda.gov/cder/drug/antid epressants/default.htm.
- 5.Freis ED. Mental depression in hypertensive patients treated for long periods with large doses of reserpine. N Engl J Med. 1954;251:1006–1008. doi: 10.1056/NEJM195412162512504. [DOI] [PubMed] [Google Scholar]
- 6.Pies R. Persistent bipolar illness after steroid administration. Arch Intern Med. 1981;141:1087. [PubMed] [Google Scholar]
- 7.Yourlawyer.com Injured by Cymbalta? [November 6, 2008]. http://www.yourlawyer.com/topics/overview/cymbalta.
- 8.Papakostas GI. Tolerability of modern antidepressants. J Clin Psychiatry. 2008;69(E1):8–13. [PubMed] [Google Scholar]
- 9.Meyer JM. Antipsychotic safety and efficacy concerns. J Clin Psychiatry. 2007;68(14):20–26. [PubMed] [Google Scholar]
- 10.Marken P, Pies R. Emerging treatments for bipolar disorder: safety and side effect profiles. Ann Pharmacother. 2006;40:276–285. doi: 10.1345/aph.1G112. [DOI] [PubMed] [Google Scholar]
- 11.Mojtabai R, Olfson M. National patterns in antidepressant treatment by psychiatrists and general medical providers: results from the National Comorbidity survey Replication. J Clin Psychiatry. 2008;69:1064–1074. doi: 10.4088/jcp.v69n0704. [DOI] [PubMed] [Google Scholar]
- 12.Ried LD, McFarland BH, Johnson RE, et al. Beta-blockers and depression: the more the murkier? Ann Pharmacother. 1998;32:699–708. doi: 10.1345/aph.17185. [DOI] [PubMed] [Google Scholar]
- 13.Steffensmeier JJ, Ernst ME, Kelly M, et al. Do randomized controlled trials always trump case reports? A second look at propranolol and depression. Pharmacotherapy. 2006;26:162–167. doi: 10.1592/phco.26.2.162. [DOI] [PubMed] [Google Scholar]
- 14.Naranjo’s algorithm. Pharmacovigilence. [November 6, 2008]. http://www.pharmacovigilance.co.in/casualityassesment.html.
- 15.Dhondt ADF. Iatrogenic Origins of Depression in the Elderly. Proefschrift, Amsterdam: Vrije Universiteit; 2003. [Google Scholar]
- 16.Thiessen BQ, Wallace SM, Blackburn JL, et al. Increased prescribing of anti-depressants subsequent to beta-blocker therapy. Arch Int Med. 1990;150:2286–2290. [PubMed] [Google Scholar]
- 17.Nash MC. Substance-induced mood disorders: depression and mania. [July, 2008];emedicine. Article Last Updated: Jul 23, 2008 www.emedicine.com/med/byname/substance-induced-mood-disorder-with-depressive-features.htm. [Google Scholar]
- 18.Maricle RA, Kinzie JD, Lewinsohn P. Medication-associated depression: a two and one-half year follow-up of a community sample. Int J Psychiatry Med. 1988;18:283–292. doi: 10.2190/5mwp-ejb2-l81k-jtyt. [DOI] [PubMed] [Google Scholar]
- 19.Turner E. Drug-induced depression [undated lecture] [August 7, 2008]. http://www.fda.gov/ohrms/dockets/ac/00/slides/3639s2b.pdf.
- 20.Dhondt TD, Beekman AT, Deeg DJ, et al. Iatrogenic depression in the elderly. Results from a community-based study in the Netherlands. Soc Psychiatry Psychiatr Epidemiol. 2002;37:393–398. doi: 10.1007/s00127-002-0573-4. [DOI] [PubMed] [Google Scholar]
- 21.Natarajan S, Lipsitz SR, Rimm E. A simple method of determining confidence intervals for population attributable risk from complex surveys. Stat Med. 2007;26:3229–3239. doi: 10.1002/sim.2779. [DOI] [PubMed] [Google Scholar]
- 22.Baumeister AA, Hawkins MF, Uzelac SM. The myth of reserpine-induced depression: role in the historical development of the monoamine hypothesis. J Hist Neurosci. 2003;12:207–220. doi: 10.1076/jhin.12.2.207.15535. [DOI] [PubMed] [Google Scholar]
- 23.Broderick PA. Alprazolam, diazepam, yohimbine, clonidine: in-vivo CA1 hippocampal norepinephrine and serotonin release profiles under chloral hydrate anesthesia. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1117–1140. doi: 10.1016/s0278-5846(97)00103-6. [DOI] [PubMed] [Google Scholar]
- 24.Kreider ML, Tate CA, Cousins MM, et al. Lasting effects of developmental dexamethasone treatment on neural cell number and size, synaptic activity, and cell signaling: critical periods of vulnerability, dose-effect relationships, regional targets, and sex selectivity. Neuropsychopharmacology. 2006;31:12–35. doi: 10.1038/sj.npp.1300783. [DOI] [PubMed] [Google Scholar]
- 25.Pumariega AJ, Nelson R, Rotenberg L et al. Varenicline-induced mixed mood and psychotic episode in a patient with a past history of depression. CNS Spectr. 2008;13(6):511–514. doi: 10.1017/s1092852900016746. [DOI] [PubMed] [Google Scholar]
- 26.Dahlof B, Lindholm LH, Hansson L, et al. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension) Lancet. 1991;338:1281–1285. doi: 10.1016/0140-6736(91)92589-t. [DOI] [PubMed] [Google Scholar]
- 27.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 28.Krumholz HM, Radford MJ, Wang Y, et al. National use and effectiveness of β-blockers for the treatment of elderly patients after acute myocardial infarction: National Cooperative Cardiovascular Project. JAMA. 1998;280:623–629. doi: 10.1001/jama.280.7.623. [DOI] [PubMed] [Google Scholar]
- 29.Sokolski KN, Conney JC, Brown BJ, DeMet EM. Once-daily high-dose pindolol for SSRI-refractory depression. Psychiatry Res. 2004;125(2):81–86. doi: 10.1016/j.psychres.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Waal HJ. Propranolol-induced depression. Br Med J. 1967;2(5543) doi: 10.1136/bmj.2.5543.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzgerald JD. Propranolol-induced depression. Br Med J. 1967;2(5548):372–373. doi: 10.1136/bmj.2.5548.372-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nemeroff C, Evans DL. Concurrent use of antidepressants and propranolol: case report and theoretical considerations. Biol Psychiatry. 1983;18:237–241. [PubMed] [Google Scholar]
- 33.Nolan B. Acute suicidal depression associated with use of timolol (case report) JAMA. 1982;247 [PubMed] [Google Scholar]
- 34.Kurtz S, Ashkenazi I, Melamed S. Major depressive episode secondary to antiglaucoma drugs. Am J Psychiatry. 1993;150:523–525. doi: 10.1176/ajp.150.3.524b. [DOI] [PubMed] [Google Scholar]
- 35.Russel J, Schuckit MA. Anxiety and depression in patient on nadolol. Lancet. 1982;2(8310):1286–1287. doi: 10.1016/s0140-6736(82)90148-9. [DOI] [PubMed] [Google Scholar]
- 36.Hansteen V, Moinichen E, Lorensten E, et al. One year’s treatment with propranolol after myocardial infarction: preliminary report of Norwegian multicentre trial. BMJ. 1982;284:155–160. doi: 10.1136/bmj.284.6310.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beta Blocker Heart Attack Trial Research Group. A randomized trial of propranolol in patients with acute myocardial infarction. JAMA. 1982;247:1707–1714. doi: 10.1001/jama.1982.03320370021023. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Stable E, Gardiener PS, Baron RB, et al. The effects of propranolol on cognitive function and quality of life: a randomized trial among patients with diastolic hypertension. Am J Med. 2000;108:359–365. doi: 10.1016/s0002-9343(00)00304-1. [DOI] [PubMed] [Google Scholar]
- 39.Julian D, Prescott RJ, Jackson FS, Szekely P. Controlled trial of sotalol for one year after myocardial infarction. Lancet. 1982;1:1142–1147. doi: 10.1016/s0140-6736(82)92225-5. [DOI] [PubMed] [Google Scholar]
- 40.Packer M, Bristow MR, Chon JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 41.Ko DT, Hebert PR, Coffey CS, et al. B-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA. 2002;288(3):351–357. doi: 10.1001/jama.288.3.351. [DOI] [PubMed] [Google Scholar]
- 42.Hullet FJ, Potkin SG, Levy AB, Ciasca R. Depression associated with nifedipine-induced calcium channel blockade. Am J Psychiatry. 1988;145(10):1277–1279. doi: 10.1176/ajp.145.10.1277. [DOI] [PubMed] [Google Scholar]
- 43.Patalia AH, Rathod NR, Gandhi RR, et al. Depression—an adverse event with nifedipine. J Assoc Physicians India. 2002;50:1432–1434. [PubMed] [Google Scholar]
- 44.Biriell C, McEwen J, Sanz E. Depression associated with diltiazem. BMJ. 1989;299 doi: 10.1136/bmj.299.6702.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dassylva B. Verapamil may cause depression. Can J Psychiatry. 1993;38:299–300. doi: 10.1177/070674379303800420. [DOI] [PubMed] [Google Scholar]
- 46.Hallas J. Evidence of depression provoked by cardiovascular medication: a prescription sequence symmetry analysis. Epidemiology. 1996;7(5):478–484. [PubMed] [Google Scholar]
- 47.Lindberg G, Bingefors K, Ranstam J, et al. Use of calcium channel blockers and risk of suicide: ecological findings confirmed in population based cohort study. BMJ. 1998;316:741–745. doi: 10.1136/bmj.316.7133.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunn NR, Freemantle SN, Mann RD. Cohort study on calcium channel blockers, other cardiovascular agents, and the prevalence of depression. Br J Clin Pharmacol. 1999;48(2):230–233. doi: 10.1046/j.1365-2125.1999.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hertzman m, Adler LW, Arling B, Kern M. Lisinopril may augment antidepressant response. J Clin Psychopharmacol. 2005;25:681–620. doi: 10.1097/01.jcp.0000186736.99523.1d. [DOI] [PubMed] [Google Scholar]
- 50.Germain L, Chouinard G. Captopril treatment of major depression with serial measurements of blood cortisol concentrations. Biol Psychiatry. 1989;25:489–493. doi: 10.1016/0006-3223(89)90203-5. [DOI] [PubMed] [Google Scholar]
- 51.Germain L, Chouinard G. Treatment of recurrent unipolar major depression with captopril. Biol Psychiatry. 1988;23:637–641. doi: 10.1016/0006-3223(88)90010-8. [DOI] [PubMed] [Google Scholar]
- 52.Ullrich H, Passenberg P, Agelink MW. Episodes of depression with attempted suicide after taking valsartan with hydrochlorthiazide. Dtsch Med Wochenschr. 2003;128:2534–2536. doi: 10.1055/s-2003-44949. [DOI] [PubMed] [Google Scholar]
- 53.Gard PR, Mandy A, Sutcliffe MA. Evidence of a possible role of altered angiotensin function in the treatment, but not etiology, of depression. Biol Psychiatry. 1999;45(8):1030–1034. doi: 10.1016/s0006-3223(98)00101-2. [DOI] [PubMed] [Google Scholar]
- 54.Christensen R, Kristensen PK, Bartels H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: A meta-analysis of randomized trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 55.US Food and Drug Administration. Endocrinologic and Metabolic Drugs Advisory Committee. Jun 13, 2007. [August 31, 2008]. Briefing information. NDA 21-888. Zimulti® (rimonabant). http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4306b1-00-index.htm.
- 56.Loftus P. Merck ends development of obesity drug. [October 3, 2008];The Wall Street Journal. October 3, 2008. http://online.wsj.com/article/SB122297743887899291.html?mod=rss_w_hats_news_us_business. [Google Scholar]
- 57.Bonaccorso S, Marino V, Biondi M, et al. Depression induced by treatment with interferon=alpha in patients affected by hepatitis C virus. J Affect Disord. 2002;72:237–241. doi: 10.1016/s0165-0327(02)00264-1. [DOI] [PubMed] [Google Scholar]
- 58.Castera L, Zigante F, Bastie A, et al. Incidence of interferon alfa-induced depression in patients with chronic hepatitis C. Hepatology. 2002;35:978–979. doi: 10.1053/jhep.2002.32104. [DOI] [PubMed] [Google Scholar]
- 59.Hosoda S, Takimura H, Shibayama M, et al. Psychiatric symptoms related to interferon therapy for chronic hepatitis C. Clonical features and prognosis. Psychiatry Clin Neurosc. 2000;54:565–572. doi: 10.1046/j.1440-1819.2000.00754.x. [DOI] [PubMed] [Google Scholar]
- 60.Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;77:942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 61.Okanoue T, Sakamoto S, Itoh Y, et al. Side effects of high-dose interferon therapy for chronic hepatitis C. J Hepatol. 1996;25:283–291. doi: 10.1016/s0168-8278(96)80113-9. [DOI] [PubMed] [Google Scholar]
- 62.Jacobs LD, Beck RY, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. N Engl J Med. 2000;343:898–904. doi: 10.1056/NEJM200009283431301. [DOI] [PubMed] [Google Scholar]
- 63.Patten SB, Metz LM. Interferon beta-1a and depression in relapsing-remitting multiple sclerosis: An analysis of depression data from the PRISMS clinical trial. Mult Scler. 2001;7:243–248. doi: 10.1177/135245850100700406. [DOI] [PubMed] [Google Scholar]
- 64.Patten SB, Metz LM. Interferon beta-1a and depression in secondary progressive MS: Data from the SPECTRIMS Trial. Neurology. 2002;59:744–746. doi: 10.1212/wnl.59.5.744. [DOI] [PubMed] [Google Scholar]
- 65.Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol. 1996;39:285–294. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- 66.European Study Group on interferon B-1b in secondary progressive MS: placebo-controlled multicentre randomized trial of interferon B-1b in treatment of secondary progressive multiple sclerosis. Lancet. 1998;352:1491–1497. [PubMed] [Google Scholar]
- 67.Patten SB, Fridhandler S, Beck CA, Metz LM. Depressive symptoms in a treated multiple sclerosis cohort. Mult Scler. 2003;9(6):616–620. doi: 10.1191/1352458503ms960oa. [DOI] [PubMed] [Google Scholar]
- 68.Walff AA, Sumida K, Frye CA. Inhibiting 5alpha-reductase in the amygdale attenuates antianxiety and antidepressive behavior of naturally receptive and hormone-primed ovariectomized rats. Psychopharmacology. 206186(3):302–311. doi: 10.1007/s00213-005-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Altomare G, Capella GL. Depression circumstantially related to the administration of finasteride for andrgenetic alopecia. J Dermatol. 2002;29:665–669. doi: 10.1111/j.1346-8138.2002.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 70.Rahimi-Ardabili B, Pourandarjani R, Habibollahi P, Mualeki A. Finasteride induced depression: a prospective study. BMC Clin Pharmacol. 2006;6 doi: 10.1186/1472-6904-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wysowski DK, Pitts M, Beitz J. An analysis of reports of depression and suicide in patients treated with isotretinoin. J Am Acad Dermatol. 2001;45:515–519. doi: 10.1067/mjd.2001.117730. [DOI] [PubMed] [Google Scholar]
- 72.Wysowski DK, Pitts M, Beitz J. Depression and suicide in patients treated with isotretinoin. N Engl J Med. 2001;344 doi: 10.1056/NEJM200102083440616. [DOI] [PubMed] [Google Scholar]
- 73.Azoulay L, Blais L, Koren G, et al. Isotretinoin and the risk of depression in patients with acne vulgaris: a case-crossover study. J Clin Psychiatry. 2008;69:526–532. doi: 10.4088/jcp.v69n0403. [DOI] [PubMed] [Google Scholar]
- 74.Hersom K, Neary MP, Levaux HP, et al. Isotretinoin and antidepressant pharmacotherapy: a prescription sequence symmetry analysis. J Am Acad Dermatol. 2003;49:424–432. doi: 10.1067/s0190-9622(03)02087-5. [DOI] [PubMed] [Google Scholar]
- 75.No authors Listed. Manufacturer moves to settle Norplant claims. Contracept Technol Update. 1999;20(11):129–130. [PubMed] [Google Scholar]
- 76.Olsson SE, Odlind V, Johansson ED, Sivin I. Contraception with NORPLANT implants and NORPLANT-2 implants (two covered rods). Results from a comparative clinical study in Sweden. Contraception. 1988;37(1):61–73. doi: 10.1016/0010-7824(88)90149-7. [DOI] [PubMed] [Google Scholar]
- 77.Wagner KD, Berenson AB. Norplant-associated major depression and panic disorder. J Clin Psychiatry. 1994;55(11):478–480. [PubMed] [Google Scholar]
- 78.Wagner KD. Major depression and anxiety disorders associated with Norplant. J Clin Psychiatry. 1996;57(4):152–157. [PubMed] [Google Scholar]
- 79.Westhoff C, Truman C, Kalmuss D, et al. Depressive symptoms and Norplant contraceptive implants. Contraception. 1998;57(4):241–245. doi: 10.1016/s0010-7824(98)00022-5. [DOI] [PubMed] [Google Scholar]
- 80.Stevens-Simon C, Kelly L, Wallis J. The timing of Norplant insertion and postpartum depression in teenagers. J Adolesc Health. 2000;26(6):408–413. doi: 10.1016/s1054-139x(99)00091-9. [DOI] [PubMed] [Google Scholar]
- 81.Elovainio M, Teperi J, Aalto AM, et al. Depressive symptoms as predictors of discontinuation of treatment of menorrhagia by levonorgestrel-releasing intrauterine system. Int J Behav Med. 2007;14(2):70–75. doi: 10.1007/BF03004171. [DOI] [PubMed] [Google Scholar]
- 82.Netherlands Pharmacovigilence Center. Montelukast and depressive symptoms. [August 31, 2008]. http://www.lareb.nl/documents/kwb_2006_4_montel.pdf.
- 83.US Food and Drug Administration. Early communication about an ongoing safety review of montelukast (Singulair) [September 10, 2008]. http://www.fda.gov/cder/drug/early_comm/montelukast.htm.
- 84.Holbrook JT, Harik-Khan R. Montelukast and emotional well-being as a marker for depression: results from 3 randomized, double-masked clinical trials. J Allergy Clin Immunol. 2008;122(4):828–829. doi: 10.1016/j.jaci.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gift AF, Wood RM, Cahill CA. Depression, somatization and steroid use in chronic obstructive pulmonary disease. Int J Nurs Stud. 1989;26(3):281–286. doi: 10.1016/0020-7489(89)90009-6. [DOI] [PubMed] [Google Scholar]
- 86.Patten SB, Williams JV, Love EJ. Self-reported depressive symptoms following treatment with corticosteroids and sedative-hypnotics. Int J Psychiatry Med. 1996;26:15–24. doi: 10.2190/BL97-BWFR-4QR0-CEY7. [DOI] [PubMed] [Google Scholar]
- 87.Patten SB, Williams JV, Love EJ. A case-control study of corticosteroid exposure as a risk factor for clinically diagnosed depressive disorders in a hospitalized population. Can J Psychiatry. 1995;40(7):396–400. [PubMed] [Google Scholar]
- 88.Feng L, Tan CH, Merchant RA, Ng TP. Association between depressive symptoms and use of HMG-CoA reductase inhibitors (statins), corticosteroids and histamine H2 receptor antagonists in community-dwelling older patients. Drugs Aging. 2008;25(9):795–805. doi: 10.2165/00002512-200825090-00005. [DOI] [PubMed] [Google Scholar]
- 89.US Food and Drug Administration Varenicline (marketed as Chantix) information. [September 30, 2008]. http://www.fda.gov/cder/drug/infop age/varenicline/default.htm.
- 90.Federal Aviation Administration. Anti-smoking medicine Chantix banned. [September 30, 2008]. http://www.faa.gov/news/updates/?newsId=56363.
- 91.Popkin MK. Exacerbation of recurrent depression as a result of treatment with varenicline. Am J Psychiatry. Am J Psychiatry. 2008;165(6) doi: 10.1176/appi.ajp.2008.07111735. [DOI] [PubMed] [Google Scholar]
- 92.Tsai ST, Cho HJ, Cheng HS, et al. A randomized, placebo-controlled trial of varenicline, a selective alpha4beta2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers. Clin Ther. 2007;29:1027–1039. doi: 10.1016/j.clinthera.2007.06.011. [DOI] [PubMed] [Google Scholar]


