Abstract
Background and Aims
Competition among genetically different pollen donors within one recipient flower may play an important role in plant populations, increasing offspring genetic diversity and vigour. However, under field conditions stochastic pollen arrival times may result in disproportionate fertilization success of the first-arriving pollen, even to the detriment of the recipient plant's and offspring fitness. It is therefore critical to evaluate the relative importance of arrival times of pollen from different donors in determining siring success.
Methods
Hand pollinations and genetic markers were used to investigate experimentally the effect of pollination timing on seed paternity, seed mass and stigmatic wilting in the the dioecious plant Silene latifolia. In this species, high prevalence of multiply-sired fruits in natural populations suggests that competition among different donors may often take place (at fertilization or during seed development); however, the role of variation due to pollen arrival times is not known.
Key Results
First-arriving pollen sired significantly more seeds than later-arriving pollen. This advantage was expressed already before the first pollen tubes could reach the ovary. Simultaneously with pollen tube growth, the stigmatic papillae wilted visibly. Individual seeds were heavier in fruits where one donor sired most seeds than in fruits where both donors had more even paternity shares.
Conclusions
In field populations of S. latifolia, fruits are often multiply-sired. Because later-arriving pollen had decreased chances of fertilizing the ovules, this implies that open-pollinated flowers often benefit from pollen carry-over or pollinator visits within short time intervals, which may contribute to increase offspring genetic diversity and fitness.
Key words: Reproduction, reproductive success, pollen, siring success, microsatellite DNA, paternity, pollen tube growth, seed mass, Silene alba, stigma wilting
INTRODUCTION
Variation in pollen donor reproductive success could be a selective force in plants, especially for species in which multiple paternity within fruits frequently occurs (Marshall and Ellstrand, 1985; Campbell, 1998; Bernasconi, 2003). Indeed, how many donors ‘compete’ – i.e. have an effective chance of being in the pool of pollen that can contribute to fertilization – and the relative shares of paternity will determine reproductive success via the male function. In addition, this will also determine the degree of genetic heterogeneity among seeds within the same fruit, and the opportunity for pre- or post-zygotic selection (e.g. Teixeira et al., 2009). However, the degree to which variation in pollen donor reproductive success is important in natural populations remains controversial, because both predictable, heritable factors and stochastic factors may contribute to it (Mulcahy, 1979). Predictable determinants of fertilization success of competing donors may include attractiveness to pollinators (e.g. Mitchell and Waser, 1992; Kudo and Harder, 2005), pollen competitive ability (e.g. pollen germination, longevity and tube growth rate; Stephenson et al. 1992; Walsh and Charlesworth, 1992; Snow and Spira, 1991, 1996; Arthur et al., 2003; Jolivet and Bernasconi, 2007b; Teixeira and Bernasconi, 2008), or traits affecting the outcome of post-pollination selection, either among pollen tubes before fertilization (Cruzan and Barrett, 1993; Skogsmyr and Lankinen, 2000; Snow, 1994; Stanton, 1994; Marshall, 1988) or among embryos after fertilization (Marshall and Ellstrand, 1988). In the field, however, pollen vectors introduce a stochastic component, unpredictably determining the timing, quantity and genetic diversity of pollen deposited on the stigma (Spira et al., 1992; Skogsmyr and Lankinen, 1999; Mitchell et al., 2005; Karron et al., 2006).
To understand the determinants of variation in male reproductive success in animal-pollinated plants, it is therefore important to evaluate the relative magnitude of stochastic versus predictable effects (e.g. Skogsmyr and Lankinen 1999). To disentangle the relative roles of stochastic versus predictable effects in explaining field paternity, the influence of the timing between deposition of pollen from competing donors on paternity shares was investigated in the dioecious plant Silene latifolia. In this species within-fruit multiple paternity is frequent in natural populations (Teixeira and Bernasconi, 2007). On the other hand, it is known that pollen deposition induces rapid wilting of female flowers in several species (e.g. Lankinen et al., 2006; Abdala-Roberts et al., 2007; Castro et al., 2008) including S. latifolia (Young and Gravitz, 2002). It is therefore relevant to explore which pollen arrival times (compared with the timing of post-pollination wilting and pollen tube growth) are consistent with the observed levels of multiple paternity.
The pollen of two males, either immediately after each other or at increasing time intervals, was experimentally applied on female plants of the same population. Then paternity was analysed with genetic markers, pollen tube growth rates and stigmatic wilting were monitored, and seed mass examined to address the following questions. Does the first-arriving pollen sire more seeds than the later-arriving pollen? For which time interval does first-male advantage become significant? How does this time delay compare with the time needed by the pollen tube to reach the ovary, and to the timing of stigmatic wilting? Additionally, to explore the effects on offspring provisioning (Bañuelos and Obeso, 2003) it was also questioned whether individual seed mass is correlated with unequal paternity shares within fruits. It was expected that for longer time intervals between pollinations, the proportion of seeds sired by the first male would increase. Also, if competition among seeds from different fathers affects seed provisioning a correlation was expected between differences in paternity shares and individual seed mass. Depending on the mechanism, this correlation could be either positive (‘complementarity’; Bernasconi et al., 2003) or negative (sibling rivalry; Bañuelos and Obeso, 2003). Finally, it was questioned whether the observed effects of pollen arrival time on paternity can shed light on the mechanisms leading to frequent multiple paternity within open-pollinated fruits and for the maintenance of genetic variability for pollen competitive ability in this species.
MATERIALS AND METHODS
Study species, field collection and rearing
The white campion, Silene latifolia Poir., is a dioecious, short-lived perennial plant native to Europe and parts of Asia (Prentice, 1979) and introduced in North America (Baker, 1948). In Europe, it flowers from May to October and is pollinated mainly by nocturnal moths (Jurgens et al., 1996). Males and females are dimorphic for several traits: male plants produce more numerous, smaller and shorter-lived flowers than female plants (Primack, 1985; Carroll and Delph, 1996; Young and Gravitz, 2002; Burkhardt et al., 2009), and start flowering slightly later (Jolivet and Bernasconi, 2007a). Female flowers usually have five stigmatic lobes (Teixeira et al., 2008), covered with papillae receptive to pollen germination (Lassere et al., 1996). Each flower produces a fruit with around 200 seeds (Jolivet and Bernasconi, 2007b; Young, 2002).
In 2003, one fruit of each of 50 plants (inter-plant distance ≥2 m) was collected in a natural S. latifolia population in Sesto Calende, northern Italy (45°44′08″N/8°37′00″E). Twenty seeds/fruit were germinated in Petri dishes (90 mm diameter) lined with cotton and filter paper, moistened with 1 mm gibberellic acid solution (16 h day/8 h night, 21 °C, 70 % RH). The seedlings were planted in pots (10 cm diameter) with 420 mL of a 1 : 2 mixture of sand and soil (Tref-De Baat BF4; GVZ-Bolltec AG, Zurich, Switzerland), and grew them in a growth chamber under the same conditions.
Hand-pollination with pollen from two males at varying time intervals
To investigate how the timing of sequential pollen deposition affects paternity by competing males and individual seed mass, one flower on each of 30 females was hand-pollinated with pollen from two males (n = 60 males; i.e. 30 two-donor crosses). All plants were derived from 14 field-collected seed families, whereby crossing plants from the same fruit was avoided. To manipulate time experimentally between pollinations, the pollen from the second male was applied at different time intervals after pollination with pollen from the first male, i.e. after 0, 2, 4, 8 or 24 h. For each of the five time intervals, six different females were pollinated [6 crosses per time interval (replicates) × 5 intervals (treatments) = 30 crosses]. One anther of each male was brushed uniformly over the entire stigmatic surface (i.e. all five lobes). To find out if one anther (pollen load of the first male) resulted in a full seed set, a preliminary test was carried out (see below). All female flowers were of the same age (36 h old). After the second pollination, each flower was bagged. The seeds were collected when ripe and each seed weighed individually to the nearest microgram (individual seed mass; Mettler Toledo MT5) for a subsample of 20 seeds per cross (n = 30 fruits from 30 two-donor crosses). Germination success of these seeds on an agar substrate (16 h day/8 h night, 21 °C, 70 % RH) was recorded. Finally, the seedlings were transplanted in 2·5 × 16 cm tubular pots (Cone-tainers™; Stuewe and Sons, Corvallis, OR, USA) with 1 : 2 sand : soil mixture (Tref-De Baat BF4; GVZ-Bolltec AG) for subsequent paternity analysis.
Preliminary experiment: pollen dose effects on seed set
To address pollen dose effects on seed set and ensure that the pollen load of the first donor was not merely preempting the stigmatic surface, seed set following pollination of 15 females with three unrelated males was examined with a variable pollen dose (one, two, three, four and five anthers). Five females were randomly assigned to each male and to a different pollen dose treatment, and one flower per female was hand-pollinated with the relevant number of anthers. A marginally significant effect of pollen dose on seed set was found (ANOVA, F4,14 = 2·2, P = 0·1). However, the mean seed set indicates that after applying pollen from the first pollen donor (one anther), the female has not yet received enough pollen for full seed set (mean ± s.e. seed set following pollination with: one anther, 57 ± 25; two anthers, 142 ± 29; three anthers, 185 ± 52; four anthers, 186 ± 40; five anthers, 181 ± 30).
Paternity analysis
Parents and offspring were genotyped using four microsatellite DNA marker loci (Sl6, Sl8, Sl14 and Sl15; Teixeira and Bernasconi, 2007). DNA from dried leaves of parents was extracted using Macherey–Nagel Nucleospin® Plant Kit (Düren, Germany) and from 20 offspring/cross from freeze-dried leaves, using the CTAB method (Doyle and Doyle, 1988). The final sample of offspring genotyped was n = 438 (i.e. 14·73 ± 0·45 offspring per fruit, n = 30 fruits) due to failures at germination, DNA extraction or amplification. The amplification conditions described in Teixeira and Bernasconi (2007) were followed. The amplified fragments were scored using GeneMapper® v3·7 (Applied Biosystems, Foster City, CA, USA). Paternity was assigned to the first or the second male by comparing the microsatellite patterns from each offspring with those of its mother, and of both putative fathers.
Pollen tube growth and post-pollination changes in stigmatic papillae
Since paternity shares may depend on pollen tube growth speed, how fast pollen tubes grow through the pistil after pollen deposition was estimated. Sixteen flowers, four on each of four different plants were hand-pollinated using pollen from an unrelated donor. At 2, 4, 8 or 24 h after pollination, three stigma-style lobes per flower were fixed in formalin acetic acid (FAA) following the protocol described in Bernasconi et al. (2007). After 24 h, they were placed in 70 % ethanol, then softened in NaOH (4 n, 1·5–2 h), and stained for 2 h using 1 %-aniline blue solution in phosphate buffer (pH = 7·8; after Martin, 1959). Each stigma lobe was placed on a microscope slide with two drops of 1 % aniline blue solution and the length of the longest pollen tube for each lobe and the length of the stigma lobe to the nearest 0·5 mm was measured in an epi-fluorescence microscope (Axioskop 2 Mot Zeiss; HBO 50-W burner; excitation filter BP362/150, dichroic mirror FT395, barrier filter LP397; Plan Neofluar/Fluar objective; 10×10 magnification). Since the ovary is at the lower end of the stigma lobes, it was thus possible to determine how close to the ovary the pollen tubes had arrived at 2, 4, 8 and 24 h after pollination. This estimates the minimum time needed for pollen tubes to reach the site of fertilization (ovary) under the experimental conditions.
To monitor changes in the stigmatic papillae after pollen deposition and during pollen tube growth qualitatively, the stigma lobes of another set of flowers were examined in the scanning electron microscope (SEM) for the same time intervals. To this end, four female flowers (36 h old) on four different plants were hand-pollinated by brushing the pollen of all anthers of one male per female plant uniformly over all stigma lobes. Each of the stigma lobes was allocated to a different treatment and cut 0, 2, 4, 8 or 24 h after pollination. The stigma lobes were fixed in FAA for 2 d, stored in 70 % acetone for 3–4 d, transferred to 100 % acetone, desiccated and covered with gold following standard procedures for SEM examination.
Statistical analyses
The effect of time between pollinations on the number of seeds sired by the first male (i.e. seed paternity; n = 6 replicates, i.e independent female plants, per time interval × 5 intervals = 30 crosses, involving 30 independent female plants and 60 independent male plants) was analysed in a linear model with the number of seeds genotyped per cross as a covariable. Since the residuals violated the assumptions of normality and homoscedasticity, and also showed overdispersion when using binomial errors, significance was tested in the linear model with permutation tests on the mean squares (Manly, 1997). The effects of time between pollinations and of the number of seeds sired by the first male were estimated by permutating the levels of these factors separately. P-values for each factor were calculated as the proportion of permutated mean-square estimates (out of 1000) that were larger than or equal to the observed mean-square (Manly, 1997). Further, post-hoc tests were conducted to compare the different time intervals among each other. Finally, for each time interval between pollinations, it was tested whether the proportion of seeds sired by the first male differed from equal paternity (i.e. expected mean of 0·5; separate Wilcoxon tests for each time interval).
To analyse the effect of time between pollinations on individual seed mass Generalized Linear Mixed Models (GLMMs) were run with time between pollinations as fixed factor, and cross as a random factor (n = 30 crosses) to account for non-independence among seeds arising from the same cross. In a second step, the analysis was restricted to the subset of crosses where both males sired at least one of offspring (n = 15 crosses; i.e. the crosses in which all genotyped seeds stemmed from the same father were excluded) to investigate better whether genetic variation within seed families was associated with variation in individual seed mass. GLMMs were performed with individual seed mass as response variable, cross as random factor and Ke (the effective number of donors, calculated as Ke = 1/Σpi2, where pi = proportion of seeds in a fruit sired by the ith male; Bernasconi, 2003) as an explanatory variable. Ke is a useful measure of the evenness in the proportion of offspring sired by several pollen donors, and a single value of Ke is calculated for each cross. In a two-donor cross, a value of Ke close to one means that the majority of the offspring were sired by one male; a value of Ke close or equal to two means that each male sired approximately half of the offspring, i.e. more even paternity.
To quantify pollen tube growth over time, the distance to the ovary for each pollen tube was calculated as the length of the stigma lobe minus the length of the longest pollen tube. The earliest time interval when the distance to the ovary was not significantly different from zero was determined, and this was considered to be a minimum time for fertilization to occur. For each time interval since pollination (except for 24 h when all pollen tubes had reached the base of the stigma lobe) whether the distance to the ovary differed from 0 was tested using one-sample t-tests.
Data were analysed with the R 2·6·2 or SPSS 13. Unless specified, data are given as mean ± standard deviation.
RESULTS
Seed paternity
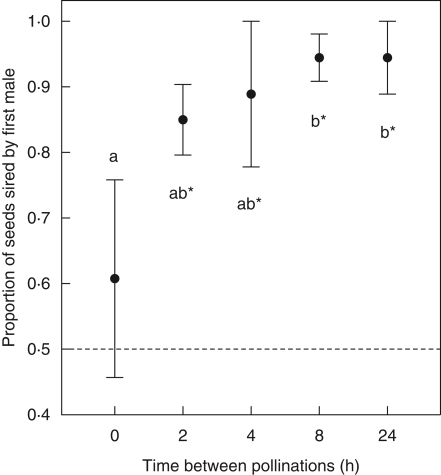
The proportion of crosses with double paternity (i.e. with offspring sired by both males) decreased significantly with increasing time between pollinations (0 h, 6/6; 2 h, 5/6; 4 h, 1/6; 8 h, 2/6; 24 h: 1/6; Fisher's exact test: P = 0·004). Accordingly, time between pollinations significantly affected seed paternity (linear model with permutation tests; d.f. = 1, MS = 50·45, P = 0·046; Fig. 1): the longer the time between pollinations, the higher the proportion of offspring sired by the first male, from 0·61 ± 0·15 when pollen of the second male was applied immediately after pollen from the first male (0 h delay) to 0·94 ± 0·06 with a 24 h delay between pollinations (n = 6 replicates per time interval). Post-hoc tests comparing pairwise each time interval revealed that seed paternity after 8 h (intervals of 8 h and 24 h) was significantly more biased towards the first male compared with 0 h delay between pollen depositions (0 h; Fig. 1). Seed paternity was not significantly different from equal paternity when the pollen from the second male was applied immediately after the pollen from the first male (0-h interval; Wilcoxon test, V = 12·5, P = 0·75), but was significantly >50 % in all other crosses in which the pollen from the second male was applied with a delay (Wilcoxon tests, all V ≥ 20, all P < 0·05, n = 6 crosses per time interval; Fig. 1).
Fig. 1.
Paternity in two-donor crosses (mean ± s.e. proportion of offspring sired by the first male) as a function of time between pollinations in Silene latifolia. Six female plants were hand-pollinated for each time interval. Asterisks denote significant (P < 0·05) deviations from equal paternity in Wilcoxon signed rank test (dashed line = equal paternity). Different letters denote time intervals that differed significantly (P < 0·05) in pairwise post-hoc tests (see Materials and Methods).
Pollen tube growth and post-pollination changes in stigmatic papillae
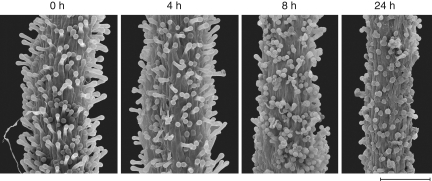
Pollen tubes reached the style/ovary junction 8 h after applying pollen to the stigma, as indicated by the fact that, for this time, the distance between the tip of the longest pollen tube and the style-to-ovary junction was no longer significantly different from zero (0·5 ± 1·1 mm; one-sample t-test, t = 1·0, d.f. = 3, P = 0·39). From this it was inferred that fertilization had to occur later than 8 h after pollen deposition under the experimental conditions. By contrast, for shorter times after pollen deposition the mean distance to the style-to-ovary junction was significantly greater than zero (2 h after pollen deposition, 9·9 ± 1 mm; t = 19·2, d.f. = 3, P < 0·001; 4 h, 3·9 ± 1·5 mm, t = 5·2 d.f. = 3, P = 0·01). In parallel, changes in turgidity of the stigmatic papillae were observed (Fig. 2).
Fig. 2.
Scanning electron micrographs showing post-pollination loss of turgidity of the stigmatic papillae in Silene latifolia flowers at different time intervals after pollen deposition. Scale bar = 300 µm.
Individual seed mass
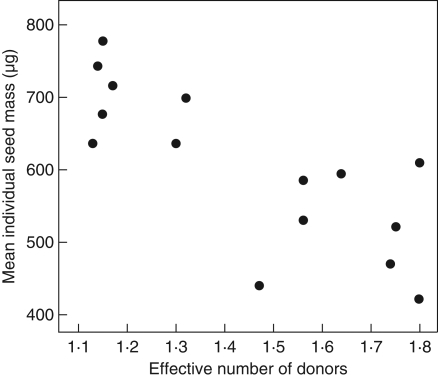
Time between pollinations did not affect significantly the mass of individual seeds (GLMM, F4,25 = 0·57, P = 0·69; n = 30 crosses; mass (μg): 0 h, 624 ± 84; 2 h, 625 ± 129; 4 h, 528 ± 228; 8 h, 602 ± 96; 24 h, 574 ± 151) nor the total number of seeds (ANOVA, F4,25 = 0·49, P = 0·74; number of seeds/fruit: 0 h, 297 ± 91; 2 h, 278 ± 72; 4 h, 235 ± 105; 8 h, 285 ± 88; 24 h, 245 ± 108), nor the total seed mass (ANOVA, F4,25 = 0·94, P = 0·46; total seed mass/fruit (mg): 0 h, 185 ± 58; 2 h, 177 ± 73; 4 h, 123 ± 58; 8 h, 177 ± 64; 24 h, 139 ± 86). Interestingly, in the 15 crosses with offspring from both males (i.e. fruits with half sibs), it was found that individual seed mass increased significantly as the proportions of offspring sired by the two competing males became more unequal (i.e. for decreasing Ke, GLMM, F1,13 = 24·2, P < 0·001; Fig. 3).
Fig. 3.
Mean individual seed mass (μg) as a function of the effective number of donors (Ke) in crosses with double paternity [estimated regression line: individual seed mass = 1086 (±100) μg – 334 (±68) × Ke]. Each dot is the mean seed mass of one cross (n = 15 crosses). If Ke is close to 1, most offspring have been sired by one male, when Ke is close to 2 both males each sired approximately half of the offspring.
DISCUSSION
In animal-pollinated plants, male reproductive success and the occurrence and outcome of competition among pollen donors are likely to depend on several factors, including inflorescence, floral and pollen traits. However, to what extent competition among pollen donors will impose selection on plant traits depends on the relative role of unpredictable determinants of variation in male reproductive success, among which an important factor is the timing of pollinator visits.
The present study shows that in Silene latifolia the pollen arrival times significantly affect siring success of competing pollen donors. Following expectations, later-arriving pollen sired a decreasing proportion of seeds with increasing time. The paternity success of the second male was in fact significantly lower than 50 % after a 2-h delay, significantly lower for 8-h delay compared with 0-h delay, and close to zero 24 h after applying pollen from the first male. Studies in several other species showed similar effects of pollen arrival times on fertilization success of pollen from competing donors (Marshall and Ellstrand, 1985; Mitchell and Marshall, 1995; Spira et al., 1996; Snow et al., 2000). For example, in Hibiscus moscheutos pollen from later visits could compete with pollen from earlier visits, but the success of late-arriving pollen declined steeply after arrival of the first pollen (Spira et al., 1996). In Raphanus sativus (Marshall and Ellstrand, 1985), a delay of 15 min between pollinations already resulted in increased paternity of the first male. In S. latifolia multiple paternity within fruits is frequent in natural populations (Teixeira and Bernasconi, 2007). Therefore, field data (Teixeira and Bernasconi, 2007) in combination with the results of hand pollination in the present study indicate that there is only a short time window after deposition of the first pollen for the later-arriving pollen to participate in fertilization. This suggests that multiple paternity within fruits is likely to arise from pollen loads which contain pollen from several males (pollen carry-over; e.g. Campbell, 1998; Morris et al., 1994) or from pollinator visits that occur within a short time interval. Pollen carry-over is consistent with observations of the behaviour of Hadena bicruris, the main pollinator of S. latifolia in Europe (A.-M. Labouche and G. Bernasconi, University of Neuchâtel, Switzerland, unpubl. res.). Thus, pollen arrival times under natural pollination apparently do not entirely impair competition among pollen donors and its potential benefits for female and offspring fitness (Teixeira et al., 2009). Consistent with this, there is variation among males in siring success in S. latifolia (Wright and Meagher, 2004).
The extent to which the timing of pollen deposition results in one father siring most of the seeds can be influenced by several pre-fertilization mechanisms, such as depletion of resources within the style, rapid growth and head-start by the first-arriving pollen tubes (Dickinson, 1965; Tadege and Kuhlemeier, 1997), stigma clogging by pollen of the first male (Cowan et al., 2000) or post-pollination wilting of the stigma (Lankinen et al., 2006, Lankinen and Kiboi, 2007). The present experiment was not designed to discriminate among these hypotheses, and future work is needed to investigate the mechanism involved. However, stigma clogging seems unlikely given that in our preliminary experiment it was found that the pollen load used for the first donor was not saturating. Arrival of the first pollen modifies the residual longevity of the female flowers of S. latifolia (Young and Gravitz, 2002), and changes in the turgidity of stigmatic papillae following pollination were observed (Fig. 2). However, it is not known whether germination of later arriving pollen was compromised by the changes of the stigma/style tissue following pollen germination from the previous male, as has been suggested (Lankinen et al., 2006). It would be interesting in future work to investigate whether female traits such as responses to post-pollination wilting can explain variation in paternity, as suggested by similar paternity responses of competing donors across related females (Teixeira et al., 2008).
Paternity shares within fruits are not only important for male fitness but may also influence offspring genetic diversity, number and quality (Mazer et al., 1986; Quesada et al., 1996; Bernasconi et al., 2003, 2004; Aizen and Harder, 2007) and the intensity of sib competition (Bañuelos and Obeso, 2003). However, the correlation between paternity shares and individual seed mass could be either positive or negative depending on the mechanism [‘complementarity’ (Bernasconi et al., 2003) or sibling rivalry (Bañuelos and Obeso, 2003), respectively]. Consistent with an effect of genetic diversity on competition among developing seeds, it was found that the more unequal the paternity shares (i.e. in fruits consisting mostly of full sibs), the higher the individual seed mass. Seed mass can correlate to several traits expressed later in life. For instance, in some species heavier seeds have increased emergence probability (Gross, 1984; Lehtila and Ehrlen, 2005), or are more likely to establish in shaded or crowded habitats (Venable and Brown, 1988; Westoby et al., 1992). However, further study is needed to determine the causes and consequences of variation in seed mass in response to average within-fruit relatedness in this species.
In conclusion, it was found that the first-arriving pollen sired more seeds than later-arriving pollen in S. latifolia. This advantage was expressed already for intervals between arrival of pollen from the first and second donors of 2 h, i.e. intervals that were shorter than the time needed for pollen tubes to reach the ovary. This suggests that there is a narrow window of opportunity for pollen to initiate development once pollination occurs in this species. In the field, multiple paternity within fruits is frequent. Taken together, experimental and field results suggest that open-pollinated flowers must often benefit from pollen carry-over or pollinator visits within short time intervals. Thus, ‘stochastic’ effects such as pollen arrival times are not entirely preventing multiple within-fruit paternity or competition among genetically diverse donors over fertilization of the same set of ovules.
ACKNOWLEDGEMENTS
We thank Katharina Foerster, Nicole Galland, Mélanie Glaettli, Anne-Marie Labouche and the reviewers for comments, Carol Goodwillie for discussion, Sara Teixeira, Philippe Busso, Rui Candeias, Jelmer Elzinga, Yann Hautier, Urs Jauch and Ana Ribeiro for practical help. This work was supported by the Swiss National Science Foundation (grant numbers 3100A0-122004/1 to G.B. and PBLAA-122727 to A.I.).
LITERATURE CITED
- Abdala-Roberts L, Parra-Tabla V, Navarro J. Is floral longevity influenced by reproductive costs and pollination success in Cohniella ascendens (Orchidaceae)? Annals of Botany. 2007;100:1367–1371. doi: 10.1093/aob/mcm219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizen MA, Harder LD. Expanding the limits of the pollen-limitation concept: effects of pollen quantity and quality. Ecology. 2007;88:271–281. doi: 10.1890/06-1017. [DOI] [PubMed] [Google Scholar]
- Arthur KM, Vejlupkova Z, Meeley RB, Fowler JE. Maize rop2 gtpase provides a competitive advantage to the male gametophyte. Genetics. 2003;165:2137–2151. doi: 10.1093/genetics/165.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker HG. Stages in invasion and replacement demonstrated by species of Melandrium. Journal of Ecology. 1948;36:96–119. [Google Scholar]
- Bañuelos M, Obeso J. Maternal provisioning, sibling rivalry and seed mass variation in the dioecious shrub Rhamnus alpinus. Evolutionary Ecology. 2003;17:19–31. [Google Scholar]
- Bernasconi G. Seed paternity in flowering plants: an evolutionary perspective. Perspectives in Plant Ecology, Evolution and Systematics. 2003;6:149–158. [Google Scholar]
- Bernasconi G, Paschke M, Schmid B. Diversity effects in reproductive biology. Oikos. 2003;102:217–220. [Google Scholar]
- Bernasconi G, Ashman TL, Birkhead TR, et al. Evolutionary ecology of the prezygotic stage. Science. 2004;303:971–975. doi: 10.1126/science.1092180. [DOI] [PubMed] [Google Scholar]
- Bernasconi G, Lang DJ, Schmid B. Microgametophyte population sizes and plant reproductive output in the insect-pollinated Prunella grandiflora (Lamiaceae) New Phytologist. 2007;173:393–400. doi: 10.1111/j.1469-8137.2006.01920.x. [DOI] [PubMed] [Google Scholar]
- Burkhardt A, Delph LF, Bernasconi G. Oecologia. 2009. Benefits and costs to pollinating, seed eating insects: the effect of flower size and fruit abortion on larval performance. (in press) [DOI] [PubMed] [Google Scholar]
- Campbell D. Multiple paternity in fruits of Ipomopsis aggregata (Polemoniaceae) American Journal of Botany. 1998;85:1022–1027. [PubMed] [Google Scholar]
- Carroll SB, Delph LF. The effects of gender and plant architecture on allocation to flowers in dioecious Silene latifolia (Caryophyllaceae) International Journal of Plant Sciences. 1996;157:493–500. [Google Scholar]
- Castro S, Silveira P, Navarro L. Effect of pollination on floral longevity and costs of delaying fertilization in the outcrossing Polygala vayredae Costa (Polygalaceae) Annals of Botany. 2008;102:1043–1048. doi: 10.1093/aob/mcn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan AA, Marshall AH, Michaelson-Yates TPT. Effect of pollen competition and stigmatic receptivity on seed set in white clover (Trifolium repens L.) Sexual Plant Reproduction. 2000;13:37–42. [Google Scholar]
- Cruzan MB, Barrett SCH. Contribution of cryptic incompatibility to the mating system of Eichhornia paniculata (Pontederiaceae) Evolution. 1993;47:925–934. doi: 10.1111/j.1558-5646.1993.tb01245.x. [DOI] [PubMed] [Google Scholar]
- Dickinson D. Germination of lily pollen: respiration and tube growth. Science. 1965;150:1818–1819. doi: 10.1126/science.150.3705.1818. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. Natural interspecific hybridization in eastern North-American Claytonia. American Journal of Botany. 1988;75:1238–1246. [Google Scholar]
- Gross KL. Effects of seed size and growth form on seedling establishment of 6 monocarpic perennial plants. Journal of Ecology. 1984;72:369–387. [Google Scholar]
- Jolivet C, Bernasconi G. Molecular and quantitative genetic differentiation in European populations of Silene latifolia (Caryophyllaceae) Annals of Botany. 2007;a 100:119–127. doi: 10.1093/aob/mcm088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivet C, Bernasconi G. Within/ between population crosses reveal genetic basis for siring success in Silene latifolia (Caryophyllaceae) Journal of Evolutionary Biology. 2007;b 20:1361–1374. doi: 10.1111/j.1420-9101.2007.01344.x. [DOI] [PubMed] [Google Scholar]
- Jurgens A, Witt T, Gottsberger G. Reproduction and pollination in central European populations of Silene and Saponaria species. Botanica Acta. 1996;109:316–324. [Google Scholar]
- Karron JD, Mitchell RJ, Bell JM. Multiple pollinator visits to Mimulus ringens (Phrymaceae) flowers increase mate number and seed set within fruits. American Journal of Botany. 2006;93:1306–1312. doi: 10.3732/ajb.93.9.1306. [DOI] [PubMed] [Google Scholar]
- Kudo G, Harder LD. Floral and inflorescence effects on variation in pollen removal and seed production among six legume species. Functional Ecology. 2005;19:245–254. [Google Scholar]
- Lankinen A, Kiboi S. Pollen donor identity affects timing of stigma receptivity in Collinsia heterophylla (Plantaginaceae): a sexual conflict during pollen competition? American Naturalist. 2007;170:854–863. doi: 10.1086/522839. [DOI] [PubMed] [Google Scholar]
- Lankinen A, Hellriegel B, Bernasconi G. Sexual conflict over floral receptivity. Evolution. 2006;60:185–196. [PubMed] [Google Scholar]
- Lassere TB, Carroll SB, Mulcahy DL. Effect of pollen competition on offspring quality at varying stages of the life cycle in Silene latifolia Poiret (Caryophyllaceae) Bulletin of the Torrey Botanical Club. 1996;123:175–179. [Google Scholar]
- Lehtila K, Ehrlen J. Seed size as an indicator of seed quality: a case study of Primula veris. Acta Oecologica. 2005;28:207–212. [Google Scholar]
- Manly BFJ. Randomization, bootstrap and Monte Carlo methods in biology. London: Chapman and Hall; 1997. [Google Scholar]
- Marshall DL. Postpollination effects on seed paternity: mechanisms in addition to microgametophyte competition operate in wild radish. Evolution. 1988;42:1256–1266. doi: 10.1111/j.1558-5646.1988.tb04185.x. [DOI] [PubMed] [Google Scholar]
- Marshall DL, Ellstrand NC. Proximal causes of multiple paternity in wild radish, Raphanus sativus. American Naturalist. 1985;126:596–605. [Google Scholar]
- Marshall DL, Ellstrand NC. Effective mate choice in wild radish:evidence for selective seed abortion and its mechanism. American Naturalist. 1988;131:739–756. [Google Scholar]
- Martin FW. Staining and observing pollen tubes in the style by means of fluorescence. Stain Technology. 1959;34:125–128. doi: 10.3109/10520295909114663. [DOI] [PubMed] [Google Scholar]
- Mazer SJ, Snow AA, Stanton ML. Fertilization dynamics and parental effects upon fruit development in Raphanus raphanistrum: consequences for seed size variation. American Journal of Botany. 1986;73:500–511. [Google Scholar]
- Mitchell RJ, Marshall DL. Effects of pollination method on paternal success in Lesquerella fendleri (Brassicaceae) American Journal of Botany. 1995;82:462–467. [Google Scholar]
- Mitchell RJ, Waser NM. Adaptive significance of Ipomopsis aggregata nectar production: pollination success of single flowers. Ecology. 1992;73:633–638. [Google Scholar]
- Mitchell RJ, Karron JD, Holmquist KG, Bell JM. Patterns of multiple paternity in fruits of Mimulus ringens (Phrymaceae) American Journal of Botany. 2005;92:885–890. doi: 10.3732/ajb.92.5.885. [DOI] [PubMed] [Google Scholar]
- Morris WF, Price MV, Waser NM, Thomson JD, Thomson B, Stratton DA. Systematic increase in pollen carryover and its consequences for geitonogamy in plant populations. Oikos. 1994;71:431–440. [Google Scholar]
- Mulcahy DL. The rise of the angiosperms: a genecological factor. Science. 1979;206:20–23. doi: 10.1126/science.206.4414.20. [DOI] [PubMed] [Google Scholar]
- Prentice HC. Numerical analysis of infraspecific variation in European Silene alba and Silene dioica (Caryophyllaceae) Botanical Journal of the Linnean Society. 1979;78:181–212. [Google Scholar]
- Primack RB. Longevity of individual flowers. Annual Review of Ecology and Systematics. 1985;16:15–37. [Google Scholar]
- Quesada M, Winsor JA, Stephenson AG. Effects of pollen selection on progeny vigor in a Cucurbita pepo × C. texana hybrid. Theoretical and Applied Genetics. 1996;92:885–890. doi: 10.1007/BF00221902. [DOI] [PubMed] [Google Scholar]
- Skogsmyr I, Lankinen Å. Selection on pollen competitive ability in relation to stochastic factors influencing pollen deposition. Evolutionary Ecology Research. 1999;1:971–985. [Google Scholar]
- Skogsmyr I, Lankinen A. Potential selection for female choice in Viola tricolor. Evolutionary Ecology Research. 2000;2:965–979. [Google Scholar]
- Snow AA. Postpollination selection and male fitness in plants. The American Naturalist. 1994;144:S69–S83. [Google Scholar]
- Snow AA, Spira TP. Pollen vigor and the potential for sexual selection in plants. Nature. 1991;352:796–797. [Google Scholar]
- Snow AA, Spira TP. Pollen-tube competition and male fitness in Hibiscus moscheutos. Evolution. 1996;50:1866–1870. doi: 10.1111/j.1558-5646.1996.tb03573.x. [DOI] [PubMed] [Google Scholar]
- Snow AA, Spira TP, Liu H. Effects of sequential pollination on the success of ‘fast’ and ‘slow’ pollen donors in Hibiscus moscheutos (Malvaceae) American Journal of Botany. 2000;87:1656–1659. [PubMed] [Google Scholar]
- Spira TP, Snow AA, Whigham DF, Leak J. Flower visitation, pollen deposition, and pollen-tube competition in Hibiscus moscheutos (Malvaceae) American Journal of Botany. 1992;79:428–433. [Google Scholar]
- Spira TP, Snow AA, Puterbaugh MN. The timing and effectiveness of sequential pollinations in Hibiscus moscheutos. Oecologia. 1996;105:230–235. doi: 10.1007/BF00328551. [DOI] [PubMed] [Google Scholar]
- Stanton ML. Male–male competition during pollination in plant populations. The American Naturalist. 1994;144:S40–S68. [Google Scholar]
- Stephenson AG, Lau TC, Quesada M, Winsor JA. Factors that affect pollen performance. In: Wyatt R, editor. Ecology and evolution of plant reproduction. New York, NY: Chapman & Hall; 1992. [Google Scholar]
- Tadege M, Kuhlemeier C. Aerobic fermentation during tobacco pollen development. Plant Molecular Biology. 1997;35:343–354. doi: 10.1023/a:1005837112653. [DOI] [PubMed] [Google Scholar]
- Teixeira S, Bernasconi G. High prevalence of multiple paternity within fruits in natural populations of Silene latifolia, as revealed by microsatellite DNA analysis. Molecular Ecology. 2007;16:4370–4379. doi: 10.1111/j.1365-294X.2007.03493.x. [DOI] [PubMed] [Google Scholar]
- Teixeira S, Bernasconi G. Effects of inbred/outbred crosses on progeny sex ratio in Silene latifolia (Caryophyllaceae) New Phytologist. 2008;178:448–456. doi: 10.1111/j.1469-8137.2007.02366.x. [DOI] [PubMed] [Google Scholar]
- Teixeira S, Burkhardt A, Bernasconi G. Genetic variation among females affects paternity in a dioecious plant. Oikos. 2008;117:1594–1600. [Google Scholar]
- Teixeira S, Foerster K, Bernasconi G. Evidence for inbreeding depression and post-pollination selection against inbreeding in the dioecious plant Silene latifolia. Heredity. 2009;102:101–112. doi: 10.1038/hdy.2008.86. [DOI] [PubMed] [Google Scholar]
- Venable DL, Brown JS. The selective interactions of dispersal, dormancy, and seed size as adaptations for reducing risk in variable environments. American Naturalist. 1988;131:360–384. [Google Scholar]
- Walsh NE, Charlesworth D. Evolutionary interpretations of differences in pollen tube growth rates. Quarterly Review of Biology. 1992;67:19–37. [Google Scholar]
- Westoby M, Jurado E, Leishman M. Comparative evolutionary ecology of seed size. Trends in Ecology & Evolution. 1992;7:368–372. doi: 10.1016/0169-5347(92)90006-W. [DOI] [PubMed] [Google Scholar]
- Wright J, Meagher T. Selection on floral characters in natural Spanish populations of Silene latifolia. Journal of evolutionary Biology. 2004;17:382–395. doi: 10.1046/j.1420-9101.2003.00671.x. [DOI] [PubMed] [Google Scholar]
- Young HJ. Diurnal and nocturnal pollination of Silene alba (Caryophyllaceae) American Journal of Botany. 2002;89:433–440. doi: 10.3732/ajb.89.3.433. [DOI] [PubMed] [Google Scholar]
- Young H, Gravitz L. The effects of stigma age on receptivity in Silene alba (Caryophyllaceae) American Journal of Botany. 2002;89:1237–1241. doi: 10.3732/ajb.89.8.1237. [DOI] [PubMed] [Google Scholar]