Abstract
Background and Aims
Erythrina speciosa is a Neotropical tree that grows mainly in moist habitats. To characterize the physiological, morphological and growth responses to soil water saturation, young plants of E. speciosa were subjected experimentally to soil flooding.
Methods
Flooding was imposed from 2 to 4 cm above the soil surface in water-filled tanks for 60 d. Non-flooded (control) plants were well watered, but never flooded. The net CO2 exchange (ACO2), stomatal conductance (gs) and intercellular CO2 concentration (Ci) were assessed for 60 d. Soluble sugar and free amino acid concentrations and the proportion of free amino acids were determined at 0, 7, 10, 21, 28 and 45 d of treatments. After 28, 45 and 60 d, dry masses of leaves, stems and roots were determined. Stem and root cross-sections were viewed using light microscopy.
Key Results
The ACO2 and gs were severely reduced by flooding treatment, but only for the first 10 d. The soluble sugars and free amino acids increased until the tenth day but decreased subsequently. The content of asparagine in the roots showed a drastic decrease while those of alanine and γ-aminobutyric increased sharply throughout the first 10 d after flooding. From the 20th day on, the flooded plants reached ACO2 and gs values similar to those observed for non-flooded plants. These events were coupled with the development of lenticels, adventitious roots and aerenchyma tissue of honeycomb type. Flooding reduced the growth rate and altered carbon allocation. The biomass allocated to the stem was higher and the root mass ratio was lower for flooded plants when compared with non-flooded plants.
Conclusions
Erythrina speciosa showed 100 % survival until the 60th day of flooding and was able to recover its metabolism. The recovery during soil flooding seems to be associated with morphological alterations, such as development of hypertrophic lenticels, adventitious roots and aerenchyma tissue, and with the maintenance of neutral amino acids in roots under long-term exposure to root-zone O2 deprivation.
Key words: Erythrina speciosa, aerenchyma, amino acid content, biomass allocation, photosynthesis, flooding adaptations, stomatal conductance, O2 deficiency, γ-aminobutyric acid (GABA)
INTRODUCTION
The occurrence of flooding events is common in several Brazilian ecosystems. Their effect on physiological and morphological responses in plants is a consequence of a partial decline of O2 concentration in the soil. Under this condition, the decrease in the rates of aerobic root respiration is detectable and hence ATP production is impaired and metabolism is disrupted (Lambers et al., 1998). Among the main consequences of oxygen depletion on plants are a decrease in stomatal conductance and photosynthesis, changes in metabolite levels, plant hormonal imbalances, and deficient water and nutrient uptake with deleterious effects on plant growth and survival (Lopez and Kursar, 2003).
However, some plants have acquired some characteristics to survive and grow under anaerobic conditions. The mechanisms of tolerance to flooding conditions are multiple and complex, including responses at all levels of organization from biochemical to anatomical and morphological alterations.
Some works have pointed out a decrease in shoot growth in several species submitted to flooding conditions, with flooding suppressing leaf formation and expansion of leaves and internodes, and inducing premature leaf senescence and abscission (Tang and Kozlowski, 1982; Tsukahara and Kozlowski, 1986; Kozlowski, 1997), while others (Lobo and Joly, 1998; Joly, 1994; Lopez and Kursar, 1999; Andrade et al., 1999) have shown that flooding did not alter growth responses, in either tolerant or non-tolerant species. Lenticel and stem hypertrophy, aerenchyma formation and adventitious roots are among the main morphological alterations that allow woody plants to continue growing and surviving in flooding environments (Parolin, 2001). Water, nutrients and hormones supplied by adventitious roots to aerial organs provide the conditions for continued growth, and in some species the concomitant lenticel hypertrophy will further contribute to a porosity increment at the insertion of adventitious roots, enhancing the rhizosphere oxidation (Armstrong et al., 1994; Vartapetian and Jackson, 1997; Colmer, 2003).
From a physiological perspective, reduced CO2 assimilation has been shown for a great number of species submitted to anaerobic conditions (Mielke et al., 2003; Fernandez, 2006). Such reductions are often associated with stomatal limitations, which restricted CO2 availability in the mesophyll and at the sites of carboxylation (Lopez and Kursar, 2003). Non-stomatal limitations are also observed which have been attributed to starch accumulation in the leaves and/or a drop in RUBISCO activities (Pezeshki, 2001). In addition, pronounced changes in the pool of several metabolites, particularly free amino acids, have been detected (Sousa and Sodek, 2003; Koppitz et al., 2004; Thomas et al., 2005), as well as in the carbohydrate availability to the root system under hypoxic conditions (Huang and Johnson, 1995).
Although the literature on plant responses to flooding is abundant for temperate species, there are few reports about the flooding effect on net CO2 assimilation and growth in tropical wild species (Lopez and Kursar, 1999, 2003; Parolin, 2001; Mielke et al., 2003). For example, Genipa americana (Rubiaceae), a widely distributed neotropical species found in flood-prone habitats in Brazil, but most commonly occurring on ‘terra firme’ elsewhere, is able to survive and to grow under soil waterlogging despite decreases in net carbon uptake (Mielke et al., 2003). Moreover, analogies between flooding tolerance mechanisms in temperate and tropical plants may be misleading since plants in temperate areas typically experience flooding during the winter and early spring, a period of minimal physiological activity, while tropical plants have to face flooding events during summer when air temperature is higher and physiological activities more intense (Joly, 1994; Lopez and Kursar, 1999).
Erythrina speciosa, a neotropical tree species distributed throughout southern and south-eastern Brazil (Krukoff, 1939; Lorenzi, 1992), is typical in fluvial forest and moist plains as well as in flooded-prone habitats, always in open and secondary formations. In the last two decades, studies of the flooding effects on some species of the genus Erythrina have been related to metabolic features at early seedling establishment and with physiological aspects of growth, carbon assimilation and the photosynthetic machinery itself (Small et al., 1989; Muthuchelian et al., 1995). For E. speciosa, Kolb et al. (2002) came to the conclusion that the maintenance of an adequate fermentative metabolic energy is important for seedling adaptation to flooded habitats. The objective of the present study was to evaluate concomitantly leaf gas exchange, soluble sugar content, free amino acid content and the proportion of free amino acids in root tissue, and morphological responses and growth in young individuals of E. speciosa under soil flooding. Taking into account the distribution pattern of the species, it was hypothesized that E. speciosa is able to show morphological and physiological alterations to cope with soil flooding.
MATERIALS AND METHODS
Experimental conditions
The experiment was carried out from March to May 1999, in a glasshouse at Universidade Estadual de Campinas, São Paulo State, Brazil, at 22°54′S; 47°05′W and 674 m a.s.l. According to Köppen, the climate is Cwa, with a warm and rainy season from October to March (temperature range from 18 to 34 °C), and a drier season from April to September (temperature range from 12 to 27 °C). The mean annual rainfall in the region is 1057 mm precipitation (Ortolani et al., 1995). Seeds of Erythrina speciosa Andrews from ripe fruits were collected from adult individuals growing in a flood area near the University. After cleaning, the seeds were scarified and germinated in Petri dishes at 25 °C under continuous white fluorescent light. Germinated seeds were transplanted to plastic pots (1 L) filled with sand and supplied with 200 mL of Hoagland solution per week. After 45 d the seedlings with one pair of cotyledonary leaves and three fully expanded leaves were selected and assigned to one of two treatments: flooding and non-flooding (control). Flooding was imposed in water-filled tanks for 60 d, providing a water level from 2 to 4 cm above the soil surface. Non-flooded plants were well watered but never flooded.
Leaf gas exchange measurements
CO2 and H2O gas exchange was measured from 0900 to 1100 h. Two fully expanded and healthy leaves from nodes 2–3 from the apex of the shoots from eight different individuals in each treatment (flood and non-flooding) were used. The net CO2 uptake per unit leaf area (ACO2), stomatal conductance to water vapour (gs), transpiration rate (EH20), leaf temperature (Tleaf) and intercellular CO2 concentration (Ci) were determined with a portable open-system infrared gas analyser (IRGA) (LCA-4; Analytical Development Co. Ltd, Hoddesdon, UK) connected to a 6·25 cm2 PLC4 (B) leaf chamber. The air entering by the leaf chamber was drawn from 3 m above-ground and passed through a 1500 cm3 buffer vessel to avoid ambient CO2 concentration (Ca) fluctuation. Water use efficiency (WUE) was calculated as the ratio ACO2/EH2O (Nobel, 2005). The IRGA allowed the measurement of Tleaf and photosynthetic photon flux density (PPFD, λ = 400–700 nm). During the measurements the plants were transferred to an open area where temperature and relative humidity ranged from 24 to 25 °C, and from 75 to 78 %, respectively. The PPFD was about 1100 µmol m−2 s−1, high enough to saturate photosynthesis, as previously determined in an additional experiment (data not shown).
Amino acids and soluble sugars in roots
Immediately after harvest, the root system was ground with liquid N2 and lyophilized. For the metabolite analyses, only the original, pre-existing roots were sampled. Amino acids and soluble sugars were extracted with MCW (methanol : chloroform : water, 12 : 5 : 3, v/v/v) (adapted from Bieleski and Turner, 1966), for 24 h, using 10 mL g−1 fresh weight of roots. The aqueous phase was recovered following phase separation on standing after addition of chloroform (1 vol.) and water (1·5 vol.) to 4 vols of supernatant. The aqueous phase was then reduced to a known volume by evaporation at 38 °C and kept frozen until analysis. Soluble sugars were determined according to Graham and Smydzuc (1965). Amino acids were determined using the method of Moore and Stein (1948) and separated by reverse-phase HPLC of their o-phthalaldehyde (OPA) derivatives, as described by Puiatti and Sodek (1999). The total dissolved amino acids are given as the sum of all detected and quantified amino acids. The contents are given in absolute (μmol g−1 d. wt.) and in relative mol percentage of total units. All these evaluations were made using three plants per treatment, at 0 (non-flooded plants), 7, 10, 21, 28 and 45 d of the experiment.
Growth and morphological measurements
The stem height and the diameter at 2 cm above the soil were measured weekly for ten flooded and ten non-flooded plants. After 28, 45 and 60 d, six plants from each treatment were harvested, the roots were thoroughly washed and the plants were separated into leaves, stems and roots. These samples were dried at 70 °C for 48 h for dry mass determination. From the primary data the following parameters were derived: root mass ratio (RMR: root mass per whole plant mass, units g g−1), stem mass ratio (SMR: stem and petiole mass per unit whole plant mass, units g g−1) and leaf mass ratio (LMR: leaf mass per whole plant mass, units g g−1). The relative growth rate (RGR: dry mass increment per unit total plant mass per unit time) was calculated according to Hunt (1982) as: RGR = [ln (final dry mass) – ln (initial dry mass)]/time.
For histological analysis, small pieces of root and stem were cut with a lamina razor from flooded and non-flooded plants and fixed in 50 FAA (formaldehyde : 50 % ethanol : acetic acid, 18 : 1 : 1) and stored in 70 % alcohol. Hand-cut transverse sections were taken, stained by astra blue-basic fuchsin, and photographed using a compound microscope optic.
Testing for oxygen diffusion from the shoot to the root system was performed after 10 d on flooded and non-flooded plants by the submergence of the root system into gel agar (1 %), 150 mg L−1 sodium dithionite and 10 mg L−1 methylene blue for colorimetric determination according to Joly (1991).
Statistics
The effects of flooding on gas exchange, soluble sugars, free amino acids, proportion of amino acids, and growth were analysed in a fully randomized design. The data were subject to an analysis of variance (ANOVA), and when F was significant the treatment means were compared by Tukey's test at 5 % probability (Snedecor and Cochran, 1967). The data of free amino acids and soluble sugars were expressed as the means and standard error.
RESULTS
Leaf gas exchange responses
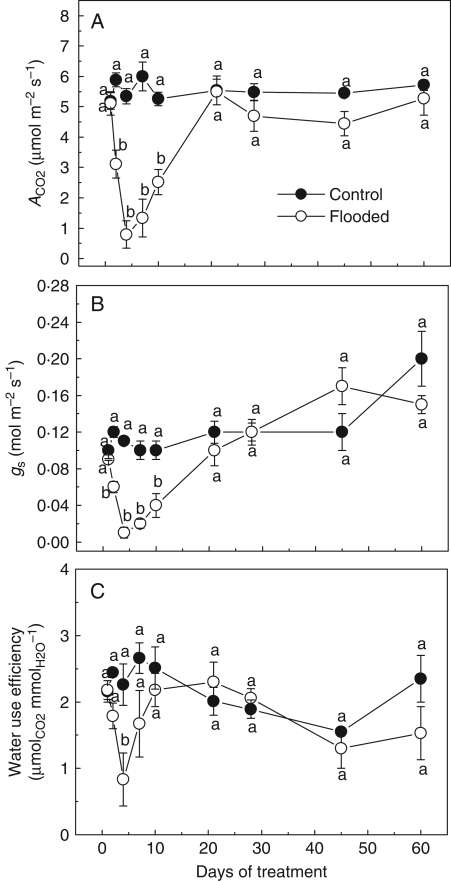
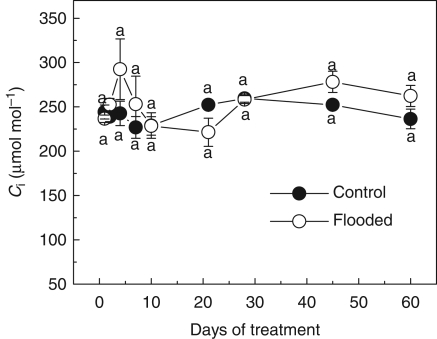
The leaf gas exchange variables were drastically affected by flooding treatments. After 4 d of flooding, ACO2 assimilation rates were reduced by 90 % from control values. The ACO2 values from control plants were about 5·88 µmol m−2 s−1 while the minimum ACO2 value from flooded plants was 0·79 µmol m−2 s−1. The stomatal conductance and WUE closely paralleled changes in ACO2 assimilation rates. Leaves from control plants exhibited a maximum gs value of 0·12 mmol m−2 s−1 while for flooded plants it was 0·01 mol m−2 s−1. After the fifth day a gradual recuperation was detected, and at the end of the experiment flooded and non-flooded plants showed similar values of ACO2 and gs. However, the WUE presented a tendency to decline at the end of the experiment, indicating a possible non-stomatal limitation of the photosynthesis (Fig. 1). A tendency for increases in Ci values was observed at the beginning of the experiment (Fig. 2).
Fig. 1.
Photosynthetic rates (ACO2), stomatal conductance (gs) and water use efficiency (WUE) in Erythrina speciosa under control and flooding conditions. Data are expressed as the mean ± s.e.; n = 4 leaves per treatment, each leaf from a different individual. Different letters indicate a significant difference between means (P < 0·05 %; Tukey test).
Fig. 2.
Internal CO2 concentration (Ci) in Erytrina speciosa under control and flooding conditions. Data are expressed as the mean ± s.e.; n = 4 leaves per treatment, each leaf from a different individual. Different letters indicate a significant difference between means (P < 0·05 %; Tukey test).
Amino acids and soluble sugars in the root system
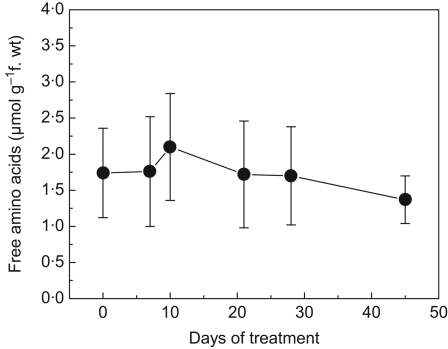
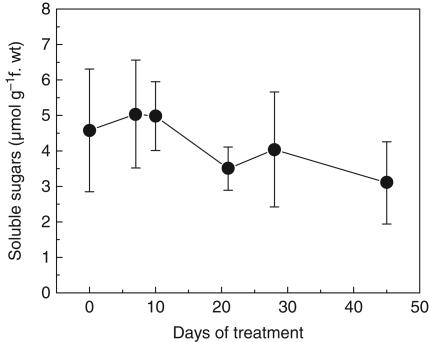
The total free amino acid concentration and the soluble sugars in the roots of E. speciosa are shown in Figs 3 and 4. Although the values of free amino acids and soluble sugars were not significantly different, both increased slightly until the tenth day of imposition of the flooding treatment and after that presented a tendency to decrease until day 45. The composition of free amino acids is presented in Table 1. At day 0 (non-flooded plants), asparagine (Asn) was the amino acid found in the highest proportion in roots of E. speciosa, followed by γ-aminobutyric acid (GABA), glutamine (Glu), aspartic acid (Asp) and alanine (Ala). Although GABA is a non-protein amino acid, it already showed elevated values on day 0. Under flooded conditions, Asn levels fell sharply and remained low until day 21 of the experiment. Concomitantly increases were observed in Ala content that lasted until day 28. However, the GABA content was high when compared with that of other amino acids at day 45, and Ala recovered values close to its initial values. In contrast to GABA and Ala, the Glu and Asp levels decreased on the tenth day of flooding imposition, but after this a recovery of the content of these amino acids was observed.
Fig. 3.
Changes in total free amino acids in root tissue of E. speciosa plants subjected to flooding of the root system. Data are the means ± s.e. of three replicates.
Fig. 4.
Changes in soluble sugars in root tissue of E. speciosa plants subjected to flooding of the root system. Data are the means ± s.e. of three replicates.
Table 1.
Amino acid composition (mol %) of roots of Erythrina speciosa plants subjected to flooding of the root system
| Time after induction of flooding (d) |
||||||
|---|---|---|---|---|---|---|
| Amino acid* | 0 | 7 | 10 | 21 | 28 | 45 |
| Asp | 10·6 ± 1·54ab | 6·7 ± 0·85b | 6·7 ± 0·27b | 7·0 ± 1·44b | 9·6 ± 1·07b | 17·6 ± 1·13a |
| Glu | 12·5 ± 2·34a | 8·1 ± 0·46a | 8·1 ± 0·71a | 11·1 ± 1·67a | 8·2 ± 1·01a | 19·2 ± 1·61a |
| Asn | 36·3 ± 1·79a | 17·6 ± 1·65ab | 10·0 ± 0·46b | 11·2 ± 1·19b | 23·7 ± 3·57ab | 13·1 ± 2·91ab |
| Ser | 2·9 ± 0·06a | 3·8 ± 0·05a | 4·5 ± 0·53a | 2·2 ± 0·10a | 2·3 ± 0·44a | 3·6 ± 0·52a |
| Ala | 5·3 ± 0·52c | 15·5 ± 0·82a | 18·4 ± 0·59a | 8·0 ± 1·26bc | 10·8 ± 0·92b | 8·5 ± 1·03bc |
| GABA | 19·2 ± 1·91a | 25·0 ± 2·89a | 38·0 ± 0·94a | 44·3 ± 5·20a | 40·5 ± 1·45a | 30·8 ± 4·41a |
For the metabolite analyses, only the original pre-existing roots were sampled. n = 3 replicates.
* The following amino acids were detected: Gln, His, Gly, Thr, Arg, Tyr, Met, Phe, Ile and Leu; however at concentrations lower than 3%. Means followed by same letter in a row do not differ by Tukey test at the 5 % level of probability.
Growth and morphological responses
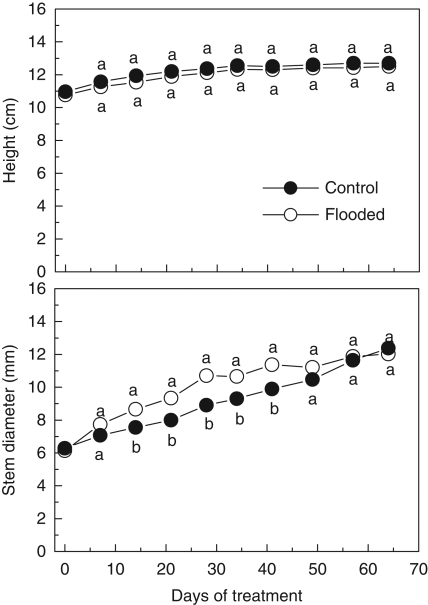
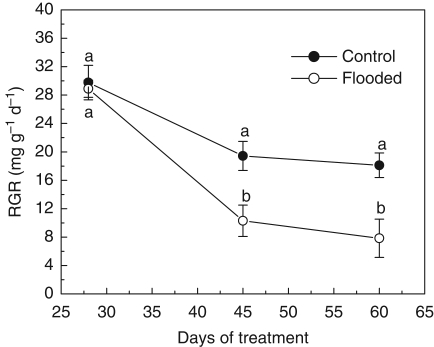
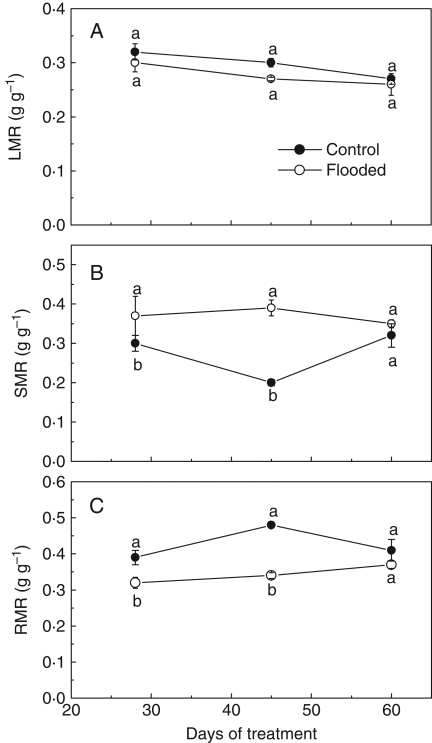
Flooding had no effect on growth height. Both control and flooded plants had reached heights of about 13 cm by the end of the experiment. In contrast, from the 14th to the 40th day, growth in stem diameter was 20 % higher in the flooding treatments compared with the control (Fig. 5). The RGR was not affected until the 28th day of treatment, but significant decreases with flooding were observed between the 45th and the 60th day (Fig. 6). The pattern of carbon allocation was also affected by flooding treatment. The biomass fraction allocated to the stem was higher on the 28th and 45th days, and the RMR and LMR were lower on the 45th day for flooded plants when compared with the non-flooded controls (Fig. 7). In general, the leaf, root and total biomass, as well as the root/shoot ratio, were affected by flooding around the 45th day; after that only root and total biomass differed between control and flooded plants (Table 2).
Fig. 5.
Height and stem diameter for E. speciosa under control and flooding conditions. Data are expressed as mean ± s.e.; n = 10 plants per treatment. Different letters indicate a significant difference between means (P < 0·05 %; Tukey test).
Fig. 6.
Relative growth rate of E. speciosa under control and flooding conditions. Data are expressed as the mean ± s.e.; n = 6 plants in each treatment. Different letters indicate a significant difference between means (P < 0·05 %; Tukey test).
Fig. 7.
(A) Leaf mass ratio (LMR; leaf dry mass/total dry mass); (B) stem mass ratio (SMR; stem dry mass/total dry mass); (C) root mass ratio (RMR; root dry mass/total dry mass) in E. speciosa under control and flooding conditions. Data are expressed as the mean ± s.e.; n = 6 plants in each treatment. Different letters indicate a significant difference between means (P < 0·05 %; Tukey test).
Table 2.
Growth and biomass characteristics of Erythrina speciosa seedlings grown under soil flooding and control conditions
| Time (d) |
||||
|---|---|---|---|---|
| Parameter | Treatment | 28 | 45 | 60 |
| Stem biomass (g) | Control | 1·36 ± 0·06a | 1·08 ± 0·15a | 1·52 ± 0·17a |
| Flooded | 1·30 ± 0·11a | 0·91 ± 0·05a | 1·03 ± 0·18a | |
| Leaf biomass (g) | Control | 1·26 ± 0·11a | 1·40 ± 0·10a | 1·33 ± 0·21a |
| Flooded | 1·27 ± 0·16a | 0·70 ± 0·03b | 0·82 ± 0·17a | |
| Root biomass (g) | Control | 1·72 ± 0·21a | 2·27 ± 0·26a | 2·18 ± 0·41a |
| Flooded | 1·24 ± 0·03a | 0·97 ± 0·11b | 1·05 ± 0·19b | |
| Total biomass (g) | Control | 4·30 ± 0·35a | 4·59 ± 0·40a | 5·04 ± 0·53a |
| Flooded | 3·86 ± 0·16a | 2·76 ± 0·24b | 2·92 ± 0·72b | |
| Root/shoot ratio | Control | 0·66 ± 0·03a | 0·92 ± 0·06a | 0·75 ± 0·07a |
| Flooded | 0·48 ± 0·07b | 0·53 ± 0·08b | 0·57 ± 0·03a | |
n = 6 replicates.
Means followed by same letter in a column do not differ by Tukey test at the 5 % level of probability.
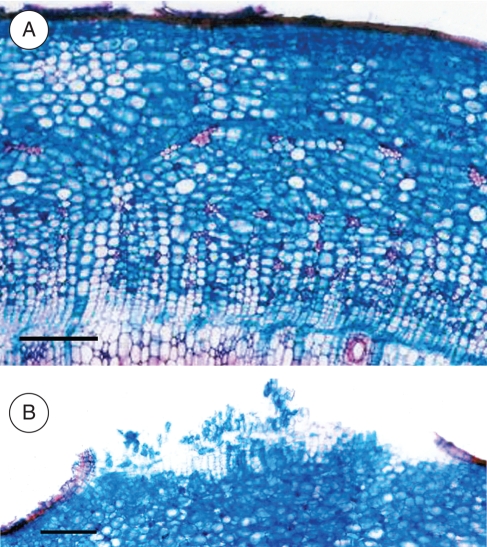
Increases in carbon allocation to the stem observed at the 40th day of flooding were coupled with the formation of hypertrophic lenticels (Fig. 8), adventitious roots and aerenchyma tissue (Fig. 9). The latter seems to be of the honeycomb type (Justin and Armstrong, 1987; Seago et al., 2005). The development of lenticels was observed 2 d after the treatment imposition and was paralleled by the emergence of adventitious roots, which proliferated throughout the experiment. Fifteen days after the beginning of the experiment, the aerenchyma tissue on flooded plants was already widely developed (Fig. 9). Oxygen diffusion from roots in gel agar was detected 10 d after flooding, meaning that the morphological modifications observed in flooded plants were contributing to a better aeration of the root system.
Fig. 8.
Stem transverse sections of E. speciosa under (A) control conditions and (B) after 60 d of flooded conditions. Note the lenticel hypertrophy on plants under flooded conditions. Scale bars = 100 µm.
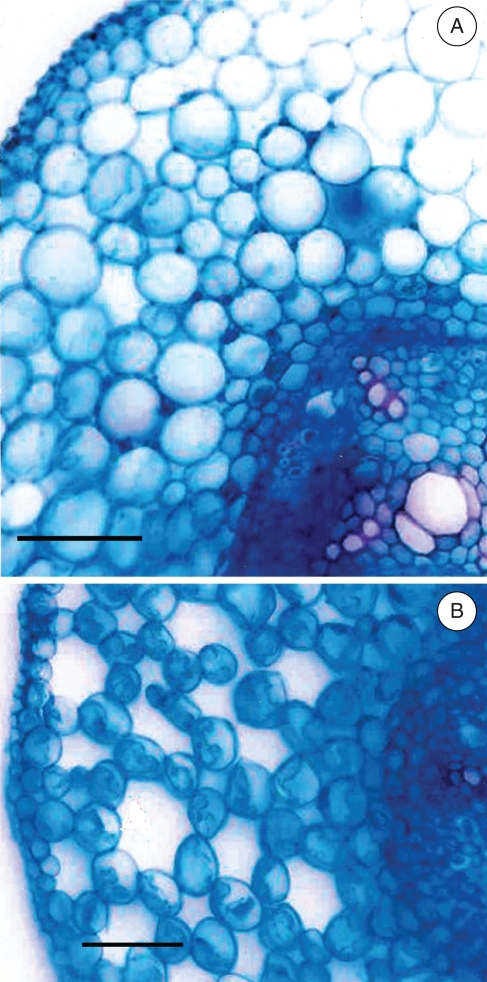
Fig. 9.
Root transverse sections of Erythrina speciosa under (A) control conditions and (B) after 60 d of flooded conditions. Root sections were made between 2 and 3 cm from the root tip, in roots with a length of no more than 5–6 cm. Note the aerenchyma development in flooded plants. Scale bars = 100 µm.
DISCUSSION
Under flood, E. speciosa went through intricate processes involving biochemical and anatomical, as well as morphological effects. This species was highly sensitive to root oxygen deprivation considering that a 90 % decrease in gs values was observed immediately after exposure to treatments. Decreases in gs and ACO2 assimilation rates are a frequent response to soil flooding conditions in both temperate (Gravatt and Kirby, 1998; Cao and Korner, 1999) and tropical species (Lopez and Kursar, 1999, 2003; Núñez-Elisea et al., 1999; Mielke et al., 2003). According to Gravatt and Kirby (1998), a sharp decline of stomatal conductance at the beginning of exposure to low O2 availability is typical for sensitive species. These results indicate that E. speciosa experienced some level of stress, even though it is normally a plant of flood-prone habitats. The stomatal closure under flooded conditions might be related to a decrease in permeability and root hydraulic conductance under anaerobic conditions, which could have induced internal water stress, leading to a decreasing leaf turgor and stomatal conductance (Else et al., 2001; Pezeshki, 2001; Mielke et al., 2003). The decrease in root hydraulic conductance may be related to fewer water channel proteins in the plasma membranes of the roots, due to a lack of metabolic energy to sustain their synthesis (Lambers et al., 1998). The other possibility is related to some chemical signals, particularly abscisic acid (ABA), which may be synthesized in the root tips submitted to hypoxia and transported to leaves, or in wilted leaves and subsequently transported to young turgid leaves (Zhang and Zhang, 1994; Jackson, 2002).
The marked reduction in gs values was followed by a pronounced decline in net ACO2 uptake and WUE. Stomatal closure impairs the CO2 diffusion from the atmosphere into the mesophyll, leading to the reduction of substrate availability (Ci) for RUBISCO activities and thus reductions in photosynthetic rates (Chaves, 1991). Nevertheless, a tendency for an increase in Ci was detected 5 d after flooding, indicating also that decreases in net CO2 uptake might be related to non-stomatal limitations. The increasing Ci coincided with a phase when decreases in ACO2 assimilation were more severely affected by flooding treatment. At the same time, the flooding treatment also adversely affected the WUE once the flooding treatment decreased net CO2 uptake to a greater extent than transpiration rates. Furthermore, in some circumstances stem photosynthesis may become a substantial component of a carbon gain during the period of acute stress imposed by flooding (Armstrong and Armstrong, 2005; Teskey et al., 2008). This aspect deserves future investigation, since some adult individuals of E. speciosa exhibits green woody tissues just below the outer bark.
In their study with several trees from seasonally flooded forest at Venezuela, Fernandez et al. (1999) showed that the reduction in net CO2 uptake induced by flooding was associated with a general inhibition of metabolism caused by hypoxic soil conditions. As a consequence of energy shortage, the general inhibition of metabolism in E. speciosa was perceived by increases in free amino acid content in their root tissue. The decline in the rate of protein synthesis leading to a lower demand for amino acids in the hypoxic conditions could explain the accumulation of free amino acids observed during early treatment imposition (Reggiani and Bertani, 2003).
From the tenth to the 20th day after the beginning of the experiment, E. speciosa was able to recover stomatal conductance and the net CO2 uptake. The same pattern was described for Taxodium distichum (Pezeshki, 1993; Pezeshki et al., 1996) and Melaleuca quinquinervia (Senna-Gomes and Kozlowski, 1980), but not for G. americana (Mielke et al., 2003), which did not exhibit complete stomatal reopening and net CO2 assimilation recovery after 63 d of hypoxia, in spite of the high rate of seedling survival under these conditions (Andrade et al., 1999; Mielke et al., 2003).
Some authors consider the capacity for leaf conductance (gs) resumption after flooding to be the physiological trait that confers flood tolerance, once it allows the net CO2 exchange to continue, with the consequent plant growth and survival under hypoxic conditions (Pezeshki, 1993; Li et al., 2004). This fact was observed for Prioria copaifera, a tree species that forms monodominant stands in seasonally flooded habitats in Panama tropical forests (Lopez and Kursar, 1999). In parallel with photosynthesis recovery, on the 21st day decreases in amino acid concentrations were observed. It is possible that these decreases were caused by a greater demand for amino acids during flooding for the biosynthesis of anaerobically induced enzymes (Vartapetian and Jackson, 1997; Kreuzwieser et al., 2002).
Likewise, quantitative changes, i.e. a shift in composition of amino acids, are usually observed in plants under O2 deprivation (Kreuzwieser et al., 2002; Reggiani and Bertani, 2003; Sousa and Sodek, 2003; Thomas et al., 2005). Under flooding, the Asn, Asp and Glu concentrations decreased while the Ala and GABA concentrations increased on the seventh day of the experiment. For instance, on the tenth day, the Ala and GABA composition represented up to 56 % of the total amino acid content. These results reflect the interconversions between amino acids that help the maintenance of cell pH (Reggiani et al., 1988; Crawford et al., 1994; Fan et al., 1997; Reggiani and Bertani, 2003). These amino acids formed were neutral or less acidic, and therefore this could counteract the elevation of the acid metabolite pool produced by fermentative reactions, such as that of lactate. Our findings also indicate that increases in Ala were proportionally higher when compared with increases in GABA on the tenth day of flooding imposition. Indeed, several studies have reported that a pronounced increase in Ala in root tissues is a typical plant response to O2 deprivation (Drew, 1997; Sousa and Sodek, 2003). Considering that Ala is produced from the reaction of pyruvate with amino-N (Good and Muench, 1993; Reggiani and Bertani, 2003), increases in Ala in roots of E. speciosa may be a consequence of pyruvate accumulation, which could reflect poorly coordinate reactions between glycolysis and alcoholic fermentation (Kreuzwieser et al., 2002). In addition, it is noteworthy that even the initial values of GABA concentration were high. The amount of GABA declined on the 28th day and thereafter remained at elevated values until the 45th day. GABA accumulation has also been observed in several trees and herbaceous species submitted to flooding, and in some cases has been considered as an important physiological response to tolerance to hypoxia (Drew, 1997, Kreuzwieser et al., 2002; Koppitz et al., 2004).
Besides the changes in photosynthetic activities and in the concentration of free amino acids, E. speciosa was able to increase, although at a lower rate, the availability of soluble sugars in roots at the onset of flooding imposition. We suggested that in E. speciosa the increase in soluble sugars could have resulted from a degradation of reserves present in roots or as a function of cellular pH acidification caused by l-lactate accumulation (data not shown). According to Roberts et al. (1992), the l-lactate accumulation during hypoxia is enough to depress the metabolism due to cytoplasmic acidosis and consequently to reduce the consumption of the substrates used in respiration.
The soluble sugars are of special importance for plants growing under O2 deprivation conditions due to their direct relationship with respiration. The switch from aerobic toward anaerobic metabolism yields very few molecules of ATP. Hence a larger sugar pool that can be readily metabolized is crucial for fermentative metabolism to be carried out properly (Gravatt and Kirby, 1988; Chen et al., 2002; Sairam et al., 2008). This hypothesis was elucidated in several experiments where plants kept under O2 shortage fed with exogenous sugars could maintain glycolysis, fermentation and root longevity (Vartapetian and Jackson, 1997, and references therein). Islam et al. (2004) observed that although root sugars declined in Picea americana and Larix laricina, the latter maintained a higher sugar content throughout the experiment. These differences coincided with the production of adventitious roots during flooding in L. laricina but not in P. americana, conferring greater tolerance to hypoxia in the former species (Islam et al., 2004). Probably a similar event may have happened in E. speciosa since the content of soluble sugars declined after morphological modifications were induced by flooding. These results indicate that the reserves were used to form adventitious roots and hypertrophied lenticels as well as to sustain fermentative metabolism.
The adventitious roots, the hypertrophied lenticels and the aerenchyma may alleviate the hypoxia stress, by facilitating oxygen diffusion through a low resistant internal pathway between shoot and root extremities, as well as a higher flow of water, nutrients and hormones to the shoot offsetting the reduced metabolic activities in the original root system (Joly, 1996; Vartapetian and Jackson, 1997; Colmer, 2003; Rengifo et al., 2005). The honeycomb aerenchyma as observed on E. speciosa under flooded conditions (Fig. 9) was probably developed by expansion of intercellular spaces into lacunae by cell division, wall separations and cell expansion, with no lysigeny or cell death (Justin and Armstrong, 1987; Seago et al., 2005). Note that some intercellular spaces are also observed in root cortex tissue from control plants. This aspect shows that the precursor of aerenchyma structure is present even before the occurrence of the flooding events. This might be an important trait in E. speciosa, indicating its likely tolerance to hypoxic conditions. In some species, aerenchymatous adventitious roots became woody even during the flooding events (Armstrong and Armstrong, 2005). Based on our field observations, the adventitious roots in E. speciosa are ephemeral, i.e. after the flooding episode they shrivel and are lost.
The development of hypertrophied lenticels, adventitious roots and aerenchyma coincided with the stomatal reopening and the recovery of photosynthetic rates. It was observed that the lenticels do not promote enough aeration to resume the aerobic metabolism in plants subjected to flooding conditions (Joly, 1991), but for E. speciosa in the present study, the lenticels' role in aeration was verified using the gel agar technique. The O2 diffusion from the base of the stem into the root system through aerenchyma is a common trait for many flood-tolerant plants (Colmer, 2003; Armstrong and Armstrong, 2005).
Flooding generally decreases the relative growth rate and affects the partitioning of carbon (Lopez and Kursar, 2003; Mielke et al., 2003), and indeed the present study shows reductions in the RGR of the flooded plants. These reductions were due to a differential pattern of photoassimilate distribution. The RMR were lower in flooded compared with control plants, which could have been due to alterations in metabolic activities caused by O2 deprivation in the root environment, since a lack of oxygen blocks mitochondrial electron transport, the oxidation of NADH + H+ and ATP synthesis (Drew, 1997; Mielke et al., 2005). On the other hand, increases in SMR could be explained by the increment in stem diameter in flooded plants compared with the control. The increase in the stem diameter is contributed to by the production of the hypertrophied lenticels. The pattern of allocation of photosynthates to root biomass (RMR) in E. speciosa was higher when compared with G. americana, a neotropical species that shows a wide distribution in flood-prone habitats (Mielke et al., 2003). The root/shoot ratio was also higher in E. speciosa when compared with several other tropical tree species, from seasonally flooded and flood-free forests (Lopez and Kursar, 1999, 2003). As pointed out by these authors, the higher investment in the root biomass in plants subjected to flooding could be an important morphological adaptation. In some ecosystems, drought may follow flooding, and species that are able to withstand some level of water limitation after a period of flooding will probably successfully occupy environments which are seasonally flooded (Lopez and Kursar, 1999).
Erythrina speciosa showed 100 % survival throughout 60 d under soil flooding. Although decreases of 86 % in the photosynthetic rates were observed at the beginning of the experiment, E. speciosa showed complete recovery of leaf gas exchange rates. The proportion between acidic amino acids and GABA was maintained under long-term exposure to O2 deprivation, which probably contributed to avoidance of cell acidification. Probably these responses could explain why the plants continue their growth under soil flooding, although at lower rates. It is important to emphasize that the tolerance mechanisms exhibited by the young sapling stage studied here may not be sufficient to sustain mature trees, which certainly require further studies.
In conclusion, the coordinated changes in physiological responses together with the development of typical morphological alterations (lenticels, adventitious roots, aerenchyma) when plants were exposed to deprivation of O2 at the rhizosphere indicated that E. speciosa showed the ability to thrive in water saturation conditions, consistent with its occurrence in flood-prone habitats (Parolin, 2001). The high levels of GABA and the presence of some aerenchyma even in root tissues of control plants indicates that seedlings of E. speciosa are ready to cope with flooding episodes.
ACKNOWLEDGEMENTS
We thank Dr. Sandra M. C. Guerreiro from the Department of Plant Biology/State University of Campinas (Unicamp) for the laboratory facilities in the morphological studies, Miss Janete Mayumi Okamoto for technical assistance during all the experiments and Dr Valdenir Queiroz Ribeiro from the Embrapa Meio-Norte for the statistical analyses. We are also grateful to Professor Dr Tim Colmer and anonymous reviewers for constructive comments on the manuscript. The authors gratefully acknowledge the financial support of the Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (grant 03/12595-7). Carlos A. Joly was supported by a CNPq Productivity Fellowship (grant 520334/99-0).
LITERATURE CITED
- Andrade ACS, Ramos FN, Souza AF, Loureiro MB, Bastos R. Flooding effects in seedlings of Cytharexyllum myrianthum Cham. and Genipa americana L.: responses of two neotropical lowland tree species. Revista Brasileira de Botânica. 1999;22:281–285. [Google Scholar]
- Armstrong W, Armstrong J. Stem photosynthesis not pressurized ventilation is responsible for light-enhanced oxygen supply to submerged roots of alder (Alnus glutinosa) Annals of Botany. 2005;96:591–612. doi: 10.1093/aob/mci213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong W, Brandle R, Jackson MB. Mechanisms of flood tolerance in plants. Acta Botanica Neerlandica. 1994;43:307–358. [Google Scholar]
- Bieleski RL, Turner NA. Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Analytical Biochemistry. 1966;17:278–293. doi: 10.1016/0003-2697(66)90206-5. [DOI] [PubMed] [Google Scholar]
- Cao FL, Conner WH. Selection of flood-tolerant Populus deltoides clones for reforestation projects in China. Forest Ecology and Management. 1999;117:211–220. [Google Scholar]
- Chaves MM. Effects of water deficits on carbon assimilation. Journal of Experimental Botany. 1991;42:1–16. [Google Scholar]
- Chen H, Qualls RG, Blank RR. Effect of soil flooding on photosynthesis, carbohydrate partitioning and nutrient uptake in the invasive exotic Lepidium latifolium. Aquatic Botany. 2002;82:250–268. [Google Scholar]
- Colmer TD. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell and Environment. 2003;26:17–36. [Google Scholar]
- Crawford LA, Bown AW, Breitkreuz KE, Guinel FC. The synthesis of γ-aminobutyric acid in response to treatments reducing cytosolic pH. Plant Physiology. 1994;104:865–871. doi: 10.1104/pp.104.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:223–250. doi: 10.1146/annurev.arplant.48.1.223. [DOI] [PubMed] [Google Scholar]
- Else MA, Coupland D, Dutton L, Jackson MB. Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinnus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap. Physiologia Plantarum. 2001;111:46–54. [Google Scholar]
- Fan TWM, Higashi RM, Frenkiel TA, Lane AN. Anaerobic nitrate and ammonium in flood-tolerant rice coleoptiles. Journal of Experimental Botany. 1997;48:1655–1666. [Google Scholar]
- Fernandez MD, Pieters A, Donoso C, et al. Seasonal changes in photosynthesis of trees in the flooded forest of the Mapire River. Tree Physiology. 1999;19:79–85. doi: 10.1093/treephys/19.2.79. [DOI] [PubMed] [Google Scholar]
- Fernandez MD. Changes in photosynthesis and fluorescence in response to flooding in emerged and submerged leaves of Pouteria orinocoensis. Photosynthetica. 2006;44:32–38. [Google Scholar]
- Good AG, Muench DG. Long-term anaerobic metabolism in root tissue. Plant Physiology. 1993;101 doi: 10.1104/pp.101.4.1163. 1163 1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D, Smydzuc J. Use of anthrone in the quantitative determination of hexose phosphates. Analytical Biochemistry. 1965;11:246–255. doi: 10.1016/0003-2697(65)90012-6. [DOI] [PubMed] [Google Scholar]
- Gravatt DA, Kirby CJ. Patterns of photosynthesis and starch allocation in seedlings of four bottomland hardwood tree species subjected to flooding. Tree Physiology. 1998;18:411–417. doi: 10.1093/treephys/18.6.411. [DOI] [PubMed] [Google Scholar]
- Huang B, Johnson JW. Root respiration and carbohydrate status of two wheat genotypes in response to hypoxia. Annals of Botany. 1995;75:427–432. [Google Scholar]
- Hunt R. Plant growth curves. The functional approach to growth analysis. London: Edward Arnold; 1982. [Google Scholar]
- Islam MA, Macdonald SE. Ecophysiological adaptations of Black spruce (Picea mariana) and tamarack (Larix laricina) seedlings to flooding. Trees. 2004;18:35–42. [Google Scholar]
- Jackson MB. Long-distance signalling from roots to shoots assessed: the flooding story. Journal of Experimental Botany. 2002;53:175–181. doi: 10.1093/jexbot/53.367.175. [DOI] [PubMed] [Google Scholar]
- Joly CA. Flooding tolerance in tropical trees. In: Jackson MB, Davies DD, Lambers H, editors. Plant life under oxygen deprivation. Ecology, physiology and biochemistry. The Hague: SPB Academic Publishing; 1991. pp. 23–34. [Google Scholar]
- Joly CA. Flooding tolerance: a reinterpretation of Crawford's metabolic theory. Proceedings of the Royal Society of Edinburgh. 1994;102B:343–354. [Google Scholar]
- Joly CA. The role of oxygen diffusion to the root system on the flooding tolerance of tropical trees. Revista Brasileira de Biologia. 1996;56:375–382. [Google Scholar]
- Justin SHFW, Armstrong W. The anatomical characteristics of roots and plant response to soil flooding. New Phytologist. 1987;106:465–495. [Google Scholar]
- Kolb RM, Rawler A, Braendle R. Parameters affecting the early seedling development of four Neotropical trees under oxygen deprivation stress. Annals of Botany. 2002;89:551–558. doi: 10.1093/aob/mcf092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppitz H, Dewender M, Ostendorf W, Schmieder K. Amino acids as indicators in comom reed Phragmites australis affected by extreme flood. Aquatic Botany. 2004;79:277–294. [Google Scholar]
- Kozlowski TT. Responses of woody plants to flooding and salinity. Tree Physiology Monograph. 1997;1:1–29. [Google Scholar]
- Kreuzwieser J, Fürniss J, Rennenberg H. Impact of waterlogging on the N-metabolism of flood tolerant and non-tolerant tree species. Plant, Cell and Environment. 2002;25:1039–1049. [Google Scholar]
- Krukoff BA. The American species of Erythrina. Brittonia. 1939;3:205–337. [Google Scholar]
- Lambers H, Chapin ST, III, Pons TJ. Plant physiological ecology. Berlin: Springer-Verlag; 1998. [Google Scholar]
- Li S, Pesezki SR, Goodwin S, Shields FDJ. Physiological responses of black willow (Salix nigra) cuttings to a range of soil moisture regimes. Photosynthetica. 2004;42:585–590. [Google Scholar]
- Lobo PC, Joly CA. Tolerance to hypoxia and anoxia in neotropical tree species. In: Scarano FR, Franco AC, editors. Ecophysioloical strategies of xerophytic and amphybious plants in the neotropics. Series Oecologia Brasiliensis. vol. IV. Rio de Janeiro: PPGE-UFRJ; 1998. pp. 137–156. [Google Scholar]
- Lopez OR, Kursar TA. Flood tolerance of four tropical tree species. Tree Physiology. 1999;19:925–932. doi: 10.1093/treephys/19.14.925. [DOI] [PubMed] [Google Scholar]
- Lopez OR, Kursar TA. Does flood tolerance explain tree species distribution in tropical seasonally flooded habitats? Oecologia. 2003;136:193–204. doi: 10.1007/s00442-003-1259-7. [DOI] [PubMed] [Google Scholar]
- Lorenzi H. Árvores Brasileiras. Manual de identificaçãp e cultivos de plantas arbóreas nativas do Brasil. Nova Odessa: Editora Plantarum; 1992. [Google Scholar]
- Mielke MS, Almeida AAF, Gomes FP, Aguilar MAG, Mangabeira PAO. Leaf gas exchange, chlorophyll fluorescence and growth responses of Genipa americana seedlings to soil flooding. Environmental and Experimental Botany. 2003;50:221–231. [Google Scholar]
- Mielke MS, Matos EL, Couto VB, Almeida AAF, Gomes FP, Mangabeira PAO. Some photosynthetic and growth responses of Annona glabra L. seedlings to soil flooding. Acta Botanica Brasilica. 2005;19:905–911. [Google Scholar]
- Moore S, Stein WH. Photometric ninhydrin method for use in the chromatography of amino acids. Journal of Biology Chemistry. 1948;176:367–388. [PubMed] [Google Scholar]
- Muthuchelian K, Murugan C, Harigovindan R, Nedunchezhian N, Kulandaivelu G. Effect of triacontanol in flooded Erythrina variegata seedlings. 1. Changes in growth, photosynthetic pigments and biomass productivity. Photosynthetica. 1995;31:269–275. [Google Scholar]
- Nobel PS. Physicochemical and environmental plant physiology. San Diego: Elsevier/Academic Press; 2005. [Google Scholar]
- Núñez-Elisea R, Schaffer B, Fischer JB, Collis AM, Crane JH. Influence of flooding on net CO2 assimilation, growth and stem anatomy of Annona species. Annals of Botany. 1999;84:771–780. [Google Scholar]
- Ortolani AA, Camargo MBP, Pedro MJ., Jr . Normas climatológicas dos pontos meteorológicos do Instituto Agronômico: 1. Centro Exeprimental de Campinas. Campinas: Instituto Agronômico; 1995. p. 13. Boletim Técnico, 155. [Google Scholar]
- Parolin P. Morphological and physiological adjustments to waterlogging and drought in seedlings of Amazonian floodplain trees. Oecologia. 2001;128:326–335. doi: 10.1007/s004420100660. [DOI] [PubMed] [Google Scholar]
- Pezeshki SR. Differences in patterns of photosynthetic responses to hypoxia in flood tolerant and flood sensitive tree species. Photosynthetica. 1993;28:423–430. [Google Scholar]
- Pezeshki SR. Wetland plant responses to soil flooding. Environmental and Experimental Botany. 2001;46:299–312. [Google Scholar]
- Pezeshki SR, Pardue JH, Delaune RD. Leaf gas exchange and growth of flood-tolerant and flood sensitive tree species under low soil redox conditions. Tree Physiology. 1996;16:453–458. doi: 10.1093/treephys/16.4.453. [DOI] [PubMed] [Google Scholar]
- Puiatti M, Sodek L. Waterlogging affects nitrogen transport in the xylem of soybean. Plant Physiology and Biochemistry. 1999;37:767–773. [Google Scholar]
- Reggiani R, Bertani A. Anaerobic amino acid metabolism. Russian Journal of Plant Phsysiology. 2003;50:733–736. [Google Scholar]
- Reggiani R, Cantú CA, Brambilla I, Bertani A. Accumulation and interconversion of amino acids in rice roots under anoxia. Plant and Cell Physiology. 1988;9:982–987. [Google Scholar]
- Rengifo E, Tezara W, Herrera A. Water relations, chlorophyll a fluorescence, and contents of saccharides in tree species of a tropical forest in response to flood. Photosynthetica. 2005;43:203–210. [Google Scholar]
- Roberts JKM, Hooks MA, Miaullis AP, Edwards S, Webster C. Contribution of malate and amino acid metabolism to cytoplasmic pH regulation in hypoxic maize root tips studied using nuclear magnetic resonance spectroscopy. Plant Physiology. 1992;98:480–487. doi: 10.1104/pp.98.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairam RK, Kumutha D, Ezhilmathi K, Deshmukh PS, Srivastava GC. Physiology and biochemistry of waterlogging tolerance in plants. Biologia Plantarum. 2008;52:401–412. [Google Scholar]
- Seago JL, Jr, Marsh LC, Stevens KJ, Soukup A, Votrubová O, Enstone DE. A re-examination of the root cortex in wetland flowering plants with respect to aerenchyma. Annals of Botany. 2005;96:565–579. doi: 10.1093/aob/mci211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senna-Gomes AR, Kozlowski TT. Growth responses and adaptations of Fraxinus pennsylvanica seedlings to flooding. Plant Physiology. 1980;66:267–271. doi: 10.1104/pp.66.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JGC, Potgieter GP, Botha FC. Anoxic seed germination of Erythrina caffra: ethanol fermentation and response to metabolic inhibitors. Journal of Experimental Botany. 1989;40:375–381. [Google Scholar]
- Snedecor GW, Cochrane WG. Statistical methods. 6th edn. Ames, IA: Iowa State University Press; 1967. [Google Scholar]
- Sousa CAF, Sodek L. Alanine metabolism and alanine aminotransferase activity in soybean (Glycine max) during hypoxia of the root system and subsequent return to normoxia. Environmental and Experimental Botany. 2003;50:1–8. [Google Scholar]
- Tang ZC, Kozlowski TT. Some physiological and morphological responses of Quercus macrocarpa seedlings to flooding. Canadian Journal of Forest Research. 1982;12:196–202. [Google Scholar]
- Teskey RO, Saveyn A, Stepper K, McGuire MA. Origin, fate and significance of CO2 in tree stems. New Phytologist. 2008;177:17–32. doi: 10.1111/j.1469-8137.2007.02286.x. [DOI] [PubMed] [Google Scholar]
- Thomas AL, Guerreiro SMC, Sodek L. Aerenchyma formation and recovery from hypoxia of the flooded root system of nodulated soybean. Annals of Botany. 2005;96:1191–1198. doi: 10.1093/aob/mci272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T, Kozlowski TT. Importance of adventitious roots to growth of flooded Platanus occidentalis seedlings. Plant and Soil. 1986;88:123–132. [Google Scholar]
- Vartapetian BB, Jackson MB. Plant adaptations to anaerobic stress. Annals of Botany. 1997;79(Supplement A):3–20. [Google Scholar]
- Visser EJW, Voesenek ACJ, Vartapetian BB, Jackson MB. Flooding and plant growth. Annals of Botany. 2003;91:107–109. [Google Scholar]
- Zhang J, Zhang X. Can early wilting of old leaves account for much of the ABA accumulation in flooded pea plants? Journal of Experimental Botany. 1994;45:1335–1342. [Google Scholar]