Abstract
Background and Aims
There is a widely used crude method to estimate the age of hedgerows (Hooper's rule) based on species' richness. The aim of this study was to try and establish a similar field method for estimating the age of grasslands based on the accumulation of macro-somatic mutations.
Methods
A countrywide survey was carried out by the British public to investigate the relationship between grassland age and the number of Ranunculus repens (creeping buttercup) plants with extra petals. In addition the relationship between grassland age and R. repens pollen viability was also investigated.
Key Results
Each plant with flowers with additional petals in a sample of 100 was found to equate to approx. 7 years. A higher significant correlation was observed between pollen viability and population age; however, this is not amenable to providing field estimates.
Conclusions
The age of British grasslands can be easily and reliably estimated in the field by counting the number flowers with additional petals in R. repens in meadows up to 200 years old. An attempt to estimate the heritability of extra petals suggests that the phenotype results from the slow accumulation of somatic mutations in a species that primarily reproduces vegetatively.
Key words: Conservation, meadow, Müller's ratchet, petal number, pollen viability, Ranunculus repens, buttercup, somatic mutation
INTRODUCTION
The correlation between the rate of colonization of hedges by woody species and time has long been used to estimate the age of hedgerows (Pollard et al., 1974). Estimates of age based on this simple ecological relationship are very crude but the method has been widely used to inform conservation activity by identifying potentially ancient hedgerows. ‘Hooper's rule’ as the relationship is known, has become part of country folk-lore (Rackham, 1986). Unfortunately no similar simple field-based methods exist to estimate the age of other habitats such as grasslands.
The accumulation of micro-mutations at the molecular level has been widely used, criticised and refined to date various evolutionary events (Kimura, 1968; Ayala, 1999; Schwartz and Maresca, 2006). As micro-mutation events are known to be rare (Drake et al., 1998), their occurrence has only been considered useful in dating very distant events such as speciation. If micro-mutations are rare, logic might suggest that macro-mutations are even rarer and would therefore only be of use in dating very ancient history. However, in species with long-lived primarily asexual life-histories the entire genome can effectively act as one linkage group that may rapidly accumulate somatic mutations. If they are recessive, only slightly deleterious or neutral, these mutations fail to be purged by somatic selection. Thus, significant chromosomal abnormalities can accumulate in asexual species by the process described as Müller's ratchet (Müller, 1964).
The occurrence of macro-chromosomal changes expressed phenotypically as reduced fertility is well documented in several clonal plants: Potentilla anserine (Ockendon and Walters, 1970), Holcus mollis (Harberd, 1967) and Crocus spp. (Brighton et al., 1983).
Similarly, the phenotypic expression of easily identified somatic mutations is well known in asexually propagated plants and has been important in generating variation in horticulture and agriculture, e.g. the production of pink and red grapefruit (Cameron et al., 1964). If such easily identified macro-mutations occur frequently then they may be utilized to estimate the time since recent ecological events rather than distant evolutionary ones.
Here the possibility of using the phenotypic frequency of occurrence of additional petals and reduced pollen viability is investigated in the primarily vegetatively reproducing creeping buttercup Ranunculus repens in order to estimate the age of the recent the ecological event of pasture establishment.
MATERIALS AND METHODS
During May and June 2008 a high profile public appeal to collect data was made across the UK via a wide range of print, broadcast and web-based media. Participants were asked to locate meadows of known age (time since last reseeded) and then randomly sample 100 R. repens L. flowers and record the number of flowers with more than the standard five petals. Ranunculus repens predominantly reproduces by vegetative means, whereas R. acris and R. bulbosus (two similar common sympatric species) primarily regenerate from seed (Sarukhan, 1974); for this reason, R. repens was thought to be more likely to be long-lived and thus accumulate somatic mutations. Simple guidance notes were therefore provided to help distinguish R. repens from R. acris and R. bulbosus. Over the next two months a total of 204 usable responses were received, several with multiple records. A few records were omitted from the analysis because of anomalies in the supporting text, and no records older than 200 years were included because of their rarity and the difficulties associated with accurately establishing their age. Records taken after July were also discarded because petal number is known to increase over the season in some species (Donnison and Francis, 2002) and flowering is more sporadic.
Over the same period a parallel survey was carried out in a range of different grasslands of known ages around Aberystwyth. In each site, ten flowers were collected randomly, and stored in individual tubes in 70 % ethanol. Subsequently the flowers were dissected and one or two randomly selected anthers placed on a microscope slide. Following staining with Alexander's triple stain (Alexander, 1969) the total numbers of viable and non-viable pollen grains were scored in a sample of between 200 and 300 pollen grains.
Heritability estimates
To estimate the heritability of additional petal production and pollen viability traits, ripe seed samples were collected from open-pollinated R. repens plants growing in an unimproved meadow at Aberystwyth University (52°24′34·5″N and 4°2′52·0″W). The seeds were collected in June 2007 when the meadow was known to be 19 years old. Seeds were collected from ten R. repens plants each with flowers with additional petals and from ten R. repens with no additional petals. The parent plants were collected at the same time and transferred into pots. The seeds were sown in July and ten progeny plants from each of the 20 female parents were grown outside in pots along with the 20 parent plants in a fully randomized design until flowering in April 2008. On flowering all of the progeny and parents were scored for the presence of flowers with additional petals and the proportion of viable to non-viable pollen grains scored as above.
RESULTS
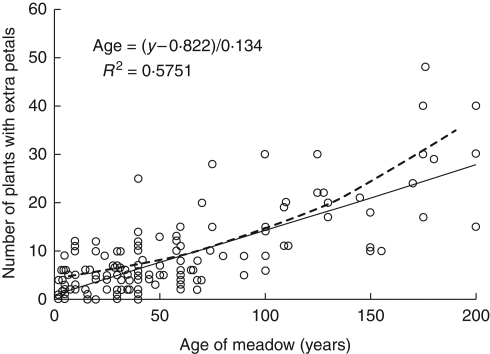
In order to establish the relationship between the production of extra petals and age of the pasture, the results collected by the public survey were plotted and both a logistic and linear regressions carried out (Fig. 1). As the response variable is binary in form, with extra petals either being present or not, then technically a logistic regression should be used. However, the aim of the study was to produce a simple field tool for estimating the age of meadows, so linear regression was also applied. The results reveal a significant correlation (P < 0·001 with both logistic and linear regression) between the number of flowers with extra petals and the known age of the grassland. Over the mid-age range (50–100 years) the logistic and linear regressions were very similar. As a crude estimation every flower with additional petals equates to roughly seven years. Consequently a sample of 100 R. repens flowers taken in a 100-year-old meadow would be expected to contain about 14 flowers with extra petals. There was very little effect of constraining the linear regression to pass through the origin; the equation of the line becomes x = (y – 0·571)/0·142 with R2 = 0·571. In contrast, the logistic regression provides a poor fit to the data close to the origin, with the age of a meadow containing a single flower with extra petals being estimated as –115 years. Over the timescale plotted there was no evidence of a plateau in the data and fitting a polynomial function to the results has virtually no effect on the R2 value.
Fig. 1.
Relationship between the age of a meadow and the number of R. repens flowers from a random sample of 100 with more than five petals. The continuous line is the linear regression, with the equation given; the dashed line is the logistic regression of the form: age = 1/0·013[ln(p/1 – p) + 3·092] (P < 0·001 with both logistic and linear regression).
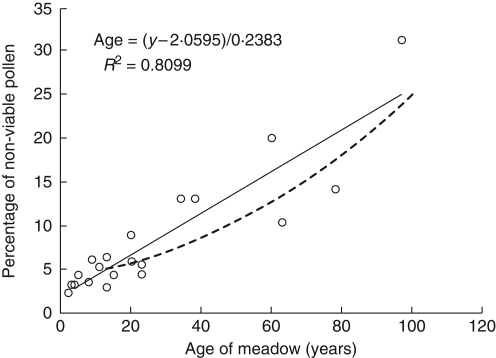
Logistic and linear regressions were also carried out on the pollen viability data and the known age of the pasture (Fig. 2). Again a positive significant correlation (P < 0·001 with both logistic and linear regression) was found between the two variables, with older pastures being associated with a higher proportion of non-viable pollen grains. In this case, the simple linear regression appeared to give a better fit to the data than did the logistic regression. A smaller age range of grasslands was investigated and again there was no evidence of a plateau in the data and the R2 value was not increased by fitting a more complex model to the data.
Fig. 2.
Relationship between the age of a meadow and the average percentage of non-viable pollen found in random sample of ten R. repens flowers per meadow. The continuous line is the linear regression, with the equation given; the dashed line is the logistic regression of the form: age = 1/0·021[ln(p/1 – p) + 3·201] (P < 0·001 with both logistic and linear regression).
Heritability estimates
The majority of the 200 progeny plants survived and produced several flowers through April and May 2008. Of these 200 plants, none were observed to produce flowers with extra petals, irrespective of their parentage. However, the parent plants retained their original petal number phenotypes with 100 % fidelity. The heritability of the trait can therefore be assumed to be zero or nearly so.
When the pollen viability of the two progeny classes were compared by one-way analysis of variance of the arcsine-transformed proportions, no significant difference was found. However, a similar comparison of the parent groups revealed that the parents with additional petals produced significantly more non-viable pollen (P < 0·001). A female parent offspring regression also indicated that the narrow sense heritability (h2) of the trait was not significant (0·02 ± 0·05).
DISCUSSION
Floral traits are generally considered to be highly conserved in evolution, so much so that they frequently form the basis of taxonomic differentiation. Even so the nature of mutations affecting floral development has been extensively researched (Crone and Lord, 1993; Nagasawa et al., 1996; Xu et al., 2008). These studies have focused on floral development in sexually reproducing species and have not correlated mutations with clone age. The link between increased frequency of pollen abortion and clone age (Fig. 2) is well established in many asexually reproducing species (Ockendon and Walters, 1970; Harberd, 1967; Brighton et al., 1983). However, this character is hardly amenable for adaptation to produce a field assessment of habitat age.
The relationship between time and the frequency of floral mutations revealed in Fig. 1 is novel and provides a simple and quick field method to estimate the approximate age of grasslands. Although the linear regression has a large standard error (5·54), it is of similar strength to the commonly used ‘Hooper's rule’ which estimates the age of hedges (Pollard et al., 1974). Although it has been criticized, Hooper's rule, has proved of considerable value as a rough tool in estimating the age of hedges, and with some refinement, many of the criticisms have been addressed (Rackham, 1986). As with Hooper's rule, the correlation reported here may be refined with greater ecological/historical knowledge. For example, in Fig. 2, many of the pastures that fall below the regression line are used for grazing horses. Swards grazed by horses are likely to be frequently damaged by their feet, unlike mown grasslands. Under these conditions recruitment from seed is likely to be more frequent (Diemer and Schmid, 2001). Increased seedling recruitment in horse pastures will make them appear to be younger than mown grassland of a similar age and so they tend to occur below the regression line. Thus, if the true age of the pasture is known, then an assessment of the number of flowers with additional petals may provide an estimate of the long-term rate of seedling establishment. Ranunculus repens is common and native to Europe, temperate Asia and northern Africa, and it is widely naturalized to the point of invasiveness across much of Australasia, southern Africa, North and South America, (USDA, 2009). Thus there is every reason to expect that this relationship will hold across many temperate grasslands.
Over much of the relationship, the logistic and linear regressions are similar and both provide a reasonable fit to the data. While it may be technically more correct to use a logistic regression, the linear regression is more amenable to providing a field method to estimate the age of meadows. With older meadows, however, the two regressions methods diverge. Here the exact nature of the relationship is less certain, because there are fewer data points and the documented ages of meadows are likely to be unreliable. Furthermore, while the rate of mutation is expected to be a constant, the vegetative spread of older clones may mean that the occurrence of extra petals may be over recorded. However, even with very ancient meadows, it seems unlikely that the proportion of plants with additional petals will reach in excess of 50 %, because there will be an equilibrium of mutation rates and seedling recruitment.
The lack of evidence for heritability of additional petals and increased pollen abortion, suggest that both traits are the result of somatic mutations accumulating over time in a predominantly vegetatively reproducing species. The accumulation of chromosomal mutations by Müller's ratchet in long-lived primarily asexually reproducing species is known to result in reduced levels of fertility (Ockendon and Walters, 1970; Harberd, 1967; Brighton et al., 1983). Such mutations are not removed by selection, if regeneration from seed is rare. Similarly, the production of extra petals may be close to selective neutrality because (a) it may not affect seed production and (b) seed production may have little effect on fitness if most reproduction occurs vegetatively. It is difficult to measure the somatic mutation rate in R. repens precisely from the results reported here, because clonal plants are known to develop as complex mosaics of genetic variation. However, the levels observed here are similar to those reported in several other clonal species such as blackberry, potato and cultivated citrus (Whitham and Slobodchikoff, 1981).
The fact that floral morphology and pollen viability were both restored in the progeny plants, suggests that the meiotic sieve was operating and removing the chromosomal mutations responsible for these traits in the parents (Richards, 1996). This is important if either trait is to be used as a tool for estimating the age of grasslands. It is reasonable to assume that following cultivation and re-establishment, there is a significant increase in the proportion of the population of R. repens plants derived from seed rather than vegetatively. Thus having a new population of plants regenerating from seed will effectively reset the mutation clock to zero (or nearly so) and allow the cultivation event to be dated until the next catastrophe in the population. However, in fields with complex terrains, cultivation may be less effective at removing vegetative plants and the age of such fields may be overestimated. Additional ecological knowledge of this kind will help refine the use of this tool for estimating the age of grasslands.
ACKNOWLEDGEMENTS
I would like to thank The Times and many other publishers and broadcasters for publicizing this study and the British public for collecting data. I am also grateful to Penri James and Mike Rose for commenting on the manuscript and to David Causton, Silvia Lutkins and Tony Hyde for statistical advice.
LITERATURE CITED
- Alexander MP. Differential staining of aborted and nonaborted pollen. Stain Technology. 1969;44:117–122. doi: 10.3109/10520296909063335. [DOI] [PubMed] [Google Scholar]
- Ayala FJ. Molecular clock mirages. Bioessays. 1999;21:71–75. doi: 10.1002/(SICI)1521-1878(199901)21:1<71::AID-BIES9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Brighton CA, Mathew B, Rudall P. A detailed study of Crocus speciosus and its ally C. pulchellus (Iridiceae) Plant Systematics and Evolution. 1983;142:187–206. [Google Scholar]
- Cameron JW, Soost RK, Olson EO. Chimeral basis for color in pink and red grapefruit. Journal of Heredity. 1964;55:23–28. [Google Scholar]
- Crone W, Lord EM. Flower development in the organ number mutant clavata1-1 of Arabidopsis thaliana (Brassicaceae) American Journal of Botany. 1993;80:1419–1426. [Google Scholar]
- Diemer M, Schmid B. Effects of biodiversity loss and disturbance on the survival and performance of two Ranunculus species with differing clonal architectures. Ecography. 2001;24:59–67. [Google Scholar]
- Donnison IS, Francis D. Models of floral pattern in detached flowers of Silene coeli-rosa Godr. (Caryophyllaceae) Botanical Journal of the Linnean Society. 2002;140:229–235. [Google Scholar]
- Drake JW, Charlesworth B, Charlesworth D, Crow JF. Rates of spontaneous mutation. Genetics. 1998;148:1667–1686. doi: 10.1093/genetics/148.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harberd DJ. Observations on natural clones of Holcus mollis. New Phytologist. 1967;66:401–408. [Google Scholar]
- Kimura M. Evolutionary rate at a molecular level. Nature. 1968;217:624–625. doi: 10.1038/217624a0. [DOI] [PubMed] [Google Scholar]
- Müller HJ. The relation of recombination to mutational advance. Mutation Research. 1964;106:2–9. doi: 10.1016/0027-5107(64)90047-8. [DOI] [PubMed] [Google Scholar]
- Nagasawa N, Miyoshi M, Kitano H, Satoh H, Yasuo Nagato Y. Mutations associated with floral organ number in rice. Planta. 1996;198:627–633. doi: 10.1007/BF00262651. [DOI] [PubMed] [Google Scholar]
- Ockendon DJ, Walters SM. Studies in Potentilla anserine L. Watsonia. 1970;8:135–144. [Google Scholar]
- Pollard E, Hooper MD, Moore NW. Hedges. London: Collins; 1974. [Google Scholar]
- Rackham O. The history of the countryside. London: Dent and Sons; 1986. [Google Scholar]
- Richards AJ. Genetic variability in the obligate apomicts of the genus Taraxacum. Folia Geobotanica. 1996;31:405–414. [Google Scholar]
- Schwartz JH, Maresca B. Do molecular clocks run at all? A critique of molecular systematics. Biological Theory. 2006;1:357–371. [Google Scholar]
- Sarukhan J. Studies on plant demography: Ranunculus repens L., R. bulbosus L. and R. acris L. II. Reproductive strategies and seed population dynamics. Journal of Ecology. 1974;62:151–157. [Google Scholar]
- USDA. Germplasm Resources Information Network. Taxonomy for plants. 2009. http://www.ars-grin.gov/cgi-bin/npgs/html/taxon.pl?30841 .
- Whitham TG, Slobodchikoff CN. Evolution by individuals, plant–herbivore interactions, and mosaics of genetic variability; the adaptive significance of somatic mutations in plants. Oecologia. 1981;49:287–292. doi: 10.1007/BF00347587. [DOI] [PubMed] [Google Scholar]
- Xu B, Li Z, Zhu Y, et al. Arabidopsis genes AS1, AS2, and JAG negatively regulate boundary-specifying genes to promote sepal and petal development. Plant Physiology. 2008;146:566–575. doi: 10.1104/pp.107.113787. [DOI] [PMC free article] [PubMed] [Google Scholar]