Abstract
Background and Aims
The major economic product of Hevea brasiliensis is a rubber-containing cytoplasm (latex), which flows out of laticifers (latex cells) when the bark is tapped. The latex yield is stimulated by ethylene. Sucrose, the unique precursor of rubber synthesis, must cross the plasma membrane through specific sucrose transporters before being metabolized in the laticifers. The relative importance of sucrose transporters in determining latex yield is unknown. Here, the effects of ethylene (by application of Ethrel®) on sucrose transporter gene expression in the inner bark tissues and latex cells of H. brasiliensis are described.
Methods
Experiments, including cloning sucrose transporters, real time RT-PCR and in situ hybridization, were carried out on virgin (untapped) trees, treated or untreated with the latex yield stimulant Ethrel.
Key Results
Seven putative full-length cDNAs of sucrose transporters were cloned from a latex-specific cDNA library. These transporters belong to all SUT (sucrose transporter) groups and differ by their basal gene expression in latex and inner soft bark, with a predominance of HbSUT1A and HbSUT1B. Of these sucrose transporters, only HbSUT1A and HbSUT2A were distinctly increased by ethylene. Moreover, this increase was shown to be specific to laticifers and to ethylene application.
Conclusion
The data and all previous information on sucrose transport show that HbSUT1A and HbSUT2A are related to the increase in sucrose import into laticifers, required for the stimulation of latex yield by ethylene in virgin trees.
Key words: Hevea brasiliensis, laticifers, latex production, ethylene, sucrose transporters
INTRODUCTION
Natural rubber (cis-polyisoprene) is the main economic product of Hevea brasiliensis and is widely used industrially. cis-Polyisoprene synthesis takes place in the cytoplasm of highly specialized cells, known as laticifers (latex cells), and is their main metabolic function. Because sucrose is the unique precursor for rubber synthesis, its import into the laticifers may be an important limiting factor in latex production.
Laticifers are periodically differentiated from the cambium and arranged in an isolated network in the inner bark of H. brasiliensis (Hébant and de Faÿ, 1980; de Faÿ and Jacob, 1989). The cytoplasm of laticifers, known as latex, is expelled when the bark is wounded or deliberately regularly cut (tapped) to obtain the latex (Gomez, 1976; Gomez and Moir, 1979). Rubber particles represent 30–50 % of the latex in volume and 90 % in dry weight, and therefore laticifers have to regenerate their cytoplasm between two consecutive tappings. Much research has shown that rubber synthesis is brought about through the mevalonate-dependent metabolic pathway (Hepper and Audley, 1969), but more recently, transcriptome studies have suggested that synthesis could also follow a mevalonate-independent pathway (Ko et al., 2003). Both pathways coexist in laticifers and need sucrose as a precursor (d'Auzac, 1964; Chow et al., 2007). In addition, sucrose contributes, together with polyols and potassium, to the generation of the high turgor pressure (0·9–1·5 MPa) in the laticifers and their surrounding cells in the soft bark (liber), which is important for latex flow (Buttery and Boatman, 1966). Moreover, the cytosolic sucrose concentration is used to evaluate metabolic activity of laticifers (Jacob et al., 1988, 1989).
Laticifers are regarded as a strong sink for sucrose and need to be adequately supplied exogenously with sucrose to meet their high carbon and energy demands (Tupy, 1973; Eschbach et al., 1986; Silpi et al., 2007). Based on a few circumstantial observations, active transport across the plasma membrane of laticifers had been hypothesized as the main route of sugar uptake. Ultrastructural studies on mature laticifers showed that they have no plasmodesmata and are therefore apoplastically isolated from adjacent cells (Hébant, 1981; de Fay and Jacob, 1989). Electrophysiological research carried out on isolated laticifers and protoplasts indicates that sucrose is able to depolarize the plasma membrane and this process is sensitive to H+-ATPase inhibitors (Bouteau et al., 1991, 1992, 1999), suggesting the existence of an energy-dependent, H+/sucrose symporter in the plasma membrane. However, there are no reports, based on molecular evidence, of the presence of such sucrose transporters in the laticifers.
Sucrose transporter (SUT) cDNAs have been isolated mainly from herbaceous plants, but also from some woody plants (Lemoine, 2000; Sauer, 2007). Generally, these sucrose transporters belong to the major facilitative superfamily (MFS; Marger and Saier, 1993) and are encoded by a multigenic family. Their activity was reported to be finely controlled (Sakr et al., 1997; Chiou and Bush, 1998; Roblin et al., 1998; Vaughn et al., 2002; Schneidereit et al., 2008) and modulated by exogenous (Juergensen et al., 2003; Meyer et al., 2004; Decourteix et al., 2006) as well as endogenous signals such as sucrose (Chiou and Bush, 1998) and hormones (Chincinska et al., 2008). Phylogenetic analyses have shown that sucrose transporter proteins fall into three independent phylogenetic clades, called SUT1, SUT4 and SUT2 (Barker et al., 2000; Weise et al., 2000; Barth et al., 2003).
Sucrose transporters play a key role in many physiological processes. This is true both for source and for sink organs. For example, AtSUC5, specifically expressed in the endosperm, is required for the supply of sucrose to seeds during early stages of development (Baud et al., 2005). Based on comparison of expression patterns of three sucrose transporters of the grape berry (VvSUC11, VvSUC12 and VvSUC27), Davies et al. (1999) suggested that only two sucrose transporters (VvSUC11, VvSUC12) might have a significant role in ripening-related sucrose import. Inhibition of LeSUT2 in tomato by antisense expression resulted in reduction of the number of fruit and the number of seed fertilized per fruit. This phenotype is due to the inability of these organs to download sucrose from phloem, and supports the central role of this sucrose transporter in fruit and seed development (Hackel et al., 2006). JrSUT1 (from Juglans regia sucrose transporter), isolated from xylem parenchyma cells, is involved in sucrose retrieval from the xylem vessel after freezing and thawing (Decourteix et al., 2006) and in providing sucrose to bursting vegetative buds (Decourteix et al., 2008). Although sucrose is the predominant soluble sugar in H. brasiliensis latex and is required for rubber synthesis and for latex cell cytoplasm regeneration between tappings, no study has yet focused on the involvement of sucrose transporters in these processes.
As ethylene was shown to be an efficient stimulant of latex yield (Abraham et al., 1968; d'Auzac and Ribaillier 1969), Ethrel® and ethylene gas are commonly used to increase rubber yield in plantations worldwide. This stimulation may occur in several ways: through an increase in sugar metabolism (Tupy, 1973; Tupy and Primot, 1976; Silpi et al., 2006) and the consecutive increase in energy (ATP) availability (Amalou et al., 1992), by increased sugar import (Lacrotte et al., 1985) and by a hyperpolarization of the laticifer's plasma membrane (Bouteau et al., 1992), suggesting a possible stimulation of H+-ATPase plasma membrane by ethylene application, activity of which is required for a plasma membrane H+-symporting sucrose transporter.
Rubber biosynthesis is the major metabolic feature of laticifers, and requires exclusively sucrose as precursor. These cells are a metabolically active sink but are apoplastically isolated from neighbouring cells. Therefore, to meet adequately their substantial carbon and energy needs, they have to import sucrose through hypothetical plasma membrane sucrose/H+ symporters. Our hypothesis is that these kinds of transporters (sucrose/H+ symporters) may be localized on the plasma membrane of laticifers and are positively regulated by ethylene application to increase sucrose import, required for the stimulation of latex production by ethylene. The present study was carried out on unexploited (virgin) trees treated or untreated with the latex yield stimulant Ethrel. Mature virgin trees were used to analyse independently the direct effects of ethylene per se upon a first tapping, and then upon a second tapping to study the combined effects of ethylene and of the metabolic changes due to the previous tapping (latex regeneration demand) on sucrose transporter gene expression. In addition, study was made of the clone PB217, which is characterized by medium rubber production and metabolic activity without ethylene stimulation, but with high latex yield when treated with ethylene (Gohet, 1996; Gohet et al., 1997, 2003).
Among seven sucrose transporter cDNAs identified, the relative transcript level of two isoforms (HbSUT1A and HbSUT2A) is found to be increased by ethylene application. Further characterization showed that they were induced specifically by ethylene. In addition, their localization in the inner bark tissues and especially in the latex cells was confirmed by in situ hybridization. The data show that these two isoforms could play an essential role in sucrose import into laticifers of virgin trees, necessary for ethylene-stimulated latex production.
MATERIALS AND METHODS
Plant material
Latex and bark samples were collected from the trunk of mature virgin rubber trees (about 8 years old) of the PB217 clone in the Bongo/SAPH plantation (Côte d'Ivoire). Latex samples were collected as described by Pujade-Renaud et al. (1994). Briefly, after discarding the first 20 drops, the latex samples were collected as a mixture of 2 mL from three trees per treatment in an equal volume of 2× fixation buffer. The samples were immediately deep-frozen in liquid nitrogen and stored at −80 °C.
Back-cutted trees (cut 6 months earlier) grown in a controlled environment chamber at CPN (Composants Naturels, Michelin Group, Clermont-Ferrand, France) were used to analyse the gene expression patterns of the sucrose transporter in different organs (mature leaves, bark and xylem) and for in situ hybridization (stems). These young trees were grown with 12 h daylight of 40 µmol m−2 s−1 photosynthetically active radiation, at 27–30 °C, with 70 ± 10 % relative humidity, and at 25 °C, with 90 % relative humidity (night). Each pot of 160 L, filled with potting mix, was connected to a watering system and sprayed to maintain high atmosphere and soil moisture.
Field experiments of ethylene treatments
Seven batches of three homogeneous (in growth) mature virgin trees were selected as described by Pujade-Renaud et al. (1994) and Sookmark et al. (2002): two as a control (treated with palm oil only) and five treated with 1 mL of 2·5 % Ethrel® emulsion (approx. 70 mm ethephon) in crude palm oil on a 1-cm-wide, slightly scraped bark band and just beneath the next tapping cut, for 4, 8, 16, 24 and 40 h, respectively, before the first sampling. To avoid the influence of possible weather variations, in all experiments the trees were opened (tapped for the first time) on a half spiral with a tapping knife, and the latex and the bark samples were collected for analysis on the same day and at the same time. The homogenous depth (approx 1 mm from the cambium) of tapping was tested with a sharp calibrated punch.
After a 3-d rest (without further stimulation), latex and bark samples from the same trees were collected again and analysed as the second tapping (Tap2).
Hormones and other bark treatments
As the relative transcript level of sucrose transporters was reported to be regulated by hormonal treatments such as gibberellins (Chincinska et al., 2008), ethylene (Chincinska et al., 2008), auxin (Harms et al., 1994) and cytokinin (Harms et al., 1994; Ehness and Roitsch, 1997), we wanted to check whether various hormone or wounding treatments could induce any effect on laticifer SUT expression. To do this, a protocol similar to that for ethylene application was used. Briefly, two batches were used as controls (treated with 1 g palm oil containing 0·05 % Tween 20), and four others were treated with 1 g of 2 % jasmonic acid (JA), 1 % salicylic acid (SA), 1·5 % 4-chlorophenoxyacetic acid (CPA, auxin analog) and 2 % abscissic acid (ABA) palm oil emulsion with 0·05 % Tween 20, and a last batch was mechanically wounded by sealing 15 nails up to the cambium (Nails), on an approx. 1-cm-wide, slightly scraped bark band, just beneath the next tapping cut, for 4, 8 and 16 h, respectively, before the first tapping. The latex and bark samples were collected on the same day and analysed as the first tapping (Tap1) in the same way as for the Ethrel experiment. After a 3-d rest, samples from the batches of the 4-h-treated trees were collected again and analysed as the second tapping (Tap2).
Molecular cloning of sucrose transporter (HbSUTs) full-length cDNA
Four latex and bark sucrose transporter probes were obtained by polymerase chain reaction (PCR) directly on latex or bark cDNA libraries, kindly provided by the team of Dr P. Kongsawadworakful, Unchera Viboonjun and H. Chrestin (Mahidol University, Bangkok, Thailand). For this purpose, degenerate primers were designed from conserved regions of published plant sucrose transporter cDNAs (EMBL data library). One primer couple was used for each SUT group: SUT1 group [primer SUT1F (5′-TA/TC/TA/GA/GC/TACA/C/TGAC/TTGGA/TTG/TGG-3′) and primer SUT1R (5′-TA/TC/TYACA/C/TGAYTGGA/TTKGG-3′)], SUT2 group [primer SUT2F (5′-CATTTA/GCCA/C/TCCC/G/TGCA/TATGCA-3′) and primer SUT2R (5′-ACTCCG/TATA/C/TGCCAAA/G/TCCTTGC/TCC-3′)] and SUT4 group [primer SUT4F (5′-AA/GATC/TTATGGCGGTGAACC-3′) and primer SUT4R (ACA/GCCCAA/TA/TGAC/TAAGCCTTGA/TCC)]. Amplifications were carried out in a thermal cycler (PTC-200; MJ Research, Watertown, MA, USA) using standard protocols (Decourteix et al., 2006). The different amplified fragments were cloned and sequenced, and then used as SUT homologous probes to screen the latex cDNA libraries under low-stringency conditions. Seventeen clones were obtained and fully sequenced. Their nucleotide sequence alignment showed that they corresponded to seven different isoforms, referred to as HbSUT1A, HbSUT1B, HbSUT2A, HbSUT2B, HbSUT2C, HbSUT4 and HbSUT5 (H. brasiliensis sucrose transporters). In addition, the sequences of five of them (HbSUT1A, HbSUT2A, HbSUT2B, HbSUT4 and HbSUT5) were identical to H. brasiliensis SUT sequences, concomitantly registered in the EMBL/GenBank/DDBJ under accession numbers DQ985466, DQ985467, DQ985465, EF067335 and EF067333, respectively (Yang et al., 2006, direct submission). The HbSUT1B and HbSUT2C sequences were registered in the EMBL/GenBank/DDBJ under accession numbers AM492537 and AM491808, respectively.
RNA extraction
Total RNAs were extracted from latex as described by Pujade-Renaud et al. (1994, 1997) and developed by Sookmark et al. (2002). Total inner bark RNA extraction from mature trees used the caesium chloride cushion method, adapted from Sambrook et al. (1989), using approx. 2 g of inner bark ground under liquid nitrogen.
Deep-frozen leaves, bark and xylem samples collected from back-cutted trees were ground in liquid nitrogen and total RNAs were extracted using CTAB extraction buffer (Chang et al., 1993) and then treated with RNase-free RQ1 DNase (Promega, Madison, WI, USA). mRNAs were spectrophotometrically quantified and checked by agarose gel electrophoresis.
Specific primer for real-time RT-PCR
Primer couples specific to each isoform were designed for real-time RT-PCR: HbSUT1A [primer SUT1AF (5′-CAGCTTTTGTGGTGGGGGCGA-3′) and primer SUT1AR (5′-TGCACATCTATATGATCACATCCA-3′)], HbSUT1B [primer SUT1BF (5′-CAGCTTTTGTGGTGGGAGGGG-3′) and primer SUT1BR (5′-CCAATTTTTGGCCATTGATGCCC-3′)], HbSUT2A [primer SUT2AF (5′-GGTTTTCATTTTGGCTAACGACTG-3′) and primer SUT2AR (5′-TGATAAAGCACTCATCTTTTTACA-3′)], HbSUT2B [primer SUT2BF (5′-GGCTTTCCTCTTGCTATTACG-3′) and primer SUT2BR (5′-GTAAACTCAATTGAAGTGTTTCAGTC-3′)], HbSUT2C [primer SUT2CF (5′-TCCTTTGAAAGCATGCGCTAAT-3′) and primer SUT2CR (5′-GTAAACTCAATTGAAGTGTTTCAGTC-3′)], HbSUT4 [primer SUT4F (5′-GCAGTTCTTGGTGTTCCGT-3′) and primer SUT4R (5′-TCAATGGACTGTTATCTGCAAA-3′)], HbSUT5 [primer SUT5F (5′-GCAGTTCTTGGTGTTCCAC-3′) and primer SUT5R (5′-ATGCTGGCATCCAATCGGATG-3′)]. The specificity of each primer pair was tested by semi-quantitative and real-time RT-PCR on each full-length cDNA cloned in pGEM-T easy vector (Promega). PCR reactions were performed as described below for real-time RT-PCR.
Besides sucrose transporters, the expression pattern of the glutamine synthetase gene (HbGS) was monitored by using the specific primer pair HbGS_F (5′-GCTGGCATCAACATTAGTGG-3′) and HbGS_R (5′-CAACGCCCCATAAGAAAGTG-3′)]. HbGS has been reported to be a good marker of the ethylene effect on gene expression in latex (Pujade-Renaud et al., 1994, 1997). For bark tissue, the expression pattern of the ethylene-induced gene ACC oxidase (1-aminocyclopropane-1-carboxylate oxidase) was determined as a positive control of the response to ethylene (Kongsawadworakul et al., 2004). The specific primer pair used was: HbAccOx_F (5′-ATGGACACAGTTGAGAGGATGAC-3′) and HbAccOx_R (5′-AGGTGGCGGAGGAAGAAGG-3′)].
Quantification of sucrose transporter transcripts by real-time RT-PCR
After treatment with DNase I, 2 µg of total RNA was used as the template in the first-strand cDNA synthesis reaction, according to the manufacturer's instructions (SuperScript-III, Invitrogen, Carlsbad, CA, USA). Real-time PCR was performed using the generated cDNA as target template, a fluorescent dye SYBR-Green and an iCycler system (Bio-Rad, Hercules, CA, USA). cDNA encoding actin was used as the internal control gene. PCR reactions were performed as follows: 5 min at 95 °C for denaturation, 20 s at 95 °C, 20 s at 58 °C and 20 s at 72 °C for amplification, and 10 min at 72 °C for final extension. ΔCt was calculated from the formula ΔCt = Ct(treated sample) − Ct(control sample). The normalized expression ratio (Qr) was calculated using the comparative Ct method, with the formula: Qr = E−ΔΔCt (E = efficiency of the primer couple, ΔΔCt = ΔCtsucrose transporter − ΔCtactin). This method makes it possible to visualize the increase (Qr > 1) and decrease (Qr < 1) of genes (up- and downregulation, respectively). The efficiency of each primer pair was previously evaluated and found to be between 1·75 and 1·85. To compare the relative abundance of transcripts in different organs, expression was calculated from: Expression = ECt(actin)−Ct(sugar transporter) as given in the Bio-Rad real-time PCR Application Guide.
Statistical analysis
As empirical errors in Qr increased with Qr values, consistent with the above exponential formulation, statistical procedures were performed on log-transformed data. Two samplings, each consisting of two independent plants, were used to estimate an unbiased biological error (with two degrees of freedom) for comparison of means using Student's t-test. Results were considered to be statistically different at P < 0·01.
In situ hybridization
Tissue preparation was done according to Brunel et al. (2002). Transverse sections, 12 µm thick, were cut with a rotary microtome, mounted on SuperFrost Plus slides (Fisher Scientific, Elancourt, France) and dried at 42 °C for 2 d. Paraffin was removed via two baths (10 min each) in Histoclear II. Slides were progressively rehydrated in an ethanol series and then washed for 5 min in DEPC water and twice in 1× phosphate-buffered saline (PBS). Slides were treated with proteinase K at 10 µg ml−1 in 1× PBS at 37 °C for 15 min and then incubated in glycine at 0·2 % in 1× PBS at room temperature for 2 min. After two washes in 1× PBS, slides were fixed in 4 % paraformaldehyde (in 1× PBS) for 10 min, rinsed in 1× PBS and then acetylated using 0·5 % acetic anhydride in 0·1 m triethanolamine for 10 min and rinsed in 1× PBS. Before hybridization, sections were dehydrated in an ethanol series. Sections were hybridized with equal concentrations (1·5 ng μL−1) of either sense or antisense probes in 1× Denhart's, 10 % dextran sulfate, 1 mg ml−1 tRNA, 50 % formamide, 1× salts (300 mm NaCl, 10 mm Tris–HCl, pH 6·8, 10 mm NaH2PO4, 10 mm Na2HPO4 and 5 mM EDTA), and incubated at 50 °C overnight in a humid chamber. Sections were washed twice in 2× saline sodium citrate (SSC), six times in 0·1× SSC for 15 min at 42 °C each and twice in 1× PBS for 10 min at room temperature. Detection of digoxigenin (DIG)-labelled probes used an anti-digoxigenin alkaline phosphatase conjugate (Poupard et al., 2001). After suitable colour development, the reaction was stopped by rinsing in water and the sections were dried and mounted in Eukitt. Sections were observed under a Zeiss Axioplan 2 microscope and with an AxioCam HR camera (Zeiss) with AxioVision digital imaging software.
RESULTS
Latex production
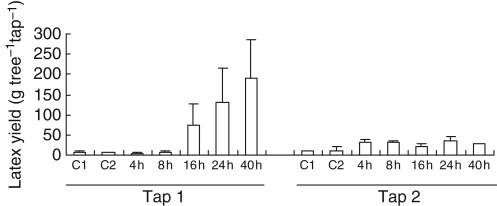
Application of Ethrel® under the tapping-cut significantly stimulated latex yield (Fig. 1), especially for the first tapping (Tap1). In comparison with the control of Tap1, latex production increased strongly by 16 h and reached its maximum value 40 h after treatment (27-fold more than in the control). The standard deviation for the marked increase in yield was very large, suggesting substantial heterogeneity in the response between trees. Nevertheless, the treatment differed significantly from the control. For the second tapping (Tap2), latex production increased only weakly at 4 h (three-fold) and remained constant until 40 h after treatment.
Fig. 1.
Latex yield of virgin PB217 trees after ethylene stimulation. Batches of three homogenous mature virgin trees were treated with 5 % Ethrel® (ethylene) 4, 8, 16, 24 or 40 h before the first simultaneous tapping (Tap1). After 3 d without any further treatment, the same trees were tapped again (Tap2). C1 and C2 represent two batches of three trees, which were un-stimulated as controls. Latex yield is expressed in grams of fresh latex per tree per tapping. Bars represent the biological standard deviation.
Cloning cDNA of putative sucrose transporters from latex
Degenerated oligonucleotide primers from conserved regions of each group of sucrose transporters (SUT1, SUT2 and SUT4) were used and allowed cloning of four different amplification products from latex and bark libraries. These four probes were used for screening a latex cDNA library, which led to the identification of seven full-length cDNA-encoding putative sucrose transporters. Based on their sequence homologies, two isoforms (HbSUT1A and HbSUT1B) were found to belong to the SUT1 group, three (HbSUT2A, HbSUT2B and HbSUT2C) to the SUT2 group and two others (HbSUT4 and HBSUT5) to the SUT4 group.
Further investigations showed that HbSUT1A, HbSUT2A, HbSUT2B, HbSUT4 and HBSUT5 were identical to those concomitantly registered by Yang et al. (direct submission, 2006) in the EMBL/GenBAnk/DDBJ under accession numbers DQ985466, DQ985467, DQ985465, EF067335 and EF067333, respectively. The new HbSUT1B and HbSUT2C full-length nucleotide sequences were registered in the EMBL/GenBank/DDBJ under accession numbers AM492537 and AM491808, respectively.
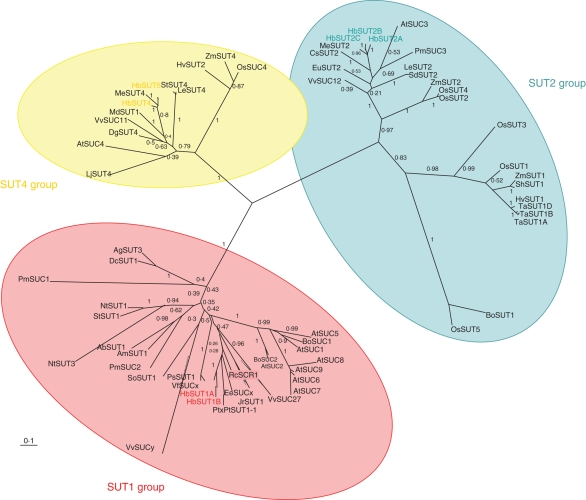
All these putative transporters belonged to the MFS and shared main characteristics with the previously cloned sucrose transporters. Their nucleotide sequence contained an open reading frame ranging from 1·5 to 1·9 kb that encoded 500 to 633 amino-acid polypeptides. In addition, their predicted amino-acid sequences exhibited highest identity to those of Ricinus communis (about 83 %) and Manihot esculenta (about 90 %), both of which belong, like H. brasiliensis, to the Euphorbiaceae family. According to the proposed classification of sucrose transporters (Sauer, 2007), these were divided into three groups (Fig. 2; accession numbers of the sequences presented are given in the Appendix). HbSUT1A and HbSUT1B belong to Group II, which contains sucrose transporters involved in phloem loading and sucrose import into different sink organs. HbSUT2A, HbSUT2B and HbSUT2C fall into Group III, which contains more amino acids than all the other sucrose transporters, due to the presence of an extended N-terminal domain and a central cytoplasmic loop. HbSUT4 and HbSUT5 belong to Group IV, with sucrose transporters characterized by low affinity/high capacity and specific localization in either the plasma membrane (Weise et al., 2000) or the tonoplast (Endler et al., 2006). For the following, we refer to the classification comprising the SUT1, SUT2 and SUT4 groups.
Fig. 2.
Phylogenetic tree of plant sucrose transporters. Confirmed or predicted plant sucrose transporter sequences from public databases (EMBL/GenBank/DDBJ) were used to construct a phylogenetic tree. Deduced protein sequences were aligned using MUltiple Sequence Comparison by Log-Expectation (http://www.ebi.ac.uk/Tools/muscle/). The tree was constructed using the program PhyML (http://www.phylogeny.fr/version2_cgi/one_task.cgi?task_type=phyml) and Treeview. Internal labels indicate bootstrap values (100 bootstraps).
Basal expression of putative sucrose transporters in latex and bark of untreated virgin trees
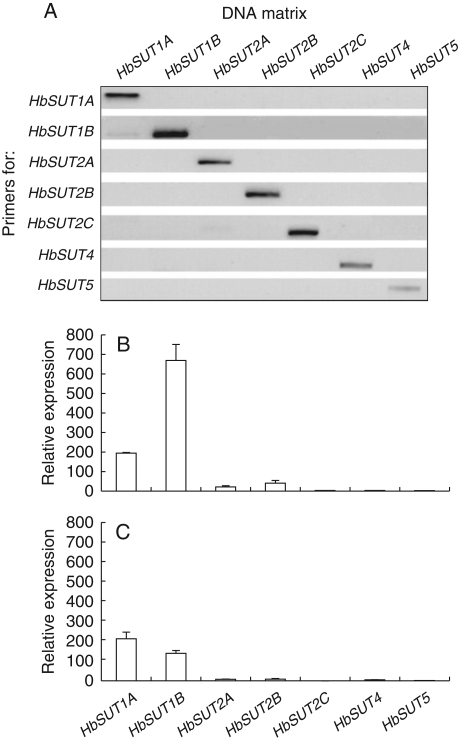
Because 3′ untranslated region (UTR) sequences are the most divergent regions within isoforms (Lerchl et al., 1995; Duval et al., 2002; Morey et al., 2002; Sakr et al., 2003; Decourteix et al., 2006, 2008) most of the eight primer pairs were designed in this region to specifically amplify each of the eight isoforms. The specificity of each primer pair was verified by PCR amplification using the purified cDNA clone of each different isolated sucrose transporter from latex, such as templates. Figure 3A shows that each primer pair only recognized and amplified the cDNA of the sucrose transporter isoform that they were designed from. Such specificity allowed us to investigate the expression of each sucrose transporter in the latex and bark, in response to tapping and ethylene treatment.
Fig. 3.
Specificity of the different primers and basal expression of different sucrose transporters in latex and bark of virgin trees. (A) Specificity, and (B) basal expression of different HbSUT isoforms in latex and (C) in bark. The relative expression of HbSUTs was determined by real-time RT-PCR in bark and latex, using actin expression as control.
First, the basal expression of different sucrose transporters from untreated virgin trees was investigated in latex. The sucrose transporter isoforms of the SUT1 group (HbSUT1A and HbSUT1B) were predominantly expressed in the resting latex cells (untapped, unstimulated), with HbSUT1B as the most abundant sucrose transporter isoform in latex (Fig. 3B). Compared with the SUT1 group, members of the SUT2 group, mainly HbSUT2A and HbSUT2B, showed very low basal expression. However, lowest expression was for the two isoforms (HbSUT4 and HbSUT5) of the SUT4 group and one isoform (HbSUT2C) of the SUT2 group.
As latex comes from the laticifers that are embedded in the inner soft bark tissues, basal expression of these transporters in bark was also investigated (Fig. 3C). All the transporters were detected, to varying degrees, in the inner bark. Again, HbSUT1A and HbSUT1B were the most highly expressed isoforms. HbSUT1B was weakly expressed in the inner bark compared with latex, suggesting a preferential expression of these two transporters in the laticifers.
Ethylene effect on sucrose transporter expression profiles in latex
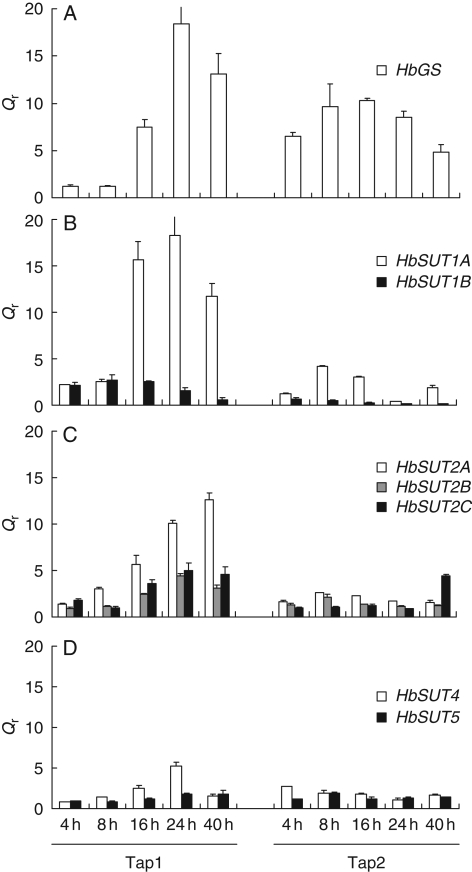
To confirm that ethylene treatment of bark stimulated gene expression in the laticifers, the relative transcript level of HbGS was investigated as a positive ethylene-responsive gene in these cells (Pujade-Renaud et al., 1994, 1997). HbGS increased in the latex cells, starting within 16 h of ethylene treatment, with a maximum (18-fold) after 24 h (Tap1, Fig. 4A). This response was prolonged for the second tapping, regardless of when the ethylene treatment was applied, with a maximum (ten-fold expression compared with the control) at 8 and 16 h (Tap2, Fig. 4A). As shown in Fig. 4, the effect of ethylene treatment not only differed between the sucrose transporter isoforms, but also between the first (Tap1) and the second (Tap2) tapping. The transcripts of HbSUT1A (Fig. 4B) and, to a lesser extent, of HbSUT2A (Fig. 4C) were found to be the most responsive to ethylene treatment.
Fig. 4.
Transcript accumulation of glutamine synthetase and different sucrose transporters after ethylene stimulation in virgin tree latex. Relative transcript levels of glutamine synthetase (A), and sucrose transporters of SUT1 group (B), SUT2 group (C) and SUT4 group (D) were monitored in latex of ethylene-treated virgin trees. Latex mRNA from mature virgin trees (PB217) was used for real-time RT-PCR. Qr was obtained by the E−ΔΔCt methods. Bars represent the technical standard deviation.
No significant effect was observed at the Tap1 within the first 8 h following ethylene treatment. At 16 h, the transcripts of HbSUT1A and HbSUT2A increased 16- and six-fold, respectively (Fig. 4B, C). This accumulation was abrupt and transient for HbSUT1A, whereas it was progressive and maximum after 40 h for HbSUT2A. Transcripts of HbSUT2B and HbSUT2C were very slightly and transiently increased by ethylene. No significant ethylene effect was detected for HbSUT4 and HbSUT5 (Fig. 4D). Of all these sucrose transporters, HbSUT1B was the only isoform for which expression was decreased by ethylene stimulation after 24 h treatment (Fig. 4B).
In contrast to Tap1, there was hardly any regulation of the sucrose transporter transcripts during Tap2. However, a slight and transient induction was observed for HbSUT1A between 8 and 16 h after ethylene treatment (Fig. 4B–D).
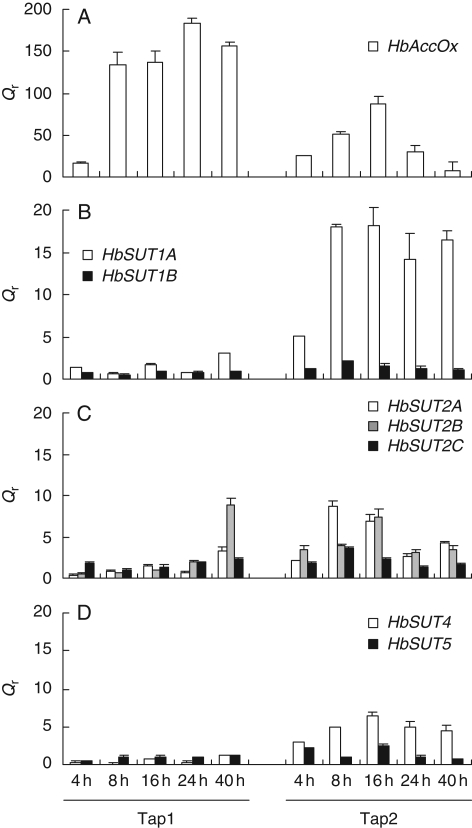
Ethylene effect on the expression profiles of the putative sucrose transporters in the inner bark
First, ethylene treatment was confirmed to induce an early (4 h) and strong (up to 200-fold) accumulation of ACC oxidase transcripts (Kongsawadworakul et al., 2004) during the first and second tappings (Fig. 5), which validated the ability of the ethylene bark treatment to regulate gene expression in the soft inner bark.
Fig. 5.
Transcript accumulation of ACC oxidase and different sucrose transporters after ethylene stimulation in bark of mature virgin trees. Relative transcript levels of ACC oxidase (A) and sucrose transporters of SUT1 group (B), SUT2 group (C) and SUT4 group (D) were monitored in bark of ethylene-treated virgin trees. Bark mRNA samples from virgin mature trees (PB217) were used for real-time RT-PCR. Qr was obtained by the E−ΔΔCt method. Bars represent the technical standard deviation.
At Tap1, most of the sucrose transporter transcripts either remained constant or decreased (Fig. 5B–D). Only the HbSUT2B isoform was weakly and transiently increased at 40 h (Fig. 5C). During Tap2, HbSUT1A, HbSUT2B and HbSUT4 were transiently increased with a maximum around 16 h (Fig. 5C, D). The increase in transcripts was strongest for the HbSUT1A isoform, which was markedly increased at 4 h, and reached a plateau between 8 and 40 h (Fig. 5B).
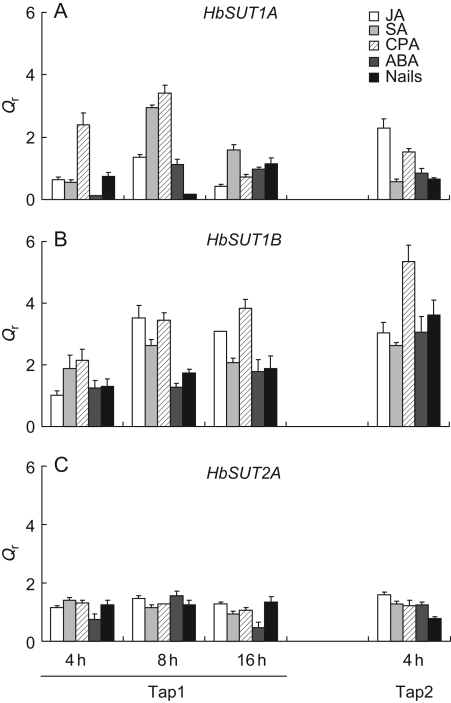
Effect of hormones and wounding on expression of HbSUT1A, HbSUT2A and HbSUT2B in the latex
As HbSUT1A, HbSUT1B and HBSUT2A were found to be significantly regulated by ethylene, the effects of various hormonal and wounding treatments applied to bark on their respective expression in latex were also investigated. For HbSUT2A, no significant increase was observed, regardless of the treatment, whereas HbSUT1A exhibited an early, transient and low stimulation in the presence of SA and CPA (Fig. 6A, C). CPA and, to a lesser extent, the other treatments (especially JA) were found to induce a significant accumulation of HbSUT1B at Tap1 (from 8 h after treatment) and Tap2 (Fig. 6B).
Fig. 6.
Transcript accumulation of ethylene-modulated HbSUT1A, HbSUT1B and HbSUT2A after hormonal treatment or wounding in latex of mature virgin trees. Relative transcript levels of HbSUT1A (A), HbSUT1B (B) and HbSUT2A (C) were monitored in latex of virgin trees treated with jasmonic acid (JA), salicylic acid (SA), 4-chlorophenoxyacetic acid (CPA, an auxin analogue), abscissic acid (ABA) or nails planting. Latex mRNA samples from these trees were used for real-time RT-PCR analysis of HbSUT1A, HbSUT1B and HbSUT2A transcripts. Qr was obtained by the E−ΔΔCt method. Bars represent the technical standard deviation.
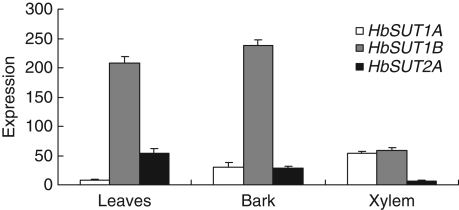
HbSUT1A, HbSUT1B and HbSUT2A expression in different organs
HbSUT1A, HbSUT1B and HbSUT2A gene expression analysis was carried out by real-time PCR, using RNAs from leaves, bark and xylem of young back-cutted trees. These three transporters were detectable to varying degrees in the source and sink organs (Fig. 7). HbSUT1A was more highly expressed in sink organs (xylem and bark) than in leaves. HbSUT1B was predominantly expressed in leaves and bark, but relatively weakly in xylem. A similar pattern but of a reduced magnitude was found for HbSUT2A.
Fig. 7.
Transcript accumulation of HbSUT1A, HbSUT1B and HbSUT2A in different organs of young virgin trees. cDNA from leaves, bark and xylem of young back-cutted trees (cut-back 6 months earlier) were used as matrix for real-time RT-PCR analysis of HbSUT1A, HbSUT1B and HbSUT2A transcripts. Expression = ECt(actin)−Ct(sugar transporter). Bars represent the technical standard deviation.
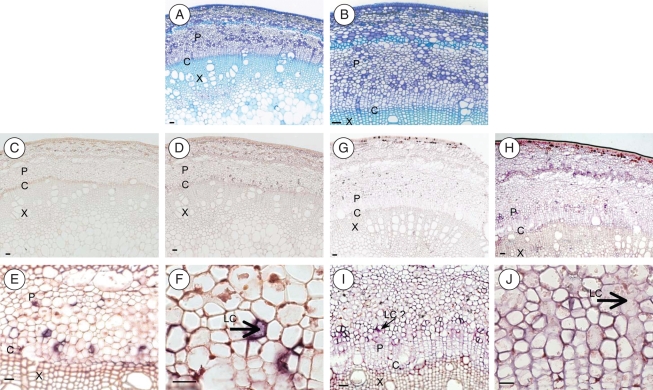
In situ hybridization
Experiments were carried out with two types of probes: the first was specific to HbSUT1A and HbSUT1B (SUT1 group), which exhibit strong sequence identity, and the second was quite HbSUT2A-specific. As shown in Fig. 8A and B, young stems of H. brasiliensis contain some isolated laticifers exclusively localized in the phloem region, near cambial cells.
Fig. 8.
In situ localization of HbSUT1 and HbSUT2A transcripts in stems of young virgin trees. Transverse sections (12 µm thick) were obtained and hybridized with antisense probes specific for HbSUT1 mRNA (E, F) and HbSUT2A mRNA (I, J); or sense probes as a negative control (HbSUT1: C, D; HbSUT2A: G, H). Positive hybridization signals are visualized by violet staining using a digoxigenin-labelled RNA immunodetection system. Other sections were stained with toluidine blue, to identify the cellular structures (A, B). Abbreviations: P, phloem; C, cambium; X, xylem; LC, laticiferous cells. Scale bar = 50 µm.
A typical colouration (violet staining) was only detected with the antisense probe (Fig. 8E, F, I, J), whereas no signal was observed with the sense probe (Fig. 8C, D, G, H), suggesting that the colouration indicates the presence of transcripts of HbSUT1s or HbSUT2A.
With regard to HbSUT1 mRNAs, colouration was found in inner bark tissue, more precisely in young phloem, laticifers and the cambial zone. No colouration was found in xylem tissue, implying limited or no expression of these transporters. Similar data were found for HbSUT2A, for which a positive hybridization signal was also mainly confined to the cambial zone and the phloem tissue. However, a weaker colouration than for the HbSUT1 probe was observed in the fusing young latex cells.
DISCUSSION
Due to their active metabolism in the synthesis of rubber, laticifers are a strong sink and require substantial and rapid sucrose import to meet their carbon and energy demands. Ethrel® is often used as latex yield stimulant (Tupy, 1973). A series of field and molecular experiments were conducted, for the first time, to assess if sucrose transporters are involved in the ethylene-induced increase in latex yield. These experiments were performed with mature virgin trees, at the very first tapping, to study the direct effect of ethylene, and then upon a second tapping. The PB217 rubber clone was used because it responds well to Ethrel yield stimulation.
Laticifers contain transcripts of many putative sucrose transporters
It is normally very difficult to access the pure cytoplasm of one single cell type. This is not the case for laticifers, from which the out-flowing cytoplasm (latex) can be collected by simple bark tapping. Moreover, the presence of polysomes and mRNA in the expelled latex (Coupé et al., 1977; Kush et al., 1990; Pujade-Renaud et al., 1994; Ko et al., 2003; Chow et al., 2007) makes it possible to study the expression of any gene expressed in this kind of cell. Therefore, to identify the sucrose transporters expressed in laticifers, a latex-specific cDNA library was screened using four PCR-amplified homologous probes. Seven putative sucrose transporter isoforms were cloned from these cells, indicating that sucrose transporters are also encoded by a multigenic family in H. brasiliensis, as is the case for many other species. For example, nine sucrose transporter isoforms were identified from Arabidopsis thaliana (Sauer and Stolz, 1994; Barker et al., 2000; Gottwald et al., 2000; Ludwig et al., 2000; Meyer et al., 2000, 2004; Sauer et al., 2004), three from tobacco (Burkle et al., 1998; Ward et al., 1998; Lemoine et al., 1999), three from tomato (Barker et al., 2000; Weise et al., 2000), four from Vitis vinifera (Davies et al., 1999; Ageorges et al., 2000), two from citrus (Li et al., 2003) and three from Oryza sativa (Scofield et al., 2007; Sun et al., 2008).
Based on their sequence homology, these sucrose transporters were found to be closely related to previously identified sucrose transporters from herbaceous as well as woody species (Lemoine, 2000; Sauer, 2007). The predicted amino-acid sequences of Hevea SUTs exhibited greatest identity (more than 83 %) with those of Ricinus communis and Manihot esculenta, which also belong to the Euphorbiaceae family. In addition, these sucrose transporters belong to all previously characterized groups of sucrose transporters, with two isoforms (HbSUT1A and HbSUT1B) in the SUT1 group, three (HbSUT2A, HbSUT2B and HbSUT2C) in the SUT2 group and two (HbSUT4 and HBSUT5) in the SUT4 group.
Analysis of their basal transcript expression showed that these sucrose transporters were expressed to varying degrees in the latex, with greater expression of the SUT1 group (HbSUT1A and HbSUT1B) isoforms (Fig. 3B, C). Two isoforms of the SUT2 group (HbSUT2A and HbSUT2A) exhibited very low expression, while expression of the other SUT2 (HbSUT2C) and of the two SUT4 isoforms could barely be detected. On the basis of this transcript accumulation pattern, HbSUT1A and HbSUT1B may be considered as the main sucrose transporters involved in sucrose importation into the laticifers. Such a physiological role was supported by the in situ hybridization data, showing that these two transporters were expressed in the inner soft bark (liber) of young stems, including the young laticifers (Fig. 8).
To our knowledge, the presence of many isoforms of sucrose transporters in one single type of cell has only been reported once: this was for sieve elements with three isoforms (LeSUT1, LeSUT2 and LeSUT4) proposed to be involved in sucrose retrieval from the apoplastic compartment (Barker et al., 2000; Weise et al., 2000; Kühn, 2003). Laticifers would therefore be expected to function as highly active sites of sucrose absorption.
Ethylene-induced increase in HbSUT1A and HbSUT2A transcripts parallels the ethylene-induced stimulation in latex yield
Much evidence in the literature indicates that an increase in sucrose transporter transcripts is often associated with a higher activity of the corresponding transporters, suggesting a major transcriptional regulation of these proteins (Sakr et al., 1993, 1997; Chiou and Bush, 1998; Lemoine et al., 1999; Decourteix et al., 2006, 2008). Hence, as ethylene-stimulated latex production has been reported to occur through an increase in sugar metabolism (Tupy, 1973; Tupy and Primot, 1976; Silpi et al., 2006), sugar influx (Lacrotte et al., 1985) and hyperpolarization of the plasma membrane of laticifers (Bouteau et al., 1999), an increased sucrose influx should be partly linked to an ethylene-induced stimulation of, at least, some of the putative cloned sucrose transporters. This should be particularly true for laticifers, as they are a very active sink cell type completely devoid of any starch reserve (Tupy, 1989).
In accordance with previously published data (Pujade-Renaud et al., 1994), ethylene treatment below the tapping cut led to an increase in HbGS expression in laticifers (Fig. 4A). This result confirmed that the ethylene signal was perceived by these cells. The expression pattern of the seven identified sucrose transporters was thus monitored on the same latex samples, using highly specific primer couples, designed from the UTR of their cDNAs (Fig. 3A). Only two transporters (HbSUT1A, HbSUT2A) were found to be, to varying degrees, significantly stimulated by ethylene. This stimulation was time-dependent as it peaked at 24 and 48 h after treatment for HbSUT1A and HbSUT2A, respectively. Note that HbSUT1B, which has the highest expression of any sucrose transporter isoform in the latex of untreated virgin trees, was significantly decreased when treated with ethylene. Under the same experimental conditions, the other isoforms of sucrose transporters were almost insensitive to ethylene. Taken together, these data suggest that the ethylene effect cannot be extended to all sucrose transporters initially cloned from the latex, and that the ethylene-induced sucrose influx into the laticifers (Lacrotte et al., 1985) may be due, at least in part, to the ethylene stimulation of HbSUT1A and HbSUT2A. Moreover, this ethylene-induced transcript accumulation of HbSUT1A and HbSUT2A was correlated with the ethylene-stimulated latex production (Fig. 1). This potential role of HbSUT1A and HbSUT2A in ethylene-induced stimulation of latex production in virgin trees could be related to the fact that none of the sucrose transporters was found to be greatly increased during Tap2 (Fig. 4), which caused much less latex production (Fig. 1).
The ethylene effect is tissue-specific
As ethylene applied to the bark tapping area induced a pronounced increase in some SUTs in the laticifers, we verified whether a similar effect could be found in the surrounding inner liber tissues. In contrast to laticifers, no sucrose transporter was found to be significantly regulated by ethylene treatment on the first tapping (Fig. 5B–E). This lack of an ethylene effect could not be due to a failure of bark cells to perceive the ethylene signal (Fig. 5A), as ethylene did induce a marked stimulation of an ACC oxidase gene expression, which has been reported to behave as a positive marker of the response of bark tissues to ethylene treatment (Kongsawadworakul et al., 2004). These data suggested that the expression of HbSUT1A and HbSUT2A induced by ethylene was limited to the laticifers, which are devoted to rubber synthesis and, thereby, latex production. A tissue-specific regulation was recently reported for JrSUT1, a putative plasma membrane-localized sucrose transporter, isolated from walnut tree xylem (Decourteix et al., 2006). Analysis of its expression showed that JrSUT1 was induced by freezing and thawing in xylem tissue but not in bark, indicating the occurrence of differential regulation mechanisms between these tissues.
Laticifers are considered to be apoplastic sinks which import sucrose from the phloem. The efficiency of phloem unloading is strongly related to the sink strength, which is defined as the capacity of cells to attract photoassimilates (Ho, 1988). This sink strength closely depends on the abundance and activity of plasma membrane transporters. Moreover, a close relationship between the transcript changes and the activity of sucrose transporters has been reported in some species (Chiou and Bush, 1998; Decourteix et al., 2006, 2008). As ethylene induces a stimulation of two sucrose transporters in latex without having any effect on those of bark, this situation might reflect a diversion of carbon assimilates (sucrose) in favour of laticifers, where latex production is strongly stimulated by ethylene. This scenario emphasizes coordinated regulation between different sink organs of the stem.
Stimulation of HbSUT1A and HbSUT2A in latex is ethylene-specific
The transcript abundance of sucrose transporters in plants has been reported to be regulated by exogenous (Sakr et al., 1993; Matsukura et al., 2000; Meyer et al., 2004; Decourteix et al., 2006) and endogenous (Ehness and Roitsch, 1997; Chiou and Bush, 1998; Ward et al., 1998; Matsukura et al., 2000; Li et al., 2003) stimuli. We have shown here that the ethylene effect was restricted to two sucrose transporters (HbSUT1A and HbSUT2A) and correlated with ethylene-induced stimulation production. It is noteworthy that ethylene decreased HbSUT1B expression (Fig. 4A) but it was significantly increased by most treatments, especially JA and CPA (an auxin-like chemical; Fig. 6B).
With regard to HbSUT1A and HbSUT2A, none of the hormonal treatments induced a stimulation of their respective transcript during Tap1 (Fig. 6B, C). In addition, as wounding (nail sealing, without latex exudation) did not lead to upregulation of either HbSUT1A or HbSUT2A, it can be hypothesized that either their response to ethylene is not due to a cross-talk between the ethylene and wounding pathway, or that the concentration of endogenous ethylene, probably induced by wounding in the rubber tree bark, might be too low to induce pronounced regulation of these two genes, as compared with the application of exogenous ethylene. This differential response of sucrose transporters means that laticifers are able to adapt the transcript amount of their sugar transporters to different environmental or developmental conditions.
The present study provides insight into the physiological role that sucrose transporters play in sucrose import to laticifers, in relation to ethylene-induced stimulation of latex production. We have described the potential involvement of two sucrose transporters, HbSUT1A and HbSUT2A, in this process. Indeed, HbSUT1A and HbSUT2B were increased by ethylene and this effect was positively correlated with ethylene stimulation of latex production. Moreover, this ethylene effect is specific to laticifers, in which cis-polyisoprene synthesis takes place. To elucidate how the expression of these transporters is stimulated by ethylene, their respective promoters will be isolated in future experiments to determine whether they harbour an ethylene-specific cis-element. Because latex yield is the most important trait in breeding programmes for H. brasiliensis, and rubber synthesis depends on sucrose importation in laticifers, the above two sucrose transporters may constitute a useful molecular tool to underpin latex yield in this plant. Thus, single nucleotide polymorphisms (SNPs) and microsatellites will be looked for in these SUT cDNA clones to map them in the Hevea gene maps (Low et al., 1996; Guen et al., 2004) and verify if they may co-localize with latex yield and/or growth quantitative trait loci. This would be of major help in rubber breeding programmes.
ACKNOWLEDGEMENTS
We are grateful to the staff of the SIPH Headquarters and of the Bongo Rubber Plantation in Côte d'Ivoire (West Africa) for their logistical and technical help in the preparation and monitoring of the field experiments, as well as for allowing access to all their plant materials and laboratory facilities. We are also grateful to Drs Rémi Lemoine, Valérie Pujade-Renaud and Laurence Maurousset for critically reading earlier versions of the manuscript. This work was supported by Manufacture Française des Pneumatiques Michelin.
APPENDIX
Accession numbers of sucrose transporter sequences presented in Fig. 2: AbSUT1 (Asarina barclaiana; AAF04294), AgSUT3 (Apium graveolens; ABB89051), AmSUT1 (Alonsoa meridionalis; AAF04295), AtSUC1 (Arabidopsis thaliana; At1g71880), AtSUC2 (Arabidopsis thaliana; At1g22710), AtSUC3 (Arabidopsis thaliana; At2g02860), AtSUC4 (Arabidopsis thaliana; At1g09960), AtSUC5 (Arabidopsis thaliana; At1g71890), AtSUC6 (Arabidopsis thaliana; At5g43610), AtSUC7 (Arabidopsis thaliana; At1g66570), AtSUC8 (Arabidopsis thaliana; At2g14670), AtSUC9 (Arabidopsis thaliana; At5g06170), BoSUC1 (Brassica oleracea; AAL58071), BoSUC2 (Brassica oleracea; AAL58072), BoSUT1 (Bambusa oldhamii; AAY43226), CsSUT2 (Citrus sinensis; AAM29153), DgSUT4 (Datisca glomerata; CAG70682), DcSUT1 (Daucus carota; BAA89458), EeSUCx (Euphorbia esula; AAF65765), EuSUT2 (Eucommia ulmoides; AAX49396), HbSUT1A (Hevea brasiliensis; DQ985466), HbSUT1B (Hevea brasiliensis; AM492537), HbSUT2A (Hevea brasiliensis; ABJ51934), HbSUT2B (Hevea brasiliensis; ABJ51932), HbSUT2C (Hevea brasiliensis; AM491808), HbSUT4 (Hevea brasiliensis; EF067335), HbSUT5 (Hevea brasiliensis; ABK60189), HvSUT1 (Hordeum vulgare; CAB75882), HvSUT2 (Hordeum vulgare; CAB75881), JrSUT1 (Juglans regia; AAU11810), LeSUT2 (Lycopersicum esculentum; AAG12987), LeSUT4 (Lycopersicum esculentum; AAG09270), LjSUT4 (Lotus japonicus; CAD61275), MdSUT1 (Malus domestica; AAR17700), MeSUT2 (Manihot esculenta; ABA08445), MeSUT4 (Manihot esculenta; ABA08443), NtSUT1 (Nicotiana tabacum; X82276), NtSUT3 (Nicotiana tabacum; AAD34610), OsSUT1 (Oryza sativa; AAF90181), OsSUT2 (Oryza sativa; AAN15219), OsSUT3 (Oryza sativa; BAB68368), OsSUT4 (Oryza sativa; BAC67164), OsSUC4 (Oryza sativa; Q2QLI1), OsSUT5 (Oryza sativa; BAC67165), PmSUC1 (Plantago major; CAI59556), PmSUC2 (Plantago major; X75764), PmSUC3 (Plantago major; CAD58887), PtSUT1-1 (Populus tremula × Populus tremuloides; CAJ33718), PsSUT1 (Pisum sativum; AAD41024), RcSCR1 (Ricinus communis; CAA83436), SdSUT2 (Solanum demissum; AAT40489), ShSUT1 (Saccharum hybridum; AAV41028), SoSUT1 (Spinacea oleracea; Q03411), StSUT1 (Solanum tuberosum; CAA48915), StSUT4 (Solanum tuberosum; AAG25923), TaSUT1A (Triticum aestivum; AAM13408), TaSUT1B (Triticum aestivum; AAM13409), TaSUT1D (Triticum aestivum; AAM13410), VfSUCx (Vicia faba; CAB07811), VvSUCy (Vitis vinifera; AAL32020), VvSUC11 (Vitis vinifera; AAF08329), VvSUC12 (Vitis vinifera; AAF08330), VvSUC27 (Vitis vinifera; AAF08331), ZmSUT1 (Zea mays; BAA83501), ZmSUT2 (Zea mays; AAS91375), ZmSUT4 (Zea mays; AAT51689).
LITERATURE CITED
- Abraham PD, Wycherley PR, Pakianathan SW. Stimulation of latex flow in Hevea brasiliensis of 4-amino-3,5,6-trichloropicolinic acid and 2-chloroethane-phosphonic acid. Journal of Rubber Research. 1968;20:291–305. [Google Scholar]
- Ageorges A, Issalya N, Picaudb S, Delrot S, Romieu C. Identification and functional expression in yeast of a grape berry sucrose carrier. Plant Physiology and Biochemistry. 2000;38:177–185. [Google Scholar]
- Amalou Z, Bangratz J, Chrestin H. Ethrel® (ethylene releaser) induced increase in the adenylate pool and transtonoplast pH within latex cells. Plant Physiology. 1992;98:1270–1276. doi: 10.1104/pp.98.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Auzac J. Mise en evidence de la glycolyse et de ses relations avec la biosynthèse du caoutchouc au sein du latex d' Hevea brasiliensis. Revue Générale des Caoutchoucs et Plastiques. 1964;41:1831–1834. [Google Scholar]
- d'Auzac J, Ribaillier D. L'ethylène, un nouveau stimulant de la production de latex chez l'Hevea brasiliensis. Compte-rendus de l' Acadadémie des Sciences Série D. 1969;268:3046–3049. [Google Scholar]
- Barker L, Kuhn C, Weise A, et al. SUT2, a putative sucrose sensor in sieve elements. Plant Cell. 2000;12:1153–1164. doi: 10.1105/tpc.12.7.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth I, Meyer S, Sauer N. PmSUC3: characterization of a SUT2/SUC3-type sucrose transporter from Plantago major. Plant Cell. 2003;15:1375–1385. doi: 10.1105/tpc.010967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud S, Wuilleme S, Lemoine R, et al. The AtSUC5 sucrose transporter specifically expressed in the endosperm is involved in early seed development in Arabidopsis. Plant Journal. 2005;43:824–836. doi: 10.1111/j.1365-313X.2005.02496.x. [DOI] [PubMed] [Google Scholar]
- Bouteau F, Lacrotte R, Cornel D, et al. Electrogenic active proton pumps in Hevea brasiliensis laticiferous cells: its role in activating sucrose/H+ and glucose/H+ symports at the plasma membrane. Bioelectrochemistry and Bioenergetics. 1991;26:223–236. [Google Scholar]
- Bouteau F, Bousquet U, Lacrotte R, Cornel D, Monestiez M, Rona JP. Sucrose/H+ and glucose/H+ symports at the plasma membrane of laticiferous cells and protoplasts of Hevea brasiliensis. Indian Journal of Natural Rubber Research. 1992;5:25–37. [Google Scholar]
- Bouteau F, Dellis O, Bousquet U, Rona JP. Evidence of multiple sugar uptake across the plasma membrane of laticifer protoplasts from Hevea. Bioelectrochemistry and Bioenergetics. 1999;48:135–139. doi: 10.1016/s0302-4598(98)00214-1. [DOI] [PubMed] [Google Scholar]
- Brunel N, Leduc N, Poupard P, Simoneau P, Mauget J-C, Viémont J-D. KNAP2, a class I KN1-like gene is a negative marker of bud growth potential in apple trees (Malus domestica [L.] Borkh.) Journal of Experimental Botany. 2002;53:2143–2149. doi: 10.1093/jxb/erf063. [DOI] [PubMed] [Google Scholar]
- Burkle L, Hibberd JM, Quick WP, Kuhn C, Hirner B, Frommer WB. The H + -sucrose cotransporter NtSUT1 is essential for sugar export from tobacco leaves. Plant Physiology. 1998;118:59–68. doi: 10.1104/pp.118.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery BR, Boatman SG. Manometric measurement of turgor pressures in laticiferous phloem tissues. Journal of Experimental Botany. 1966;17:283–296. [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Molecular Biology Reporter. 1993;11:113–116. [Google Scholar]
- Chincinska IA, Liesche J, Krügel U, Michalska J, Geigenberger P, Grimm B, Kühn C. Sucrose Transporter StSUT4 from Potato Affects Flowering, Tuberization, and Shade Avoidance Response. Plant Physiology. 2008;146:515–528. doi: 10.1104/pp.107.112334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Bush DR. Sucrose is a signal molecule in assimilate partitioning. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4784–4788. doi: 10.1073/pnas.95.8.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow K-S, Wan K-L, Isa MNM, et al. Insights into rubber biosynthesis from transcriptome analysis of Hevea brasiliensis latex. Journal of Experimental Botany. 2007;58:2429–2440. doi: 10.1093/jxb/erm093. [DOI] [PubMed] [Google Scholar]
- Coupé M, Chrestin H. Physico-chemical and biochemical mechanisms of the hormonal (ethylene) stimulation: early biochemical events induced, in Hevea latex, by hormonal bark stimulation. In: d'Auzac J, Jacob JL, Chrestin H, editors. Physiology of rubber tree latex. Boca Raton, FL: CRC Press Inc; 1989. pp. 295–319. [Google Scholar]
- Davies C, Wolf T, Robinson SP. Three putative sucrose transporters are differentially expressed in grapevine tissue. Plant Science. 1999;147:93–100. [Google Scholar]
- Decourteix M, Alves G, Brunel N, et al. JrSUT1, a putative xylem sucrose transporter, could mediate sucrose influx into xylem parenchyma cells and be up-regulated by freeze-thaw cycles over the autumn-winter period in walnut tree (Juglans regia L.) Plant, Cell and Environment. 2006;29:36–47. doi: 10.1111/j.1365-3040.2005.01398.x. [DOI] [PubMed] [Google Scholar]
- Decourteix M, Alves G, Bonhomme M, et al. Sucrose (JrSUT1) and hexose (JrHT1 and JrHT2) transporters in walnut xylem parenchyma cells: their potential role in early events of growth resumption. Tree Physiology. 2008;28:214–224. doi: 10.1093/treephys/28.2.215. [DOI] [PubMed] [Google Scholar]
- Duval FD, Renard M, Jaquinod M, Biou V, Montrichard F, Macherel D. Differential expression and functional analysis of three calmodulin isoforms in germinating pea (Pisum sativum L.) seeds. Plant Journal. 2002;32:481–493. doi: 10.1046/j.1365-313x.2002.01409.x. [DOI] [PubMed] [Google Scholar]
- Ehness R, Roitsch T. Co-ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. Plant Journal. 1997;11:539–548. doi: 10.1046/j.1365-313x.1997.11030539.x. [DOI] [PubMed] [Google Scholar]
- Endler A, Meyer S, Schelbert S, et al. Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiology. 2006;141:196–207. doi: 10.1104/pp.106.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschbach J-M, Tupy J, Lacrotte R. Photosynthate allocation and productivity of latex vessels in Hevea brasiliensis. Biologia Plantarum. 1986;28:321–328. [Google Scholar]
- de Faÿ E, Jacob J-L. Anatomical organization of the laticiferous system in the bark. In: d'Auzac J, Jacob JL, Chrestin H, editors. Physiology of rubber tree latex. Boca Raton, FL: CRC Press Inc; 1989. pp. 3–14. [Google Scholar]
- Gohet E. Montpellier: Université Montpellier II; 1996. La production de latex par Hevea brasiliensis, relations avec la croissance. Influence de différents facteurs: origine clonale, stimulation hormonale, réserves hydrocarbonées; p. 343. Thesis. [Google Scholar]
- Gohet E, Kouadio D, Prévôt JC, et al. IRRDB 1997. Tapping Panel Dryness. Montpellier: CIRAD-CP; 1997. Relation between clone type, latex sucrose content and the occurence of tapping panel dryness in Hevea brasiliensis. [14] p. IRRDB Tapping Panel Dryness Workshop, 1997-04-29/1997-04-30, (Danzhou, Chine) [Google Scholar]
- Gohet E, Chantuma P, Lacote R, et al. Latex clonal typology of Hevea brasiliensis. Physiological modelling of yield potential and clonal response to Ethephon stimulation. IRRDB Workshop on Exploitation Technology; 15–18 December 2003; Kottayam, India. 2003. s.l.: s.n., 14 p.. IRRDB Workshop on Exploitation Technology, 2003-12-15/2003-12-18, Kottayam, Inde. [Google Scholar]
- Gomez JB. Proceedings of the International Rubber Conference. Kuala Lumpur: Rubber Research Institute; 1976. Comparative ultracytology of young and mature latex vessels in Hevea brasiliensis; pp. 43–164. [Google Scholar]
- Gomez JB, Moir GFJ. Kuala Lumpur: 1979. The ultracytology of latex vessels in Hevea brasiliensis. Malaysian Rubber Research and Development Board, Monograph no. 4. [Google Scholar]
- Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR. Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13979–13984. doi: 10.1073/pnas.250473797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guen V, Lespinasse D, Oliver G, Rodier-Goud M, Pinard F, Seguin M. Molecular mapping of genes conferring field resistance to South American Leaf Blight (Microcyclus ulei) in rubber tree. Theoretical and Applied Genetics. 2004;108:160–167. doi: 10.1007/s00122-003-1407-9. [DOI] [PubMed] [Google Scholar]
- Hackel A, Schauer N, Carrari F, Fernie AR, Grimm B, Kuhn C. Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant Journal. 2006;45:180–192. doi: 10.1111/j.1365-313X.2005.02572.x. [DOI] [PubMed] [Google Scholar]
- Harms K, Wöhner RV, Schulz B, Frommer WB. Isolation and characterization of P-type H + -ATPase genes from potato. Plant Molecular Biology. 1994;26:1045–1053. doi: 10.1007/BF00028864. [DOI] [PubMed] [Google Scholar]
- Hébant C. Ontogénie des laticifères du système primaire de l' Hevea brasiliensis, une etude ultra structurale et cytochimique. Canadian Journal of Botany. 1981;59:974–985. [Google Scholar]
- Hébant C, de Faÿ E. Functional organizationof the bark of Hevea brasiliensis (rubber tree): a structural and histoenzymological study. Pflanzenphysiology. 1980;97:391–398. [Google Scholar]
- Hepper CM, Audley BG. The biosynthesis of rubber from hydroxy-methyl-glutaryl coenzyme A in Hevea brasiliensis latex. Biochemistry Journal. 1969;114:379. doi: 10.1042/bj1140379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L. Metabolism and compartmentation of imported sugars in sink organs in relation to sink strength. Plant Physiology Plant Molecular Biology. 1988;39:355–378. [Google Scholar]
- Jacob JL, Serres E, Prévôt JC, et al. Development of Hevea latex diagnosis. Agritrop. 1988;12:97–115. [Google Scholar]
- Jacob JL, Prévôt JC, Roussel D, et al. Hevea brasiliensis yield-limiting factors, latex physiological parameters, latex diagnosis and clonal typology. In: d'Auzac J, Jacob JL, Chrestin H, editors. Physiology of rubber tree latex. Boca Raton, FL: CRC Press Inc; 1989. pp. 345–382. [Google Scholar]
- Juergensen K, Scholz-Starke J, Sauer N, Hess P, Bel AJK, Grundler FM. The companion cell-specific Arabidopsis disaccharide carrier AtSUC2 is expressed in nematode-induced syncytia. Plant Physiology. 2003;131:61–69. doi: 10.1104/pp.008037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Chow KS, Han K. Transcriptome analysis reveals novel features of the molecular events occurring in the laticifers of Hevea brasiliensis (para rubber tree) Plant Molecular Biology. 2003;53:479–492. doi: 10.1023/B:PLAN.0000019119.66643.5d. [DOI] [PubMed] [Google Scholar]
- Kongsawadworakul P, Peret B, Pellegrin F, Nandris D, Chrestin H. The ethylene/cyanide metabolic crossroad in Hevea bark and latex; Proceedings of IRRDB Seminar on Hevea Physiology and Breeding; China: 2004. pp. 331–345. [Google Scholar]
- Kühn C. A comparison of the sucrose transporter systems of different plant species. Plant Biology. 2003;5:215–232. [Google Scholar]
- Kush A, Goyvaerts E, Chye ML, Chua NH. Laticifer-specific gene expression in Hevea brasiliensis (rubber tree) Proceedings of the National Academy of Sciences of the United States of America. 1990;87:1787–1790. doi: 10.1073/pnas.87.5.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacrotte R, Sype HVd, Chrestin H. Influence de l'éthylène sur l'utilisation du saccharose exogène par les laticifères d' Hevea brasiliensis. Proposition d'un mécanisme d'action. Physiologie Vegetale. 1985;23:187–198. [Google Scholar]
- Lemoine R. Sucrose transporters in plants: update on function and structure. Biochimica et Biophysica Acta, Biomembranes. 2000;1465:246–262. doi: 10.1016/s0005-2736(00)00142-5. [DOI] [PubMed] [Google Scholar]
- Lemoine R, Burkle L, Barker L, et al. Identification of a pollen-specific sucrose transporter-like protein NtSUT3 from tobacco. FEBS Letters. 1999;454:325–330. doi: 10.1016/s0014-5793(99)00843-1. [DOI] [PubMed] [Google Scholar]
- Lerchl J, König S, Zrenner R, Sonnewald U. Molecular cloning, characterization and expression analysis of isoforms encoding tonoplast-bound proton-translocating inorganic pyrophosphatase in tobacco. Plant Molecular Biology. 1995;29:833–840. doi: 10.1007/BF00041172. [DOI] [PubMed] [Google Scholar]
- Li C, Shi J, Weiss D, Goldschmidt EE. Sugars regulate sucrose transporter gene expression in citrus. Biochemical and Biophysical Research Communications. 2003;306:402–407. doi: 10.1016/s0006-291x(03)00978-1. [DOI] [PubMed] [Google Scholar]
- Low FC, Atan S, Hafsah Jaafar Tan H. Recent advances in the development of molecular markers for Hevea studies. Journal of Natural Rubber Research. 1996;11:32–44. [Google Scholar]
- Ludwig A, Stolz J, Sauer N. Plant sucrose-H+ symporters mediate the transport of vitamin H. Plant Journal. 2000;24:503–509. doi: 10.1046/j.1365-313x.2000.00900.x. [DOI] [PubMed] [Google Scholar]
- Marger MD, Saier MH. A major superfamily of transmembranes facilitators that catalyse uniport, symport and antiport. Trends in Biochemical Sciences. 1993;18:13–20. doi: 10.1016/0968-0004(93)90081-w. [DOI] [PubMed] [Google Scholar]
- Matsukura C, Saitoh T, Hirose T, Ohsugi R, Perata P, Yamaguchi J. Sugar uptake and transport in rice embryo. Expression of companion cell-specific sucrose transporter (OsSUT1) induced by sugar and light. Plant Physiology. 2000;124:85–93. doi: 10.1104/pp.124.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Melzer M, Truernit E, et al. AtSUC3, a gene encoding a new Arabidopsis sucrose transporter, is expressed in cells adjacent to the vascular tissue and in a carpel cell layer. Plant Journal. 2000;24:869–882. doi: 10.1046/j.1365-313x.2000.00934.x. [DOI] [PubMed] [Google Scholar]
- Meyer S, Lauterbach C, Niedermeier M, Barth I, Sjolund RD, Sauer N. Wounding enhances expression of AtSUC3, a sucrose transporter from Arabidopsis sieve elements and sink tissues. Plant Physiology. 2004;134:684–693. doi: 10.1104/pp.103.033399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey KJ, Ortega JL, Sengupta-Gopalan C. Cytosolic glutamine synthetase in soybean is encoded by a multigene family, and the members are regulated in an organ-specific and developmental manner. Plant Physiology. 2002;128:182–193. [PMC free article] [PubMed] [Google Scholar]
- Poupard P, Brunel N, Leduc N, Viémont JD, Strullu DG, Simoneau P. Expression of a Bet v1 homologue gene encoding a PR 10 protein in birch roots: induction by auxin and localization of the transcripts by in situ hybridization. Australian Journal of Plant Physiology. 2001;28:57–63. [Google Scholar]
- Pujade-Renaud V, Clement A, Perrot-Rechenmann C, et al. Ethylene-induced increase in glutamine synthetase activity and mRNA levels in Hevea brasiliensis latex cells. Plant Physiology. 1994;105:127–132. doi: 10.1104/pp.105.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujade-Renaud V, Perrot-Rechenmann C, Chrestin H, Lacrotte R, Guern J. Characterization of a full-length cDNA clone encoding glutamine synthetase from rubber tree latex. Plant Physiology and Biochemistry (Paris) 1997;35:85–93. [Google Scholar]
- Roblin G, Sakr S, Bonmort J, Delrot S. Regulation of a plant plasma membrane sucrose transporter by phosphorylation. FEBS Letters. 1998;424:165–168. doi: 10.1016/s0014-5793(98)00165-3. [DOI] [PubMed] [Google Scholar]
- Sakr S, Lemoine R, Gaillard C, Delrot S. Effect of cutting on solute uptake by plasma membrane vesicles from sugar beet (Beta vulgaris L.) leaves. Plant Physiology. 1993;103:49–58. doi: 10.1104/pp.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr S, Noubahni M, Bourbouloux A, et al. Cutting, ageing and expression of plant membrane transporters. Biochimica et Biophysica Acta. 1997;1330:207–216. doi: 10.1016/s0005-2736(97)00169-7. [DOI] [PubMed] [Google Scholar]
- Sakr S, Alves G, Morillon R, et al. Plasma membrane aquaporins are involved in winter embolism recovery in walnut tree. Plant Physiology. 2003;133:630–641. doi: 10.1104/pp.103.027797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sauer N. Molecular physiology of higher plant sucrose transporters. FEBS Letters. 2007;581:2309–2317. doi: 10.1016/j.febslet.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Sauer N, Stolz J. SUC1 and SUC2: two sucrose transporters from Arabidopsis thaliana; expression and characterization in baker's yeast and identification of the histidine-tagged protein. Plant Journal. 1994;6:67–77. doi: 10.1046/j.1365-313x.1994.6010067.x. [DOI] [PubMed] [Google Scholar]
- Sauer N, Ludwig A, Knoblauch A, Rothe P, Gahrtz M, Klebl F. AtSUC8 and AtSUC9 encode functional sucrose transporters, but the closely related AtSUC6 and AtSUC7 genes encode aberrant proteins in different Arabidopsis ecotypes. Plant Journal. 2004;40:120–130. doi: 10.1111/j.1365-313X.2004.02196.x. [DOI] [PubMed] [Google Scholar]
- Scofield GN, Aoki N, Hirose T, Takano M, Jenkins CLD, Furbank RT. The role of the sucrose transporter, OsSUT1, in germination and early seedling growth and development of rice plants. Journal of Experimental Botany. 2007;58:483–495. doi: 10.1093/jxb/erl217. [DOI] [PubMed] [Google Scholar]
- Schneidereit A, Imlau A, Sauer N. Conserved cis -regulatory elements for DNA-binding-with-one-finger and homeo-domain-leucine-zipper transcription factors regulate companion cell-specific expression of the Arabidopsis thaliana SUCROSE TRANSPORTER 2 gene. Planta. 2008;228:651–662. doi: 10.1007/s00425-008-0767-4. [DOI] [PubMed] [Google Scholar]
- Silpi U, Chantuma P, Kasemsap P, et al. Sucrose and metabolism distribution patterns in the latices of three Hevea brasiliensis clones: effects of tapping and stimulation on the tree trunk. Journal of Rubber Research. 2006;9:115–131. [Google Scholar]
- Silpi U, Lacointe A, Kasempsap P, et al. Carbohydrate reserves as a competing sink: evidence from tapping rubber trees. Tree Physiology. 2007;27:881–889. doi: 10.1093/treephys/27.6.881. [DOI] [PubMed] [Google Scholar]
- Sookmark U, Pujade-Renaud V, Chrestin H, et al. Characterization of polypeptides accumulated in the latex cytosol of rubber trees affected by the tapping panel dryness syndrome. Plant and Cell Physiology. 2002;43:1323–1333. doi: 10.1093/pcp/pcf161. [DOI] [PubMed] [Google Scholar]
- Sun AJ, Xu HL, Gong WK, et al. Cloning and expression analysis of rice sucrose transporter genes OsSUT2M and OsSUT5Z. Journal of Integrative Plant Biology. 2008;50:62–75. doi: 10.1111/j.1744-7909.2007.00596.x. [DOI] [PubMed] [Google Scholar]
- Tupy J. The level and distribution pattern of latex sucrose along the trunk of Hevea brasiliensis Mull.Arg. as affected by the sink region induced by latex tapping. Physiologie Vegetale. 1973;11:1–11. [Google Scholar]
- Tupy J, Primot L. Control of carbohydrate metabolism by ethylene in latex vessels of Hevea brasiliensis Muel. Arg. in relation to rubber production. Biologia Plantarum. 1976;18:373–383. [Google Scholar]
- Vaughn MW, Harrington GN, Bush DR. Sucrose-mediated transcriptional regulation of sucrose symporter activity in the phloem. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10876–10880. doi: 10.1073/pnas.172198599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Kühn C, Tegeder M, Frommer WB. Sucrose transport in higher plants. International Review of Cytology-A Survey Of Cell Biology. 1998;178:41–71. doi: 10.1016/s0074-7696(08)62135-x. [DOI] [PubMed] [Google Scholar]
- Weise A, Barker L, Kuhn C, et al. A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell. 2000;12:1345–1355. doi: 10.1105/tpc.12.8.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Huang D, Liu S, Tang C. Molecular cloning and sequence analysis of six sucrose transporter genes from Hevea brasiliensis (para rubber tree) Re Dai Zuo Wu Xue Bao. 2007;28:32–38. [Google Scholar]