Abstract
Background and Aims
Seed survival in the soil contributes to population persistence and community diversity, creating a need for reliable measures of soil seed bank persistence. Several methods estimate soil seed bank persistence, most of which count seedlings emerging from soil samples. Seasonality, depth distribution and presence (or absence) in vegetation are then used to classify a species' soil seed bank into persistent or transient, often synthesized into a longevity index. This study aims to determine if counts of seedlings from soil samples yield reliable seed bank persistence estimates and if this is correlated to seed production.
Methods
Seeds of 38 annual weeds taken from arable fields were buried in the field and their viability tested by germination and tetrazolium tests at 6 month intervals for 2·5 years. This direct measure of soil seed survival was compared with indirect estimates from the literature, which use seedling emergence from soil samples to determine seed bank persistence. Published databases were used to explore the generality of the influence of reproductive capacity on seed bank persistence estimates from seedling emergence data.
Key Results
There was no relationship between a species' soil seed survival in the burial experiment and its seed bank persistence estimate from published data using seedling emergence from soil samples. The analysis of complementary data from published databases revealed that while seed bank persistence estimates based on seedling emergence from soil samples are generally correlated with seed production, estimates of seed banks from burial experiments are not.
Conclusions
The results can be explained in terms of the seed size–seed number trade-off, which suggests that the higher number of smaller seeds is compensated after germination. Soil seed bank persistence estimates correlated to seed production are therefore not useful for studies on population persistence or community diversity. Confusion of soil seed survival and seed production can be avoided by separate use of soil seed abundance and experimental soil seed survival.
Key words: Arable weeds, Bifora testiculata, Carthamus lanatus, Centaurea solstitialis, longevity index, seed bank persistence, soil seed bank
INTRODUCTION
Soil seed banks are a key to understanding the dynamics of plant populations, species and ecosystems (Silvertown, 1982; Kalisz, 1991; Kalisz and McPeek, 1992; Günter, 1997; Bekker et al., 1998a; Cabin et al., 1998). Notably, seed persistence in soil has been shown to be an important correlate of population persistence (Stöcklin and Fischer, 1999; Rees et al., 2002). The importance of high seed survival in soil seed banks to ensure persistence of local populations has also been demonstrated in theoretical models (Pake and Venable, 1995, 1996). Species coexistence in communities is enhanced by the ‘storage effect’ of seeds (Chesson and Warner, 1981; Warner and Chesson, 1985; Levine and Rees, 2004; Facelli et al., 2005). Thus, seed bank attributes such as seed persistence or survival account for a considerable part of diversity of plant communities via coexistence and may be one of the traits corresponding to α-niche differentiation (Silvertown et al., 1999, 2006). Additionally, it has been shown that soil seed banks are important for community composition in open and highly disturbed habitats (Thompson et al., 1997; Hopfensberger, 2007) and on a smaller scale for bare soil communities in particular habitats (Peco et al., 1998; Wellstein et al., 2007). This explains the substantial practical use of soil seed banks for restoration of these communities (van der Valk and Pederson, 1989; Bakker et al., 1996a). The correct identification of transient, short- and long-term persistent species and levels of seed survival is therefore crucial for the feasibility and success of restoration efforts for plant communities (Poschlod, 1993; Hutchings and Booth, 1996; Willems and Bik, 1998; Dutoit et al., 2003; von Blanckenhagen and Poschlod, 2005) and populations (Adams et al., 2005), and for understanding the maintenance of rare species in man-made ecosystems. Evidently, the same is true for more basic questions on vegetation and population dynamics as well as on species coexistence.
There are various methods to study soil seed bank persistence of seeds, which can be classified into: (a) direct age determination by 14C dating (McGraw et al., 1991; Moriuchi et al., 2000); (b) burial experiments of seeds and subsequent testing of germinability or viability (e.g. Telewski and Zeevart, 2002); (c) determination of the depth distribution of germinable seeds in the soil (Bekker et al., 1998b); (d) determination of soil seed banks along successional seres (Poschlod et al., 1998; Wäldchen et al., 2005); and (e) comparative analysis of seasonal dynamics of seed rain and seed bank (Thompson and Grime, 1979; Poschlod and Jackel, 1993). However, the methods are not equivalent with respect to quality of results. Whereas methods (a) and (b) accurately identify soil seed bank survival, methods (c) to (e) produce results which are not accurate for several reasons. First, they may be affected by seed input – only species which are frequent and/or have a high seed production will be found. Secondly, the results of using depth distribution will depend on the importance of soil movement and disturbances. Finally, methods (c) to (e) are based on the so-called seedling emergence method, where soil samples are exposed to ‘favourable’ conditions for germination in order to identify and count seedlings. Since ungerminated but viable seeds are not quantified, levels of dormancy can influence the results of these methods. For data from the indirect methods (c–e) based on seedling emergence we use the term ‘seed bank persistence’ and for direct measures coming from burial experiments (b), we use the term ‘soil seed survival’.
Methods that determine seed survival (a and b) are expensive and time consuming, therefore Thompson et al. (1998) proposed the calculation of a ‘longevity index’ (LI) which summarizes seed bank persistence and soil seed survival data from different studies (methods b–e) for a species and is measured on a continuous scale. LI is the proportion of the number of records in a database that report species as having a persistent seed bank relative to all records, including those classifying the species' seed bank as transient. LI is now widely used in fundamental ecological studies when a single continuous value is needed to describe the soil seed bank type for a given species, e.g. to study ecological correlates of seed bank persistence at species (Hodkinson et al., 1998; Thompson et al., 1998) and community levels (Thompson et al., 1998) or even searching trade-offs to other traits (Ozinga et al., 2007).
For several local floras, the use of seedling emergence data to determine soil seed bank persistence has revealed that persistent seeds tend to be smaller, more compact, dormant and dependent on light for germination, while transient seeds are larger, often elongated or bear appendages (Thompson and Grime, 1979; Thompson et al., 1993; Bekker et al., 1998b; Moles et al., 2000; Cerabolini et al., 2003; Peco et al., 2003; Funes et al., 2007). In contrast, no seed size–seed longevity relationship was demonstrated for the Australian flora by Leishman and Westoby (1998), who used dormancy patterns to estimate soil seed bank persistence.
The seedling emergence method to study seed bank persistence can, even in intensive studies, fail to pick up species with short dispersal distance, short seed shedding period or short germination season and with primary dormancy (Thompson and Grime, 1979). Indeed, Bakker et al. (1996a) and Thompson et al. (1997) have already pointed out that rare species can be absent in seed bank studies even if the species is present in the above-ground vegetation and although its seed bank may be persistent. These aspects raise the question of whether seed bank persistence measured by seed counts from soil seed samples is reliable, and thus correlated to independent measures of seed longevity, such as soil seed survival in burial experiments.
It is widely acknowledged that seed size is related to seed production by a fundamental trade-off (Shipley and Dion, 1992; Turnbull et al., 1999; Jakobsson and Eriksson, 2000). High seed production enhances dispersal efficiency (Tackenberg et al., 2003; Poschlod et al., 2005; Bruun and Poschlod, 2006) and it has also been suggested to increase seed bank persistence (Thompson, 2000). Surprisingly, this has never been tested directly and it has not been asked if the different measures of seed bank persistence all relate to levels of seed production. This is especially interesting because the trade-off supposes that many small seeds are equally efficient for reproduction as a few large seeds. The latter compensate for their lower number at other life stages, beginning with the seedling (Leishman et al., 2000b; Moles et al., 2004). In order to understand population dynamics and community diversity it is important to distinguish between number and survival of seeds, and to know whether seed bank persistence estimates can be influenced by seed number. Seed production influences seed rain (Jackel and Poschlod, 1994); therefore, we can hypothesize that it also influences seed bank persistence estimates which are based on seedling emergence from soil samples but not so for soil seed survival measured in burial experiments.
The understanding of soil seed bank persistence is based primarily on works from arable fields since they contribute a large part of available data (Thompson et al., 1997). The difficulty of detecting rare species in seed bank studies using soil samples (Bakker et al., 1996a; Thompson et al., 1997) means that the work of conservation biologists is hampered by the lack of reliable information on the longevity of seeds for the rarest arable weed species (Schneider et al., 1994; Wäldchen et al., 2005). Thus, rare arable weeds are ideal candidates to study the importance of seed counts in soil samples for the estimation of seed bank persistence together with data on seed survival from burial experiments. For these reasons and because annuals depend on long-term persistent soil seed banks for their persistence, we explicitly studied a mixed set of rare and more common annual arable weeds in an experimental study (Appendix) and more generally in a wide set of habitats and species.
Our questions were studied using two different approaches. First, an ‘experimental approach’ (a) was used to gather reliable data on survival of seed in the soil for a quantified seed population. This experiment was complemented by an analysis of seed production and seed bank persistence from literature for the same species to (b) answer the question on the reliability of seed bank persistence estimated by seedling emergence from soil samples in the light of experimental soil seed survival and to (c) explore whether experimental soil seed survival is related to seed production.
In a second ‘database approach’, questions (b) and (c) were studied further in a more general way using databases on a wider set of species. This allowed us (d) to analyse whether published soil seed survival data from burial experiments show a relationship to literature data on seed production and (e) to determine whether the LI based on published burial experiments and seed production from the literature are related.
MATERIALS AND METHODS
Experimental approach
Study system
Annual arable weeds were chosen as the study system because of the well-known interspecific differences in seed bank persistence and their short life cycle, making them heavily dependent on mortality in the seed bank. A burial experiment was carried out at Cucuron (43°46′5′′N, 5°21′2′′E, south-eastern France). The surrounding agricultural landscape in the Luberon area was chosen as the study region because, here, traditional agriculture has maintained a high diversity of rare arable weeds that are extinct elsewhere in Europe. This region is characterized by Mediterranean climate (autumn rain and summer drought).
Seed material was collected in the study region between June and September 2005. For each species, ripe seeds were taken from at least ten individuals of a single large population and mixed. Seed material was stored under dry conditions in paper bags until October 2005, when the burial experiment and the initial viability test were started. We cannot exclude a loss of viability or a loss of dormancy due to after-ripening because seeds were not studied directly after harvesting (Baskin and Baskin, 1998). However, this is what normally happens under Mediterranean climate, where seeds after-ripen in dry summer and germinate in autumn after the first rains or after ploughing (Baskin and Baskin, 1998). Every seed sample was randomly taken from a single well-mixed seed lot.
Experimental design of the burial experiment
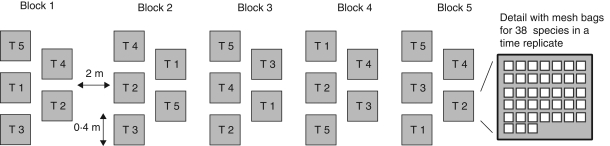
A burial experiment was set up using 38 annual arable weed species (Appendix), for which seed samples were buried for at maximum 2·5 years. Viability was tested every 6 months to capture the two main germination periods, in autumn and spring. The burial experiment was carried out in young fallow land with no disturbance during the time of the experiment. The seed samples were divided into 30 sub-samples with 25–50 seeds for most species (see Appendix). For each species, five samples were assigned at random to each of five retrieval dates (T1–T5), and five samples were kept for the initial test (T0). The experiment was set up as a randomized block design with each block containing groups of samples for each of five time intervals (T1–T5), placed at random in the block (Fig. 1). Each of these time interval groups contained 38 nylon mesh bags, one for each of the 38 species. Samples were buried at 10 cm depth. At each retrieval date, one group of 38 mesh bags per block was removed and studied in the laboratory. Seeds were retrieved twice a year: in spring (T1, T3, T5) and in autumn (T2, T4); the initial test (T0) was done in autumn 2005. In the burial experiment, 39 400 seeds were buried, and an additional 7880 seeds were tested in the initial test. In all tests, 9802 seeds germinated in the three germination test phases (see below), 16 574 ungerminated seeds were tested after the germination tests for viability using a tetrazolium test and 20 897 seeds presumably died during burial. The burial experiment was started in October 2005 and the last tetrazolium tests were finished in September 2008.
Fig. 1.
Experimental layout: position of blocks, time step replicates (T1–T5) and mesh bags for each species inside blocks.
Testing experimental seed survival in the burial experiment
Germinability was tested using a sequence of germination conditions standardized for all seed retrieval dates. After seeds were exhumed, the empty seeds were counted. These were apparent by their shape or colour, or by being soft when they were pressed with a needle (Ter Heerdt et al., 1996). Firm seeds were then incubated at 22 °C for 14 h in the light (cool white fluorescent tubes, ± 10 000 lux; approx. ± 250 µmol m−2 s−1 PPFD) and at 14 °C for 10 h in darkness in a growth chamber on moist filter paper. After 28 d, seeds were cold stratified for 6 weeks at 4 °C in darkness. Seed samples were then again subjected to 22 /14 °C (14 /10 h) for 28 d. The positions of Petri dishes were randomized in the growth chamber. Seeds were counted as they germinated and discarded when the tip of the radicle emerged.
Seeds that did not germinate were tested for viability with tetrazolium chloride (ISTA, 1996). Seeds of Consolida regalis, Legousia hybrida and Legousia speculum-veneris stained well without previous bisection. Seeds of Papaver rhoeas, P. argemone, P. hybridum and Roemeria hybrida did not stain in the tetrazolium test. However, the embryos were firm and white, and thus the seeds were classified as viable. In some cases (e.g. Adonis annua; morphological dormancy in Ranunculaceae), a very small, underdeveloped embryo made the use of tetrazolium impossible in the first stages of the experiment. Thus, the highest number of viable seeds (germinable + dormant) detected from a later seed retrieval date were used as the initial number of living seeds.
Compiling seed bank persistence estimates from the literature: the longevity index
The LI for the species in the burial experiment was calculated using literature data. Thus, we compiled a database using the entries for our species in Thompson et al. (1997) and the results of a survey of the recent literature. Records on seed bank persistence were classified into one of the following soil seed bank types Thompson et al. (1997): (a) transient: species persist for <1 year; (b) short-term persistent: seeds persist for >1 year but <5 years after dispersal; and (c) long-term persistent: seeds persist in a viable state in the soil for at least 5 years.
The LI was calculated for each species (Thompson et al., 1998):
| 1 |
where LI is the proportion of the number of records (R) classifying a species as short- (sp) and long-term persistent (lp) to the sum of all records, including the number of transient records (Rt) for a given species. Initally all types of data were used. We then tested if using data from seedling emergence from soil samples alone changed the reliability of the LI. Due to limited data in the literature on our initial 38 species, we only had LI values for 26 species using all data and for 21 species using only data from seed bank persistence estimates by seedling emergence from soil samples.
Seed production
Seed production was determined for the 38 species, i.e. mean individual seed production of ten individuals in the field. Some species had multiseeded fruits (e.g. Papaver sp. pl.), whereas others had many fruits per infructescence (e.g. Apiaceae); therefore, we counted the number of fruits or infructescences per individual for these species. Then the number of seeds per fruit or infructescence was counted in two fruits or infructescences. Seed production per individual was calculated as the mean number of seeds per fruit or infructescence multiplied by the number of fruits or infructescences counted per individual.
Database approach
Data on seed bank studies
A second approach compared seed bank persistence with seed production and completed the (necessarily) limited data set on arable weeds. Here, a larger database on seed bank studies (i.e. Thompson et al., 1997) was explored, together with another published database on seed production in the field (Šera and Šery, 2004). All species for which there were data on both seed bank persistence and seed production were extracted. The database of Thompson and co-workers (1997) includes a large number of seed bank studies using seedling emergence from soil seed samples and a relatively small number of burial experiments. Each record included information on the seed bank type for the species (transient, short- or long-term persistent) according to the key in Thompson et al. (1997). The data were sub-divided into those from seed burial experiments and those from seedling emergence studies. For the latter, only species with at least five entries were used (Thompson et al., 1998). For burial experiments, all species were used because seed bank type is more reliable with this method. LI was calculated for all species in both sub-sets as explained above.
Data on reproductive capacity
Šera and Šery (2004) measured reproductive capacity by counting seeds per surface of sampled vegetation and using cover percentages of a species to calculate the potential seed production of a species at 100 % cover; they provide data for 492 species. For 227 of these species there were seed bank data using the seedling emergence method and 174 species with data from burial experiments in the database of Thompson et al. (1997). Five of these species were also used in our own burial experiment.
Statistical analysis
The data were analysed using linear regression to test relationships between continuous parameters. All analyses were run in the R statistical environment (R Development Core Team, 2008).
RESULTS
Experimental approach
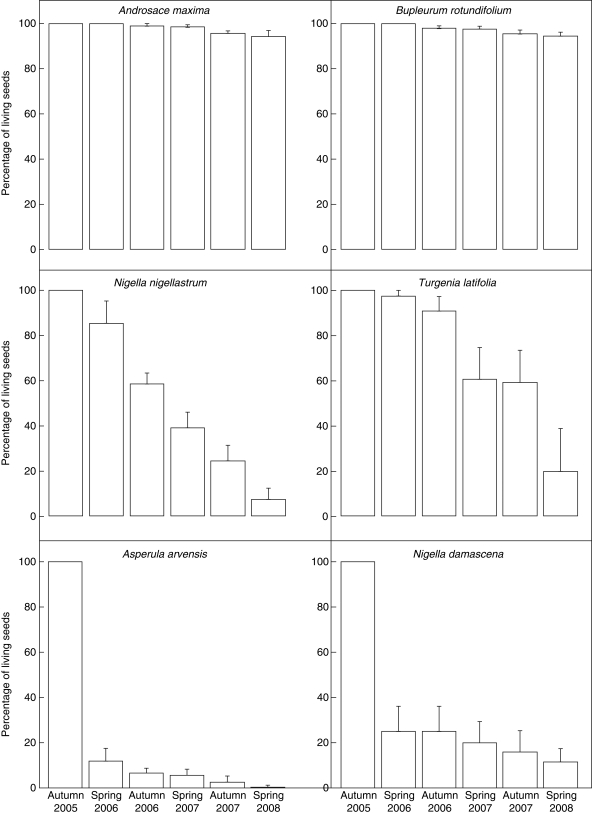
Mortality of buried seeds at the end of the experiment ranged from very high, reaching 100 % in some cases, as exemplified by Agrostemma githago, Asperula arvensis and Nigella arvensis (Fig. 2, Appendix), to very low (down to 3·5 %) for species such as Androsace maxima, Bupleurum rotundifolium, Adonis annua and Carthamus lanatus (Fig. 2, Appendix). Other species had intermediate mortalities. There were marked differences in the proportion of surviving seeds and shape of the mortality curve between species. In some cases, final mortalities were similar but mortality curves were different; compare, for example, Nigella nigellastrum and N. damascena in Fig. 2.
Fig. 2.
Percentage survival for five retrieval dates for six representative species. Initial viability in autumn 2005 is presented as 100 % to give a scale among species; the survival percentages are relative to this initial viability. Bars are s.e.
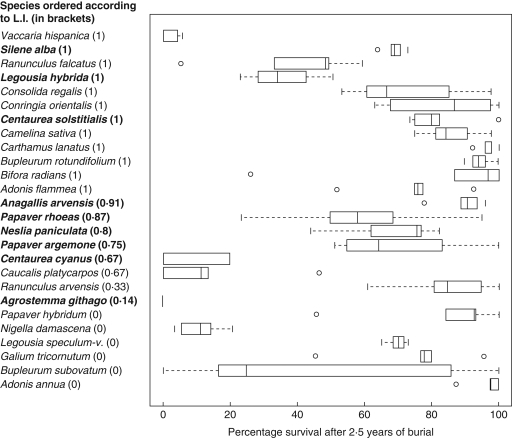
There was no relationship between soil seed mortality in the burial experiment and the longevity index of the same species (R2 = 0·02, F1,25 = 0·58, P = 0·45; Fig. 3). When the analysis was restricted to LI calculated from seedling emergence from soil sample data only, still no relationship to experimental soil seed survival was found (R2 = 0·02, F1,20 = 0·50, P = 0·49). Clearly, seed mortality under field conditions is not related to seed bank persistence determined using the seedling emergence method in soil seed samples. Furthermore, there was no significant relationship between individual seed production and experimental seed survival after 2·5 years (R2 = 0·01, F1,36 = 0·46, P = 0·50). This indicates the independence of the two parameters.
Fig. 3.
Boxplots of percentage survival of seeds for 26 species after 2·5 years of burial (five replicates per species). Boxes and central bars represent the interquartile range and median, dashed lines represent the range of sample, and dots are outliers. Species are ordered according to their longevity index (LI). Species in bold are those for which at least five records were used for calculation of the LI.
Database approach
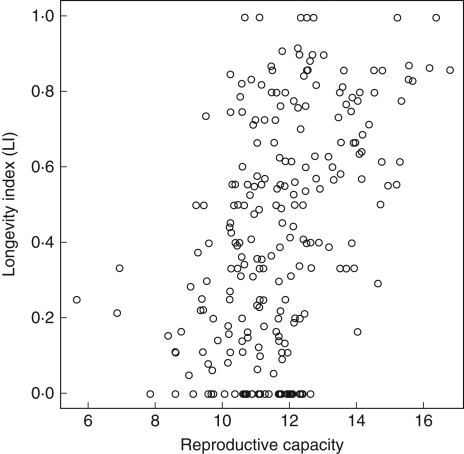
The relationship between reproductive capacity (seed production) and LI using counts of emerging seedlings in soil samples was significant and positive (R2 = 0·10, F1,225 = 25·23, P < 0·001; T = 5·02, P < 0·001; Fig. 4), indicating that soil seed bank persistence determined in this manner can be related to the number of seeds produced per surface unit. However, the parallel analysis of soil seed bank persistence using only burial experiments yielded no significant relationship (R2 < 0·01, F1,172 = 0·12, P = 0·73), indicating that maximum longevity in burial experiments is not related to the number of seeds produced per surface unit. The joint analysis of the two sub-sets is not shown because the results were completely dominated by the data from seedling emergence studies since they are the majority in the studied data sets.
Fig. 4.
Relationship of a species' reproductive capacity (logarithm of seeds produced per m2, Šera and Šery, 2004) and its longevity index (LI; Thompson et al., 1997) based on ≥5 studies per species using seedling emergence from soil seed bank samples; LI is high when many studies classify the species as persistent, and low when there are many transient records; for details, see text (R2 = 0·10, F1,225 = 25·23, P < 0·001).
DISCUSSION
Experimental approach
This study shows that seed survival measured from a burial experiment is not correlated to the commonly used seed bank persistence estimates from the literature when it is estimated from seedling emergence. The absence of a relationship leads us to the following questions. How have these seed bank persistence estimates been generalized as a measure for seed longevity? What can explain the differences between our experimental ‘soil seed survival’ and seed bank persistence from other studies? What affects seed survival in the soil?
Bekker et al. (1998b) tested the general validity of seed bank persistence estimates based on the depth distribution of viable seeds; they detected seeds with the seedling emergence method. In order to show that their ‘depth-derived’ method reflects soil seed longevity, they used a database without ‘depth-derived’ data. However, their database still contained many entries using the seedling emergence method mixed with entries using seed burial experiments. The mixture which Bekker et al. (1998b) used in their validation database makes it difficult to know whether the seedling emergence method is related to experimental soil seed survival, and therefore it is also not clear if data from ‘depth-derived’ methods are related to experimental soil seed bank survival. There is, to our knowledge, no other analysis that tested the generality of seed bank persistence estimated on seedling emergence from soil samples in the light of experimental seed survival in soil.
The differences between our ‘soil seed survival’ and ‘seed bank persistence’ estimates based on seedling emergence from soil samples can be interpreted by methodological differences. Classically, the seedling emergence method uses ten plots, each with ten soil samples of 4 cm diameter, yielding a total sampled surface of just 0·125 m2 to represent a community (Hutchings, 1986; Bakker et al., 1996a; Bekker et al., 2005). Thompson and Grime (1979) argued that species with low seed production are difficult to detect in the soil seed bank even if seeds are long lived in the soil. Consequently, there is a strong risk of an erroneous classification since species present in the vegetation but absent in the seed bank are classified as transient. Especially rare species or species with low seed production are absent from samples, although they have long-lived seeds in the soil.
In addition to this, environmental factors acting on soil seed mortality can also explain the differences between our experimental data and the literature data. For example, studies on fungi indicate that there are differences in soil seed mortality within species related to soil properties (Blaney and Kotanen, 2001; Schafer and Kotanen, 2003; Wagner and Mitschunas, 2007; but consider also Leishman et al., 2000a). Dry habitat species have higher seed mortality under moist than under dry conditions due to attack by pathogenic fungi (Blaney and Kotanen, 2001; Schafer and Kotanen, 2003). Thus, soil seed survival varies greatly from one site to another for a given species, and differences among sites may contribute to the differences between our experimental data and the data from the literature. Moreover, the conditions in our mesh bags may not reflect conditions in natural seed banks; this point was addressed by van Mourik et al. (2005). This might imply that we overestimated seed depletion, but overall we found rather high survival rates and in addition we did not have particularly wet conditions compared with the fields from which the seeds originated. Furthermore, marked differences in seed decay among species appeared in our burial experiment, as exemplified by Fig. 2. This suggests that our experimental seed survival is realistic and that the seed bank persistence estimates from the literature may reflect another aspect rather than only seed survival. According to suggestions of Thompson (e.g. Thompson and Grime, 1979; Thompson, 2000), seed production is a possible candidate to influence it. However, absence of a significant relationship between seed production and experimental seed survival in our work suggests that both are independent. We can only draw limited conclusions with our experimental data because they represent only a single habitat and a limited number of species.
Database approach
Use of two larger databases on soil seed bank studies and on reproductive capacity from the literature, including many species from many different habitats, explored whether reproductive capacity is related to seed bank persistence based on seedling emergence (Fig. 4). The regression showed that seed production influences seed bank persistence estimates. In the light of seed depletion models (e.g. Murdoch and Ellis, 2000), it becomes clear that the more seeds a species produces, the higher the chance that at least some seeds survive for a longer time and are detected. It is not surprising that this relationship to reproductive capacity disappears when soil seed survival from burial experiments is used, a finding confirmed by our experimental data. No study so far has explored the relationship between seed bank persistence and reproductive capacity. This leads to the conclusion that the seed bank persistence estimates used until now do not represent seed longevity alone but that they mix seed production with soil seed survival. This has to be considered for all studies using the seedling emergence method without an estimate of the total initial seed population. Furthermore, this also concerns studies that directly count seeds in soil samples (e.g. Moriuchi et al., 2000). Here empirical data are added showing that seed production is an important factor for the formation of a persistent seed bank (Parker et al., 1989; Simpson et al., 1989; Thompson, 2000). Bruun and Poschlod (2006) showed that seed production is a relevant component of dispersal through space, and, therefore, seed production may also be related to dispersal through time (see also Poschlod et al., 2005). Our data suggest that seed production and seed mortality are two independent processes, since there is no relationship between experimental seed survival and seed production. We think both contribute to soil seed bank formation. In contrast to seed production, seed mass and shape have been frequently used to explain soil seed bank formation (Bekker et al., 1998b). This should be reconsidered in the light of our findings – which emphasize the role of seed number – and a fortiori in the light of the seed size–seed number trade-off (Shipley and Dion, 1992; Turnbull et al., 1999; Jakobsson and Eriksson, 2000).
The correlation between seed production and persistence reported here suggests that the size and detectability of the soil seed bank of smaller seeds are probably in the same trade-off with seed size than is seed number. This offers a new and parsimonious explanation for the seed size–seed bank persistence relationship (Thompson et al., 1993; Bekker et al., 1998b; Moles et al., 2000; Cerabolini et al., 2003; Peco et al., 2003; Funes et al., 2007). Using the seedling emergence method, seed longevity estimates for smaller seeds (i.e. more numerous) are higher without a higher soil seed survival, because mechanisms that compensate larger seeds for their lower number act after germination, at the seedling stage (McGinley et al., 1987; Louda, 1989; Jakobsson and Eriksson, 2000; Leishman et al., 2000b; Coomes and Grubb, 2003; Moles et al., 2004; Pizo et al., 2006; Bladé and Vallejo, 2008). This has the consequence that species with a higher seed bank persistence estimate do not yield higher numbers of established plants (Hillier et al., 1990). Seed bank persistence estimates based on seedling emergence methods are therefore potentially meaningless to explain population persistence or community diversity.
Conclusions
Our results question the use of seed bank persistence estimates based on seedling emergence in the current literature (Bekker et al., 1998; Thompson et al., 1998; Ozinga et al., 2007). The strong relationship between seed production and seed bank persistence estimates based on seedling emergence presented here should encourage us to re-evaluate this literature carefully. Moreover, we think that a clear distinction between seed quantity-related parameters and seed age-related parameters can significantly increase our understanding of mechanisms generating soil seed banks and give new insights into what role seed banks play in vegetation and population dynamics. In any case, seed production should be included in future models on seed bank dynamics (Parker et al., 1989).
Finally, there is a need to describe the two fundamental characteristics of soil seed banks, i.e. longevity and abundance, in future studies. For longevity, differences in survival of seeds between species already become apparent after 1·5 years (Fig. 2). A longer burial period (>2·5 years) would confound transient and short-term persistent species because – at least in our data – both have similar final mortalities (Fig. 2) and additionally would greatly limit available data. Soil seed viability determined after only 1 year of seed burial does not discriminate between transient and persistent species. We therefore propose that two parameters should be used: (1) classes of soil seed abundance; and (2) mean percentage survival of seeds after 1·5 years of burial. These two parameters are independent in the data sets studied here and represent two main factors for the formation of soil seed banks.
ACKNOWLEDGEMENTS
We thank Franck Torre and Pascal Campagne for advice in experimental design and statistics; Giacomo Gazzaniga, Nadia Bertagne, Frédéric Henry, Clémentine Coiffait, Florence Fraisse, Mariannick Juin, Maryse Alvitre, Inge Lauer and Elise Buisson for help with experimental and field work; Myriam Virevaire (Conservatoire Botanique National Méditerranéen) for seed material; and Louis-Michel Bremond for permission to use the study site. This work was supported by Bayerisch-Französisches Hochschulzentrum and Parc Naturel Régional du Luberon. We thank two anonymous referees for many helpful comments on a previous version.
APPENDIX
List of tested seed lots, number of replicates, origins and mortality percentages
| Percentage survival |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Family | Code | Rarity | Location: Commune/Département | No. of seeds | 0·5 year | 1·5 years | 2·5 years | Mean individual seed production | Mean seed weight (mg) |
| Adonis annua | Ranunculaceae | Adan | RR | Rustrel/84 | 40 | 100 | 99·5 | 96·5 | 161·2 | 11 |
| Adonis flammea | Ranunculaceae | Adfl | RR | Vachères/04 | 40 | 100 | 88·7 | 74·6 | 672 | 9·88 |
| Agrostemma githago | Caryophyllaceae | Aggi | RR | Lagarde d'Apt/84 | 50 | 0 | 0 | 0 | 337·8 | 16·98 |
| Anagallis arvensis | Primulaceae | Anar | CCC | Istres/13 | 50 | 99·6 | 98·9 | 89·2 | 54·7 | 0·404 |
| Androsace maxima | Primulaceae | Anma | RR | Lagarde d'Apt/84 | 50 | 100 | 98·6 | 94·4 | 41·5 | 1·422 |
| Asperula arvensis | Rubiaceae | Asar | RRR | Lagarde d'Apt/84 | 50 | 11·5 | 5·3 | 0 | 94·7 | 7·233 |
| Bifora radians | Apiaceae | Bira | R | Pierrevert/04 | 50 | 100 | 95·4 | 83·8 | 46·7 | 12·88 |
| Bifora testiculata | Apiaceae | Bits | RRR | Pertuis/84 | 50 | 100 | 77 | 72·1 | 75·4 | 7·593 |
| Bupleurum rotundifolium | Apiaceae | Buro | RR | Aubenas/04 | 50 | 100 | 97·6 | 94·5 | 429·6 | 3·573 |
| Bupleurum subovatum | Apiaceae | Busu | RRR | Mirabeau/04 | 50 | 100 | 87·1 | 45·2 | 69·3 | 4·403 |
| Camelina sativa | Brassicaceae | Casa | RR | Lagarde d'Apt/84 | 50 | 98·2 | 94·1 | 85·8 | 1279·5 | 0·44 |
| Carthamus lanatus | Asteraceae | Cala | C | Vauvenargues/13 | 50 | 100 | 99·6 | 96·4 | 499·6 | 13·008 |
| Caucalis platycarpos | Apiaceae | Capl | R | Pierrevert/04 | 20 | 88·7 | 52·5 | 13·7 | 8·0 | 25·454 |
| Centaurea cyanus | Asteraceae | Cecy | C | Lagarde d'Apt/84 | 20 | 21·1 | 10·5 | 10·5 | 629·0 | 4·331 |
| Centaurea solstitialis | Asteraceae | Ceso | C | Ventabren/13 | 20 | 97·4 | 92·2 | 81·8 | 710·8 | 1·682 |
| Ceratocephala falcata | Ranunculaceae | Rafl | RR | Pertuis/84 | 50 | 100 | 67·9 | 39·6 | 85·5 | 14·436 |
| Cnicus benedictus | Asteraceae | Cnbe | R | La Bastidonne /84 | 30 | 100 | 83·7 | 49·6 | 50·8 | 30·897 |
| Conringia orientalis | Brassicaceae | Coor | RR | Lagarde d'Apt/84 | 50 | 100 | 89·5 | 83·1 | 280 | 2·543 |
| Consolida regalis | Ranunculaceae | Core | R | Céreste/04 | 50 | 100 | 98·3 | 72·2 | 227·1 | 0·862 |
| Galeopsis ladanum | Lamiaceae | Gala | R | Céreste/04 | 20 | 100 | 95·2 | 61·9 | 95·2 | 1·2 |
| Galium tricornutum | Rubiaceae | Gatr | R | Pertuis/04 | 50 | 96·8 | 88·1 | 75·2 | 109·4 | 12·419 |
| Glaucium flavum | Papaveraceae | Glfl | – | Méjean/13 | 50 | 89·2 | 89·2 | 74·3 | 18577·6 | 1·148 |
| Hypecoum pendulum | Papaveraceae | Hype | RRR | Pertuis/13 | 30 | 98·6 | 89·9 | 82 | 174·0 | 2·77 |
| Legousia hybrida | Campanulaceae | Lehy | R | Pertuis/13 | 10 | 96 ·4 | 82·1 | 35·7 | 299·9 | 0·18 |
| Legousia speculum-veneris | Campanulaceae | Lesv | C | Vachères/04 | 50 | 99·2 | 90·3 | 69·8 | 805·8 | 0·194 |
| Myagrum perfoliatum | Brassicaceae | Mype | RR | Céreste/04 | 5 | 100 | 81·2 | 31·2 | 76·1 | 20·88 |
| Neslia paniculata | Brassicaceae | Nepa | RR | Lagarde d'Apt/84 | 50 | 100 | 86·5 | 67 | 76·4 | 3·02 |
| Nigella damascena | Ranunculaceae | Nida | R | Buoux/84 | 50 | 24·8 | 19·6 | 11·1 | 166·1 | 1·386 |
| Nigella nigellastrum | Ranunculaceae | Gani | RRR | Mérindol/04 | 10 | 85·4 | 39 | 7·3 | 69·3 | 1·78 |
| Papaver argemone | Papaveraceae | Paar | C | Buoux/84 | 50 | 96·4 | 91·1 | 69·3 | 3488 | 0·168 |
| Papaver hybridum | Papaveraceae | Pahy | R | Lourmarin/84 | 50 | 95·9 | 95 | 82·1 | 2079 | 0·109 |
| Papaver rhoeas | Papaveraceae | Parh | CCC | Buoux/84 | 50 | 93·5 | 91·7 | 55·6 | 1762·8 | 0·088 |
| Ranunculus arvensis | Ranunculaceae | Raar | C | Lagarde d'Apt/84 | 25 | 96·2 | 90·4 | 83·7 | 151 | 13·496 |
| Rapistrum rugosum | Brassicaceae | Raru | C | Céreste/04 | 5 | 50 | 0 | 0 | 647·8 | 3·13 |
| Roemeria hybrida | Papaveraceae | Rohy | RRR | Pertuis/84 | 50 | 99·6 | 90·4 | 85·8 | 169·2 | 0·158 |
| Silene latifolia | Caryophyllaceae | Sial | C | Buoux/84 | 50 | 80 | 73·5 | 68·8 | 4619·7 | 1·24 |
| Turgenia latifolia | Apiaceae | Tula | RRR | Pierrevert/04 | 20 | 97·4 | 60·5 | 19·7 | 1873·6 | 33·783 |
| Vaccaria hispanica | Caryophyllaceae | Vahi | RR | Lagarde d'Apt/84 | 50 | 4·4 | 2·8 | 2 | 443·6 | 7·525 |
Species' names (according to Jauzein, 1995); families; species code; rarity according to Jauzein (1995): CCC, very common; C, common; R, moderately rare; RR, rare; RRR, very rare; location; number of seeds per replicate used for the burial experiment (N); percentage survival after 6 months. 1·5 years and 2·5 years of burial, mean individual seed production for ten individuals; code for French Départements (counties) in the location column: 04, Alpes de Haute Provence; 13. Bouches du Rhône; 84. Vaucluse.
LITERATURE CITED
- Adams VM, Marsh DM, Knox JS. Importance of the seed bank for population viability and population monitoring in a threatened wetland herb. Biological Conservation. 2005;124:425–436. [Google Scholar]
- Bakker JP, Poschlod P, Strykstra RJ, Bekker RM, Thompson K. Seed banks and seed dispersal: important topics in restoration ecology. Acta Botanica Neerlandica. 1996;a 45:461–490. [Google Scholar]
- Bakker JP, Bakker ES, Rosén E, Verweij GL, Bekker RM. Soil seed bank composition along a gradient from dry alvar grassland to Juniperus-shrubland. Journal of Vegetation Science. 1996;b 7:165–176. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography and evolution of dormancy and germination. San Diego: Academic Press; 1998. [Google Scholar]
- Bekker RM, Schaminée JHJ, Bakker JP, Thompson K. Seed bank characteristics of Dutch plant communities. Acta Botanica Neerlandica. 1998;a 47:15–26. [Google Scholar]
- Bekker RM, Bakker JP, Grandin U, et al. Seed size, shape and vertical distribution in the soil: indicators of seed longevity. Functional Ecology. 1998;b 12:834–842. [Google Scholar]
- Bekker RM, Bakker JP, Thompson K. Seed longevity. In: Knevel IC, Bekker RM, Kunzmann D, Stadler M, Thompson K., editors. The LEDA traitbase collecting and measuring standards. Groningen, The Netherlands: University of Groningen; 2005. pp. 104–111. [Google Scholar]
- Bladé C, Vallejo VR. Seed mass effects on performance of Pinus halepensis Mill. seedlings sown after fire. Forest Ecology and Management. 2008;255:2362–2372. [Google Scholar]
- von Blanckenhagen B, Poschlod P. Restoration of calcareous grasslands: the role of the soil seed bank and seed dispersal for recolonisation processes. Biotechnology, Agronomy, Society and Environment. 2005;9:143–149. [Google Scholar]
- Blaney CS, Kotanen PM. Effects of fungal pathogens on seeds of native and exotic plants: a test using congeneric pairs. Journal of Applied Ecology. 2001;38:1104–1113. [Google Scholar]
- Bruun HH, Poschlod P. Why are small seeds dispersed through animal guts: large numbers or seed size per se? Oikos. 2006;113:402–411. [Google Scholar]
- Cabin RJ, Mitchell RJ, Marshall DL. Do surface plant and soil seed bank populations differ genetically? A multipopulation study of the desert mustard Lesquerella fendleri (Brassicaceae) American Journal of Botany. 1998;85:1098–1109. [PubMed] [Google Scholar]
- Cerabolini B, Ceriani RM, Caccianiga M, De Andreis R, Raimondi B. Seed size and shape and persistence in soil: a test on Italian flora from Alps to Mediterranean coasts. Seed Science Research. 2003;13:75–85. [Google Scholar]
- Chesson PL, Warner RR. Environmental variability promotes coexistence in lottery competitive systems. American Naturalist. 1981;117:923–943. [Google Scholar]
- Coomes DA, Grubb PJ. Colonization, tolerance, competition and seed-size variation within functional groups. Trends in Ecology and Evolution. 2003;18:283–291. [Google Scholar]
- Dutoit T, Gerbaud E, Buisson E, Roche P. Dynamique d'une communauté d'adventices dans un champ de céréales créé après le labour d'une prairie semi-naturelle: rôle de la banque de graines permanente. Ecoscience. 2003;10:225–235. [Google Scholar]
- Facelli JM, Chesson P, Barnes N. Differences in seed biology of annual plants in arid lands: a key ingredient of the storage effect. Ecology. 2005;86:2998–3006. [Google Scholar]
- Funes G, Basconcelo S, Días S, Cabido M. Seed size and shape are good predictors of seed persistence in soil in temperate mountain grasslands of Argentina. Seed Science Research. 2007;9:341–345. [Google Scholar]
- Günter G. Populationsbiologie seltener Segetalarten. Scripta Geobotanica. 1997;XXII:220. [Google Scholar]
- Hillier SH, Walton DHW, Wells DA., editors. Calcareous grasslands: ecology and management. Huntingdon, UK: Bluntisham Books; 1990. [Google Scholar]
- Hodkinson DJ, Askew AP, Thompson K, Hodgson JG, Bakker JP, Bekker RM. Ecological correlates of seed size in the British flora. Functional Ecology. 1998;12:762–766. [Google Scholar]
- Hopfensberger K. A review of similarity between seed bank and standing vegetation across ecosystems. Oikos. 2007;116:1438–1448. [Google Scholar]
- Hutchings MJ, Booth KD. Studies on the feasibility of re-creating chalk grassland vegetation on ex-arable land I. The potential roles of the seed bank and the seed rain. Journal of Applied Ecology. 1996;33:1171–1181. [Google Scholar]
- Hutchings MJ. Plant population biology. In: Moore PD, Chapman SB., editors. Methods in plant ecology. 2nd edn. Oxford: Blackwell; 1986. pp. 377–435. [Google Scholar]
- ISTA. Seed Science and Technology. suppl. Vol. 24. Zurich: 1996. International rules for seed testing. International Seed Testing Association (ISTA) [Google Scholar]
- Jackel A-K, Poschlod P. Diaspore production and the influence of the size of diaspore traps on the quantitative result of seasonal diaspore rain in two calcareous grassland sites. Berichte des Institutes für Landschafts- und Pflanzenökologie der Universität Hohenheim. 1994;3:123–132. [Google Scholar]
- Jakobsson A, Eriksson O. A comparative study of seed number, seed size, seedling size and recruitment in grassland plants. Oikos. 2000;88:494–502. [Google Scholar]
- Jauzein P. Flore des champs cultivés. Paris: INRA; 1995. [Google Scholar]
- Kalisz S. Experimental determination of seed bank age structure in the winter annual Collinsia verna. Ecology. 1991;73:575–585. [Google Scholar]
- Kalisz S, McPeek MA. The demography of an age-structured annual: resampled projection matrices, elasticity analyses and seed bank effects. Ecology. 1992;73:1082–1093. [Google Scholar]
- Leishman MR, Westoby M. Seed size and shape are not related to persistence in soil in Australia in the same way as in Britain. Functional Ecology. 1998;12:480–485. [Google Scholar]
- Leishman MR, Masters GJ, Clarke IP, Brown VK. Seed bank dynamics: the role of fungal pathogens and climate change. Functional Ecology. 2000;a 14:293–299. [Google Scholar]
- Leishman MR, Wright IJ, Moles AT, Westoby M. The evolutionary ecology of seed size. In: Fenner M, editor. Seeds: the ecology of regeneration in plant communities. 2nd edn. Wallingford, UK: CABI; 2000b. pp. 31–57. [Google Scholar]
- Levine JM, Rees M. Effects of temporal variability on rare plant persistence in annual systems. American Naturalist. 2004;164:350–363. doi: 10.1086/422859. [DOI] [PubMed] [Google Scholar]
- Louda SM. Predation in the dynamics of seed regeneration. In: Leck MA, Parker VT, Simpson RL., editors. Ecology of soil seed banks. London: Academic Press; 1989. pp. 25–51. [Google Scholar]
- McGraw JB, Vavrek MC, Bennington CC. Ecological genetic variation in seed banks. I. Establishment of a time transect. Journal of Ecology. 1991;79:617–625. [Google Scholar]
- McGinley MA, Temme DH, Geber MA. Parental investment in offspring in variable environments: theoretical and empirical considerations. American Naturalist. 1987;130:370–398. [Google Scholar]
- Moles AT, Hodson DW, Webb CJ. Seed size and persistence in the soil in the New Zealand flora. Oikos. 2000;89:541–545. [Google Scholar]
- Moles AT, Falster DS, Leishman MR, Westoby M. Small-seeded species produce more seeds per square metre of canopy per year, but not per individual per lifetime. Journal of Ecology. 2004;92:384–396. [Google Scholar]
- Moriuchi KS, Venable DL, Pake CE, Lange T. Direct measurement of the seed bank age structure of a Sonoran desert annual plant. Ecology. 2000;81:1133–1138. [Google Scholar]
- Murdoch AJ, Ellis RH. Dormancy, viability and longevity. In: Fenner M., editor. Seeds: the ecology of regeneration in plant communities. 2nd edn. Wallingford, UK: CABI; 2000. pp. 183–214. [Google Scholar]
- Ozinga WA, Hennekens SM, Schaminée JHJ, et al. Local above-ground persistence of vascular plants: life-history trade-offs and environmental constraints. Journal of Vegetation Science. 2007;18:489–497. [Google Scholar]
- Pake CE, Venable DL. Is coexistence of Sonoran desert annuals mediated by temporal variability in reproductive success? Ecology. 1995;76:246–261. [Google Scholar]
- Pake CE, Venable DL. Seed banks in desert annuals: implications for persistence and coexistence in variable environments. Ecology. 1996;77:1427–1436. [Google Scholar]
- Parker VT, Simpson RL, Leck MA. Pattern and process in the dynamics of seed banks. In: Leck MA, Parker VT, Simpson RL, editors. Ecology of soil seed banks. London: Academic Press; 1989. pp. 367–384. [Google Scholar]
- Peco B, Ortega M, Levassor C. Similarity between seed bank and vegetation in Mediterranean grasslands: a predictive model. Journal of Vegetation Science. 1998;9:815–828. [Google Scholar]
- Peco B, Traba J, Levassor C, Sánchez AM, Azcárate FM. Seed size, shape and persistence in dry Mediterranean grass and scrublands. Seed Science Research. 2003;13:87–95. [Google Scholar]
- Pizo MA, von Allmen C, Morellato LPC. Seed size variation in the palm Euterpe edulis and the effects of seed predators on germination and seedling survival. Acta Oecologica. 2006;29:311–315. [Google Scholar]
- Poschlod P. Die Dauerhaftigkeit von generativen Diasporenbanken in Böden am Beispiel von Kalkmagerrasenpflanzen und deren Bedeutung für den botanischen Arten- und Biotopschutz. Verhandlungen der Gesellschaft für Ökologie. 1993;22:229–240. [Google Scholar]
- Poschlod P, Jackel AK. Untersuchungen zur Dynamik von generativen Diasporenbanken von Samenpflanzen in Kalkmagerrasen. I. Jahreszeitliche Dynamik des Diasporenregens und der Diasporenbank auf zwei Kalkmagerrasenstandorten der Schwäbischen Alb. Flora. 1993;188:48–71. [Google Scholar]
- Poschlod P, Kiefer S, Tränkle U, Fischer S, Bonn S. Plant species richness in calcareous grasslands as affected by dispersability in space and time. Applied Vegetation Science. 1998;1:75–90. [Google Scholar]
- Poschlod P, Tackenberg O, Bonn S. Plant dispersal potential and its relation to species frequency and coexistence. In: van der Maarel E., editor. Vegetation ecology. Oxford: Blackwell; 2005. pp. 147–171. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. URL http://www.R-project.org . [Google Scholar]
- Rees JM, Mills LS, Dunning JB, et al. Emerging issues in population viability analysis. Conservation Biology. 2002;16:7–19. doi: 10.1046/j.1523-1739.2002.99419.x. [DOI] [PubMed] [Google Scholar]
- Salisbury EJ. The reproductive capacity of plants. London: Bell.; 1942. [Google Scholar]
- Šera B, Šery M. Relation between number and weight of diaspores and reproductive strategies of herbacaeous plants. Folia Geobotanica. 2004;39:27–42. [Google Scholar]
- Schafer M, Kotanen PM. The influence of soil moisture on losses of buried seeds to fungi. Acta Oecologica. 2003;24:255–263. [Google Scholar]
- Schneider C, Sukopp U, Sukopp H. Biologisch-ökologische Grundlagen des Schutzes gefährdeter Segetalpflanzen. Schriftenreihe für Vegetationskunde. 1994;26:1–356. [Google Scholar]
- Shipley B, Dion J. The allometry of seed production in herbaceous angiosperms. American Naturalist. 1992;139:467–483. [Google Scholar]
- Silvertown JW. Introduction to plant population ecology. London: Longman; 1982. [Google Scholar]
- Silvertown J. Seed ecology, dormancy, and germination: a modern synthesis from Baskin and Baskin. Book review. American Journal of Botany. 1999;86:903–905. [Google Scholar]
- Silvertown J, Dodd ME, Gowing D, Mountford O. Hydrologically-defined niches reveal a basis for species-richness in plant communities. Nature. 1999;400:61–63. [Google Scholar]
- Silvertown J, McConway K, Gowing D, et al. Absence of phylogenetic signal in the niche structure of meadow plant communities. Proceedings of the Royal Society B: Biological Science. 2006;273:39–44. doi: 10.1098/rspb.2005.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RL, Leck MA, Parker VT. Seed banks: general concepts and methodological issues. In: Leck MA, Parker VT, Simpson RL, editors. Ecology of soil seed banks. London: Academic Press; 1989. pp. 3–8. [Google Scholar]
- Stöcklin J, Fischer M. Plants with longer-lived seeds have lower local extinction rates in grassland remnants 1950–1985. Oecologia. 1999;120:539–543. doi: 10.1007/s004420050888. [DOI] [PubMed] [Google Scholar]
- Tackenberg O, Poschlod P, Bonn S. Assessment of wind dispersal potential in plant species. Ecological Monographs. 2003;73:191–205. [Google Scholar]
- Telewski FW, Zeevart JAD. The 120-yr period for Dr Beal's seed viability experiment. American Journal of Botany. 2002;89:1285–1288. doi: 10.3732/ajb.89.8.1285. [DOI] [PubMed] [Google Scholar]
- Ter Heerdt GNJ, Verweij GL, Bekker RM, Bakker JP. An improved method for seed bank analysis: seedling emergence after removing the soil by sieving. Functional Ecology. 1996;10:144–151. [Google Scholar]
- Thompson K. The functional ecology of seed banks. In: Fenner M., editor. Seeds. 2nd edn. Wallingford, UK: CABI; 2000. pp. 215–235. [Google Scholar]
- Thompson K, Grime JP. Seasonal variation in the seed bank of herbaceous species in ten contrasting habitats. Journal of Ecology. 1979;67:893–921. [Google Scholar]
- Thompson K, Band SR, Hodgson JG. Seed size and shape predict persistence in the soil. Functional Ecology. 1993;7:236–241. [Google Scholar]
- Thompson K, Bakker JP, Bekker RM. Soil seed banks of north west Europe: methodology, density and longevity. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Thompson K, Bakker JP, Bekker RM, Hodgson JG. Ecological correlates of seed persistence in soil in the north-west European flora. Journal of Ecology. 1998;86:163–169. [Google Scholar]
- Thompson K, Ceriani RM, Bakker JP, Bekker RM. Are seed dormancy and persistence in the soil related? Seed Science Research. 2003;13:97–100. [Google Scholar]
- Turnbull LA, Crawley MJ, Rees M. Are plant populations seed-limited? A review of seed sowing experiments. Oikos. 2000;88:225–238. [Google Scholar]
- van der Valk AG, Pederson RL. Seed banks and the management and restoration of natural vegetation. In: Leck MA, Parker VT, Simpson RL., editors. Ecology of soil seed banks. London: Academic Press; 1989. pp. 329–346. [Google Scholar]
- Van Mourik TA, Stomph TJ, Murdoch AJ. Why high seed densities within buried mesh bags may overestimate depletion rates of soil seed banks. Journal of Applied Ecology. 2005;42:299–305. [Google Scholar]
- Wagner M, Mitschunas N. Fungal effects on seed bank persistence and potential applications in weed biocontrol: a review. Basic and Applied Ecology. 2007;9:191–203. [Google Scholar]
- Wäldchen J, Pusch J, Luthardt V. Zur Diasporen-Keimfähigkeit von Segetalpflanzen – Untersuchungen in Nord-Thüringen. Beiträge zur Forstwirtschaft und Landschaftsökologie. 2005;38:145–156. [Google Scholar]
- Warner RR, Chesson PL. Coexistence mediated by the recruitment fluctuations: a field guide to the storage effect. American Naturalist. 1985;125:769–787. [Google Scholar]
- Wellstein C, Otte A, Waldhart R. Seed bank diversity in mesic grasslands in relation to vegetation type, management and site conditions. Journal of Vegetation Science. 2007;18:153–162. [Google Scholar]
- Willems JH, Bik LPM. Restoration of high species density in calcareous grassland: the role of seed rain and soil seed bank. Applied Vegetation Science. 1998;1:91–100. [Google Scholar]