Abstract
Background and Aims
Deciduous trees have a seasonal carbon dioxide exchange pattern that is attributed to changes in leaf biochemical properties. However, it is not known if the pattern in leaf biochemical properties – maximum Rubisco carboxylation (Vcmax) and electron transport (Jmax) – differ between species. This study explored whether a general pattern of changes in Vcmax, Jmax, and a standardized soil moisture response accounted for carbon dioxide exchange of deciduous trees throughout the growing season.
Methods
The model MAESTRA was used to examine Vcmax and Jmax of leaves of five deciduous trees, Acer rubrum ‘Summer Red’, Betula nigra, Quercus nuttallii, Quercus phellos and Paulownia elongata, and their response to soil moisture. MAESTRA was parameterized using data from in situ measurements on organs. Linking the changes in biochemical properties of leaves to the whole tree, MAESTRA integrated the general pattern in Vcmax and Jmax from gas exchange parameters of leaves with a standardized soil moisture response to describe carbon dioxide exchange throughout the growing season. The model estimates were tested against measurements made on the five species under both irrigated and water-stressed conditions.
Key Results
Measurements and modelling demonstrate that the seasonal pattern of biochemical activity in leaves and soil moisture response can be parameterized with straightforward general relationships. Over the course of the season, differences in carbon exchange between measured and modelled values were within 6–12 % under well-watered conditions and 2–25 % under water stress conditions. Hence, a generalized seasonal pattern in the leaf-level physiological change of Vcmax and Jmax, and a standardized response to soil moisture was sufficient to parameterize carbon dioxide exchange for large-scale evaluations.
Conclusions
Simplification in parameterization of the seasonal pattern of leaf biochemical activity and soil moisture response of deciduous forest species is demonstrated. This allows reliable modelling of carbon exchange for deciduous trees, thus circumventing the need for extensive gas exchange experiments on different species.
Key words: Carbon budget, deciduous trees, modelling, MAESTRA, soil moisture, species response, transpiration, Acer rubrum, Betula nigra, Quercus nuttallii, Q. phellos, Paulownia elongata
INTRODUCTION
Trees are a primary component of terrestrial carbon sinks. The carbon budget of deciduous tree plantations is of particular interest because they sequester carbon, particularly in the juvenile, most productive phase of a tree's life cycle (e.g. Ryan et al., 2004). Modelling carbon accumulation accurately is most important for obtaining carbon budgets over many spatial and temporal scales. However, growing season patterns in basic biochemical properties of leaves, maximum Rubisco carboxylation (Vcmax) and electron transport (Jmax), affect assimilate production and thus simulation accuracy (Wilson et al., 2001; Kosugi et al., 2003; Xu and Baldocchi, 2003). To complicate matters further, trees are genetically diverse and highly complex organisms that adjust to changes in environmental conditions, including water availability. Recently, studies have highlighted both the inadequacy with which models simulate carbon exchange over the course of a growing season (e.g. Sasai et al., 2007; Harrison et al., 2008; Ricciuto et al., 2008) and the lack of a generic set of parameters to predict soil water constraints (e.g. Van Wijk et al., 2001; Baldocchi et al., 2002; Bauerle et al., 2004; Verbeeck et al., 2007). As a result, development of forest management models still faces the challenge of integrating knowledge that describes the carbon exchange response at different temporal and spatial scales (Pretzsch et al., 2008).
Simulation of forest ecosystem carbon exchange calls for proficient models that capture the physiological behaviour of trees at the ecosystem scale (e.g. Running and Coughlan, 1988; Wang et al., 1998; Pretzsch et al., 2008). A consistent difficulty, therefore, is to determine experimentally the parameters and define the acceptable degree of equation simplification (Tardieu et al., 2005). The goal of the present study, therefore, is to report on a general Vcmax and Jmax seasonal pattern along with a standardized water stress response that describes deciduous tree carbon exchange dynamics throughout the growing season.
A process-based model (MAESTRO; Wang and Jarvis, 1990), which is spatially explicit for individual trees, was used here to simulate seasonal carbon dioxide exchange for different species using a simple leaf biochemical and soil moisture response function. The study is unique in that seasonal variation among deciduous species was controlled for. The specific objectives were to (1) examine the relationship differences between species' seasonal physiological changes of leaves, (2) investigate the use of a general soil moisture response model among five temperate deciduous tree species and (3) compare measurements made on pot-grown plants under field conditions with model estimates, made using independently derived parameters, to test a generalized growing season Vcmax and Jmax pattern and a standardized soil moisture response function. Thus, we hypothesized that deciduous species Vcmax, Jmax and soil moisture response parameters could be simplified for predicting the seasonal dynamics of deciduous species' carbon dioxide exchange.
MATERIALS AND METHODS
Site description and plant material
The duration of the study was from 15 May 2006 to 15 October 2006, most of the 2006 growing season. The field site was located at the Clemson University Calhoun Field Laboratory in Clemson, SC, USA (34°40′8″N, 82°50′40″W). A full site description is provided in Bauerle et al. (2002). Clonal 1-year-old pot-grown saplings of Acer rubrum ‘Summer Red’, Betula nigra, Quercus nuttallii, Quercus phellos and Paulownia elongata, 40 per species, were transplanted from 11-L into 114-L plastic containers of 0·67 m depth on 7 March 2006. To minimize root restrictions, the containers used were three times the size of those used for commercial production of equal dimension deciduous trees throughout a growing season (P. Parsons, Parsons Nursery, Georgetown, SC, pers. comm.). Plants were grown in a potting compost (Fafard Inc., Anderson, SC, USA), a 1:1:1:1 (v/v) mixture of peat, perlite, vermiculite and silt loam fertilized with 9 kg m−3 of Osmocoat Pro® 19-5-8 (Scotts Inc., Marysville, OH, USA). To create a common garden, plants were randomly distributed throughout a plot in a grid pattern (1·5-m spacing), initially watered to container capacity and allowed to drain for 24 h.
Soil water measurements
Micro-emitter (360° spray pattern) irrigation was applied at the base of each tree stem, delivering water three times daily. The root zone volumetric water content (VWC) was monitored and maintained within a previously determined well-watered range (0·3–0·5 m3 m−3) (Bauerle et al., 2003). To link the soil moisture to the individual trees, ECH2O probes, type EC-20 (Decagon Devices, Pullman, WA, USA) were installed at a 45° angle in the soil of eight randomly selected trees per species. The probes recorded bulk VWC every minute and hourly averages were saved in a CR7X data logger (Campbell Scientific, Logan, UT, USA). All trees were allowed to acclimate for 45 d.
Drought treatment
At the start of the experiment (after 45 d of growth), 20 trees per species were randomly assigned to the drought treatment and 20 trees to the well-watered control. Drought was achieved by restricting the water supply to 70 % less than the controls. Soil moisture measurements continued with equal numbers of ECH2O probes between the two treatments randomly distributed. Irrigation times and duration were adjusted per tree species and treatment to ensure that the VWC in the drought treatment was <0·3 m3 m−3 and the control treatment VWC remained >0·3 m3 m−3 (a predetermined value shown not to depress gas exchange of the species). In addition, container radiation load was minimized and precipitation excluded as described in Bauerle et al. (2002).
Sap-flux measurements
Sap flow gauges (Dynamax Inc., Houston, TX, USA) were installed on four or five randomly selected trees per species (two or three per treatment; models SGB13-WS, SGB16-WS, SGB19-WS, SGB25-WS and SGB35-WS). Installation and operation followed Bauerle et al. (2002).
Leaf gas exchange and light absorption measurements
Gas exchange was measured at 3-week intervals on recently fully expanded leaves of four replicate trees per species, using a portable gas exchange system (CIRAS-1, PP Systems, Haverhill, MA, USA) fitted with a light- and temperature-controlled cuvette (Model PLC (B), PP Systems). The temperature inside the cuvette was controlled at 25 ± 0·7 °C and vapour pressure deficit of 1·27 ± 0·12 kPa. Leaf net photosynthesis (Anet) versus CO2 (Anet/Ci curves, where Anet is in units of μmol m−2 s−1 and Ci is the internal CO2 concentration expressed as the molar fraction of CO2) were constructed as described in Bauerle et al. (2007). In addition, light response curves were constructed and quantum yield calculations were corrected for the percentage photosynthetically active radiation absorbed by each leaf from paired SPAD meter (model 502B, Minolta Inc., Ramsey, NJ, USA) measurements as described in Bauerle et al. (2006).
Growth and maintenance respiration measurements
Three trees of each species per treatment (30 total) were randomly selected and harvested at 3-week intervals (seven harvests in total). All leaves were removed and measured for total area (LiCor 3100, Lincoln, NE, USA). The soil was gently hand washed from the roots, blotted dry, and fine roots (diameter <3 mm) were separated from the coarse roots (diameter >3 mm). Respiration measurements were made simultaneously on each individual replicate from a separate plant. Each organ was taken, placed inside a 25 °C temperature-controlled room and allowed to equilibrate for 30 min. Stem, coarse root and fine root tissue respiration was measured (CIRAS-1 connected to a model SRC-1 respiration chamber, PP Systems). The chamber was modified with a lid to seal and enclose the organ. Respiration was logged after reaching a steady state and organ volume was determined by water displacement in a graduated cylinder. Individual organs were then dried at 70 °C for approx. 21 d and weighed. After each harvest, the remaining trees in the plot were randomly repositioned at the same spacing.
Model description
We developed a modified version of the three-dimensional photosynthesis, transpiration and absorbed radiation model MAESTRO (Wang and Jarvis, 1990). Recently, MAESTRO has been renamed MAESTRA and described and applied in several studies, for example Wang and Polglase (1995), Kruijt et al. (1999), Bauerle et al. (2007), Emhart et al. (2007) and Medlyn et al. (2007). Readers are referred to an online bibliography of model components, development and application at www.bio.mq.edu.au/maestra. We only detail the changes that applied to the present study. Bauerle et al. (2002, 2004) updated the model to run on a 15-min time step, incorporated a soil moisture response function, and parameterized and validated the model on the deciduous tree species Acer rubrum. Our version includes the Vcmax and Jmax temperature response functions of Bernacchi et al. (2001, 2003) and was parameterized and validated for estimating light transfer and interception (Bauerle et al., 2004) and transpiration (Bowden and Bauerle, 2008) within the crowns of deciduous trees.
Leaf model parameterization
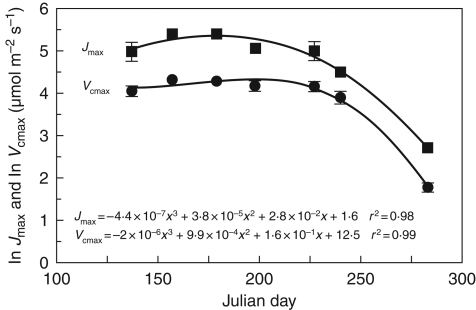
Changes in Jmax and Vcmax over the season were assessed by analysis of covariance among species differences after values were log transformed. As there were no differences between species in the seasonal trends in Jmax and Vcmax a general third-order polynomial was fitted to the seasonal change in the log-transformed values (Fig. 1), to generalize the response among species. The non-linear equations of Fig. 1 were used to parameterize the seasonal pattern of Vcmax and Jmax in the Farquhar and von Caemmerer (1982) biochemical submodel of photosynthesis. The kinetic parameters used to estimate the temperature response of Vcmax, Jmax and leaf dark respiration are described in Bauerle et al. (2007).
Fig. 1.
Seasonal change in the log-transformed mean maximum electron transport rate (Jmax) and the log-transformed mean maximum carboxylation rate (Vcmax) for five species of deciduous trees grown under optimal conditions. The solid lines are non-linear third-order polynomial regression curves fitted to the seasonal data set (r2 = 0·98, Jmax; r2 = 0·99, Vcmax), where the spread within a day is the natural log of species variation ± s.e.
Differences between species were quantified by parameters within the coupled CO2 and H2O models of Farquhar and von Caemmerer (1982) and Ball et al. (1987). The linkage between the models is described in Ball et al. (1987) and Kim and Lieth (2003). MAESTRA scaled up the leaf-level properties to the whole-tree scale.
The equations and parameters of Granier and Loustau (1994) are followed to scale leaf stomatal conductance (gs) and model the whole-tree conductance response to soil moisture, with the following changes: the mean value of soil moisture deficit was substituted for the leaf water potential (Schulze et al., 1987) and photosynthetic photon flux density (PPFD) was substituted into the gs equation, shown to be of primary importance in modelling the response of gs (Massman and Kaufmann, 1991). The final form of the response model was:
| 1 |
where k1, kr, kd1, kd2, ks1 and ks2 are estimated parameters, Ps is the PPFD (μmol m−2 s−1), δq is the water vapour deficit (g kg−1), and δM is the soil moisture deficit. Note that the parameters are not species-specific. Soil moisture deficit (δM) was estimated by
| 2 |
where Mmax, Mmin and M are the maximum, minimum and actual root zone VWC for the specific soil (Cosh et al., 2005). Other functions of the variables were tested (Ogink-Hendriks, 1995), but the estimates derived by Granier and Loustau (1994) gave the best fit (Table 1).
Table 1.
Estimates of the model parameters from Granier and Loustau (1994)
| Parameter | Estimates | Units |
|---|---|---|
| k1 | 0·02017 | mm s−1 |
| kr | 497·791 | W m−2 |
| kd1 | 0·0360 | kg g−1 |
| kd2 | 0·389 | kg g−1 |
| ks1 | 0·0156 | – |
| ks2 | 4·269 | – |
Transpiration of each sub-volume of the crown was calculated by applying an inverse form of the Penman–Monteith equation (Granier and Loustau 1994). A detailed description of how MAESTRA links transpiration of individual leaves to the whole crown and the subsequent leaf energy balance calculations can be found in Medlyn et al. (2007).
Meteorological data to drive MAESTRA were collected on a 15-min time step by a weather station at the site, linked to a CR10X data logger (Campbell Scientific) and the data together with soil moisture data were input into the drought response equation (eqn 2).
Stem and root parameterization
Seven times during the growing season (1 d before each harvest), total tree height (HT), trunk diameter (DT), trunk length and three-dimensional live crown size (x, y and z direction, in m) were measured. MAESTRA used the DT and HT data to interpolate incremental woody biomass across the season, where the total woody stem and root biomass are calculated from the general allometric relation
| 3 |
where k, Dexp and WI are organ-specific parameters calculated from HT and DT and fine root biomass is assumed to be a constant fraction of total root biomass. To subtract carbon lost via respiration, individual organ respiration (Ro) was calculated on a 15-min time step from dry mass (DM), respiration rate at 25 °C and temperature:
| 4 |
where Ta is air temperature (°C), Rot is organ temperature (°C), Q10W is the temperature response factor, and WB is the woody biomass (kg) calculated from HT and DT. Respiration rates of organs from each species (Rmw) were calculated from the stem surface area:dry mass ratio according to Valentini et al. (1996). In addition, linear interpolation of leaf area development was applied. The online MAESTRA manual (http://www.bio.mq.edu.au/maestra/) provides greater detail.
Model simulations and functional group analysis
Net primary production (NPP) was estimated for each species every 15 min from 15 May 2006 to 15 October 2006. Output consisted of 15-min, hourly, and daily carbon and water exchange. Although our main focus was on the simulation of physiological processes between species and scaling up leaf behaviour to the whole plant, convergence in the response of the physiology of leaves was also investigated. Species were lumped into functional groups based on their physiological responses and the mean physiological parameters were calculated. Individual species' estimates were compared with that of the functional group. Quercus species were lumped as a potential functional group and B. nigra and P. elongata as a second functional group. On a physiological basis, A. rubrum was significantly different from the other species and/or functional groups and therefore was not included in the functional group analysis.
Validation tests
Estimates of carbon balance under well-watered and drought conditions from MAESTRA were compared with the independently observed values every 3 weeks. In addition, estimates of diurnal patterns of transpiration were compared with independent measured values.
Performance of the model was evaluated by comparing observed values with predicted values, where a paired sample t-test for differences in carbon exchange between (1) measured and modelled control ‘well-watered’ trees of each species, (2) measured and modelled water-stressed trees of each species, and (3) watered and water-stressed trees (treatment effect) at each harvest time was used to test the null hypothesis that the average of the differences between measured and modelled paired observations is zero at α = 0·05 (SAS Institute, Cary, NC, USA). In addition, a t-test was performed to compare the organ-specific estimates versus measurements in carbon differences among species at the end of the season, again at α = 0·05.
RESULTS
Testing the species response against a generalized seasonal response function for predicting leaf-level Vcmax and Jmax
The seasonal trend in Vcmax and Jmax rates was non-linear for all species (Fig. 1), and there were no differences between the responses between species when the log-transformed values were compared (P = 0·99; Fig. 1). In fact, a general seasonal pattern of Jmax and Vcmax was observed for all species (Fig. 1) and was represented by a third-order polynomial with an r2 = 0·98 and 0·99 for Jmax and Vcmax, respectively (Fig. 1).
Impact of water stress on dry matter production and partitioning
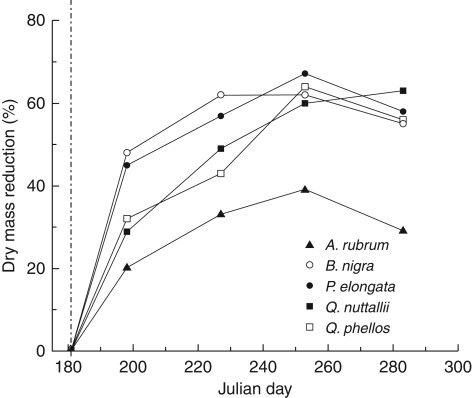
Over the course of the season, dry matter production differed significantly between the irrigation treatments (P < 0·05; Fig. 2). After 17 d of irrigation deficit, carbon sequestration decreased by 20–48 % compared with the well-watered controls. Except for A. rubrum, dry matter accumulation over the season was on average 37–46 % less than well-watered values. A. rubrum produced more dry matter under water deficit than the other species, 71 % of the well-watered control. Dry matter allocation to organs differed among species; however, the general percentage of allocation to organs within a species was not significantly different across the season and in response to water stress. One notable exception was A. rubrum, where water stress caused fine root dry matter to increase by 46 % relative to the well-watered control.
Fig. 2.
Decrease in carbon sequestration by trees caused by drought treatment compared with the well-watered controls: Acer rubrum, Betula nigra, Paulownia elongata, Quercus nuttallii and Q. phellos, as indicated. The dashed line indicates the start of the drought treatment (Julian day 181).
Comparison between observed and simulated estimates of carbon exchange under well-watered and water stress conditions
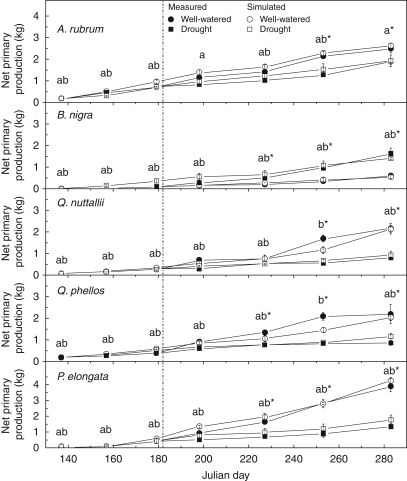
Parameter values for species' seasonally variable and invariable physiology are given in Tables 2 and 3, respectively. Carbon sequestration was compared over 3 weeks of growth related to the destructive harvests. Figure 3 illustrates species-specific measured and modelled NPP under both well-watered and drought conditions across the season. Comparisons were made between the carbon accumulated in the well-watered and drought treatments to indicate when a significant treatment effect was observed and the model predicted intra-seasonal NPP in well-watered and water-stressed deciduous tree species (Fig. 3). Validation of the carbon accumulation over the growing season showed that the model predicted carbon sequestration compared with measured values within 6 % for A. rubrum, 12 % for B. nigra, 8 % for P. elongata, 2 % for Q. nuttallii and 7 % for Q. phellos in the well-watered control treatment and within 2 % for A. rubrum, 9 % for B. nigra, 24 % for P. elongata, 13 % for Q. nuttallii and 25 % for Q. phellos in the drought treatment.
Table 2.
Control treatment seasonal variation in Jmax, maximum rate of electron transport (μmol m−2 s−1) and Vcmax, maximum rate of Rubisco activity (μmol m−2 s−1).
| Julian day |
|||||||
|---|---|---|---|---|---|---|---|
| 137 | 157 | 179 | 198 | 227 | 240 | 283 | |
| Acer rubrum | |||||||
| Jmax | 186·3 | 257·8 | 192·8 | 192·3 | 154·0 | 85·0 | 13·6 |
| Vcmax | 62·4 | 75·4 | 72·6 | 62·3 | 66·1 | 57·5 | 5·2 |
| Betula nigra | |||||||
| Jmax | 223·8 | 244·8 | 342·3 | 201·5 | 245·5 | 131·2 | 11·4 |
| Vcmax | 69·5 | 87·6 | 74·7 | 86·5 | 97·7 | 76·2 | 4·3 |
| Paulownia elongata | |||||||
| Jmax | 215·0 | 301·5 | 262·0 | 181·5 | 245·8 | 91·9 | 13·8 |
| Vcmax | 71·5 | 78·6 | 88·7 | 65·1 | 77·2 | 46·0 | 5·9 |
| Quercus nuttallii | |||||||
| Jmax | 79·2 | 175·8 | 195·5 | 149·0 | 114·6 | 70·6 | 16·0 |
| Vcmax | 42·1 | 69·7 | 65·7 | 64·2 | 50·5 | 41·5 | 7·5 |
| Quercus phellos | |||||||
| Jmax | 96·2 | 165·8 | 160·0 | 119·0 | 123·1 | 75·8 | 22·1 |
| Vcmax | 45·9 | 66·2 | 63·9 | 47·7 | 51·2 | 42·1 | 7·2 |
Table 3.
Organ-level photosynthetic and respiration parameters
| Species | Anet | Rd | go | g1 | Lcomp | Lsat | rStem | rCoarse root | rFine root |
|---|---|---|---|---|---|---|---|---|---|
| Acer rubrum | 16·2 ± 2·4CD | 3 ± 0·5A | 0·14 ± 0·0A | 1·7 ± 0·3D | 21·1 ± 4·2AB | 157·3 ± 20·4A | 44·4 ± 7·6A | 92·2 ± 12·2AB | 217·6 ± 31·8 |
| Betula nigra | 26·3 ± 3·5A | 3·1 ± 0·5A | 0·69 ± 0·1B | 2·5 ± 0·7CD | 11·7 ± 3·8A | 321·9 ± 41·8BC | 59·8 ± 13·9A | 65·1 ± 7·2AB | 215·7 ± 19·7A |
| Paulownia elongata | 19·9 ± 2·9BC | 3·5 ± 0·6A | 0·14 ± 0·0A | 5·8 ± 1·1AB | 29·2 ± 5·3B | 467·8 ± 34·7C | 46·7 ± 11·1A | 101·8 ± 13·7AB | 223·3 ± 38·9AB |
| Quercus nuttallii | 18·5 ± 1·7BC | 2·6 ± 0·4A | 0·15 ± 0·0A | 3·3 ± 0·9BC | 17·8 ± 4·6AB | 201·3 ± 21·9AB | 41·7 ± 4·1A | 80·1 ± 10·2AB | 135·9 ± 13·7A |
| Quercus phellos | 12·8 ± 1·4D | 2·7 ± 0·4A | 0·11 ± 0·0A | 2·4 ± 0·7CD | 16·1 ± 4·1AB | 192·0 ± 18·4AB | 48·2 ± 9·5A | 45·9 ± 4·3AB | 182·7 ± 25·5AB |
Average seasonal photosynthetic rate (Anet, μmol m−2 s−1), dark respiration rate (Rd, μmol m−2 s−1), minimum stomatal conductance (go, μmol m−2 s−1), stomatal opening slope coefficient (g1), light compensation point (Lcomp, μmol m−2 s−1), light saturation point (Lsat), stem respiration rate (rStem, nmol g−1 s−1), coarse root respiration rate (rCoarse root, nmol g−1 s−1) and fine root respiration rate (rFine root, nmol g−1 s−1). Different letters within a parameter indicate differences among species (P < 0·05). Note: parameters were used to model both the control and drought treatment response.
Fig. 3.
Measured versus modelled net carbon accumulation throughout the study period. Solid symbols represent well-watered (circles) and drought treatment (squares) measured values and open symbols represent simulated well-watered (circles) and drought treatment (squares) net carbon accumulation. Data are the mean of three trees per harvest date ± s.e. ‘a’, no significant difference between modelled and measured data in the well-watered treatment at α = 0·05; b, no significant difference between modelled and measured data in the drought treatment at α = 0·05; *, a significant treatment effect between measured data at α = 0·05. The dashed line indicates the start of the drought treatment (Julian day 181).
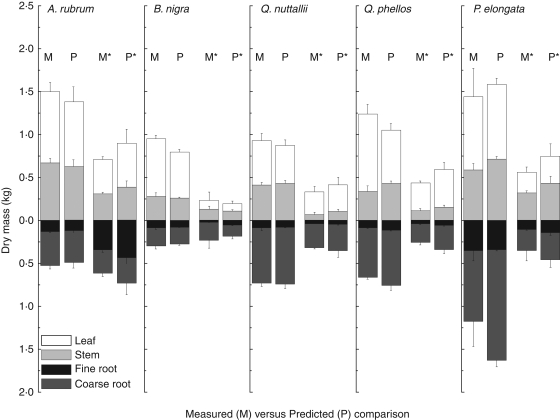
Although MAESTRA only calculates NPP for the whole tree, carbon partitioning averaged over the season was not significantly different from that during the season, and therefore seasonal averages of organ carbon allocation were used to partition the estimates of whole-tree NPP among organs. Figure 4 illustrates the organ-specific accumulation of carbon in control and drought trees, as compared with model estimates. In the well-watered control, the model predictions under-estimated carbon in leaves of B. nigra by 7 % and of Q. phellos by 20 % and over-estimated carbon in coarse roots of Q. phellos by 13 % and of P. elongata by 27%. Modelled estimates of fine root production were within 0·13 kg of actual accumulation of carbon in fine roots in all species and estimated carbon in stems was not significantly different from the measured data for A. rubrum, B. nigra and Q. nuttallii. The measured versus modelled carbon accumulated in organs under drought (Fig. 4) from seasonal NPP estimates overestimated carbon in stems and leaves in P. elongata by 34 and 35 %, respectively, and carbon in stems of Q. phellos by 36 %. Carbon in fine roots and leaves in A. rubrum were also over-estimated by 24 and 25 %, respectively. However, model predictions of carbon accumulation compared with measured values showed no significant difference for other organs under drought stress.
Fig. 4.
Carbon accumulation in organs of well-watered and drought trees. Within a species, bars below the letter M and P represent measured and predicted data ± s.e., respectively. Bars below M* and P* represent measured and predicted data in the drought treatment ± s.e., respectively.
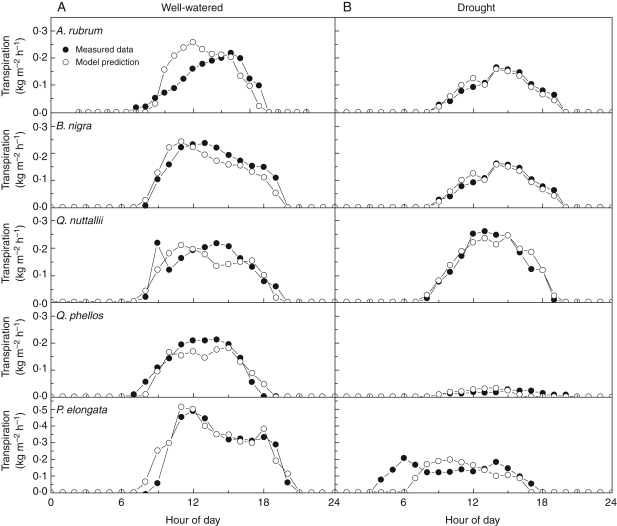
Comparison between observed and simulated estimates of transpiration under well-watered and water stress conditions
Predictions of transpiration under well-watered conditions compared with measured values from sap flux during a representative diurnal time course (Julian day 212) are shown in Fig. 5A. P. elongata transpired approximately twice as much water as the other four species, 26·7 kg m−2 leaf area compared with 12·0–14·0 kg m−2 week−1. Figure 5B shows the differences in species' diurnal transpiration under irrigation deficit, which decreased water use for all species, compared with well-watered controls. The reduction in transpiration under water-stress conditions was species specific (cf. Fig. 5A vs. 5B). Differences among species were confirmed by independent measurements made with the sap flow system (data not shown). Species-specific transpiration decreases under irrigation deficit were 33 % (Q. nuttallii), 43 % (A. rubrum), 51 % (B. nigra), 58 % (P. elongata) and 79 % (Q. phellos). Among all species, there was a 53 % reduction in transpiration compared with well-watered controls. Although there was a good agreement between the seasonal evolution of transpiration and carbon exchange, an approx. 35 % reduction in total daily transpiration occurred in September as compared with July. The decrease corresponded with the decline in Vcmax and Jmax toward the end of the season (Fig. 1).
Fig. 5.
Measured versus modelled sap flux of five species of deciduous trees during the day under well-watered (A) and drought stress (B) conditions. Data illustrate mean hourly transpiration during a representative diurnal period (Julian day 212).
Functional group predictions
Species differed physiologically (Tables 2 and 3). When Quercus sp. were grouped based on physiological characteristics of leaves, simulations under well-watered conditions over-estimated carbon exchange by 6 % compared with separate estimates for Q. phellos and Q. nuttallii. In response to drought, Quercus sp. as a functional group under-estimated carbon exchange by approx. 7 % compared with separate estimates. Estimates for the second functional group (B. nigra and P. elongata) were significantly different from those of the individual species, under-estimating carbon exchange by 13 % as compared with B. nigra and P. elongata separately. With drought the discrepancy increased, with a 15 % under-estimate.
DISCUSSION
Typically, carbon dioxide exchange of temperate deciduous trees varies over the growing season as a result of changes in the biochemical parameters that influence photosynthetic capacity (Wilson et al., 2001; Kosugi et al., 2003; Ito et al., 2006). The complexity of such changes and difficulties of measurement on the numerous species in forests has precluded analysis of the seasonal differences within and between species (Wilson et al., 2001; Kosugi et al., 2003). We found changes in biochemical parameters over the season consistent with other studies on deciduous trees and were able to test for differences between species. The seasonal change in biochemical parameters was not different between the species tested. The NPP measured and simulated in this study was adequately described with a seasonal trend in biochemical activity that was universal for the species. This finding allows simulation models to be simplified and so describe temporal trends in leaf physiological activity within heterogeneous, deciduous ecosystems. Furthermore, we agree with the conclusions of Wilson et al. (2001), Kosugi et al. (2003) and Ito et al. (2006) that it is critical to include the biochemical fluctuation in simulations of deciduous tree gas exchange.
Carbon exchange and transpiration estimates improved when parameters that characterized the seasonal change in biochemical parameters were used. To regulate gs, the direct influence of photosynthetic capacity on transpiration was a consequence of the close relationship between gs and photosynthesis (Wong et al., 1979). Describing the loss of capacity toward the end of the growing season further suggests that systematic model error can be circumvented if efforts are concentrated on improving the representation of vegetation parameters that are sensitive to seasonal changes. This finding has direct implications for efforts to improve global vegetation models that attempt to scale-up leaf-level carbon exchange and transpiration, in that global models do not usually account for adjustments of photosynthesis parameters (Thum et al., 2008).
Similar to the changes in photosynthetic capacity over the season and the effects on carbon dioxide exchange, fluctuation in soil moisture plays an important role in regulating the carbon exchange rate between vegetation and the atmosphere. In fact, drought has been shown to be a major constraint of net carbon exchange of many species (e.g. Oren and Pataki, 2001), as well as a significant limitation to carbon sequestration in both natural and managed ecosystems (e.g. Granier et al., 2006; Passioura, 2007). With regard to modelling a species' response to soil moisture deficit, studies using earlier versions of MAESTRA or similar process models applied to deciduous species have acknowledged the absence of a soil moisture response function (for a review see Hanson et al., 2004). In fact, most process models (earlier versions of MAESTRA included – Medlyn et al., 2005; Janssens et al., 2005; Ibrom et al., 2006) either assumed that soil moisture was non-limiting or did not account for the differences between species in stomatal regulation of carbon dioxide exchange rates in response to soil water deficits. Therefore, models have had a tendency to over-estimate carbon sequestration under water stress (Hanson et al., 2004). In addition, most process models are inadequate in one or more other categories too; for example, they do not describe the contributions of individual species to net ecosystem carbon fluxes (e.g. Badeck et al., 2001; Sinoquet et al., 2001; Baldocchi et al., 2002), do not allow physiological parameterization in space within canopies (Bauerle et al., 2007) and are either not responsive to water stress (e.g. Sinoquet et al., 2001; Hanson et al., 2004) or fail to accurately quantify the soil moisture response among forest species (Wullschleger et al., 2001). The results of the present study are novel in that we were able to incorporate all of the above. Notably, the study showed that a version of MAESTRA incorporating response to soil moisture that can describe carbon exchange of complete plants of individual species can estimate carbon exchange under both well-watered and water-stressed conditions. The model is appropriate to study differences in carbon exchange among species and to estimate their carbon fluxes in response to water stress with a general soil moisture response sub-model, which was originally developed to estimate the transpiration of a maritime pine forest (Granier and Loustou, 1994). Despite its origin, the model performed well on all of the deciduous species. However, future studies should investigate if it is also suited to other conifer and deciduous species.
Water stress can decrease NPP by a direct effect on photosynthetic capacity of the mesophyll or by a CO2 limitation resulting from a decrease in stomatal aperture. Although the stomatal limitation brought about by a decrease in soil moisture was central to constrain both carbon exchange and transpiration under water stress, the discrepancy between measured and modelled values increased with water-stress severity. The parameters Vcmax and Jmax might explain the discrepancy that NPP estimates became increasingly erratic and erroneous along a gradient toward severe water stress. Under these conditions, the parameter changes in Vcmax and Jmax, however, primarily influenced carbon as opposed to transpiration predictions. This observation is in agreement with the physiological parameter sensitivity analysis reported in Bowden and Bauerle (2008). Regardless of water stress, however, the importance of accounting for Vcmax and Jmax over the course of the growing season was highest when the parameter values changed toward the end of the growing season.
Two gas exchange factors that are unaccounted for may explain the increased discrepancies in model estimates and measured plant water loss under irrigation deficit. First, we constructed our Anet/Ci curves under well-watered soil moisture conditions and a moderate VPD to minimize patchy stomatal closure caused by the onset of water (e.g. Downton et al., 1988) and humidity stress (Cardon et al., 1994). Patchy stomatal closure due to either stress could lead to an over-estimation of leaf internal CO2 levels that would invalidate our calculated Ci values (e.g. Sharkey and Seemann, 1989; Terashima, 1992). Alternatively, cuticular properties may affect gas exchange (e.g. Boyer et al., 1997). The present Vcmax and Jmax calculations do not include cuticular attributes, which could result in a miscalculation of Ci if the diffusion properties of the cuticle differ from those in the stomata. Although the experimental protocol was not designed to permanently impair plant metabolism through prolonged and/or severe water stress, patchy stomatal closure and/or cuticular conductance may explain the increased separation in model estimates and actual plant water loss under water stress conditions.
The present study demonstrates that general biochemical parameters related to season and the response to soil moisture are important: when incorporated into a whole-tree model, they permit simulation of carbon exchange and transpiration for individual deciduous tree species. This conclusion builds on previous research using the MAESTRA model showing that the seasonal photosynthesis and transpiration parameters expressed intraseasonal changes (Bowden and Bauerle, 2008). The present study highlights the need to incorporate seasonal patterns in parameters that control carbon and water exchange, and provides a simple and general method to describe seasonal changes in biochemistry and response to soil moisture when simulating deciduous species carbon dioxide and water exchange.
CONCLUSIONS
Modelling the carbon and water balances of forests generally requires many parameters and a proficient means to incorporate the biochemical activity of species during the season, and the response to soil moisture is important for understanding such ecosystems. This study shows that the seasonal pattern of leaf biochemical activity and its response to soil moisture can be parameterized with straightforward general relationships. The MAESTRA model balances complex equations against simplifications that are acceptable and facilitate modelling of the carbon dioxide exchange of species at higher scales. To our knowledge, this is the first successful attempt at integrating a general seasonality and water stress approach to predict carbon exchange among species.
ACKNOWLEDGEMENTS
We thank Parsons Nursery and the American Paulownia Association for donating the trees for this study, J. Bowden, R. Klos and P. Baldwin for assistance with data collection, J. Toler and M. Shahba for statistical help, and B. Medlyn for helpful comments on an earlier draft of the manuscript. This work was funded by the United States Department of Agriculture Forest Service, Horticulture Research Institute, South Carolina Experimental Research Station, and Colorado State Experimental Research Station.
LITERATURE CITED
- Badeck FW, Lischke H, Bugmann H, et al. Tree species composition in European pristine forests: comparison of stand data to model predictions. Climatic Change. 2001;51:307–347. [Google Scholar]
- Baldocchi DD, Wilson KB, Gu L. How the environment, canopy structure and canopy physiological functioning influence carbon, water and energy fluxes of a temperate broad-leaved deciduous forest – An assessment with the biophysical model CANOAK. Tree Physiology. 2002;22:1065–1077. doi: 10.1093/treephys/22.15-16.1065. [DOI] [PubMed] [Google Scholar]
- Ball JT, Woodrow IE, Berry JA. A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In: Biggens J, editor. Progress in photosynthesis research. Dordrecht: Martinus Nijhoff; 1987. pp. 221–224. [Google Scholar]
- Bauerle WL, Post CJ, McLeod MF, Dudley JB, Toler JE. Measurement and modeling of the transpiration of a temperate red maple container nursery. Agriculture and Forest Meteorology. 2002;114:45–57. [Google Scholar]
- Bauerle WL, Whitlow TH, Setter TL, Bauerle TL, Vermeylen FM. Ecophysiology of Acer rubrum L. seedlings from contrasting hydrologic habitats: growth, gas exchange, tissue water relations, abscisic acid, and carbon isotope discrimination. Tree Physiology. 2003;23:841–850. doi: 10.1093/treephys/23.12.841. [DOI] [PubMed] [Google Scholar]
- Bauerle WL, Bowden JD, McLeod MF, Toler JE. Modeling intra-crown and intra-canopy interactions in red maple: assessment of light transfer on carbon dioxide and water vapor exchange. Tree Physiology. 2004;24:589–597. doi: 10.1093/treephys/24.5.589. [DOI] [PubMed] [Google Scholar]
- Bauerle WL, Wang GG, Bowden JD, Hong CM. An analysis of ecophysiological responses to drought in American Chestnut. Annals of Forest Science. 2006;63:833–842. [Google Scholar]
- Bauerle WL, Bowden JD, Wang GG. The influence of temperature on within-canopy acclimation and variation in leaf photosynthesis and respiration: spatial acclimation to microclimate gradients among thermally divergent Acer rubrum L. genotypes. Journal of Experimental Botany. 2007;58:3285–3298. doi: 10.1093/jxb/erm177. [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Singsass EL, Pimentel C, Portis AR, Long SP. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell & Environment. 2001;24:253–259. [Google Scholar]
- Bernacchi CJ, Pimentel C, Long SP. In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant, Cell & Environment. 2003;26:1419–1430. [Google Scholar]
- Bowden JD, Bauerle WL. Measuring and modeling the variation in species-specific transpiration in temperate deciduous hardwoods. Tree Physiology. 2008;28:1675–1683. doi: 10.1093/treephys/28.11.1675. [DOI] [PubMed] [Google Scholar]
- Boyer JS, Wong SC, Farquhar GD. CO2 and water vapor exchange across leaf cuticle (epidermis) at various water potentials. Plant Physiology. 1997;114:185–191. doi: 10.1104/pp.114.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon ZG, Mott KA, Berry JA. Dynamics of patchy stomatal movements and their contribution to steady-state and oscillating stomatal conductance calculated using gas-exchange techniques. Plant, Cell & Environment. 1994;17:995–1007. [Google Scholar]
- Cosh MH, Jackson TJ, Bindlish R, Famiglietti JS, Ryu D. Calibration of an impedance probe for estimation of surface soil water content over large regions. Journal of Hydrology. 2005;311:49–58. [Google Scholar]
- Downton WJS, Loveys BR, Grant WJR. Non-uniform stomatal closure induced by water stress causes putative non-stomatal inhibition of photosynthesis. New Phytologist. 1988;110:503–509. [Google Scholar]
- Emhart VI, Martin TA, White TL, Huber DA. Clonal variation in crown structure, absorbed photosynthetically active radiation and growth of loblolly pine and slash pine. Tree Physiology. 2007;27:421–430. doi: 10.1093/treephys/27.3.421. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S. Modeling of photosynthetic response to environmental conditions. In: Lange O, Nobel PS, Osmond C, Ziegler H, editors. Encyclopedia of plant physiology. Berlin: Springer-Verlag; 1982. pp. 549–588. [Google Scholar]
- Granier A, Loustau D. Measuring and modeling the transpiration of a maritime pine canopy from sap-flow data. Agricultural and Forest Meteorology. 1994;71:61–81. [Google Scholar]
- Granier A, Reichstein M, Bréda N, et al. Evidence for soil water control on carbon and water dynamics in European forests during the extremely dry year: 2003. Agricultural and Forest Meteorology. 2006;143:123–145. [Google Scholar]
- Hanson PJ, Amthor JS, Wullschleger SD, et al. Oak forest carbon and water simulations: model intercomparison and evaluations against independent data. Ecological Monographs. 2004;74:443–489. [Google Scholar]
- Harrison RG, Jones CD, Hughes JK. Competing roles of rising CO2 and climate change in the contemporary European carbon balance. Biogeosciences. 2008;5:1–10. [Google Scholar]
- Ibrom A, Jarvis PG, Clement R, et al. A comparative analysis of simulated and observed photosynthetic CO2 uptake in two coniferous forest canopies. Tree Physiology. 2006;26:845–864. doi: 10.1093/treephys/26.7.845. [DOI] [PubMed] [Google Scholar]
- Ito A, Muraoka H, Koizumi H, Saigusa N, Murayama S, Yamamoto S. Seasonal variation in leaf properties and ecosystem carbon budget in a cool–temperate deciduous broad-leaved forest: simulation analysis at Takayama site, Japan. Ecological Research. 2006;21:137–149. [Google Scholar]
- Janssens IA, Medlyn B, Gielen B, et al. Carbon budget of Pinus sylvestris saplings after four years of exposure to elevated atmospheric carbon dioxide concentration. Tree Physiology. 2005;25:325–337. doi: 10.1093/treephys/25.3.325. [DOI] [PubMed] [Google Scholar]
- Kim S-H, Lieth JH. A coupled model of photosynthesis, stomatal conductance and transpiration for a rose leaf (Rosa hybrida L.) Annals of Botany. 2003;91:771–781. doi: 10.1093/aob/mcg080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi Y, Shibata S, Kobashi S. Parameterization of the CO2 and H2O gas exchange of several temperate deciduous broad-leaved trees at the leaf scale considering seasonal changes. Plant, Cell & Environment. 2003;26:285–301. [Google Scholar]
- Kruijt B, Barton C, Rey A, Jarvis PG. The sensitivity of stand-scale photosynthesis and transpiration to changes in atmospheric CO2 concentration and climate. Hydrology & Earth Systems Science. 1999;3:55–69. [Google Scholar]
- Massman WJ, Kaufmann MR. Stomatal response to certain environmental factors: a comparison of models for subalpine trees in the Rocky Mountains. Agricultural and Forest Meteorology. 1991;54:155–167. [Google Scholar]
- Medlyn BE, Berbigier P, Clement R, et al. Carbon balance of coniferous forests growing in contrasting climates: model-based analysis. Agricultural and Forest Meteorology. 2005;131:97–124. [Google Scholar]
- Medlyn BE, Pepper DA, O'Grady AP, Keith H. Linking leaf and tree water use with an individual-tree model. Tree Physiology. 2007;27:1687–1699. doi: 10.1093/treephys/27.12.1687. [DOI] [PubMed] [Google Scholar]
- Ogink-Hendriks MJ. Modeling surface conductance and transpiration of an oak forest in The Netherlands. Agricultural and Forest Meteorology. 1995;74:99–118. [Google Scholar]
- Oren R, Pataki DE. Transpiration in response to variation in microclimate and soil moisture in southeastern deciduous forests. Oecologia. 2001;127:549–559. doi: 10.1007/s004420000622. [DOI] [PubMed] [Google Scholar]
- Passioura J. The drought environment: physical, biological and agricultural perspectives. Journal of Experimental Botany. 2007;58:113–117. doi: 10.1093/jxb/erl212. [DOI] [PubMed] [Google Scholar]
- Pretzsch H, Grote R, Reineking B, Rötzer Th, Seifert St. Models for forest ecosystem management: a European perspective. Annals of Botany. 2008;101:1065–1087. doi: 10.1093/aob/mcm246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciuto DM, Davis KJ, Keller K. Causes of interannual variability in ecosystem–atmosphere CO2 exchange in a northern Wisconsin forest using a Bayesian model calibration. Agricultural and Forest Meteorology. 2008;148:309–327. [Google Scholar]
- Running SW, Coughlan JC. A general model of forest ecosystem processes for regional applications: I. hydrologic balance, canopy gas exchange and primary production processes. Ecological Modelling. 1988;42:125–154. [Google Scholar]
- Ryan MG, Binkley D, Fownes JH, Giardina CP, Senock RS. An experimental test of the causes of forest growth decline with stand age. Ecological Monographs. 2004;74:393–414. [Google Scholar]
- Sasai T, Okamoto K, Hiyama T, Yamaguchi Y. Comparing terrestrial carbon fluxes from the scale of a flux tower to the global scale. Ecological Modelling. 2007;208:135–144. [Google Scholar]
- Schulze ED, Turner NC, Gollan T, Schakel KA. Stomatal response to air humidity and to soil drought. In: Zeiger E, Cowan I, Farquhar GD, editors. Stomatal function. Stanford, CA: Stanford University Press; 1987. pp. 311–321. [Google Scholar]
- Sharkey TD, Seemann JR. Mild water stress effects on carbon-reduction-cycle intermediates, ribulose bisphosphate carboxylase activity, and spatial homogeneity of photosynthesis in intact leaves. Plant Physiology. 1989;89:1060–1065. doi: 10.1104/pp.89.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinoquet H, Le Roux X, Adam B, Ameglio T, Daudet FA. RATP: a model for simulating the spatial distribution of radiation absorption, transpiration and photosynthesis within canopies: application to an isolated tree crown. Plant, Cell & Environment. 2001;24:395–406. [Google Scholar]
- Tardieu F, Reymond M, Muller B, et al. Linking physiological and genetic analyses of the control of leaf growth under changing environmental conditions. Australian Journal of Agricultural Research. 2005;56:937–946. [Google Scholar]
- Terashima I. Anatomy of non-uniform leaf photosynthesis. Photosynthesis Research. 1992;31:195–212. doi: 10.1007/BF00035537. [DOI] [PubMed] [Google Scholar]
- Thum T, Aalto T, Laurila T, et al. Assessing seasonality of boreal coniferous forest CO2 exchange by estimating biochemical model parameters from micrometeorological flux observations. Biogeosciences Discussions. 2008;5:2707–2747. [Google Scholar]
- Valentini R, De Angelis P, Matteucci G, Monaco R, Dore S, Scarascia-Mucnozza GE. Seasonal net carbon dioxide exchange of a beech forest with the atmosphere. Global Change Biology. 1996;2:199–207. [Google Scholar]
- Van Wijk MT, Dekker SC, Bouten W, Kohsiek W, Mohren GMJ. Simulation of carbon and water budgets of a Douglas-fir forest. Forest Ecology and Management. 2001;145:229–241. [Google Scholar]
- Verbeeck H, Steppe K, Nadezhdina N, et al. Stored water use and transpiration in Scots pine: a modeling analysis with ANAFORE. Tree Physiology. 2007;27:1671–1685. doi: 10.1093/treephys/27.12.1671. [DOI] [PubMed] [Google Scholar]
- Wang YP, Jarvis PG. Description and validation of an array model-MAESTRO. Agricultural and Forest Meteorology. 1990;51:257–280. [Google Scholar]
- Wang YP, Polglase PJ. The carbon balance in the tundra, boreal and humid tropical forests during climate change – scaling up from leaf physiology and soil carbon dynamics. Plant, Cell and Environment. 1995;18:1226–1244. [Google Scholar]
- Wang YP, Rey A, Jarvis PG. Carbon balance of young birch trees grown in ambient and elevated atmospheric CO2 concentrations. Global Change Biology. 1998;4:797–808. [Google Scholar]
- Wilson KB, Baldocchi DD, Hanson PJ. Leaf age affects the seasonal pattern of photosynthetic capacity and net ecosystem exchange of carbon in a deciduous forest. Plant, Cell and Environment. 2001;24:571–583. [Google Scholar]
- Wong S, Cowan I, Farquhar G. Stomatal conductance correlates with photosynthetic capacity. Nature. 1979;282:424–426. [Google Scholar]
- Wullschleger SD, Jackson RB, Currie WS, et al. Below-ground processes in gap models for simulating forest response to global change. Climatic Change. 2001;51:449–473. [Google Scholar]
- Xu L, Baldocchi DD. Seasonal trends in photosynthetic parameters and stomatal conductance of blue oak (Quercus douglasii) under prolonged summer drought and high temperature. Tree Physiology. 2003;23:865–877. doi: 10.1093/treephys/23.13.865. [DOI] [PubMed] [Google Scholar]