Abstract
Total creatine (Cr) levels are widely used as an internal reference for the quantification of other metabolites in 1H magnetic resonance spectroscopy (MRS). However, Cr plays an important role in brain energy metabolism and its levels can be modulated by conditions of energy production and demand. Therefore, abnormal Cr levels in patient vs. control populations could confound the utility of this metabolite as an internal reference. We quantified Cr levels in 22 healthy controls, 15 acutely manic patients with bipolar disorder and 15 acutely ill patients with schizophrenia using 1H MRS in the anterior cingulate cortex, and the parieto-occipital cortex at 4 Tesla. Patients with schizophrenia had a statistically significant reduction in Cr levels as compared with controls; bipolar disorder patients showed no difference in Cr as compared with controls. In addition, older age was associated with reductions in Cr in healthy controls, but not in patients with either disorder. These findings indicate that the use of Cr as an internal reference in schizophrenia MRS research is problematic unless Cr levels are shown to be normal in the study population. They also add to the literature on bioenergetic abnormalities in schizophrenia.
Keywords: anterior cingulate cortex, parieto-occipital cortex, magnetic resonance spectroscopy, internal reference
1. INTRODUCTION
In vivo proton magnetic resonance spectroscopy (1H MRS) investigations of the human brain reveal a prominent singlet at 3.03ppm arising from a combination of creatine and phosphocreatine (termed total creatine, we will abbreviate Cr). Cr concentration in the human brain varies between 6–10 mM, although it is reported to be at the higher end of this range in grey matter (Govindaraju et al., 2000). Phosphocreatine acts as a reservoir for the generation of ATP in neurons; although Cr levels are typically maintained within a narrow range, short periods of intense energy requirement can lead to rapid breakdown of phosphocreatine and possibly reduce overall Cr levels (Kato et al., 1996). Thus Cr may act as a marker of cellular energy production.
The magnitude of the Cr peak has been widely used as a concentration reference in human MRS studies, where measures of other metabolites are reported as ratios to Cr. This reduces subject-specific sources of variance inherent in 1H MRS data and would be valid if Cr levels do not change systematically across study groups. Despite the widespread use of this approach in studies of neuropsychiatric conditions, few studies have explicitly examined this assumption. Several recent studies have found reduced Cr levels in the dorsolateral prefrontal cortex in bipolar disorder (Frey et al., 2007), the anterior cingulate cortex in schizophrenia (Theberge et al., 2007), and dorsolateral prefrontal cortex of individuals at high risk of psychosis (Wood et al., 2003), but elevated Cr levels in the prefrontal cortex in major depressive disorder (Gruber et al., 2003). Cr levels have also been variably reported to increase (Saunders et al., 1999) or decrease (Charles et al., 1994) with advancing age. In this report, we examine Cr levels in acutely ill, medicated, bipolar disorder and schizophrenia patients using 1H MRS at 4 Tesla. We hypothesized based on the previous literature that we would find reductions in Cr levels in both patient groups. To test the regional specificity of our findings, we report data from two brain regions: the anterior cingulate cortex (ACC) which is implicated in psychiatric disorders (Öngür and Price 2000) and the parieto-occipital cortex (POC) as a control region.
2. METHODS
2.1. Subjects
Following approval by the McLean Hospital IRB, data were obtained from 22 healthy control (NC), 15 bipolar manic (BD) and 15 schizophrenia (SZ) subjects (7 SZ subjects diagnosed with schizoaffective disorder). An additional 24 BD and 3 SZ subjects were recruited for the study but were not able to provide usable data. Inability to tolerate the scanning environment due to psychiatric condition was the primary reason for drop-out. BD subjects who did not complete the study were somewhat more symptomatic (YMRS:26.9; MADRS:18.4; PANSS:82.0) than those who did (see Table 1). All patients were hospitalized with acute episodes. Patients were assessed using the structured clinical interview for the DSM-IV, as well as the Positive and Negative Syndrome Scale (PANSS), Young Mania Rating Scale (YMRS), and the Montgomery-Asberg Depression Rating Scale (MADRS) on the day of scan. All bipolar disorder patients were type 1 and met DSM-IV criteria for a current manic episode; three patients in this group met criteria for a mixed episode. Inclusion criteria were ages 18–65, both genders; for patients, a SCID-IV diagnosis of bipolar 1 disorder for the BD group, and schizophrenia or schizoaffective disorder currently not in a mood episode for the SZ group were required. Medications and psychiatric comorbidities were allowed. Although patients continued their regular medications, no sedative medications were prescribed for the purposes of the study. In the BD group, 8 patients had a past history of substance abuse, one each had comorbid social anxiety disorder, generalized anxiety disorder, and bulimia nervosa. In the SZ group, 8 patients had a past history of substance abuse, while two patients had comorbid post-traumatic stress disorder and one each had panic disorder and binge eating disorder. Significant neurological or medical problems, current substance abuse or history of substance dependence, and contraindications for MR scanning were exclusion criteria. Chlorpromazine (CPZ) equivalents were calculated for patients taking antipsychotic medications (Woods 2003).
Table 1.
Subject demographic and clinical information (Mean±SD where appropriate)
| Normal Control (N=22) |
Bipolar Disorder (N=15) |
Schizophrenia (N=15) |
Statistical Evaluation | |
|---|---|---|---|---|
| Age | 34.9±10.0 | 36.3±11.6 | 42.9±9.8 | F(49,2)=2.53; P=0.09 |
| Gender | 12M, 10F | 7M, 8F | 8M, 7F | χ2: 0.16 (NS) |
| Education | 6.3±0.8 | 4.9±1.7 | 5.0±1.9 | F(44,2)=3.20; P=0.05 |
| Handedness | 21 RH | 15 RH | 14 RH | χ2: 0.09 (NS) |
| Age of onset | -- | 25.5±9.5 | 23.3±8.0 | F(25,1)=0.05; P =0.82 |
| Illness duration | -- | 10.8±12.3 | 18.6±11.7 | F(25,1)=2.30; P =0.14 |
| MADRS | -- | 11.1±3.6 | 16.5±7.5 | F(28,1)=6.49; P =0.02 |
| YMRS | -- | 24.7±8.9 | 14.9±9.3 | F(28,1)=8.77; P =0.01 |
| PANSS | -- | 59.6±13.9 | 85.3±20.3 | F(28,1)=16.29; P <0.001 |
| Lithium | -- | 9 | 3 | χ2: 5.00; P =0.03 |
| Anticonvulsants | -- | 10 (8 valproate) | 6 | χ2: 2.14 (NS) |
| SGAs | -- | 15 | 14 | χ2: 0.02 (NS) |
| FGAs | -- | 0 | 2 | χ2: 1.11 (NS) |
| CPZ equivalents | -- | 244±122 | 525±419 | F(26,1)=6.16; P =0.02 |
| Benzodiazepines | -- | 7 | 8 | χ2: 0.13 (NS) |
RH: Right-handed; SGA: Second generation antipsychotic; FGA: First generation antipsychotic. Other abbreviations are as in the text.
2.2. MRI/MRS Scans
All subjects underwent a structural MRI scan in a Siemens (Erlangen, Germany) 3.0 T Trio MR scanner, and subjects with structural abnormalities were excluded from the study. All 1H MRS acquisitions and related brain imaging were conducted on a full-body MR scanner (Varian/UnityInova, Varian Inc., Palo Alto, CA) operating at 4 Tesla magnetic field strength, using a single-tuned volumetric-birdcage design coil. Following scout images to ensure optimal patient positioning, manual global shimming of unsuppressed water signal yielded an unfiltered water linewidth of ≤ 30 Hz. Next, T1-weighted sagittal and axial images were acquired as anatomical guides, and for subsequent image segmentation.
A 2×2×2cm single voxel (8cc) was next placed on the ACC along the midline (Figure 1). Manual shimming resulted in global water linewidths of less than 11 Hz. We collected 48 water-suppressed spectra from the voxel, with echo-times ranging from 30ms to 500 ms in 10ms increments (TR=2.0s, acquisition bandwidth=2000kHz, repetitions=16). The identical process was then carried out on a 2×2×2cm POC voxel, but with 8 repetitions (Figure 1). Total time in the magnet was 75 minutes.
Figure 1.
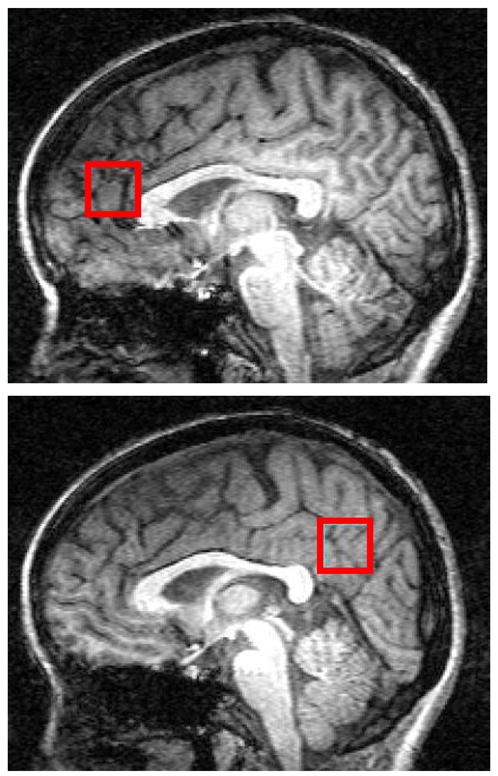
Parasagittal views of the brain from T1-weighted images illustrate ACC and POC voxel placement in one control subject.
The full width at half maximum of the water resonance was recorded as one measure of data quality. These values were 9.9±1.2 and 8.7±0.8 Hz for NC, 9.3±1.5 and 8.6±0.7 Hz for BD and 9.4±2.3 and 8.9±0.9 Hz for SZ subjects (in ACC and POC voxels, respectively). There were no group differences for this measure.
2.3. MRS Data Processing and Analysis
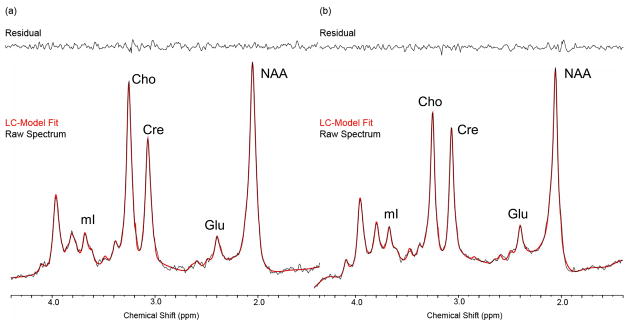
All MRS processing was carried out blinded to diagnosis using the TE-averaging approach as previously described (Prescot et al., 2006). Representative TE-averaged spectra output from the commercial spectral-fitting package LCModel (version 6.0–1) (Provencher 1993) are shown in Figure 2. The Cr values reported in this study are institutional units based on the LCModel fitting of TE-averaged spectra.
Figure 2.
Examples of spectra from the ACC in a control (left) and BD subject (right). Raw data are visible as a black line and the LCModel spectral fit is in red, with the residual shown in the top panel.
LCModel reports Cramer-Rao Lower Bounds (CRLB), the lower limit of the variance associated with the estimation procedure. The mean CRLBs for Cr were 4 and 3% for NC, 3 and 3% for BD, and 4 and 2% for SZ (in the ACC and POC, respectively). There were no between-group differences in these CRLB values.
2.4. Image Segmentation
Tissue-segmentation of the T1-weighted images used FMRIB’s Automated Segmentation Tool (FAST; Oxford, UK) which segments images into grey matter (GM), white matter (WM), and cerebrospinal fluid (CSF) based on pixel intensity, while also correcting for spatial intensity variations (RF inhomogeneities). Due to poor quality of T1-weighted images in some cases, this analysis was available for 61 out of the total 94 voxels. GM% in the ACC and POC was 79±5% and 70±8% in NC, 76±8% and 69±8% in BD, and 77±3% and 69±7% in SZ, respectively. WM% was 17±6% and 28±8% for NC, 16±8% and 29±8% in BD, and 16±4% and 29±8% in SZ, respectively.
2.5. Statistical Analysis
Demographic and clinical variables were compared across groups using one-way analysis of variance (ANOVA) and chi-square tests (Table 1). We also explored relationships between Cr and demographic and clinical variables by computing a series of correlation coefficients (corrected for multiple comparisons).
We analyzed the effects of diagnosis and age on Cr levels using a repeated measures regression model with brain region (POC and ACC), age, diagnosis (NC, BD, and SZ) as covariates. In a second analysis, we included the age by diagnosis interaction as an additional covariate. All 52 subjects with 94 total voxels were included in the analysis. Data was available only from the ACC voxel for 10 subjects who requested terminating the study due to longer-than anticipated scan times (e.g. due to difficult shimming or brief software malfunction). Five of these subjects were in the NC group, three in BD, and two in SZ. We also considered a model controlling for %GM using only the 61 voxels where %GM information was available. Finally, because clozapine can reduce cerebral metabolism (Molina et al., 2005) and possibly Cr levels, we repeated the analysis excluding schizophrenia patients receiving clozapine. Models were fit using in SAS 9.1.3 (SAS Institute, Cary, NC). All P values presented are two-tailed.
3. RESULTS
SZ subjects had lower than normal Cr in both voxels (Table 2). The largest group difference was a 21% reduction in Cr in the ACC for the NC-SZ comparison. BD subjects had slightly lower Cr levels than control subjects (Table 2).
Table 2.
Cr levels (Mean±SD)
| ACC | |
| NC | 0.889±0.206 |
| BD | 0.861±0.206 |
| SZ | 0.699±0.214 |
| POC | |
| NC | 0.908±0.290 |
| BD | 0.863±0.245 |
| SZ | 0.840±0.167 |
All abbreviations are as in the text.
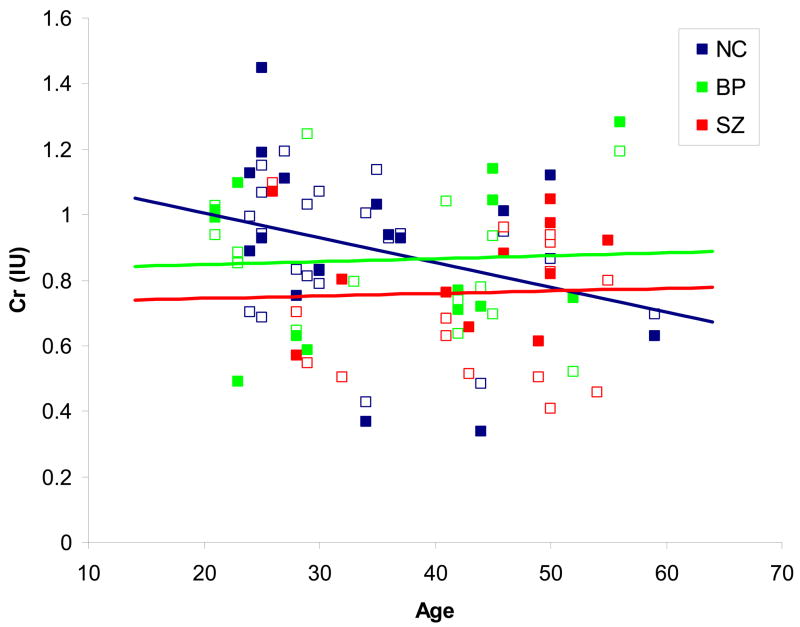
The model estimated a mean reduction of 0.15 Cr units for SZ and 0.03 units for BD relative to NC, controlling for age and brain region (Table 3). The difference was significant for SZ (t49=−2.00, P=0.05), but not BD (t49=−0.39, P=0.70). The statistical model with an interaction between age and diagnosis estimated a decline of 0.06 Cr units per decade for NC but an increase of 0.04 units per decade for BD and 0.002 units per decade for SZ (Figure 3), controlling for brain region, but this pattern was not significant (P>0.10, two-tailed). When %GM was entered into the model, the difference between SZ and NC was not significant because of the smaller sample size. When only SZ patients not taking clozapine were included in the analysis (N=12), the mean Cr levels for the non-clozapine SZ group were 0.711 for ACC and 0.829 for POC. The difference between the NC and SZ groups was still significant (t46=−1.68, P=0.05).
Table 3.
Parameter estimates for the statistical model, 52 subjects
| Effect | Estimate | SEa | DFa | t Value | P |
|---|---|---|---|---|---|
| Intercept | 0.88 | 0.04 | 50 | 19.70 | <0.001 |
| ACC (Reference) | 0.00 | -- | -- | -- | -- |
| POC | 0.04 | 0.03 | 42 | 1.45 | 0.16 |
| Age (Decades)b | −0.03 | 0.03 | 48 | −1.01 | 0.32 |
| NC (Reference) | 0.00 | -- | -- | -- | -- |
| BD | −0.03 | 0.07 | 48 | −0.39 | 0.70 |
| SZ | −0.15 | 0.07 | 49 | −2.00 | 0.05 |
DF: degrees of freedom. SE: standard error. Other abbreviations are as in the text.
Centered at 37.3 years
Figure 3.
Cr levels by age in the three study groups. Cr levels from both brain regions for each subject are shown (ACC as open squares, POC as filled squares). The trendlines are shown for ACC and POC data combined, since brain region had no effect on Cr levels in this study. See text for details of differences between groups.
Because the mean ages of the subject groups differed, especially between the NC and SZ groups, we carried out an additional analysis using a subsample consisting of pairwise age-matched subjects (10 SZ and 10 NC). Among the age-matched pairs of subjects, 5 pairs had the same age, 2 pairs differed by 1 year, 1 pair differed by 2 years, and 2 pairs differed by 4 years. These were the best possible pairings within the constraints of the age difference in our sample. The mean age of both groups was 40.6 years. In this subsample, the NC group had a mean Cr level of 0.87 and the SZ group 0.76. A t-test (paired at the subject and voxel level) showed that the SZ group still had significantly lower Cr levels than the NC group (t19=−1.80, P=0.04).
Correlation analyses revealed that Cr measures were not related to YMRS, MADRS, PANSS scores, age at disease onset, and CPZ equivalents. We also examined medication effects on Cr levels using one-way ANOVAs and found no differences between patients on or off lithium, anticonvulsants, second-generation antipsychotics and benzodiazepines.
4. DISCUSSION
We report that hospitalized patients with schizophrenia have significantly reduced Cr levels as compared with healthy control subjects. Acutely manic patients with bipolar disorder did not show reductions in Cr levels. In addition, healthy control subjects show an age related decline in Cr levels, while patients with bipolar disorder and schizophrenia do not show this pattern. Instead, younger patients in both groups have relatively low Cr levels, and these levels remain unchanged in older subjects. Given Cr’s role in brain energy production, this finding adds to reported abnormalities in energy metabolism reported in schizophrenia.
The total Cr signal we quantified arises from a combination of creatine and phosphocreatine. The latter molecule is synthesized from the former with the use of adenosine triphosphate (ATP). At times of high energy demand, phosphocreatine acts as a source of high-energy phosphates by generating ATP and Cr. Acute reductions in brain phosphocreatine are common in periods of intense neuronal activity, but sustained reductions in the phosphocreatine signal are associated with a disruption in brain energy production and a dearth of ATP (Stork and Renshaw 2005). The reduction in Cr levels in 1H MRS studies is interpreted as being consistent with this scenario (Frey et al., 2007). Therefore, our findings are suggestive of a long-term failure of energy production in the brain in schizophrenia. This interpretation is consistent with previous findings of mitochondrial dysfunction in schizophrenia (Ben-Shachar and Laifenfeld 2004), and may be related to metabolic abnormalities in the ACC in this condition (Siegel et al., 1993).
These findings indicate that the use of Cr levels as an “internal reference” to reduce variability in other 1H MRS measures is inappropriate in psychiatric research. Cr levels should not be assumed to remain constant unless explicitly and independently assessed. Our findings indicate that the divergence from the normal pattern is greatest at younger ages, making the use of Cr referencing particularly problematic in studies of patients in the first episode or early stages of illness.
The relationship of Cr levels to age in healthy populations has not been clarified, with some studies reporting increases with age (Saunders et al., 1999), others reporting no change or reductions (Charles et al., 1994). Our data are consistent with a reduction in Cr with aging in healthy controls, but our small sample size precludes any general conclusions.
This study has several limitations. First, the small sample size raises the risk of type-I and type-II error. Second, we present data using institutional units; we did not attempt internal referencing to water or absolute quantification approaches. Therefore, there may be alternative explanations of our data. For example, group differences in coil loading due to differential head size or signal changes due to differential brain atrophy may cause changes in this measure. However, several measures in our data (linewidth of the water resonance; GM/WM/CSF distribution in study voxels) indicate that our spectra were of comparable quality and composition across the groups, increasing our confidence that our findings are biologically based. In addition, this argument would not explain our finding of a reduction in Cr levels with age in healthy controls and not in patients.
Acknowledgments
The authors thank Dr. Caitlin Ravchandran for helpful discussions.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ben-Shachar D, Laifenfeld D. Mitochondria, synaptic plasticity, and schizophrenia. International Review of Neurobiology. 2004;59:273–96. doi: 10.1016/S0074-7742(04)59011-6. [DOI] [PubMed] [Google Scholar]
- Charles HC, Lazeyras F, Krishnan KR, Boyko OB, Patterson LJ, Doraiswamy PM, McDonald WM. Proton spectroscopy of human brain: effects of age and sex. Progress in Neuropsychopharmacology & Biological Psychiatry. 1994;18:995–1004. doi: 10.1016/0278-5846(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Frey BN, Stanley JA, Nery FG, Monkul ES, Nicoletti MA, Chen HH, Hatch JP, Caetano SC, Ortiz O, Kapczinski F, Soares JC. Abnormal cellular energy and phospholipid metabolism in the left dorsolateral prefrontal cortex of medication-free individuals with bipolar disorder: an in vivo 1H MRS study. Bipolar Disorders. 2007;9(Suppl 1):119–27. doi: 10.1111/j.1399-5618.2007.00454.x. [DOI] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR in Biomedicine. 2000;13:129–53. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Gruber S, Frey R, Mlynarik V, Stadlbauer A, Heiden A, Kasper S, Kemp GJ, Moser E. Quantification of metabolic differences in the frontal brain of depressive patients and controls obtained by 1H-MRS at 3 Tesla. Investigational Radiology. 2003;38:403–8. doi: 10.1097/01.rli.0000073446.43445.20. [DOI] [PubMed] [Google Scholar]
- Kato T, Murashita J, Shioiri T, Hamakawa H, Inubushi T. Effect of photic stimulation on energy metabolism in the human brain measured by 31P-MR spectroscopy. Journal of Neuropsychiatry and Clinical Neuroscience. 1996;8:417–22. doi: 10.1176/jnp.8.4.417. [DOI] [PubMed] [Google Scholar]
- Molina V, Gispert JD, Reig S, Sanz J, Pascau J, Santos A, Desco M, Palomo T. Cerebral metabolic changes induced by clozapine in schizophrenia and related to clinical improvement. Psychopharmacology (Berl) 2005;178:17–26. doi: 10.1007/s00213-004-1981-9. [DOI] [PubMed] [Google Scholar]
- Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Prescot AP, de BFB, Wang L, Brown J, Jensen JE, Kaufman MJ, Renshaw PF. In vivo detection of brain glycine with echo-time-averaged (1)H magnetic resonance spectroscopy at 4.0 T. Magnetic Resonance in Medicine. 2006;55:681–6. doi: 10.1002/mrm.20807. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance in Medicine. 1993;30:672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Saunders DE, Howe FA, van den Boogaart A, Griffiths JR, Brown MM. Aging of the adult human brain: in vivo quantitation of metabolite content with proton magnetic resonance spectroscopy. Journal of Magnetic Resonance Imaging. 1999;9:711–6. doi: 10.1002/(sici)1522-2586(199905)9:5<711::aid-jmri14>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Siegel BV, Jr, Buchsbaum MS, Bunney WE, Jr, Gottschalk LA, Haier RJ, Lohr JB, Lottenberg S, Najafi A, Nuechterlein KH, Potkin SG. Cortical-striatal-thalamic circuits and brain glucose metabolic activity in 70 unmedicated male schizophrenic patients. American Journal of Psychiatry. 1993;150:1325–36. doi: 10.1176/ajp.150.9.1325. [DOI] [PubMed] [Google Scholar]
- Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Molecular Psychiatry. 2005;10:900–19. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- Theberge J, Williamson KE, Aoyama N, Drost DJ, Manchanda R, Malla AK, Northcott S, Menon RS, Neufeld RW, Rajakumar N, Pavlosky W, Densmore M, Schaefer B, Williamson PC. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. British Journal of Psychiatry. 2007;191:325–34. doi: 10.1192/bjp.bp.106.033670. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Berger G, Velakoulis D, Phillips LJ, McGorry PD, Yung AR, Desmond P, Pantelis C. Proton magnetic resonance spectroscopy in first episode psychosis and ultra high-risk individuals. Schizophrenia Bulletin. 2003;29:831–43. doi: 10.1093/oxfordjournals.schbul.a007049. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry. 2003;64:663–7. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]