Abstract
Body composition is sparsely described in spinal muscular atrophy (SMA). Body (BMI, mass/height in m2), fat-free (FFMI, lean mass/height in m2) and fat (FMI, fat mass/height in m2) mass indexes were estimated in 25 children (ages 5–18) with SMA (2 type I, 13 type II, 10 type III) using dual-energy radiograph absorptiometry and anthropometric data referenced to gender and age-matched healthy children (NHANES III, New York Pediatric Rosetta Body Project). BMI was ≥ 50th percentile in 11 (44%) and ≥ 85th in 5 (20%). FFMI was reduced (p<0.005) and FMI was increased (P<0.005) in the overall study cohort. FMI was ≥ 50th, ≥ 85th and 95th percentiles in 19 (76%), 10 (40%) and 5 (20%) subjects, respectively. Using a receiver operator characteristic curve, BMI above 75th, 50th and 3rd percentiles maximized sensitivity and specificity for FMI ≥ 95th, ≥ 85th and ≥ 50th percentiles, respectively. Children with SMA have reduced lean and increased fat mass compared to healthy children. Obesity is a potentially important modifiable source of morbidity in SMA.
Introduction
Spinal muscular atrophy (SMA) is a progressive, recessively-inherited, neuromuscular disease characterized by weakness and muscle atrophy due to loss of spinal cord motor neurons. Traditionally this disease, particularly in its most severe type I form (Werdnig-Hoffman), has been associated with considerable morbidity and mortality[1], although there have been advances in recent years with proactive medical care that have altered its natural course[1]. Despite progress at clinical, preclinical and molecular levels, and improved understanding of its molecular pathogenesis, optimal nutritional management and ideal growth parameters remain poorly described. While there is potential morbidity associated with being overweight as well as with malnutrition in this population, few articles presently in the literature specifically address body composition, growth expectations or anthropometric measures[2]. Recently Messina et al reported a high frequency of swallowing dysfunction and subnormal weight in a cohort of patients with SMA type II[3]. While limited by available methodology (this study was based on parental estimation of weight obtained via telephone survey and did not incorporate other anthropometric measures or assessment of body composition), the authors speculated that the observed low body weight may reflect malnutrition or failure to thrive secondary to poor feeding specific to this population (SMA type II)[3].
Body mass index (BMI, weight in kg/height in m2) has been widely utilized to screen for overweight in otherwise healthy children[4, 5], with BMI greater than the 85th and 95th percentiles for healthy children[6] connoting “at risk for overweight” and “overweight”, respectively. Studies have called into question, however, the applicability of BMI for a number of groups, including Asians[7], postmenopausal women[8] and persons with spinal cord injury[9–12]. Recent literature has eschewed relatively crude measures of excess body fat such as BMI and skin-fold based estimates of percentage body fat (%BF) in favor of direct descriptions of body composition such as the fat-free mass index (FFMI, kg of lean mass/height in m2) and fat mass index (FMI, kg of fat mass/height in m2) [4, 13, 14], applying similar percentile cutoffs (above 85th percentile for “at risk of overweight”, above 95th for “overweight”[6]) to FMI to define overweight status. Fat mass estimation using dual energy x-ray absorptiometry (DXA) has emerged as a viable and reproducible technique for directly assessing relative body composition in the pediatric population[15–18]. Recently this technique has been utilized by the New York Pediatric Rosetta Body Project to describe body composition in a large sample of healthy children and define body composition parameters for normal children based on age, race and gender[4, 13, 14]. While it has not been widely studied in SMA for feasibility, reproducibility, and correlation with other measures over time, DXA may represent a useful technique for measuring body composition, and hence muscle atrophy and excess adiposity, in this population.
Utilizing DXA to assess absolute fat and fat-free mass and incorporating anthropometric data to determine BMI, FMI and FFMI values in a cohort of 25 children and adolescents with SMA (including types I, II, and III) enrolled in a natural history study of the disease, we postulate that application of standard BMI percentiles obtained from cohorts of normal subjects underestimates total body fat in these patients, thus underestimating overweight in this population. Moreover, we hypothesize that children with SMA have reduced FFMI and increased FMI when compared to gender and age-matched reference data obtained from the National Health and Nutrition Examination Survey (NHANES III; 1988–1994) and the New York Pediatric Rosetta Body Project.
Patients and Methods
Subjects
25 consecutive pediatric-aged (5–18 years) patients with SMA (11 girls, 14 boys, age 5 to 17 years) for whom DXA was performed as part of an ongoing natural history study at Columbia-Presbyterian Medical center in New York City were selected for the study. This is a well described, genetically characterized cohort of patients for whom a battery of clinical, demographic and anthropometric data has been recorded on an ongoing basis as part of scheduled follow-up assessments and care. Demographic and descriptive measures of these patients are incorporated into Table 1. The Hammersmith Scale of motor function, expanded (HSMFE) is a 66 point scale designed and validated for assessment of motor function in SMA[19, 20]. Forced vital capacity (FVC) has likewise been found reliable, valid and feasible in children above 5 years of age with SMA, using standard technique[21]. DXA was only performed on two children with SMA type I. Both children (ages 9.75 and 12.5 years) are fully BiPAP dependent (precluding pulmonary function testing) and score zero on the HSMFE. HMFSE and FVC values for these two patients were excluded from the cohort characteristics depicted in Table 1 (see footnotes).
Table 1.
Demographic and body composition data for the study cohort
| Summary Characteristics by Age (Mean ± SD) |
Summary Characteristics by SMA Type (Mean ± SD) |
||||
|---|---|---|---|---|---|
| Total Cohort |
Aged 5–11 years |
Aged 12–18 years |
SMA Types I & II |
SMA Type III |
|
| n | 25 | 16 | 9 | 15 | 10 |
| boys/girls | 14/11 | 9/7 | 5/4 | 9/6 | 5/5 |
| Age | 9.1 ±4.3 | 6.2 ±1.9 | 14.2 ±1.8 | 8.2 ±3.4 | 10.4 ±5.3 |
| Hammersmith Score of Motor Functiona | 28.1 ±15.1 | 27.6 ±14.8 | 28.7 ±16.1 | 11.0 ±7.3 | 44.7 ±14.3 |
| Forced Vital Capacity (% expected)a | 78.9% ±25.4% | 77.0% ±17.2% | 80.7% ±32.5% | 60.6% ±23.7% | 94.1% ±14.8% |
| Use of BiPAP or CPAP | 5 (20%) | 3 (19%) | 2 (22%) | 5 (33%) | 0 (0%) |
| Use of Gastrostomy Tube | 3 (12%) | 1 (6%) | 2 (22%) | 3 (20%) | 0 (0%) |
| Body Mass Index (BMI) | 16.7 ±5.4 | 16.5 ±6.0 | 17.1 ±4.5 | 16.2 ±6.4 | 17.4 ±3.7 |
| Fat-Free Mass Index (FFM in kg/m2) | 9.4 ±2.0 | 9.4 ±2.1 | 9.4 ±2.1 | 8.9 ±2.1 | 10.2 ±1.6 |
| Z-Scoreb | −4.6 ±1.9** | −4.5 ±2.0** | −4.7 ±1.6** | −5.0 ±2.1** | −3.9 ±1.2** |
| Fat-Mass Index (FM in kg/m2) | 6.2 ±3.0 | 7.1 ±4.4 | 7.2 ±2.5 | 7.4 ±4.5 | 6.7 ±2.5 |
| Z-Scoreb | 1.0 ±1.3** | 1.0 ±1.5* | 1.0 ±1.0* | 0.8 ±1.5 | 0.6 ±1.1 |
| Percentage Body Fat (%BF) | 39.7% ±8.9% | 39.2% ±10.3% | 40.6% ±6.0% | 41.4% ±10.0% | 37.2% ±6.4% |
| Subjects with BMI >25 | 1 4.0% | 1 6.3% | 0 0.0% | 1 6.7% | 0 0.0% |
| BMI above 50th percentile for age | 11 44.0% | 8 50.0% | 3 33.3% | 6 40.0% | 5 50.0% |
| BMI above 85th percentile (>1 SD above mean) for age | 5 20.0% | 5 31.3% | 0 0.0% | 5 33.3% | 0 0.0% |
| Above 50th percentile for FMI | 19 76.0% | 12 75.0% | 7 77.8% | 10 66.7% | 9 90.0% |
| Above 85th percentile (>1 S.D. above mean) for FMI | 10 40.0% | 6 37.5% | 4 44.4% | 7 46.7% | 3 30.0% |
| Above 95th percentile (> 2 S.D. above mean) for FMI | 5 20.0% | 4 25.0% | 1 11.1% | 4 26.7% | 1 10.0% |
Anthropometrics
For all subjects who were able to stand erect, height was measured while standing to the nearest centimeter with a stadiometer. For subjects unable to stand erect, stature was measured on a flat table in a supine position. Every effort was made to account for any contractures. Weight of the subject and the wheelchair was obtained to the nearest kilogram with a wheelchair balance scale (Pelstar, Bridgeview Il) with the subject in the wheelchair. Then the wheelchair was weighed by itself and subtracted from the total weight. Subjects able to sit unsupported or to stand were weighed directly on a standard balance scale. BMI was calculated from measured weight and height.
DXA
Whole body DXA scans were performed using Lunar models DPX with pediatric software 3.8G and DPX-L with pediatric software 1.5G (GE Lunar Corporation, General Electric, Madison, WI) in accordance with the manufacturer’s instructions[22]. Each scan provided estimates of subjects’ fat mass and fat-free mass in kilograms and %BF. Technique, coefficient of variation, and quality control procedures (i.e. usage of phantoms to simulate bone, fat and fat-free tissues) has been described previously[4]. FMI and FFMI was then calculated for each patient by dividing estimated fat and lean mass (kg), respectively, by measured height in meters, squared (kg/m2).
Age-and gender-specific BMI reference values developed by CDC[5] through the National Health and Nutrition Examination Survey (NHANES III; 1988–1994) were used to assign BMI percentiles based on this extensive cohort of 33,994 healthy volunteers, aged 2 months to 80 years, to study participants. Age, gender and race-controlled reference values for %BF[4], FFMI and FMI[13] were obtained from data published by the Pediatric Rosetta Study at St Luke’s-Roosevelt Hospital Center in New York (1995–2000), a cross-sectional study of pediatric body composition in 1208 healthy children and adolescents whose mean height, weight, and BMI were only slightly different from children and adolescents examined in the NHANES data[4, 5, 13] (See Table 2). Z-scores for FFMI and FMI were assigned based on available reference data from the Pediatric Rosetta Body project[13] and depicted in Table 2.
Table 2.
Normative Values for Fat Mass, Fat-mass Index, Fat-free mass and Fat-free Mass Index from New York Pediatric Rosetta Body Project by age, race and gender[13].
| Boys | Girls | |||||||
|---|---|---|---|---|---|---|---|---|
| Age (y) | Whites | Blacks | Hispanics | Asians | Whites | Blacks | Hispanics | Asians |
| 5–11 | ||||||||
| N | 90 | 57 | 36 | 101 | 80 | 77 | 32 | 97 |
| Age (y) | 9.1 +/− 2 | 8.9 +/− 2 | 9.4 +/− 2 | 9.1 +/− 2 | 9.2 +/− 2 | 9.1 +/− 2 | 9.3 +/− 2 | 9.1 +/− 2 |
| Weight-for-age Z | 0.4 | 0.8 | 0.8 | 0.6 | 0.3 | 0.8 | 0.6 | 0.1 |
| Height-for-age Z | 0.3 | 0.5 | 0.3 | 0.1 | 0.1 | 0.7 | −0.1 | −0.1 |
| BMI (kg/m2) | 18 +/− 3 | 20 +/− 5 | 19 +/− 4 | 19 +/− 3 | 18 +/− 4 | 19 +/− 4 | 20 +/− 5 | 18 +/− 3 |
| BMI-for-age Z | 0.3 | 0.8 | 0.9 | 0.7 | 0.4 | 0.7 | 0.8 | 0.2 |
| BMI-for-age P | 58 | 70 | 74 | 71 | 61 | 68 | 72 | 58 |
| Fat mass (kg) | 7 +/− 6 | 9 +/− 10 | 9 +/− 8 | 9 +/− 5 | 9 +/− 7 | 10 +/− 7 | 11 +/− 8 | 8 +/− 5 |
| FMI (kg/m2) | 3.5 +/− 3 | 4.3 +/− 4 | 4.8 +/− 4 | 4.6 +/− 3 | 4.8 +/− 3 | 4.9 +/− 3 | 5.9 +/− 4 | 4.4 +/− 2 |
| Fat-free mass (kg) | 27 +/− 6 | 28 +/− 7 | 28 +/− 7 | 26 +/− 5 | 25 +/− 6 | 28 +/− 8 | 26 +/− 7 | 24 +/− 6 |
| FFMI (kg/m2) | 14.2 +/− 1 | 15.2 +/− 2 | 14.6 +/− 1 | 14.2 +/− 1 | 13.7 +/− 1 | 14.3 +/− 2 | 14 +/− 1 | 13.3 +/− 1 |
|
| ||||||||
| 12–18 | ||||||||
| N | 72 | 73 | 58 | 91 | 61 | 63 | 47 | 69 |
| Age (y) | 15 +/− 2 | 15 +/− 2 | 14.9 +/− 2 | 15.3 +/− 2 | 14.5 +/− 2 | 15.2 +/− 2 | 14.9 +/− 2 | 15.5 +/− 2 |
| Weight for-age Z | 0.4 | 0.7 | 0.6 | 0.3 | 0.4 | 0.8 | 0.6 | 0.3 |
| Height-for-age Z | 0.1 | 0.5 | 0 | 0 | 0.2 | 0.2 | −0.3 | −0.4 |
| BMI (kg/m2) | 22 +/− 4 | 22 +/− 5 | 23 +/− 4 | 22 +/− 2 | 21 +/− 4 | 25 +/− 6 | 24 +/− 5 | 22 +/− 4 |
| BMI-for-age Z | 0.3 | 0.5 | 0.7 | 0.3 | 0.3 | 0.8 | 0.7 | 0.3 |
| BMI-for-age P | 61 | 62 | 70 | 59 | 61 | 71 | 70 | 60 |
| Fat mass (kg) | 12 +/− 9 | 11 +/− 12 | 12 +/− 8 | 12 +/− 8 | 16 +/− 8 | 22 +/− 14 | 20 +/− 10 | 17 +/− 7 |
| FMI (kg/m2) | 4.4 +/− 3 | 3.9 +/− 4 | 4.7 +/− 3 | 4.1 +/− 3 | 6.1 +/− 3 | 8.1 +/− 5 | 8.1 +/− 4 | 6.6 +/− 3 |
| Fat-free mass (kg) | 50 +/− 12 | 53 +/− 11 | 51 +/− 11 | 51 +/− 8 | 39 +/− 6 | 44 +/− 6 | 41 +/− 7 | 39 +/− 5 |
| FFMI (kg/m2) | 17.4 +/− 2 | 18.3 +/− 2 | 18.1 +/− 2 | 17.9 +/− 2 | 15.2 +/− 1 | 16.6 +/− 1 | 16.1 +/− 2 | 15.4 +/− 2 |
Receiver operating characteristic curve (ROC)
To determine the optimal cutoff points for BMI percentile corresponding to FMI above the median for age and gender (by DXA), “at risk for overweight” (FMI above 85th percentile for age and gender, by DXA) and “overweight” (FMI above 95th percentile for age and gender, by DXA), a ROC was generated for the combined cohort of SMA subjects. The optimum cutoff point was considered to be the percentile where the sum of sensitivity plus specificity was greatest.
Statistical Analysis
All results are expressed as mean ± standard deviation unless otherwise specified. Unpaired, one-sided Student’s t-test was used to compare mean differences in FFMI and FMI Z-scores between the overall SMA cohort and the Pediatric Rosetta Body cohort. P-values for FMI and FFMI Z-scores in four sub-groups of the overall cohort (ages 5–11, ages 12–18, SMA types I&II, SMA type III) were also obtained as secondary measures. Significance was established at P<0.05.
Results
BMI, FFMI, FMI and %BF data for the overall study group, age (5–11 years, 12–18 years) and SMA type (types I and II, type III) cohorts are presented in table 1.
Body Mass Index
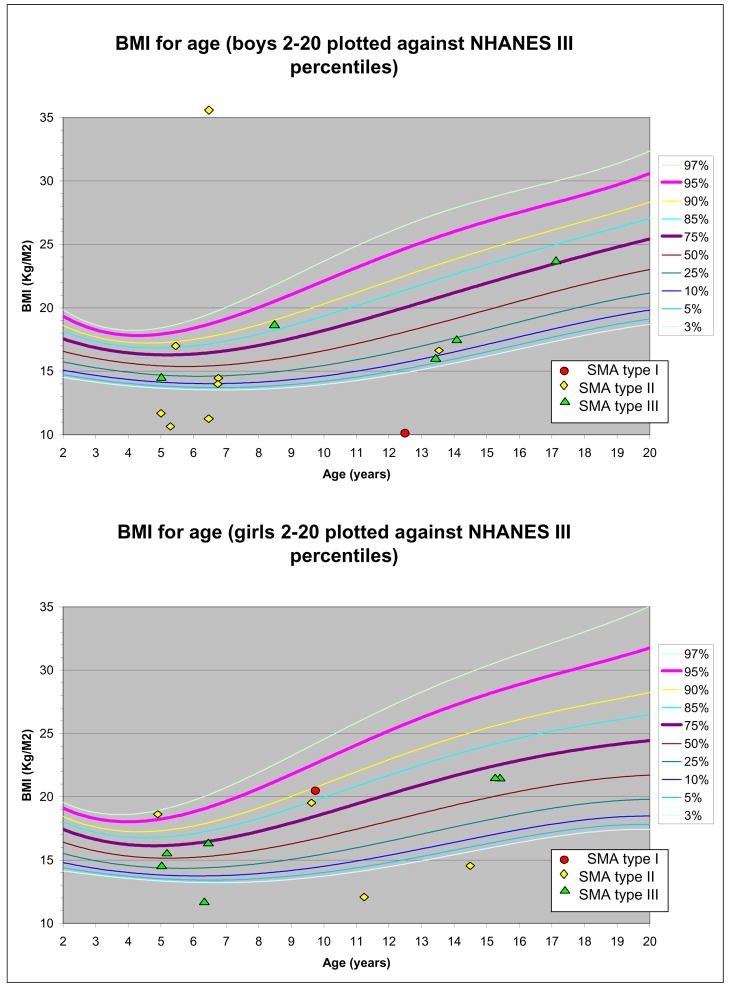
The majority of patients in the study (56%) had BMI below the 50th percentile for age, adjusted for gender, although 5 of the 25 patients in the study (20%) had BMI above the 85th percentile (>1 standard deviation above the mean) for healthy children. Only one subject had a BMI greater than 25 kg/m2, the threshold value used to screen for “at risk of overweight” in healthy adult populations. All 5 of the subjects with BMI above the 85th percentile were in the SMA type I and II cohort, representing 35.7% of these patients. Figure 1 depicts the BMI of individual study subjects, controlled for age and gender, applied to NHANES percentile curve for BMI.
Figure 1.
SMA cohort BMI. for age and gender applied to NHANES percentile curve
Fat-Free Mass Index (FFMI)
Subjects aged 5 – 11 years averaged FFMI of 9.44 kg/m2 (± 2.07), below normative values for gender, age and race-matched healthy children as depicted in Table 2[13]. A similar result was noted in the 12–18 year-old age group, with an average FFMI of 9.37 ± 2.05. FFMI was 8.9 ± 2.1 and 10.2 ± 1.6 in the SMA types I & II and −4.7(±1.6) in the aged 5–11 and 12–18 years cohorts, respectively, and −5.0(±2.1) and −3.9(±1.2) in the SMA I/II and SMA III cohorts, respectively (See table 1), significantly reduced compared to healthy peers. While there was a trend to reduced FFMI in types I and II compared to type III patients, this finding did not reach statistical significance (p=0.12). Compared with healthy patients studied in the Pediatric Rosetta Study, 96% (24/25) of study patients had FFMI more than one standard deviation below the mean (approximately 15th percentile), while 92% (23/25) fell more than 2 standard deviations below the mean (approximately 5th percentile) for age and gender.
Fat Mass Index (FMI)
FMI was significantly increased in SMA patients relative to age, gender and race-matched control subjects from the Pediatric Rosetta Study cohort, as depicted in table 2. Subjects aged 5–11 had a FMI of 7.1 ± 4.4 kg/m2; a similar result, FMI of 7.2 ± 2.5 kg/m2, was found in the 12–18 year-old age group, adiposity significantly above that seen in normal age-matched children[13]. This observation remained consistent for both the SMA type I/II and SMA type III cohorts (7.4 ± 4.5 and 6.7 ± 2.5 kg/m2, respectively), although neither reached statistical significance. As shown in table 1, FMI z-scores averaged 1.0(±1.3) for the total group, 1.0(±1.5) and 1.0(±1.0) in the aged 5–11 and 12–18 years cohorts, respectively, and 0.8(±1.5) and 0.6 (±1.1) in the SMA I/II and SMA III cohorts, respectively. Compared with the Pediatric Rosetta Study cohort, FMI was above the 50th percentile in 76% (19/25). 40% (10/25) and 20% (5/25) were above the 85th and 95th FMI percentiles for healthy children, respectively. Percentage body fat was similarly elevated across the study cohort, when referenced to children and adolescents from the Pediatric Rosetta Body Project[4].
Receiver-Operator Characteristics
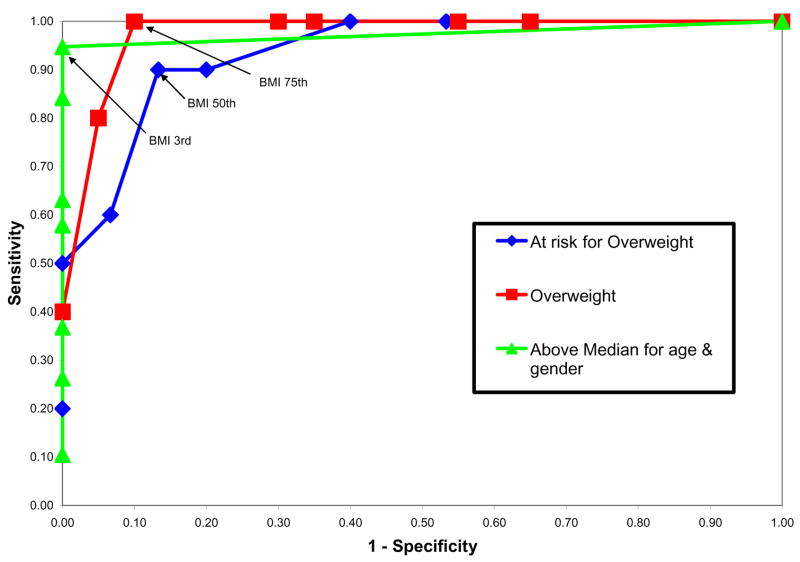
The ROC curve depicting the relationship between BMI percentile, plotted in reference to NHANES III percentiles for age accounting for gender, and FMI above the median for age, “at risk for overweight” and “overweight” status, is depicted graphically in figure 2. The BMI percentile that maximized the sensitivity and specificity corresponding to FMI greater than 85% (“at risk for overweight”) was the 50th percentile. The BMI percentile that maximized the sensitivity and specificity corresponding to FMI greater than 95% (“overweight”) was 75%, while presence of BMI above the 3rd percentile was highly specific and sensitive for FMI above the median. Table 3 shows the sensitivity (true-positive rate) and specificity (true-negative rate) used to create the ROC analysis for the above median, overweight and obese analyses.
Figure 2.
Receiver-operator curves of BMI-for-age (using NHANES III percentiles for age and gender)[5] for identification of excess body fat defined by FMI greater than 50th percentile (triangle marker), greater than 85th percentile (“at risk of overweight”, diamond marker) and greater than 95th percentile (“overweight”, square marker) compared to the reference cohort (see table 2)[13].
Table 3.
Receiver-Operator Characteristics table for BMI percentile prediction of above median FMI, overweight and obese adjusted for age and gender.
| Above median FMI |
Overweight |
Obese |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BMI above: | Sensitivity | Specificity | Sensitivity + Specificity |
Sensitivity | Specificity | Sensitivity + Specificity |
Sensitivity | Specificity | Sensitivity + Specificity |
| 0% | 1.00 | 0.00 | 1.00 | 1.00 | 0.00 | 1.00 | 1.00 | 0.00 | 1.00 |
| 3% (>3 S.D. below) | 0.95 | 1.00 | 1.95 | 1.00 | 0.47 | 1.47 | 1.00 | 0.35 | 1.35 |
| 5% (>2 S.D. below) | 0.95 | 1.00 | 1.95 | 1.00 | 0.47 | 1.47 | 1.00 | 0.35 | 1.35 |
| 10% | 0.84 | 1.00 | 1.84 | 1.00 | 0.60 | 1.60 | 1.00 | 0.45 | 1.45 |
| 25% | 0.63 | 1.00 | 1.63 | 0.90 | 0.80 | 1.70 | 1.00 | 0.65 | 1.65 |
| 50% | 0.58 | 1.00 | 1.58 | 0.90 | 0.87 | 1.77 | 1.00 | 0.70 | 1.70 |
| 75% | 0.37 | 1.00 | 1.37 | 0.60 | 0.93 | 1.53 | 1.00 | 0.90 | 1.90 |
| 85% (>1 S.D. above) | 0.26 | 1.00 | 1.26 | 0.50 | 1.00 | 1.50 | 0.80 | 0.95 | 1.75 |
| 95% (>2 S.D. above) | 0.11 | 1.00 | 1.11 | 0.20 | 1.00 | 1.20 | 0.40 | 1.00 | 1.40 |
| 97% (>3 S.D. above) | 0.11 | 1.00 | 1.11 | 0.20 | 1.00 | 1.20 | 0.40 | 1.00 | 1.40 |
Discussion
The high and rising prevalence of overweight children is a subject of increasing interest and focus in both the medical and lay press. Less widely recognized is the high prevalence of overweight children and adolescents with neuromuscular disease. Body composition has been a sparsely addressed subject in the management of spinal muscular atrophy, despite its widely acknowledged importance in the health maintenance of these children[2], at risk both for growth failure due to poor feeding as well as excessive weight gain due to decreased activity leading to a reduction in overall metabolic demand. Current dietary management is loosely based on experience with patients with spinal cord injury that has shown lower lean tissue and higher body fat percentage in these patients when compared to control subjects[9, 23–26]. Similar results have been demonstrated in patients with Duchenne muscular dystrophy[27–29].
Based on this literature, it has been inferred that children with SMA may plot as underweight based on criteria derived from cohorts of healthy children due to a marked decrease in lean body mass. Standard management strategies maintain BMI on a percentile curve, typically on the lower aspects of the “normal” range. There have been, however, no prior studies describing body composition in SMA or addressing the applicability of this approach. This study supports conventional assumptions about body composition in SMA, specifically that total non-fat mass and FFMI are reduced markedly in SMA patients when compared to healthy children and adolescents. Patients in this study, regardless of SMA type, demonstrated reduced FFMI. Unsurprisingly, this reduction was more extreme in the type I and II cohort than that seen in type III. Decreased fat-free mass is most likely due to a loss of overall muscle mass, reflective of the underlying pathophysiology of the disease.
Not unexpectedly, body fat percentage is markedly increased based on DXA imaging data, with a high proportion of study subjects falling above the 85th and even 95th percentiles for age. Elevated percentage body fat, however, is not entirely due to the expected reduction in muscle mass. There is a high incidence of elevated FMI in the study group, reflecting a significant increase in absolute fat mass adjusted for height in the study cohort. FMI reflects adiposity in isolation, and is thus independent of relative increases or decreases in non-fat mass. A large proportion of the patients in this study, 40%, had FMI above the 85th percentile while 20% were above the 95th percentile (demarking “at risk for overweight” and “overweight” using conventional definitions, respectively[6]). As seen in this study, children and adolescents with SMA are at high risk of overweight status when compared to healthy children. This is despite anthropometric data (BMI) that places them overwhelmingly in the seeming middle of the NHANES BMI percentile curves.
The American Academy of Pediatrics guidelines, published in 2003, recommend screening for overweight based on BMI as part of routine well child care, and that appropriate behavioral interventions and counseling be provided to promote sustained weight loss for overweight children[30]. A similar approach is perhaps even more essential in persons with disabilities[25], particularly neuromuscular diseases such as spinal muscular atrophy. As a result of the significant alterations in body composition, standard classification of BMI is insufficient and inaccurate for children and adolescents with SMA. In our study, 40% of subjects met criteria for “at risk for overweight” and 20% met criteria for “overweight” based on the FMI criteria proposed by the Pediatric Rosetta Body study group[13]. This trend remains consistent even for the relatively less affected SMA type III patients, who in many cases remain at least partially ambulatory and retain higher overall muscle mass. In our sample, the ROC showed that the most accurate cutoff for “at risk for overweight” (based on FMI) was the 50th percentile for age, controlling for gender, while the most accurate cutoff for “overweight” was the 75th percentile seen in healthy children.
Much attention has been placed on the increasing frequency of complications of extreme adiposity seen in the pediatric population, ranging from diabetes mellitus type II, to hypertension to the so-called “metabolic syndrome”. The risk of such complications has been cited as a significant concern in the management of spinal cord injury[24–26], with more than 90% of overweight patients from a regional spinal cord injury clinic meeting metabolic syndrome criteria[25]. Identification of overweight patients at risk for metabolic syndrome using easily obtainable clinical criteria should be a high priority for clinical care in all patients, including those with spinal muscular atrophy.
Unaddressed is the potential morbidity of even “normal” levels of adiposity in SMA. Increased fat mass is an additional burden on already severely weakened muscles, possibly leading to decreased motor function and thus worsening morbidity associated with the disease. In this study, 76% of participants had FMI above the 50th percentile for age adjusted for gender; this group was well approximated by BMI above the 3rd percentile for age and gender. This mirrors the description by Mussina et al (2008), who reported a high frequency of body weight greater than two standard deviations below the median for age and sex matched controls among a cohort of 122 patients with SMA type II[3]. The authors, noting a high frequency of chewing and swallowing difficulties, speculated a potential risk for malnutrition in these individuals[3]. Even mild elevation in fat mass, however, may limit overall motor function and thus increase morbidity in children and adolescents with spinal muscular atrophy, regardless of type. Whether achievement and maintenance of low fat composition (and commensurate to this goal, maintaining BMI below the 3rd percentile for age and gender) will improve or possibly worsen motor function and outcome in SMA remains at this point conjecture but an intriguing area of future exploration.
Spinal muscular atrophy remains a disease marked by significant morbidity and mortality, particularly in its more severe forms, despite advances in research and patient management. In this study, we have described body composition in a mixed cohort of children with spinal muscular atrophy, as assessed using DXA. This cohort, due to the limitations of this technology (age above 5 years), does not describe body composition in young children with SMA. As a further result of this limitation, only two patients with SMA type 1, the most severe form of the disease were incorporated into the study. Moreover, the cohort enrolled in this study are drawn from an ongoing natural history study; while body composition and nutrition is not a primary outcome measure nor focus of the study, there may be an unrecognized selection bias inherent to patients who choose to enroll in a clinical trial of this nature that confounds the observed results in application to the broader population of patients with SMA.
In this study we report significantly and markedly reduced fat-free mass and increased fat mass among a cohort of children 5–18 years of age with spinal muscular atrophy. We also report a high frequency of subjects either overweight or at risk for overweight, despite body mass index within the normal range described in healthy subjects. Assessment of BMI may continue to serve a role in the nutritional management of patients with SMA, with the idealized BMI falling near the 3rd percentile of the NHANES III BMI curve drawn from a cohort of healthy children. While outcome and morbidity was unaddressed by this study, it is reasonable to extrapolate that overweight status may represent a potentially modifiable component to the overall morbidity of this disease.
Acknowledgments
This research was supported by funding from the SMA foundation and a NINDS, Neurological Sciences Academic Development Award (K12 NS01698). We would like to thank the patients who participated in the study, and their parents, for their generosity of time and commitment to furthering clinical knowledge and research in SMA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oskoui M, Levy G, Garland CJ, et al. The changing natural history of spinal muscular atrophy type 1. Neurology. 2007;69(20):1931–6. doi: 10.1212/01.wnl.0000290830.40544.b9. [DOI] [PubMed] [Google Scholar]
- 2.Wang CH, Finkel RS, Bertini ES, et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22(8):1027–49. doi: 10.1177/0883073807305788. [DOI] [PubMed] [Google Scholar]
- 3.Messina S, Pane M, De Rose P, et al. Feeding problems and malnutrition in spinal muscular atrophy type II. Neuromuscul Disord. 2008;18(5):389–393. doi: 10.1016/j.nmd.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Mei Z, Grummer-Strawn LM, Wang J, et al. Do skinfold measurements provide additional information to body mass index in the assessment of body fatness among children and adolescents? Pediatrics. 2007;119(6):e1306–13. doi: 10.1542/peds.2006-2546. [DOI] [PubMed] [Google Scholar]
- 5.Kuczmarski R, Ogden C, Guo S. 2000 CDC growth charts for the United States: methods and development. . 2002; May:1–190. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 6.Ogden C, Flegal K, Carrol M, Johnson C. Prevalence and trends in overweight among US children and adolescents 1999–2000. JAMA. 2002;288(14):1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 7.Goh V, Tain C, Tong T, Mok H, Wong M. Are BMI and other anthropometric measures appropriate as indices for obesity? A study in an Asian population. J Lipid Res. 2004;45:1892–1898. doi: 10.1194/jlr.M400159-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Evans E, Rowe D, Racette S, Ross K, McAuley E. Is the current BMI obesity classification appropriate for black and white postmenopausal women? Int J Obes (Lond) 2006;30:837–843. doi: 10.1038/sj.ijo.0803208. [DOI] [PubMed] [Google Scholar]
- 9.McDonald C, Abresch-Meyer A, Nelson M, Widman L. Body mass index and body composition measures by dual x-ray absorptiometry in patients aged 10 to 21 years with spinal cord injury. J Spinal Cord Med. 2007;30(Suppl 1):S 97–104. doi: 10.1080/10790268.2007.11754612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchholz A, Bugaresti J. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord. 2005;43:513–518. doi: 10.1038/sj.sc.3101744. [DOI] [PubMed] [Google Scholar]
- 11.Spungen A, Adkins R, Stewart C. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol. 2003;95:2398–2407. doi: 10.1152/japplphysiol.00729.2002. [DOI] [PubMed] [Google Scholar]
- 12.Jones L, Legge M, Goulding A. Healthy body mass index values often underestimate body fat in men with spinal cord injury. Arch Phys Med Rehabil. 2003;84:1068–1071. doi: 10.1016/s0003-9993(03)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Freedman D, Wang J, Maynard L, et al. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes (Lond) 2005;29(1):1–8. doi: 10.1038/sj.ijo.0802735. [DOI] [PubMed] [Google Scholar]
- 14.Freedman D, Wang J, Ogden C, et al. The prediction of body fatness by BMI and skinfold thicknesses among children and adolescents. Ann Hum Biol. 2007 Mar-Apr;34(2):183–194. doi: 10.1080/03014460601116860. [DOI] [PubMed] [Google Scholar]
- 15.Chan G. Performance of dual-energy x-ray absorptiometry in evaluating bone, lean body mass, and fat in pediatric subjects. J Bone Miner Res. 1992;7:369–374. doi: 10.1002/jbmr.5650070403. [DOI] [PubMed] [Google Scholar]
- 16.Leroy-Willig A, Willig T, Henry-Feugeas M, et al. Body composition determined with MR in patients with Duchenne muscular dystrophy, spinal muscular atrophy, and normal subjects. Magn Reson Imaging. 1997;15(7):737–744. doi: 10.1016/s0730-725x(97)00046-5. [DOI] [PubMed] [Google Scholar]
- 17.Margulies L, Horlick M, Thornton J. Reproducibility of whole body bone and body composition measures by dual energy X-ray absorptiometry using GE Lunar Prodigy. J Clin Densitometry. 2005;8:298–304. doi: 10.1385/jcd:8:3:298. [DOI] [PubMed] [Google Scholar]
- 18.Schmelzle H, Fusch C. Body fat in neonates and young infants: validation of skinfold thickness versus dual-energy X-ray absorptiometry. Am J Clin Nutr. 2002 Nov;76(5):1096–1100. doi: 10.1093/ajcn/76.5.1096. [DOI] [PubMed] [Google Scholar]
- 19.Main M, Kairon H, Mercuri E, Muntoni F. The Hammersmith functional motor scale for children with spinal muscular atrophy: a scale to test ability and monitor progress in children with limited ambulation. Eur J Paediatr Neurol. 2003;7(4):155–9. doi: 10.1016/s1090-3798(03)00060-6. [DOI] [PubMed] [Google Scholar]
- 20.O’Hagen JM, Glanzman AM, McDermott MP, et al. An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromuscul Disord. 2007;17(9–10):693–7. doi: 10.1016/j.nmd.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Iannaccone ST. Outcome measures for pediatric spinal muscular atrophy. Arch Neurol. 2002;59(9):1445–50. doi: 10.1001/archneur.59.9.1445. [DOI] [PubMed] [Google Scholar]
- 22.Mazess R, Barden H, Bisek J, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–1112. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 23.Spungen AM, Wang J, Pierson RN, Jr, WA B. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol. 2000;88(4):1310–1315. doi: 10.1152/jappl.2000.88.4.1310. [DOI] [PubMed] [Google Scholar]
- 24.Liusuwan A, Abresch R, McDonald C. Altered body composition affects resting energy expenditure and interpretation of body mass index in children with spinal cord injury. J Spinal Cord Med. 2004;27(Suppl 11):S24–S28. doi: 10.1080/10790268.2004.11753781. [DOI] [PubMed] [Google Scholar]
- 25.Dopler-Nelson M, Widman L, Abresch R, et al. Metabolic syndrome in adolescents with spinal cord dysfunction. J Spinal Cord Med. 2007;30:S 130–142. doi: 10.1080/10790268.2007.11754591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abresch R, McDonald D, Widman L, McGinnis K, Hickey K. Impact of spinal cord dysfunction and obesity on the health-related quality of life of children and adolescents. J Spinal Cord Med. 2007;30(Suppl 1 ):S 112–118. doi: 10.1080/10790268.2007.11754614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willig T, Carlier L, Legrand M, Rivière H, Navarro J. Nutritional assessment in Duchenne muscular dystrophy. Dev Med Child Neurol. 1993 Dec;35(12):1074–1082. doi: 10.1111/j.1469-8749.1993.tb07925.x. [DOI] [PubMed] [Google Scholar]
- 28.Pichiecchio A, Uggetti C, Egitto M, et al. Quantitative MR evaluation of body composition in patients with Duchenne muscular dystrophy. Eur Radiol. 2002 Nov. 12;(11):2704–2709. doi: 10.1007/s00330-002-1392-4. [DOI] [PubMed] [Google Scholar]
- 29.Zanardi M, Tagliabue A, Orcesi S, et al. Body composition and energy expenditure in Duchenne muscular dystrophy. Eur J Clin Nutr. 2003 Feb;57(2):273–278. doi: 10.1038/sj.ejcn.1601524. [DOI] [PubMed] [Google Scholar]
- 30.Krebs N, Jacobson M. Nutrition AAoPCo, Policy Statement: Prevention of pediatric overweight and obesity. Pediatrics. 2003;112:424–430. doi: 10.1542/peds.112.2.424. [DOI] [PubMed] [Google Scholar]