Abstract
Objective To test a transactional model of sleep–wake development in infants born preterm or low birthweight (PT LBW), which may inform clinical practice, interventions, and future research in this at risk population. Methods One hundred and twenty-eight mother–infant dyads participated from hospital discharge to 4 months postterm. Assessments of prematurity, infant sleep–wake patterns, maternal interaction quality, depression, feeding route, and sociodemographic factors were conducted. Results Path analyses revealed that maternal interactions directly related to infant sleep patterns and family sociodemographic risks related to less optimal parenting. In addition, bottle fed infants experienced fewer night wakings and more nighttime sleep. Conclusions Two potential pathways to sleep patterns in PT LBW infants were identified. The findings suggest directions for clinical work, such as supporting healthy infant sleep through parenting interventions or supporting interpersonal relations between parents and their PT LBW infants by encouraging more daytime naps. Additionally, clinicians should assess parents’ nighttime sleep concerns within the larger sociodemographic and feeding context.
Keywords: low birthweight, preterm, sleep, transactional development
The transition from parent-led coregulation to independent self-regulation in early childhood is a crucial process in development (Kochanska, Coy, & Murray, 2001), and sleep is one of the first coregulatory behaviors that infants practice with parents. Through gradual scaffolding, sensitive responding, and limit setting, parents may promote positive sleep patterns in their children (Mindell & Owens, 2003). Using a transactional model that implicates parenting interactions as a key predictor of sleep development (Goodlin-Jones, Burnham, & Anders, 2000), this study examined early sleep regulation in a sample of infants who experience increased risk for regulatory difficulties, developmental delays, and relational problems (Vergara & Bigsby, 2004): Infants born preterm (<37 weeks gestation) or low birthweight (<2500 g). By increasing our understanding of the mechanisms through which healthy sleep–wake cycles develop, practitioners and researchers may assist parents to promote optimal sleep development in their preterm or low birthweight (PT LBW) infants. Healthy sleep patterns are essential for normal growth across several domains including emotion regulation, learning, memory, and immune function (Davis, Parker & Montgomery, 2004). Although contextual factors like parent–child relations and family sociodemographic risks are recognized as central factors in development (National Research Council and Institute of Medicine, 2000), they are not regularly included in studies of sleep, especially in sleep research conducted with PT LBW infants.
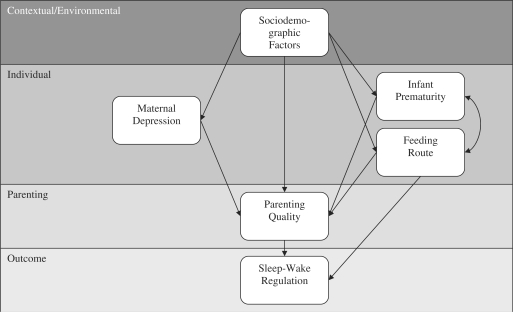
Goodlin-Jones and colleagues’ (2000) transactional model of sleep–wake development implicates contextual and individual characteristics as well as parenting interactions in the development of sleep. Within their model, dynamic processes occur between distal factors and more immediate or proximal factors over time, as each factor influences the development of infant sleep–wake regulation and later sleep problems. This model also claims that regulation of infant sleep–wake states is mediated through parent–infant interactions. In the present study, we applied this transactional model to sleep in PT LBW infants, focusing on the variables of family sociodemographic risks, maternal depressive symptoms, infant prematurity, infant feeding route, and parenting interactions (Figure 1).
Figure 1.
Transactional model of infant sleep–wake development (adapted from Goodlin-Jones et al., 2000).
PT LBW Infant Sleep and Parenting Interactions
Early in development, PT LBW infants engage in longer, lighter and more active sleep than infants born at term (Vergara & Bigsby, 2004). As PT LBW infants develop, their sleep patterns gradually begin to resemble the sleep patterns of full-term infants, although their sleep tends to be more variable and is not as consistent across the first year of life (Anders & Keener, 1985). However, factors associated with sleep in PT LBW infants, including the contributions of parent–child interaction quality, are understudied. Parenting interactions may be particularly important for PT LBW infants because they do not show the range of joint attention and interactional skills seen in full-terms (Barnard, Bee, & Hammond, 1984; Landry, 1995). PT LBW infants are less able to provide clear distress signals and are more easily stressed and over-stimulated than full-term infants (e.g., Feldman, 2006). These difficulties may contribute to less optimal parenting, which may in turn contribute to PT LBW infant sleep development. Difficulties in sleep–wake regulation are associated with a host of negative developmental outcomes, including serious behavior problems, academic difficulties, and metabolic and cardiovascular problems (Mindell et al., 2003).
Several studies of full-term infant sleep have indicated that less optimal infant–parent interactions are associated with night waking and sleep onset problems (Anders, 1994; Benoit, Zeanah, Boucher, & Minde, 1992; Mindell & Owens, 2003; Scher, 2001). Some of these studies have found that overly attentive or inconsistent parenting behaviors are associated with problems in sleep onset and night waking (Anders, 1994; Mindell & Owens, 2003). Although sleep research with full-terms has shifted from an exclusive focus on infant physiologic or maturational development toward the inclusion of parenting behaviors, this trend has not yet emerged in sleep studies of PT LBW infants. To address this gap, we hypothesized that parenting interactions characterized by more sensitivity, positive affect, and less intrusiveness and hostility would directly relate to more optimal sleep in PT LBW infants. Consistent with the proposed transactional model, we expected individual and contextual factors to relate to infant sleep indirectly, as mediated by parenting quality.
Feeding Route
Studies assessing infant sleep via maternal report sleep logs or diaries have found more night waking and less nighttime sleep among breastfed infants when compared to bottlefed infants (DeLeon & Hildebrandt Karraker, 2007; Thomas, 2000). In a study of 41 healthy 9-month-old infants, DeLeon and Hildebrandt Karraker (2007) reported that breastfed infants spent more time awake at night (i.e., more night waking and less nighttime sleep). Wolke et al.'s (1995) study of sleep in 4,427 infants born preterm and full-term reported that breastfeeding was the most robust predictor of night waking. Researchers have hypothesized that breastfed infants are awake more at night because of shorter hunger–satiety cycles.
The findings linking feeding route and infant sleep paint a slightly contradictory picture for scholars who study parent–child relations and infant development because mothers who breastfeed report greater feelings of closeness and engage in more sensitive and responsive interactions with their infants compared to mothers who bottlefeed (Mckee, Jankowski, & Zayas, 2004; Riordan, 2005), and breastfeeding is associated with a host of positive outcomes for infants and mothers (e.g., Evenhouse & Reily, 2005). Thus, we hypothesized that breastfeeding would (a) directly relate to more night waking and less nighttime sleep within the positive context of breastfeeding and (b) indirectly relate to more optimal infant sleep via positive parent–child interactions.
Individual and Contextual Factors
Several studies have documented links between elevated maternal depression and less optimal infant sleep (Dennis & Ross, 2005; Hiscock & Wake, 2002). For example, in a community survey of 738 mothers, Hiscock and Wake (2002) reported that the most robust correlate of infant sleep problems was maternal depressive symptoms. Despite these findings, processes linking maternal depression and infant sleep are unclear. In the transactional model of sleep regulation, parenting interactions are a proposed mediating mechanism through which maternal depression relates to infant sleep.
An extensive body of research has documented associations between elevated maternal depression and problematic parent–child interactions (e.g., Edhborg, Lundh, Seimyr, & Widström, 2003). Although few studies have tested a mediation model even with full-term infants, Harnish and associates (1995) found that parenting quality partially mediated the relation between maternal depressive symptoms and behavior problems in school age children. In addition, the NICHD Early Child Care Research Network (1999) found some support for their model focusing on maternal sensitivity as a mediator of the relation between maternal depression and children's outcomes. However, none of these studies examined infant sleep as an outcome, particularly in PT LBW infants.
The transactional model also highlights the potential importance of more distal factors such as family socioeconomic status (SES) for infant sleep. Several studies have documented less optimal sleep in lower SES families (e.g., Field, Diego, & Hernansez-Reif, 2002; McLaughlin-Crabtree et al., 2005). However, Bayer and associates (2007) found no association between family SES and infant sleep problems between 3 and 6 months of age. Although the role of family sociodemographic factors in infant sleep–wake regulation is unclear, especially in PT LBW infants, we expected these factors to relate to infant sleep indirectly (i.e., as mediated by parenting interactions). Because fewer SES resources are associated with elevated risk for maternal depression (Belle, 1990), preterm birth (Luo, Wilkins, & Kramer, 2006), less optimal parenting (Smith, Landry, & Swank, 2000), and bottlefeeding (Heck, Braveman, Cubbin, Chavez, & Kiely, 2006), we expected more family sociodemographic risks to relate to less optimal infant sleep as mediated via these variables.
Research Questions
Our primary research question was whether our data supported the transactional model of sleep development (Figure 1) in PT LBW infants regarding the sleep outcomes of daytime sleep, naps, nighttime sleep, night waking, and diurnal sleep consolidation. Within the transactional model, we hypothesized direct associations and several mediated relationships. Consistent with the recommendations of Holmbeck (2002), we considered mediation an indirect association wherein variable A must be associated with variable C, and the mediator (variable B) must be association with A and C and account for a significant portion of the association between A and C. We hypothesized that the distal factor of SES risk would relate to the proximal factors of infant prematurity, maternal depressive symptoms, feeding route, and less optimal parenting interactions. We expected that elevated SES risk would be associated with more prematurity, elevated maternal depressive symptoms, bottle feeding, and less optimal parenting. Additionally, we hypothesized that infants born at lower birthweights and younger gestational ages, infants of mothers with more depressive symptoms, and infants who were bottlefed would experience less optimal parenting interactions. We expected that the most proximal factor (parenting interactions) would directly predict infant sleep. Specifically, we expected that parenting interactions characterized by sensitivity, positive affect, and less intrusiveness and hostility would be associated with more optimal infant sleep–wake regulation and that parenting would mediate the association between infant sleep and infant prematurity, maternal depression, and sociodemographic risks. We also expected that infants who were breastfed at night would exhibit more night waking but would also experience more positive parent–child interactions.
Methods
Participants
Data were collected from 164 families recruited from three Wisconsin neonatal intensive care units as part of a larger longitudinal study. A research nurse from each hospital followed IRB approved procedures of informed consent if they met the following criteria: (a) infants were born less than 35 weeks gestation or weighed less than 2500 g at birth, (b) infants had no known congenital malformations or prenatal drug exposures, (c) mothers were at least 17 years of age, (c) mothers could read English, and (d) mothers self-identified as the child's primary caregiver. Because the hospitals would not allow us to be ‘first contact’ for families and they only provided us with information about families who signed consent forms for the study, we were unable to calculate a participation rate or ascertain other population descriptive statistics from each hospital (e.g., ethnicity). However, participant family characteristics paralleled the population of Wisconsin in education and poverty, although participant families were more racially diverse. For example, during our data collection period (from 2002 to 2004), 25% of adults had a bachelors degree in Wisconsin (US Census Bureau, n.d.). In our study, approximately 28% had a bachelors degree. In Wisconsin, families living in poverty during the same time frame ranged from 10% to 14%, and in our study 16% families reported incomes in the poverty range. State-wide estimates of ethnicities in Wisconsin were Caucasian (88%), African American (6%), Asian (2%), and more than one ethnicity (1–2%). Our sample was slightly more diverse with 95 (74%) Caucasian, 9 (7%) African American, 2 (1.6%) Latino, 1 (0.8%) Asian, and 21(16.5%) infants with more than one ethnicity. Additional infant descriptive statistics are provided in Table I.
Table I.
Sample Demographic Descriptive Statistics (n = 128)
| Variable | Range | M or Frequency | SD or percentage |
|---|---|---|---|
| Gender (female) | 61 | 68% | |
| Multiple birth (% yes) | 26 | 20% | |
| Birthweight (g) | 564–3328 | 1760 | 605 |
| LBW | 70 | 55% | |
| VLBW | 23 | 18% | |
| ELBW | 22 | 17% | |
| Appropriateness of weight | |||
| LGA | 6 | 5% | |
| AGA | 106 | 83% | |
| SGA | 16 | 12% | |
| Gestational age (weeks) | 23.71–37.14 | 31.53 | 3.20 |
| >36 | 5 | 4% | |
| <36–34 | 36 | 28% | |
| <34–30 | 50 | 39% | |
| <30–26 | 28 | 22% | |
| <26–23 | 9 | 7% | |
| Apgar 1 min | 1–9 | 5.86 | 2.04 |
| Apgar 5 min | 2–10 | 7.97 | 1.41 |
LBW: low birth weight, < 2500 g; VLBW: very low birth weight, < 1500 g; ELBW: extremely low birth weight, < 1000 g; LGA: large for gestational age; AGA: appropriate for gestational age; SGA: small for gestational age; Infants born preterm are born before 37 weeks gestation and infants considered low birthweight weigh less than 2500 g at birth regardless of their gestational age. Therefore, in this study infants may be only preterm, only low birthweight or both.
From hospital discharge to 4 months postterm (corrected for gestational age), 17 (10.2%) families could not be located or dropped out of the study, and 17 (11.6%) families did not return their sleep logs. An additional two families returned data that was deemed invalid, resulting in a final sample of 128 families. Attrition analyses revealed that families who could not be located or who dropped out of the study reported more sociodemographic risks at hospital discharge (i.e., unemployed, not married or living with a spouse, younger, non-white, and less educated). Missing data analyses revealed that families who did not complete all of the forms were more likely to be unmarried, non-white, received public assistance, completed less education, and tended to be more depressed. Thus, our analyses focus on families who were slightly more advantaged.
Procedure
Multiple methods were used to collect data at the infant's hospital discharge and at 4 months postterm. Infant birthweight and gestational age were collected from hospital records. When infants were 4 months postterm, additional data were collected during home visits. Mothers completed a demographic questionnaire, depression scale, and infant sleep log. Additionally, they participated in an interview and a play session. Mothers were asked to “play with your infant as you would normally at home” and dyads were videotaped during this 15-min interaction. At the end of the visit, mothers were paid $25 for their participation and infants were given a board book. Mothers were instructed to complete the infant sleep log for a minimum of three consecutive 24 h periods and then return the log in a post-paid envelope.
Materials
Sleep Parameters
Infant sleep parameters were generated via maternal report sleep logs. Sleep logs instructed mothers to shade in the times that their infants slept for a minimum of three consecutive 24-h periods (in 30-min intervals). Previous research has established this measure's reliability, with 90% agreement between parental reports and video monitoring and a .70 (p <.01) correlation between infant sleep patterns and infant sleep log reports (Elias, Nicolson, Bora, & Johnson, 1986). Infant sleep log data were used to calculate five sleep indices: number of infant night wakings that required parental assistance from 7 pm to 7 am, nighttime sleep duration, diurnal sleep consolidation (percentage of total sleep that took place at night), number of daytime naps, and daytime sleep duration. Infant nighttime sleep was defined as starting after 7 pm and daytime sleep was defined as starting after 7 am. In the present sample, most infants signaled for their parents once per night at 4 months postterm (M = 0.87; SD = 0.85). Infant nighttime sleep ranged from 368 to 710 minutes (M = 577 min; SD = 74) and diurnal sleep consolidation ranged from 49% to 100% (M = 71%; SD = 0.08). One infant did not nap; therefore, infant daytime sleep ranged from 0 to 430 min (M = 232 min; SD = 79) and naps ranged from 0 to 4.5 (M = 2.75; SD = .83).
Parenting Interactions
The first 5 min of each play interaction was coded using the parenting variables of the Parent Child Early Relational Assessment (PCERA; Clark, 1985). The PCERA is a coding system designed to assess dyads on 65 (29 parent, 28 infant, and 8 dyadic) interaction quality variables. Each variable is coded on a scale ranging from one (negative relational quality) to five (positive relational quality). The variables focus on the frequency, duration, and intensity of affect and behavioral characteristics in an attempt to assess the interactional strengths and limitations of the parent, child, and dyad. Coders completed a PCERA training workshop with Dr Roseanne Clark or a master coder and continued training until 80% inter-coder agreement was achieved. Additionally, 10% of all tapes were coded by two independent coders with 94% within-one agreement across items.
For the present study, the 28 PCERA parent items were subjected to principal components analysis with a promax rotation. Using a .50 cutoff, a three factor solution emerged. We labeled the factors: Anger, Hostility, and Criticism (nine items; M = 30.64; SD = 3.58; α = .82); Sensitivity, Connectedness, and Communication (19 items; M = 67.85; SD = 10.14; α = .94); and Intrusiveness and Unpredictability (four items; M = 16.37; SD = 2.69; α = .84). Three items loaded on both the hostility and sensitivity factors (i.e., positive affect, depressed mood, enthusiasm) and two items loaded on both the sensitivity and intrusiveness factors (i.e., sensitivity, consistency). Correlations between factors ranged from 0.40 to 0.64; however, substantive analysis revealed distinct parenting behaviors across factors. Higher scores reflected more positive parenting.
Feeding Route
At 4 months postterm, mothers completed a brief interview focusing on their infant's sleep routines and nighttime feeding route. Within this sample, 24 (19%) infants were breastfed and 105 infants were bottlefed at night. The breast feeding rates in this subsample are slightly lower than national estimates across all infants. According to the Center for Disease Control National Immunization Survey (CDC NIS) infants born between 2002 and 2004 (the data collection period of this study) 24–49% were exclusively or partially breast fed at 4 months of age (CDC NIS, 2008). However, the CDC NIS does not break down breast feeding rates by gestational age. Thus, given the high-risk nature of this sample, a lower breast feeding rate was expected. For example, Colaizy and Morriss’ (2008) study reported breast feeding at 4 weeks in only 47–50% of NICU-admitted infants which is considerably lower than national estimates (62–67%) of breast feeding at 1 month (CDC NIS, 2008). Therefore, the breast feeding rate in this study likely reflects the high-risk nature of this sample. For all analyses, breast feeding was coded (1) and bottle feeding (0).
Infant Prematurity
Infant medical records were reviewed to collect infant prematurity data. Because infant birthweight and gestational age were highly correlated (r = 0.88, p <.01), we standardized and summed them to generate an index of infant prematurity. Our infant prematurity index ranged from −4.39 to 3.90 with an average of .12 (SD = 1.98) with higher scores representing more prematurity. Within this study infant prematurity is used as a proxy for medical risks and complications. Infants born earlier and-or who weigh less generally have more medical complications and longer NICU stays (Kilbride, Thorstad, & Daily, 2004; Vergara & Bigsby, 2004).
Maternal Depressive Symptoms
Maternal depressive symptoms at 4 months postterm were assessed using the Center for Epidemiological Studies—Depression Scale (CESD; Radloff, 1977). This scale contains 20 statements which represent depressive symptoms across seven main areas including sleep disturbance, appetite, psychomotor retardation, hopelessness, helplessness, guilt, and depressed mood. Previous studies have established reasonable internal consistency, test-retest reliability and concurrent validity in the CESD (Katz, Shaw, Vallis, & Kaiser, 1995; Radloff, 1977). In the present study, CESD scores ranged from 0 to 28, with a mean of 8.43 (SD = 6.83). Cronbach's alpha was .85. Twenty-three (18%) mothers scored 16 or higher, indicating an increased likelihood of clinical depression.
Maternal Sociodemographic Risks
Mothers completed a demographic questionnaire when their infants were 4 months postterm that included information about maternal age, maternal education, and family income. Because maternal age, education and income were highly correlated, these variables were standardized and summed to generate a sociodemographic risk index. Scores were normally distributed and ranged from −5.78 to 6.53 (M = 0.04; SD = 2.43) with a Cronbach's alpha of .74. Lower scores reflected more sociodemographic risk.
Results
Model Specification
The transactional model of infant sleep development was assessed with Mplus version 5 for each sleep outcome and PCERA parenting factor. Each model was specified, indentified, and tested for assumption violations prior to model and path estimation and interpretation.
Model Identification
Before completing analyses, model identification steps were performed to confirm that each model was overidentified. Within our specified models, there were five exogenous variables (i.e., maternal depression, infant prematurity, feeding route, parenting quality, infant sleep parameter) and one endogenous variable (i.e., family sociodemographic factors) for a total of six observed variables (k). When this number was plugged into the “counting rule” equation (k*(k + 1)/2), it was apparent that this model had 21 known elements (Kaplan, 2008). Each specified model had a total of 15 estimates, and with 21 known elements, our model was left with six degrees of freedom. Models with more than zero degrees of freedom are considered overidentified; therefore, our model was overidentified.
Data Screening
The specified parameters were estimated using a Maximum Likelihood (ML) estimation procedure within Mplus. To confirm that the assumptions of ML were not violated, each variable was assessed for multicollinearity and multivariate normality using SPSS version 15. All variables had acceptable intercorrelations. One variable, night waking, did not follow a normal distribution. To account for the potential bias, each model containing night waking was run using Maximum Likelihood Robust (MLR) procedures. MLR is an estimation procedure that is not as sensitive to violations of non-normality.
Model and Parameter Estimation
To assess the overall model fit, three indices were assessed, including: chi-square (χ2), root mean square error of approximation (RMSEA), and the comparative fit index (CFI). The χ2 index is a model of misspecification; therefore, a significant χ2 means the model does not fit the sample data. Because some scholars claim that the exact fit tested in χ2 is an unrealistic standard, indices of approximate fit like RMSEA were also assessed (Kaplan, 2008). RMSEA tests whether the model fits the population approximately. In RMSEA, .00 is the best possible fit, with higher values indicating poorer fit. Acceptable fit within the RMSEA index is generally .05 or lower (Browne & Cudeck, 1992), although this cutoff is debated within the field. Within this study, the CFI compares the specified model to a null model. The null model posits that there are no relationships between the variables. CFI ranges from 0 to 1, with higher values indicating better fit. CFI values above .90 are generally interpreted as acceptable model fit (Bagozzi & Yi, 1988). Each interpreted model had acceptable model fit across the χ2, RMSEA and CFI fit indices. For the interpreted models, χ2 ranged from 1.18 to 9.16 (p > .05), RMSEA ranged from 0.00 to 0.06, and CFI ranged from 1.00 to 0.94. Two models focusing on the associations between napping and parenting hostility (χ2 = 12.80, p < .05; RMSEA = 0.09; CFI = 0.79) and intrusiveness (χ2 = 11.80, p < .05; RMSEA = 0.09; CFI = 0.84) did not have acceptable fit and were not interpreted.
Evaluation of the parameter estimates within the specified models focusing on the association between distal and proximal variables indicated consistent relations between the family sociodemographic index and maternal depressive symptoms, infant prematurity, infant feeding route, and parenting interactions.
Parenting Interactions
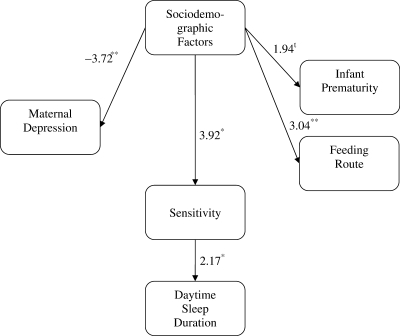
Four-month infant sleep–wake parameters were associated with maternal sensitivity (Table II), maternal anger/hostility (Table III) and infant feeding route. For the daytime sleep parameters, maternal sensitivity predicted the number of naps and amount of sleep infants completed during the day (Figure 2). Infants who experienced play interactions marked by more sensitivity, connectedness, and communication took more naps and slept more during the day. However, maternal intrusiveness and anger/hostility were not associated with infant daytime sleep parameters (i.e., number of naps and amount of daytime sleep).
Table II.
PCERA Parenting Sensitivity, Connectedness, and Communication Factor: Test Statistics for Path Parameters (n = 128)
| Sleep parameter |
|||||
|---|---|---|---|---|---|
| Night-waking | Night-time sleep | Diurnal sleep consolidation | Nap | Daytime sleep | |
| Sleep parameter on | |||||
| Sensitivity | −0.05 | 0.05 | −2.07* | 2.68** | 2.17* |
| Feeding route | 3.26** | −1.92t | |||
| Sensitivity on | |||||
| Maternal depression | −0.44 | −0.47 | −0.47 | −0.47 | −0.47 |
| Infant prematurity | −0.09 | −0.10 | −0.10 | −0.10 | −0.10 |
| Feeding route | 0.62 | 0.57 | 0.57 | 0.57 | 0.57 |
| SES | 3.64** | 3.92** | 3.92** | 3.92** | 3.92** |
| Maternal depression on SES | −3.33** | −3.72** | −3.72** | −3.72** | −3.72** |
| Infant prematurity on SES | 2.01* | 1.94t | 1.94t | 1.94t | 1.94t |
| Feeding route on SES | 3.34** | 3.04** | 3.04** | 3.04** | 3.04** |
| Infant prematurity and feeding route | 1.48 | 1.39 | 1.39 | 1.39 | 1.39 |
*p < .05, **p < .01, tp < .10.
Table III.
PCERA Parenting Anger, Hostility, and Criticism Factor: Test Statistics for Path Parameters (n = 128).
| Sleep parameter |
|||||
|---|---|---|---|---|---|
| Night-waking | Night-time sleep | Diurnal sleep consolidation | Nap | Daytime sleep | |
| Sleep parameter on | |||||
| Anger | −3.13** | −0.32 | 0.02 | 0.12 | 0.21 |
| Feeding route | 3.56** | −1.87t | −0.31 | – | – |
| Anger on | |||||
| Maternal depression | −0.34 | −0.38 | −0.38 | −0.38 | −0.38 |
| Infant prematurity | 0.42 | 0.41 | 0.41 | 0.41 | 0.41 |
| Feeding route | 1.06 | 1.02 | 1.02 | 1.02 | 1.02 |
| SES | 1.71t | 1.61 | 1.61 | 1.61 | 1.61 |
| Maternal depression on SES | −3.33** | −3.72** | −3.72** | −3.72** | −3.72** |
| Infant prematurity on SES | 2.10** | 1.94t | 1.94t | 1.94t | 1.94t |
| Feeding route on SES | 3.44** | 3.04** | 3.04** | 3.04** | 3.04** |
| Infant prematurity and feeding route | 1.48 | 1.39 | 1.39 | 1.39 | 1.39 |
*p < .05, **p < .01, tp < .10.
Figure 2.
Significant paths test statistics for daytime sleep duration and PCERA parenting factor sensitivity, connectedness, and communication. Model fit indices: X2 (df = 5) = 1.88, p > .05, RMSEA = .00, CFI = 1.00. *p < .05, **p < .01, tp < .10.
For night waking, mothers who engaged in more angry, hostile, and critical interactions during play at 4 months had infants who woke more at night (Table III, Figure 3). However, maternal sensitivity and intrusiveness were not associated with night waking or nighttime sleep duration.
Figure 3.
Significant paths test statistics for nighttime sleep and PCERA parenting factor anger, hostility and criticism. Model fit indices: X2 (df = 5) = 3.30, p > .05, RMSEA = .00, CFI = 1.00. *p < .05, **p < .01, tp < .10.
Diurnal sleep consolidation (i.e., the proportion of sleep that occurs at night) was associated with maternal sensitivity (Table II). When mothers engaged in more sensitive play interactions, infants experienced less diurnal sleep consolidation (i.e., the infants’ sleep was distributed so that more sleep took place during the day and less at night). Parental intrusiveness and hostility were not associated with diurnal sleep consolidation.
Feeding Route
For the nighttime sleep parameters of nightwaking and amount of nighttime sleep, infant feeding route was the most robust predictor. Infants who were bottlefed at night at 4 months (n = 105) woke less at night and tended to sleep more at night than infants who were breastfed at night (n = 24).
Individual and Contextual Factors
Families experiencing more sociodemographic risks (lower maternal education, younger maternal age, and less family income) had infants with lower birthweights and younger gestational ages and their infants were less likely to breastfeed at 4 months postterm. In addition, more family sociodemographic risks were associated with higher levels of maternal depressive symptoms and less sensitive and more intrusive parenting. Contrary to our expectations, however, the analyses did not reveal significant associations between parenting interactions and maternal depression, infant prematurity, or feeding route.
Although the total effects of each of the models interpreted were statistically significant, none of the models had statistically significant indirect effects. For example, in the nighttime sleep model, our results supported a direct association between SES and parenting sensitivity (Total Effect TS = 4.61, p < .01) but did not support our mediator hypotheses (Indirect Effects TS = 0.63, p = .53).
Discussion
Three key findings emerged, revealing partial support for the transactional model. First, parenting interactions were directly associated with daytime sleep (i.e., number of naps and amount of daytime sleep) and nighttime sleep (i.e., diurnal sleep consolidation). Second, infants who were bottlefed at night experienced fewer night wakings and more nighttime sleep compared to infants who were breastfed at night. Finally, family sociodemographic risks were associated with individual risks and less optimal parenting interactions. Taken together, these findings suggest that PT LBW infants’ early sleep–wake regulation patterns may develop through two contrasting routes: parenting interactions and feeding method. One path leads from less optimal parenting interactions to more night wakings but less daytime sleep and less diurnal sleep consolidation, whereas the other path leads from bottlefeeding to more nighttime sleep and fewer night wakings. We discuss these paths and their potential implications below.
Parenting Interactions
Consistent with the transactional model of sleep–wake regulation (Goodlin-Jones et al., 2000), mothers who engaged in more angry, hostile, and critical interactions with their PT LBW infants reported more infant night wakings. From an attachment perspective, angry or hostile parenting interactions may interfere with a child's emerging ability to use the parent as a secure base and safe haven (Bowlby, 1982; Ainsworth, Blehar, Water, & Wall, 1978). Certain stimuli may activate the child's fear system (e.g., waking in the dark) and increase the child's attachment behaviors, such as signaling to regain proximity to the attachment figure. Infants may signal when they rouse in the night rather than self sooth as the result of their anxiety.
Another potential mechanism that explains the association between angry parenting interactions and night waking is that caregivers who experience more disrupted sleep may feel more irritable and fatigued during the day. This irritability may be reflected in their play interactions, particularly with vulnerable infants who may be difficult to engage (e.g., Vergara & Bigsby, 2004). Our use of parental-report sleep logs support this interpretation because mothers had to notice the infant's nighttime signaling in order to record the waking episodes. Similarly, PT LBW infants who woke more at night may have been more difficult interactional partners during the day because they were tired from signaling at night, leading to increased maternal anger and criticism. Likely the relation is bidirectional, as suggested by transactional models of development. These findings suggest that clinicians could explore screening for angry parenting interactions in follow up assessments of PT LBW infants, especially if parents report a high number of infant night wakings. In addition, future research should explore interventions that link infant night time sleep with day time dyadic interactions in high-risk infants.
We also found that PT LBW infants who experienced play interactions characterized by more sensitivity, better communication, and more connectedness took more naps and slept more during the day, and their overall sleep patterns were distributed so that relatively less sleep took place at night and more during the day. At first glance, this finding may seem counterintuitive because more diurnal consolidation is often seen as “better sleep” (i.e., linked to the milestone of sleeping through the night). Although circadian periodicity becomes evident at about 3 months in full-terms (Goodlin-Jones et al., 2000), it is possible that PT LBW infants may need more daytime sleep at 4 months postterm in order to accommodate the level of stimulation provided by play interactions. Perhaps high-risk infants who nap more during the day are more relaxed and easier to engage because their daytime sleep helps them become more organized. Mothers who engage in sensitive interactions may be more likely to read their infants’ cues and provide breaks when they are overstimulated. Alternatively, mothers whose infants nap more during the day may feel more rested and able to sustain their levels of sensitivity and positive interactions with their infants during play. Again, the relation is likely bidirectional. This is the first study to apply a transactional model to sleep in PT LBW infants and lays the ground work for future interventions that may promote infant daytime naps to assist with parenting this vulnerable population. However, additional studies examining associations between daytime sleep and PT LBW infant development are needed.
Although parenting interactions related to sleep parameters in our study, they did not mediate the relation between maternal depressive symptoms or infant prematurity and sleep outcomes, as the indirect paths in the model were not statistically significant.
Feeding Route
The second path to sleep that we identified in PT LBW infants involved associations between bottlefeeding and more nighttime sleep and fewer night wakings, as recorded by parental sleep logs. We speculate that this path reflects infant hunger–satiety cycles and that breastfed infants signal more often at night to meet their hunger needs. We also found that nighttime feeding route was not associated with quality of daytime parenting, suggesting that the hunger–satiety cycle is a different route to sleep–wake regulation in PT LBW infants.
Although numerous studies have found links between feeding route and night waking (Messer & Richards, 1993), little support has emerged for an association between breast feeding and infant sleep problems. In addition, a recent study by Doan and associates (Doan, Gardiner, Gay, & Lee, 2007) found that parents of healthy 3-month old infants slept more at night than parents of formula fed or combination fed infants. For high-risk infants, the degree of infant prematurity and the nature of the child's neonatal medical course are often associated with how likely it is for infants to breastfeed successfully (Riordan, 2005). Infants born PT LBW must overcome metabolic and physiologic barriers to successfully breastfeed. In addition, lower SES mothers are more likely to bottlefeed (Heck et al., 2006). Our findings linking more SES risks with elevated maternal depressive symptoms, more infant prematurity, and increased likelihood of bottlefeeding support previous findings in the literature. Although our sample of bottlefed PT LBW infants slept more at night at 4 months postterm, it is unlikely that more nighttime sleep would protect them from these contextual risks. Consistent with transactional developmental theory (Sameroff, 2006; Sameroff & Fiese, 2000) and empirical findings (e.g., Liaw & Brooks-Gunn, 1994) the accumulation of risks would more likely predict their developmental outcomes.
Because of these factors, it is possible that more nighttime sleep in and of itself at 4 months postterm is not always optimal for PT LBW infants. For clinical and research assessments, sleep should be understood in the context of individual, dyadic, and family factors over time, consistent with transactional models (Goodlin-Jones et al., 2000; Sameroff & Fiese, 2000). As we consider possible clinical applications of these findings, it is important to note that our data do not suggest that bottle feeding in relation to more night time sleep is a preferable route in PT LBW infants, as this route was associated with multiple contextual risk factors.
Individual and Contextual Factors
Consistent associations between more family sociodemographic risks and elevated maternal depressive symptoms, more infant prematurity, infant bottle feeding, and parenting quality emerged across our models. Higher levels of SES risks, as indexed by less maternal education, younger maternal age, and lower family income, were associated with less optimal infant and maternal characteristics and less sensitive and more intrusive parenting interactions. Previous research has found that SES factors such as maternal income and age are associated with preterm birth (e.g., Zarling, Hirsch, & Landry, 1988), bottlefeeding (Heck et al., 2006), and maternal depression (Belle, 1990). In addition, poverty places children at risk for preterm birth and less optimal cognitive and social outcomes (e.g., Duncan, Brooks-Gunn, & Klebanov, 1994), setting a risky stage for development in fragile PT LBW infants (e.g., Liaw & Brooks-Gunn, 1994). Consistent with the transactional model, family SES factors are key contextual variables in infant development. Implications for these findings are twofold. Clinicians working with families raising PT LBW infants need to consider the larger family and social context, rather than focusing solely on the child's sleep patterns or level of prematurity. Second, future research should continue to incorporate family contextual variables, as they have wide ranging effects (National Research Council and Institute of Medicine, 2000), especially for vulnerable infants.
We also found that infant prematurity was not associated with infant sleep parameters. This finding may reflect the restricted range of prematurity in this sample. Previous studies reporting an association between prematurity and sleep have compared full-term and preterm infants (e.g., Scher, Steppe, Dahl, Asthana, & Guthrie, 1992). However, our finding is consistent with the results of Anders and Keener (1985) and others, suggesting that sleep variables in preterms may be similar to full-terms of the same postterm age. Similarly, Wolke and associates (1995) reported gestational age was not a predictor of night waking in preterm infants.
We also did not find an association between maternal depressive symptoms and parenting interactions. Although there is empirical evidence linking depression and less optimal parenting, a growing number of studies have found that the duration and severity of depressive symptoms are more important than the presence of elevated symptoms at one point in time (e.g., Campbell & Cohn, 1997; NICHD ECRN, 1999). Additionally, the relation between depressive symptoms and children's sleep patterns may be bidirectional, wherein early infant sleep disruptions may result in maternal fatigue and increased vulnerability for psychological distress or depression, which in turn may negatively affect children's sleep habits and other developmental processes. We are currently examining these possibilities in our longitudinal study of high-risk infants.
Limitations
Our study has several limitations, including the use of parental report sleep logs rather than actigraph or videosomnography and the lack of longitudinal sleep and parent–child interaction data. Although it is important to document whether infants signal upon waking when attempting to understand the role of parenting interactions in the coregulation of sleep and the emergence of children's sleep problems, other methods of sleep assessment may be more objective (Beecroft et al., 2008; Werner, Luciano, Guyer, & Oskar, 2008). For example, the use of actigraphy could provide more accurate information on nighttime arousals or videosomnography could provide more detailed information on parental actions during night wakings. Moreover, it is critical to study these phenomena over time to document bidirectional processes, especially when employing a transactional model.
The breast feeding rate in our study (19%) is somewhat lower than national estimates (24–49%); therefore, findings regarding feeding route and nighttime sleep should be considered exploratory (CDC NIS, 2008). We suspect that the lower rate of breast feeding in this sample reflects the high-risk nature of PT LBW infants; alternatively, it is possible that our sample included a subset of mothers who were less likely to breast feed. Information on sleep in breast fed PT LBW infants is extremely limited; therefore, disseminating this information is important but it should be interpreted with the appropriate caution.
Although our attrition rate between NICU discharge and 4 months was relatively low for a study of high-risk infants, attrition and missing data were more likely to occur when families were stressed by sociodemographic risks. Although this limits the generalizability of our results, it may have led to an underestimate of the effects of sociodemographic risks and indirect paths to sleep in our study. These factors should be taken into account when interpreting our findings.
Implications and Summary
The results of our study have implications for pediatric psychologists and programs that attempt to support parents and their PT LBW infants, particularly in the area of sleep development. Consistent with transactional models, our findings suggest that practitioners should attend not only to specific sleep parameters (e.g., night waking), but also to the family context of sleep and the sleep needs of individual infants. For example, some PT LBW infants may need more daytime naps to accommodate the level of stimulation that they receive and thus, it may be unrealistic to expect a fragile preterm infant to sleep through the night at 4 months postterm. Finally, interventions that focus on increasing parental sensitivity and decreasing anger or hostility during interactions (e.g., Berlin, Ziv, Amaya-Jackson, & Greenberg, 2005) should be examined as a means of supporting parents and emerging sleep–wake regulation in infants born PT LBW. Our results also highlight the need for longitudinal studies of sleep regulation in PT LBW infants, particularly in relation to the family context.
Acknowledgements
This research was supported by grants from the National Institutes of Health (HD044163 and HD07489) and the University of Wisconsin. Special thanks to the children and families who generously gave of their time to participate in the study.
References
- Ainsworth M, Blehar M, Water E, Wall S. Patterns of attachment: A psychological study of the strange situation. New York: Academic Press; 1978. [Google Scholar]
- Anders TF. Infant sleep, nighttime relationships, and attachment. Psychiatry: Interpersonal and Biological Processes. 1994;57:11. doi: 10.1080/00332747.1994.11024664. [DOI] [PubMed] [Google Scholar]
- Anders TF, Keener MA. Developmental course of nighttime sleep-wake patterns in full-term and premature infants during the first year of life: I. Sleep: Journal of Sleep Research & Sleep Medicine. 1985;8:173. doi: 10.1093/sleep/8.3.173. [DOI] [PubMed] [Google Scholar]
- Bagozzi R, Yi Y. On the evaluation of structural equation models. Journal of the Academy of Marketing Science. 1988;16:74–94. [Google Scholar]
- Barnard KE, Bee HL, Hammond MA. Developmental changes in maternal interactions with term and preterm infants. Infant Behavior & Development. 1984;7:101–113. [Google Scholar]
- Bayer J, Hicock H, Hampton A, Wake M. Sleep problems in young infants and maternal mental and physical health. Journal of Paediatrics and Child Health. 2007;43:66–73. doi: 10.1111/j.1440-1754.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- Beecroft JM, Ward M, Younes M, Crombach S, Smith O, Hanly PJ. Sleep monitoring in the intensive care unit: Comparison of nurse assessment, actigraphy and polysomnography. Intensive Care Medicine. 2008;34:2076–2083. doi: 10.1007/s00134-008-1180-y. [DOI] [PubMed] [Google Scholar]
- Belle D. Poverty and women's mental health. American Psychologist. 1990;45:385–387. [Google Scholar]
- Benoit D, Zeanah CH, Boucher C, Minde KK. Sleep disorders in early childhood: Association with insecure maternal attachment. Journal of the American Academy of Child & Adolescent Psychiatry. 1992;31:86–93. doi: 10.1097/00004583-199201000-00013. [DOI] [PubMed] [Google Scholar]
- Berlin LJ, Ziv Y, Amaya-Jackson L, Greenberg MT. Enhancing early attachments: Theory, research, intervention, and policy. New York, NY: Guildford; 2005. [Google Scholar]
- Bowlby J. Attachment and loss: Attachment. 2nd. Institute of Psycho-Analysis: London; 1982. [Google Scholar]
- Browne M, Cudeck R. Alternative ways of assessing model fit. Sociological Methods and Research. 1992;21:230–258. [Google Scholar]
- Campbell S, Cohn J. The timing and chronicity of postpartum depression: Implications for infant development. In: Murray L, Cooper P, editors. Postpartum depression and child development. New York, NY: Guilford Press; 1997. pp. 165–197. [Google Scholar]
- CDC NIS. Breastfeeding among US children born 1999—2005, CDC national immunization survey. 2008 Retrieved September 23, 2008 from http://www.cdc.gov/breastfeeding/data/NIS_data/index.htm.
- Clark R. The parent-child early relational assessment. University of Wisconsin Medical School, Madison, WI: Department of Psychiatry; 1985. Instrument and Manual. [Google Scholar]
- Colaizy T, Morriss F. Positive effect of NICU admission on breastfeeding of preterm US infants in 2000 to 2003. Journal of Perinatology. 2008;28:505–510. doi: 10.1038/jp.2008.32. [DOI] [PubMed] [Google Scholar]
- Davis K, Parker K, Montgomery G. Sleep in infants and young children: Part one: Normal sleep. Journal of Pediatric Health Care. 2004;18:65–71. doi: 10.1016/s0891-5245(03)00149-4. [DOI] [PubMed] [Google Scholar]
- DeLeon C, Hildebrandt Karraker K. Intrinsic and extrinsic factors associated with night waking in 9-month-old infants. Infant Behavior & Development. 2007;30:596–605. doi: 10.1016/j.infbeh.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Dennis C, Ross L. Relationships among infant sleep patterns, maternal fatigue, and developmental depressive symptomatology. Birth. 2005;32:187–193. doi: 10.1111/j.0730-7659.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- Doan T, Gardiner A, Gay C, Lee K. Breast-feeding increases sleep duration of new parents. Journal of Perinatal and Neonatal Nursing. 2007;21:200–206. doi: 10.1097/01.JPN.0000285809.36398.1b. [DOI] [PubMed] [Google Scholar]
- Duncan G, Brooks-Gunn J, Klebanov P. Economic deprivation and early childhood development. Child Development. 1994;65:296–318. [PubMed] [Google Scholar]
- Edhborg M, Lundh W, Seimyr L, Widström A. The parent-child relationship in the context of maternal depressive mood. Archives of Women's Mental Health. 2003;6:211–216. doi: 10.1007/s00737-003-0020-x. [DOI] [PubMed] [Google Scholar]
- Elias M, Nicolson N, Bora C, Johnson J. Sleep/wake patterns of breast-fed infants in the first two years of life. Pediatrics. 1986;77:322–329. [PubMed] [Google Scholar]
- Evenhouse E, Reilly S. Improved estimates of the benefits of breastfeeding using sibling comparisons to reduce selection bias. Health Services Research. 2005;40:1781–1802. doi: 10.1111/j.1475-6773.2004.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. From biological rhythms to social rhythms: physiological precursors of mother-infant synchrony. Developmental Psychology. 2006;42:173–188. doi: 10.1037/0012-1649.42.1.175. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Hernansez-Reif M. Prenatal depression effects on the foetus and neonate in different ethnic and socio-economic status groups. Journal of Reproductive and Infant Psychology. 2002;20:149–157. [Google Scholar]
- Goodlin-Jones BL, Burnham MM, Anders TF. Sleep and sleep disturbances: Regulatory processes in infancy. In: Sameroff AJ, Lewis M, editors. Handbook of developmental psychopathology. 2nd. NewYork, NY: Kluwer; 2000. p. 309. [Google Scholar]
- Harnish J, Dodge K, Valente E Conduct Problems Prevention Research Group. Mother-child interaction quality as a partial mediator of the roles of maternal depressive symptomatology and socioeconomic status in the development of child behavior problems. Child Development. 1995;66:739–753. doi: 10.1111/j.1467-8624.1995.tb00902.x. [DOI] [PubMed] [Google Scholar]
- Heck K, Braveman P, Cubbin C, Chávez G, Kiely J. Socioeconomic status and breastfeeding initiation among California mothers. Public Health Reports. 2006;121:51–59. doi: 10.1177/003335490612100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock H, Wake M. Randomized controlled trial of behavioural infant sleep intervention to improve infant sleep and maternal mood. British Medical Journal. 2002;324:1062–1065. doi: 10.1136/bmj.324.7345.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck GN. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. Journal of Pediatric Psychology. 2002;29:87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- Kaplan D. Structural equation modeling: Foundations and extensions. 2nd. Newbury Park, CA: Sage Publications; 2008. [Google Scholar]
- Katz R, Shaw B, Vallis TM, Kaiser AS. The assessment of severity and symptom patterns in Depression. In: Beckham E, Leber W, editors. Handbook of depression. New York, NY: Guilford Press; 1995. pp. 61–85. [Google Scholar]
- Kilbride H, Thorstad K, Daily D. Preschool outcome of less than 801-gram preterm infants compared with full-term siblings. Pediatrics. 2004;113:742–747. doi: 10.1542/peds.113.4.742. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Coy KC, Murray KT. The development of self-regulation in the first four years of life. Child Development. 2001;72:1091. doi: 10.1111/1467-8624.00336. [DOI] [PubMed] [Google Scholar]
- Landry SH. The development of joint attention in premature low birth weight infants: Effects of early medical complications and maternal attention-directing behaviors. In: Moore C, Dunham P, editors. Joint attention: Its origins and role in development. Hillsdale, NJ: Lawrence Erlbaum; 1995. pp. 223–250. [Google Scholar]
- Liaw F, Brooks-Gunn J. Cumulative familial risks and low-birthweight children's cognitive and behavioral development. Journal of Clinical Child Psychology. 1994;23:360–372. [Google Scholar]
- Luo Z, Wilkins R, Kramer M. Effect of neighbourhood income and maternal education on birth outcomes: a population-based study. Canadian Medical Association Journal. 2006;174:1415–1421. doi: 10.1503/cmaj.051096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee MD, Jankowski KRB, Zayas LH. Breastfeeding intention and practice in an urban minority population: Relationship to maternal depressive symptoms and mother-infant closeness. Journal of Reproductive and Infant Psychology. 2004;22:167–181. [Google Scholar]
- McLaughlin-Crabtree V, Korhonen J, Montgomery-Downs H, Jones V, O’Brien L, Gozal D. Cultural influences on the bedtime behaviors of young children. Sleep Medicine. 2005;6:319–324. doi: 10.1016/j.sleep.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Messer D, Richards M. The development of sleeping difficulites. In: James-Robers ISt, Harris G, Messer D., editors. Infant crying, feeding, and sleeping: Development, problems and treatments. New York, NY: Harvester Wheatsheaf; 1993. pp. 150–173. [Google Scholar]
- Mindell J, Owens J. A clinical guide to pediatric sleep: Diagnosis and management of sleep problems. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- National Research Council and Institute of Medicine.Committee on intergrading the science of early childhood development. Board on children, youth, and families, commission on behavioral and social sciences and education. From neuron to neighborhoods: The science of early childhood development. In: Shonkoff J, Phillips D, editors. Washington, DC: National Academy Press; 2000. [Google Scholar]
- NICHD ECRN. Chronicity of maternal depressive symptoms, maternal sensitivity, and child functioning at 36 months. Developmental Psychology. 1999;35:1297–1310. doi: 10.1037//0012-1649.35.5.1297. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Riordan J. Breastfeeding and human lactation. Sudbury, MA: Jones and Bartlett; 2005. [Google Scholar]
- Sameroff AJ. Identifying risk and protective factors for healthy child development. In: Clark-Steward A, Dunn J, editors. Families count: Effects on child and adolescent development. New York, NY: Cambridge University Press; 2006. pp. 53–78. [Google Scholar]
- Sameroff AJ, Fiese BH. Transactional regulation: The developmental ecology of early intervention. In: Shonkoff JP, Meisels SJ, editors. Handbook of early childhood intervention. Oakleigh, Melbourne: Cambridge University Press; 2000. pp. 135–149. [Google Scholar]
- Scher A. Mother-child interaction and sleep regulation in one-year-olds. Infant Mental Health Journal. 2001;22:515. [Google Scholar]
- Scher M, Steppe D, Dahl R, Asthana S, Guthrie R. Comparison of EEG sleep measures in healthy full-term and preterm infants at matched conceptual ages. Sleep. 1992;15:442–448. doi: 10.1093/sleep/15.5.442. [DOI] [PubMed] [Google Scholar]
- Smith K, Landry S, Swank P. The influence of early patterns of positive parenting on children's preschool outcomes. Early Education and Development. 2000;11:147–169. [Google Scholar]
- Thomas K. Differential effects of breast- and formula-feeding on preterm infants sleep-wake patterns. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2000;29:145–152. doi: 10.1111/j.1552-6909.2000.tb02034.x. [DOI] [PubMed] [Google Scholar]
- US Bureau of Census (n.d.) Wisconsin statistics. Retrieved April 10, 2008, from http://www.census.gov/2003-2005.
- Vergara E, Bigsby R. Developmental and Therapeutic Interventions in the NICU. Baltimore, MD: Paul H. Brookes; 2004. [Google Scholar]
- Werner H, Luciano M, Guyer C, Oskar J. Agreement rates between actigraphy, diary, and questionnaire for children's sleep patterns. Archives of Pediatrics & Adolescent Medicine. 2008;162:350–358. doi: 10.1001/archpedi.162.4.350. [DOI] [PubMed] [Google Scholar]
- Wolke D, Meyer R, Ohrt B, Riegel K. The incidence of sleeping problems in preterm and fullterm infants discharged from neonatal special care units: An epidemiological longitudinal study. Journal of Child Psychology and Psychiatry. 1995;36:203. doi: 10.1111/j.1469-7610.1995.tb01821.x. [DOI] [PubMed] [Google Scholar]
- Zarling C, Hirsch B, Landry S. Maternal social networks and mother-infant interactions in full-term and very low birthweight, preterm infants. Child Development. 1988;59:178–185. doi: 10.1111/j.1467-8624.1988.tb03205.x. [DOI] [PubMed] [Google Scholar]