Abstract
Objective Collaboration between youths with type 1 diabetes (T1D) and their adult caregivers may be central to effective management of T1D. This article includes analysis of cross-sectional associations between T1D outcomes (adherence, glycemic control, quality of life, family conflict, depression, and self-efficacy) and scores on the Collaborative Parent Involvement (CPI) Scale obtained from 309 youths with T1D about their primary and secondary caregivers. Methods MANCOVA, controlling for age, evaluated associations of diabetes outcomes with youths’ CPI scores for each caregiver. Results Diabetes outcomes were poor when both caregivers obtained CPI scores below the median. Diabetes outcomes were more strongly associated with CPI scores of primary, rather than secondary, caregivers. CPI scores at or above the median among primary caregivers were associated with more favorable status on multiple youth outcomes. When both caregivers obtained CPI scores at or above the median, children had significantly lower HbA1C and parents retained more responsibility for diabetes care. Conclusions Higher collaborative involvement, particularly among primary caregivers, was associated with favorable status along a variety of diabetes outcomes. Longitudinal studies could confirm if youth–parent collaboration is a justifiable intervention target.
Keywords: adherence, diabetes, metabolic control, parent involvement, responsibility, social support
Treatment of pediatric type 1 diabetes mellitus (T1D) requires several daily insulin injections or use of an insulin pump, daily blood glucose checks, regulating carbohydrate intake, regular exercise, and correction of abnormally high (hyperglycemia) and low (hypoglycemia) blood glucose (American Diabetes Association, 2008). Effective treatment requires parent–child teamwork, family communication, and problem solving (Anderson, Ho, Brackett, Finkelstein, & Laffel, 1999; Laffel et al., 2003; Wysocki, Buckloh, Lochrie, & Antal, 2005).
Much research has focused on the role of caregiver involvement in managing T1D and on the transfer of diabetes responsibilities from parents to children. Ingersoll, Orr, Herrold, and Golden (1985) found that parental transfer of responsibility for insulin adjustments to adolescents was influenced more by youths' age than by their cognitive maturity or willingness to assume responsibility. Anderson, Auslander, Jung, Miller, and Santiago (1990) showed that youths' responsibility for T1D tasks increases with the child's age and that parent–child disagreement about those responsibilities predicted poorer glycemic control. Wysocki et al. (1996) showed that youths with inordinate T1D self-care autonomy relative to their psychological maturity had poorer adherence and more hospitalizations compared with youths with more appropriate autonomy. Anderson et al. (1999) reported that more parental involvement in blood glucose monitoring was associated with more frequent blood glucose checks by youths and better glycemic control. Several recent studies (Ellis et al., 2008; Helgeson et al., 2008; Wiebe et al., 2005) have further confirmed that maintenance of parental involvement in T1D care was associated with more favorable psychological adjustment, treatment adherence, and glycemic control. Thus, maintenance of parental involvement has been associated consistently with more effective family management of pediatric diabetes. Most recently, Nansel & Rovner, et al. (2008) validated the Collaborative Parent Involvement Scale (CPI) for youths with T1D to measure youths' perceptions of the degree of collaborative involvement in diabetes management by a specified caregiver. CPI is defined by the present authors as the extent to which the child with T1D perceives the caregiver as providing the amount and type of support needed for diabetes management. CPI reflects parent responsiveness to the child's changing needs, which may assist the youth with problem solving, encourage safe and appropriate autonomy in diabetes management, promote emotional adjustment to diabetes, and facilitate the youth's diabetes management in school, sports, and similar settings. No studies have examined this construct in families in which two adult caregivers contribute to the youth's T1D care.
Research on families of youths without chronic medical conditions reveals beneficial effects on child development, adjustment, and health when two adult caregivers are actively involved in parenting activities (Booth & Croutier, 1998; Lamb, 1997). Studies have shown that more paternal involvement in parenting is associated with more favorable outcomes in terms of academic performance (Winquist-Nord, 1998), peer relations and psychological adjustment (Amato, 1994; Phares & Compas, 1992), prevention of substance abuse (Phares, 1998), and outcomes of behavioral parent training programs (Webster-Stratton, 1985).
There is evidence that involvement of two parents in the management of pediatric chronic medical conditions may also be associated with favorable outcomes. Youths from two-parent families have better T1D outcomes than those from single-parent homes (Auslander, Anderson, Bubb, & Jung, 1990; Hanson, Henggeler, Rodrigue, Burghen, & Murphy, 1988; Harris, Greco, Wysocki, Elder, & White, 1999; Thompson, Auslander, & White, 2001). Also, more involvement of fathers in the management of pediatric chronic diseases enhances maternal, marital, and family functioning (Gavin & Wysocki, 2006) and teens' treatment adherence and quality of life (Wysocki & Gavin, 2006). If there are two adult caregivers involved in the youths' management of T1D, there are several possible patterns of their involvement. These patterns are high collaborative involvement of both caregivers, low collaborative involvement of both caregivers, or a combination of high collaborative involvement of one caregiver and low involvement of the other. Previous research assessed youths’ perceptions either of a single caregiver or of multiple caregivers combined. No studies have examined this construct separately for each of two adult caregivers who contribute to the youth's T1D care.
Wallander, Varni, Babanis, Banis, and Wilcox (1989) proposed a risk and resistance model of chronic illness adaptation that has received extensive empirical support in pediatric psychology research. This model suggests that adaptation to the stress associated with a chronic medical condition (e.g., functional disability, treatment burden) is a function of risk factors (e.g., prior psychological adjustment, the magnitude of experienced stress) and resistance factors (e.g., coping skills and resources). According to this model, individuals who enjoy more coping resources are likely to have available a broader repertoire of coping strategies and hence be more likely to experience favorable adjustment to a chronic medical condition. Collaborative involvement of caregivers can be viewed as a coping resource as described by Wallander et al. (1989). More collaborative involvement could improve youths’ T1D outcomes by enabling more effective monitoring of the child's self-care (Ellis et al., 2008), more parental promotion of youths’ diabetes problem solving (Wysocki et al., 2008), clearer accountability for T1D tasks among family members (Anderson et al., 1990), and more frequent or timely emotional support to the child during stress (Hanson, Henggeler, & Burghen, 1987). Presumably, collaborative involvement of two caregivers would only magnify these benefits. This article includes evaluation of associations between several diabetes outcomes and the youth's perceived degree of collaborative involvement of each of two caregivers. The primary purpose was to evaluate whether greater collaborative involvement of two caregivers is associated with more favorable T1D outcomes compared with family circumstances in which either one or both caregivers demonstrate lower levels of collaborative involvement with the youth around T1D management.
Hypotheses
We hypothesized that multivariate analysis of covariance (MANCOVA), with youth age controlled, would reveal that youths’ scores on the CPI (Nansel & Weissberg-Benchell, et al., 2008) would be related to youths' diabetes outcomes (glycemic control, treatment adherence, diabetes and general quality of life, family conflict, youth depressive symptoms, fear of hypoglycemia, and family sharing of diabetes responsibilities) as follows:
Hypothesis 1: Significant multivariate main effects will be obtained for level of CPI scores for both primary and secondary caregivers. Youths whose primary or secondary caregivers' CPI scores greater than or equal to the median will have more favorable diabetes outcomes, compared to scores less than the median value.
Hypothesis 2: A significant multivariate interaction will be found between CPI scores for primary and secondary caregivers. CPI scores for a given caregiver group will have differing effects on T1D outcomes at differing levels of the other caregivers’ CPI scores. Specifically, we examined whether higher CPI scores for secondary caregivers were associated with more favorable diabetes outcomes when the primary caregivers’ CPI scores were high rather than low.
Hypothesis 3: Pairwise comparisons will show that families in which CPI scores for both caregivers being greater than or equal to the median will have significantly better outcomes than the other groups, whereas the converse will occur for families in which both caregivers CPI scores are less than the median. These comparisons permitted evaluation of the possibilities that low collaborative involvement in both caregivers was associated with poorer diabetes outcomes and that high collaborative involvement of both caregivers was associated with favorable status on those same outcomes.
Hypothesis 4: Pairwise comparisons will show that CPI scores for primary caregivers will be more strongly associated with youths' diabetes outcomes than secondary caregivers' scores. When primary caregivers' CPI scores are high, secondary caregiver's CPI scores will not significantly enhance diabetes outcomes. These pairwise comparisons were planned to evaluate the possibility that greater collaborative involvement of the primary caregiver was associated with favorable diabetes outcomes regardless of the secondary caregiver's level of collaborative involvement.
Methods
Participants
Participants had enrolled in a trial of a clinic-integrated, family-focused behavioral intervention. The objective was to enroll a representative sample of families of youths with T1D between the ages of 9 and 14.5 years. All data reported here were collected at the baseline assessment of families prior to randomization to groups. The trial is in progress but data collection is not yet complete. Families were recruited at four pediatric diabetes centers in the Northeastern, Southeastern, Midwestern, and Southwestern United States. Parents/caregivers signed IRB-approved informed consent forms, and youths signed assent forms before any study data were collected from them. Management of T1D at all sites included approximately quarterly clinic visits with a pediatric endocrinologist and diabetes nurse, thorough diabetes education to promote effective management of T1D, and referral to dietitians, social workers, psychologists, or other mental health professionals as needed. Standard treatment goals (adaptable for individuals) were to achieve age-adjusted targets for glycosylated hemoglobin (HbA1C) of less than 8.0% for youths aged below 12 years and less than 7.5% for youths aged above 12 years while minimizing hypoglycemia and promoting youths' quality of life and normal maturation (American Diabetes Association, 2008). To maintain consistency of measurement procedures and respondents throughout the trial, families identified a primary diabetes caregiver and, if feasible, a secondary diabetes caregiver. As described later in this section, this was done for logistic purposes to enable clear identification of the caregiver who would agree to be the primary adult participant throughout the 2-year study.
A total of 564 eligible families were contacted at the four sites. Of these, 404 families (72%) signed parental permission and child assent forms for the study. The number of enrolled families ranged from 87 to 121 across the four sites. Racial and ethnic minorities comprised 24% of the sample, with similar representation at the four sites. Baseline evaluations were scheduled for each enrolled family and these evaluations were completed by 390 (97%) of those who had committed to enter the study. Comparisons of characteristics of enrolled families and eligible families who declined enrollment were completed by independent samples t-tests for continuous variables and with chi-squared tests for categorical variables. The 390 enrolled families did not differ significantly from the 564 eligible families who were approached for the study in terms of race, ethnicity, HbA1C, youth age, youth gender, duration of diabetes, parental education, or family income.
Of the 390 youths who completed baseline assessments, 309 had had T1D for more than 1 year and the youth had completed the CPI about each of two adult caregivers. Each family was asked to identify a primary diabetes caregiver, who was an adult living with the youth and most involved in the youth's T1D care, and who would be the main study participant over the next 2 years. Families were encouraged but not required to nominate a secondary diabetes caregiver, who was a second adult, also involved in the youth's T1D care. The 309 families whose data were analyzed for this article identified both a primary and a secondary caregiver by consensus of the family members. No data were collected about how families made this decision. Primary diabetes caregivers were 87% mothers or stepmothers, 12% fathers or stepfathers, and 1% other. Secondary caregivers were 82% fathers or stepfathers, 13% mothers or stepmothers, 3% grandmothers, and 2% aunts. Some secondary caregivers (19%) did not live in the same home as the child with T1D. Youths with T1D duration of less than 1 year were excluded from this report because they could be continuing to secrete insulin and because their adjustment to T1D could have differed from that of youths with longer disease duration. Youths for whom no secondary diabetes caregiver could be identified were excluded because the present hypotheses required CPI scores regarding two caregivers.
The sampling plan sought to enroll a sample of families who were broadly representative of the four clinic populations and who were appropriate candidates for a low-intensity, preventive behavioral intervention. The enrollment criteria were designed to exclude those with unstable home situations (e.g., no working telephone, impending changes in parental custody, inconsistent appointment keeping) or evidence of serious psychopathology in the parent or child (e.g., recent inpatient psychiatric or substance abuse treatment, or a current diagnosis of psychosis, bipolar disorder, or substance abuse disorder). Demographic characteristics of the sample are summarized in Table I. T1D occurs much more commonly among Caucasians than among racial and ethnic minorities. Because 24% of the enrolled youths represented racial/ethnic minorities, the sample would appear to be at least as diverse on this dimension as the general clinical population of youths with T1D.
Table I.
Demographic Characteristics of the Sample
| Mean | SD | |
|---|---|---|
| Youth age (years) | 12.5 | 3.4 |
| Duration of diabetes (years) | 5.4 | 5.1 |
| HbA1C (%) | 8.5 | 1.5 |
| N | Percentage | |
| Youth gender | ||
| Female | 154 | 50 |
| Male | 155 | 50 |
| Youth race/ethnicity | ||
| Caucasian | 234 | 76 |
| African-American | 22 | 7 |
| Hispanic | 31 | 10 |
| Other/mixed | 22 | 7 |
| Diabetes regimen | ||
| Conventional fixed dose | 81 | 26 |
| Intensified (multiple daily injections) | 98 | 32 |
| Intensified (insulin pump) | 130 | 42 |
| Parental education (male/female caregivers) | ||
| Less than high school | 13/6 | 4/2 |
| High school diploma | 53/38 | 17/12 |
| Some college | 98/115 | 32/37 |
| Bachelor's degree | 90/104 | 29/34 |
| Advanced degree | 55/46 | 18/15 |
| Annual household income | ||
| <$20K | 16 | 5 |
| $20–40K | 31 | 10 |
| $40–70K | 67 | 22 |
| $70–100K | 77 | 25 |
| $100–150K | 65 | 21 |
| >$150K | 53 | 17 |
Note: HbA1C: glycosylated hemoglobin. Continuous variables are reported as means and SDs. Categorical variables are presented as number (N) and percent of the sample.
Measures
Measures obtained at baseline included the primary diabetes outcomes specified for the intervention trial (glycemic control, treatment adherence, quality of life) and potential psychosocial influences on those outcomes. Internal consistency (alpha coefficient) of all measures was calculated based on data obtained from the 309 families whose results were analyzed for this article. Each participant was paid $25 for completion of the baseline assessment. For all questionnaires and interviews described below, mean scores for all completed items were computed. These scores entered the data analyses and are reported in the table and figures to follow. This served as the method of treatment of data that was missing at the item level. There was no missing data at the scale level for the present sample.
Measures Obtained from Youths
Measures obtained from youths that entered the present analyses were those that were specifically designed for completion by youths.
CPI
The CPI has 12 items loading on one primary factor (Nansel & Weissberg-Benchell, et al., 2008) that seeks the child's rating of an adult's level of collaboration with that youth in T1D care. For the two-caregiver families, the child completed the CPI separately for each caregiver. The CPI items, listed in Table II, offered the following response options: 1 = “Almost Never,” 2 = “Sometimes,” 3 = “Often,” 4 = “Almost Always,” and 5 = “Always.” Higher total scores (possible range = 12–60) indicate that the youth perceives more collaborative involvement by that caregiver. Alpha coefficients for this sample were .93 for youths’ ratings of their primary caregivers and .96 for youths’ ratings of their secondary caregivers.
Table II.
All Items of the Collaborative Parent Involvement Scale
| I have a parent/guardian who… |
|---|
| 1. Helps me plan my diabetes care to fit my schedule. |
| 2. Knows when I need a little extra help with my diabetes. |
| 3. Helps me figure out how to change my insulin or eating to fit the amount I exercise. |
| 4. Helps me out when I am too tired or stressed to take care of my diabetes on my own. |
| 5. Knows what things are hard for me in taking care of my diabetes. |
| 6. Helps me learn how to take care of troubles I have with my diabetes. |
| 7. Knows when to let me do more to take care of myself and my diabetes. |
| 8. Helps me plan how to spend time with my friends and still take good care of my diabetes. |
| 9. Talks with me about how to adjust (change) my insulin, eating, and exercise. |
| 10. Helps me with my diabetes when I need it. |
| 11. Helps me take care of any problems I am having at school with taking care of my diabetes. |
| 12. Knows how I am taking care of my diabetes when I am with friends. |
Source: Nansel & Weissberg-Benchell, et al., 2008.
Response options for the items mentioned here from 1 = "Almost Never" to 5 = "Always."
HbA1C
HbA1C was measured at the Joslin Diabetes Center reference laboratory to index recent glycemic control. Blood samples were obtained, frozen, and shipped as whole blood to that laboratory. The samples were processed using the Tosoh 2.2 high-performance liquid chromatography device (Tosoh Corporation, Foster City, CA).
Peds QL
Youths completed both the 23-item Peds QL Generic and the 28-item Peds QL Diabetes modules to measure general and diabetes-specific quality of life, respectively (Varni et al., 2003). The form appropriate to the child's age (8–12 or 13–18 years) was administered. Higher scores indicate better quality of life. Example items are as follows: “I am embarrassed about having diabetes” (diabetes module) and “I have trouble sleeping” (generic module). A recent paper confirms that the PedsQL diabetes module consists of a single primary measurement factor, justifying analysis of a total score from this measure (Nansel & Weissberg-Benchell, et al., 2008). Based on the present sample, internal consistency of the total score was .89 for the core generic module and .83 for the diabetes module.
Diabetes Management Self-Efficacy Scale (DMSES)
Children's self-efficacy for T1D self-care was assessed using the 10-item DMSES (Iannotti, Schneider, Nansel, Haynie, & Simons-Morton, 2004). Higher scores indicate more self-efficacy. Youths are asked to rate their degree of confidence that they can effectively complete diabetes tasks such as, “Do your blood sugar checks even when you are really busy.” The alpha coefficient for this sample was .82.
Children's Depression Inventory (CDI)
The 27-item CDI was used to measure children's depressive symptoms (Kovacs, 1985). Higher scores indicate more depressive symptoms. Youths rated the degree to which they were experiencing certain symptoms such as “feeling lonely.” The alpha coefficient for the present sample was .89.
Hypoglycemia Fear Survey (HFS)
A 16-item child version of the HFS, employing the original 13 worry subscale items plus three new items concerning sleep quality and disruption, was used to measure children's fear of hypoglycemia (Cox, Irvine, Gonder-Frederick, Nowacek, & Butterfield, 1987; Green, Wysocki, & Reineck, 1990). Higher scores indicate more fear of hypoglycemia. Youths rated the degree to which they acknowledge certain fears related to hypoglycemia such as “Having a low blood sugar while alone”. The alpha coefficient for the present sample was .84.
Measures Obtained from Primary Caregivers
The measures listed below were obtained from both parents and youths, but only the parent-reported results were analyzed here. This was done to reduce monorespondent bias, to limit the number of statistical comparisons, and to permit a multimethod test of the associations between child-reported scores on the CPI (Nansel & Weissberg-Benchell, et al., 2008) and these diabetes outcomes.
Diabetes Family Conflict Scale (DFC)
Family diabetes conflict was measured by the DFC (Hood, Butler, Anderson, & Laffel, 2007). Caregivers rated the degree to which they had conflict with their children with diabetes related to 15 aspects of diabetes care such as “Remembering when to give shots.” Higher scores on the 20-item scale indicate more family conflict over T1D. The alpha coefficient for this sample was .89.
Diabetes Family Responsibility Questionnaire (DFRQ)
The DFRQ was completed by parents to measure the division of responsibility between child and parent for 17 diabetes tasks (Anderson et al., 1990). Caregivers rated the degree of parent, child, or shared responsibility for diabetes-related tasks such as “Deciding what to eat at meals and snacks.” Higher total scores indicate more parental responsibility for T1D care. An alpha coefficient of .80 was obtained for the present sample.
Diabetes Self-Management Profile (DSMP)
This 24-item structured interview assesses T1D treatment adherence (Harris et al., 2000). Different parallel versions were used for children on conventional, fixed-dose regimens and those on flexible regimens with self-adjustment of insulin doses based on current glucose levels and expected carbohydrate intake (Diabetes Research in Children Network, 2005). Caregivers rated the degree to which their children complete diabetes self-care tasks such as “In the past 3 months, how often has your child checked his/her blood sugar within 2–3 h after a meal?” The total score correlates significantly with HbA1C (Harris et al., 2000; Iannotti et al., 2006; Lewin et al., 2005). Higher scores reflect more meticulous diabetes management. Alpha coefficients for the present sample were .68 for the conventional regimen version and .75 for the flexible regimen version.
Group Designations and Statistical Analyses
Median splits on CPI total scores for primary and secondary caregivers (medians of 54.0 and 44.0, respectively) were used to create four categories consisting of either high (≥median) or low (<median) scores for the primary and secondary caregivers. These categories served as independent variables in a 2 (primary caregiver CPI score high or low) × 2 (secondary caregiver CPI score high or low) factorial multivariate analysis of covariance (MANCOVA). MANCOVA was performed using the SPSS version 14.0 General Linear Model methods. The assumptions of multivariate normality, equality of error variance, and independence of error terms were met satisfactorily. The MANCOVA was chosen over multiple regression as the main analytic method based on ease of presentation and interpretation. Preliminary analyses of continuous variables using multiple regression approaches yielded virtually identical findings, but it was more difficult to present the results concisely. After the multivariate effects were evaluated using MANCOVA, univariate analyses and pairwise comparisons were done to isolate the sources of multivariate effects. The dependent variables were the various T1D outcome measures described above. As age was correlated with many of these outcomes and with CPI scores, youths' age was treated as a covariate in these analyses. Other demographic variables were not associated significantly with CPI scores (p > .05) and were much less robustly associated with the diabetes outcomes than was youth age. Because inclusion of additional covariates was likely to diminish statistical power, age was selected as the only covariate. The four groups were as follows:
(1) H-H: Both the primary and secondary caregiver obtained a CPI score at or above the median (N = 101). The mean ± SEM CPI scores for these caregivers were as follows: primary, 57.9 ± 0.2; and secondary, 56.2 ± 0.4.
(2) L-L: Both the primary and secondary caregiver obtained a CPI score below the median (N = 111). The mean ± SEM CPI scores for these caregivers were as follows: primary, 42.3 ± 0.7; and secondary, 31.2 ± 0.8.
(3) H-L: The primary caregiver obtained a CPI score at or above the median and the secondary caregiver obtained a CPI score below the median (N = 49). The mean ± SEM CPI scores for these caregivers were as follows: primary, 57.4 ± 0.2; and secondary, 32.9 ± 1.1.
(4) L-H: The primary caregiver obtained a CPI score below the median and the secondary caregiver obtained a CPI score at or above the median (N = 48). The mean ± SEM CPI scores for these caregivers were: Primary, 44.8 ± 1.1; and Secondary, 51.0 ± 0.5.
Results
MANCOVA
The covariate effect for youth age was statistically significant: Wilks' λ = .611, F(9, 293) = 20.69, p < .0001, partial η2 = .39. Among older youths, there was significant deterioration in many diabetes outcomes, including scores on the DMSES, Peds QL Generic module, HbA1C, DFRQ, and DSMP. After controlling for age, statistically significant multivariate associations were obtained for the primary caregiver score on the CPI: Wilks' λ = .833, F(9, 293) = 6.540, p < .0001, partial η2 = .17; secondary caregiver score on the CPI: Wilks' λ = .944, F(9, 293) = 1.93, p < .05, partial η2 = .06; and the CPI-primary × CPI-secondary interaction: Wilks' λ = .937, F(9, 293) = 2.18, p < .025, partial η2 = .06. The partial η2 statistic is an index of effect size and values of .01, .06, and .14 are commonly interpreted as small, medium, and large effect sizes, respectively (Cohen, 1988). The obtained multivariate effects, therefore, ranged from moderate to large effect sizes. The corrected model yielded statistically significant between-subjects effects on all outcome variables. The multivariate hypotheses put forth earlier were all confirmed because statistically significant multivariate effects were obtained for the CPI-primary caregiver and CPI-secondary caregiver (Hypothesis 1) and the CPI-primary caregiver × CPI-secondary caregiver interaction (Hypothesis 2). Further analyses were then completed to isolate the sources of these associations.
Univariate Analyses of Variance
Table III summarizes the univariate ANCOVA between-subject effects of CPI-primary, CPI-secondary, and the CPI-primary × CPI-secondary interaction. Significant univariate between-subject effects of CPI-primary were obtained for scores on the DMSES, CDI, HFS, Peds QL Generic, Peds QL Diabetes, and DSMP scales. High CPI scores were associated with more favorable youth status on each of these dimensions. Significant univariate between-subject effects of CPI-secondary were obtained for scores on the DMSES and DFRQ. Again, high CPI scores were associated with more favorable status on these two measures. Finally, significant univariate between-subject effects attributable to the CPI-primary × CPI-secondary interaction included the HFS and Peds QL Diabetes module. The univariate analyses were followed by pairwise comparisons to further explore the sources of these significant associations.
Table III.
Results of 2 × 2 (Primary Caregiver High CPI or Low CPI × Secondary Caregiver High CPI or Low CPI) MANCOVA (Controlling for Youth Age)
| Dependent variable | F | p | Partial η2 |
|---|---|---|---|
| Child age | |||
| Diabetes Management Self-Efficacy Scale | 22.1 | .0001 | .068 |
| Children's Depression Inventory | 0.03 | .38 | .003 |
| Child Hypoglycemia Fear Survey | 1.56 | .27 | .007 |
| Peds QL Diabetes module | 2.24 | .12 | .011 |
| Peds QL Core Generic module | 10.26 | .002 | .033 |
| HbA1C | 3.85 | .05 | .013 |
| Diabetes Family Responsibility Questionnaire | 84.90 | .0001 | .220 |
| Diabetes Self-Management Profile | 15.95 | .0001 | .050 |
| Diabetes Family Conflict Scale | 16.44 | .0001 | .052 |
| CPI-primary caregiver | |||
| Diabetes Management Self-Efficacy Scale | 30.03 | .0001 | .091 |
| Children's Depression Inventory | 25.39 | .0001 | .078 |
| Child Hypoglycemia Fear Survey | 6.01 | .015 | .020 |
| Peds QL Diabetes module | 22.93 | .0001 | .072 |
| Peds QL Core Generic module | 18.86 | .0001 | .059 |
| HbA1C | 1.49 | .22 | .008 |
| Diabetes Family Responsibility Questionnaire | 0.14 | .48 | .002 |
| Diabetes Self-Management Profile | 24.26 | .0001 | .075 |
| Diabetes Family Conflict Scale | 3.66 | .06 | .012 |
| CPI-secondary caregiver | |||
| Diabetes Management Self-Efficacy Scale | 5.08 | .025 | .017 |
| Children's Depression Inventory | 1.55 | .19 | .009 |
| Child Hypoglycemia Fear Survey | 0.53 | .31 | .004 |
| Peds QL Diabetes module | 0.05 | .43 | .002 |
| Peds QL Core Generic module | 0.23 | .34 | .004 |
| HbA1C | 0.65 | .29 | .006 |
| Diabetes Family Responsibility Questionnaire | 6.99 | .01 | .023 |
| Diabetes Self-Management Profile | 0.03 | .57 | .001 |
| Diabetes Family Conflict Scale | 0.13 | .50 | .001 |
| CPI-primary caregiver × CPI-secondary caregiver | |||
| Diabetes Management Self-Efficacy Scale | 2.27 | .08 | .011 |
| Children's Depression Inventory | 0.04 | .49 | .002 |
| Child Hypoglycemia Fear Survey | 5.31 | .022 | .017 |
| Peds QL Diabetes module | 4.43 | .036 | .015 |
| Peds QL Core Generic module | 2.19 | .10 | .010 |
| HbA1C | 0.09 | .41 | .003 |
| Diabetes Family Responsibility Questionnaire | 2.60 | .06 | .012 |
| Diabetes Self-Management Profile | 0.12 | .46 | .003 |
| Diabetes Family Conflict Scale | 2.43 | .07 | .011 |
HbA1C: glycosylated hemoglobin. The multivariate analysis of covariance (MANCOVA) evaluating between-subjects effect of caregivers' Collaborative Parent Involvement Scale (CPI) scores on child outcomes. See text for results of multivariate tests.
Pairwise Comparisons
Pairwise comparisons were done to isolate the sources of these associations. Figures 1 through 4 show the results of these analyses for each of the primary study outcomes and Table IV presents the results for the other outcome measures.
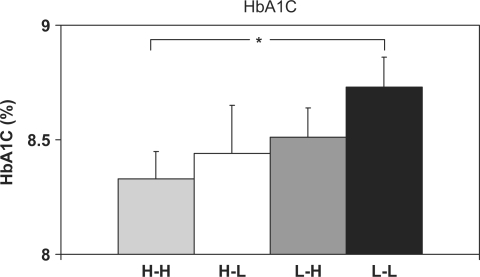
Figure 1.
Summary of pairwise comparisons for glycosylated hemoglobin (HbA1C) results. Data are estimated marginal means adjusted for youth age. Error bars indicate 1 SEM (standard error of measurement). Significance level was *p < .05. All others are nonsignificant.
Table IV.
Summaries of Pairwise Comparisons for Additional Outcome Measures
| Measure | H-H | H-L | L-H | H-L | Significant differences |
|---|---|---|---|---|---|
| DMSES | 0.39 (.01) | 0.38 (.01) | 35 (.01) | 0.33 (.01) | H-H & H-L > L-L (p < .0001) |
| H-H > L-H (p < .01) | |||||
| H-L > L-H (p < .05) | |||||
| L-H > L-L (p < .01) | |||||
| CDI | 0.13 (.01) | 0.17 (.02) | 0.28 (.04) | 0.29 (.03) | H-H < L-L (p < .0001) |
| H-H < L-H (p < .01) | |||||
| H-L < L-L (p < .01) | |||||
| H-L < L-H (p < .05) | |||||
| HFS | 1.76 (.08) | 1.65 (.12) | 1.82 (.10) | 1.95 (.09) | H-H & H-L < L-L (p < .01) |
| DCS | 1.28 (.06) | 1.25 (.05) | 1.34 (.07) | 1.36 (.08) | All nonsignificant |
| DFRQ | 2.28 (.06) | 2.12 (.04) | 2.20 (.04) | 2.14 (.03) | H-H > H-L (p < .01) |
| H-H > L-L (p < .05) |
CDI: Children's Depression Inventory; DFRQ: Diabetes Family Responsibility Questionnaire; DMSES: Diabetes Management Self-Efficacy Scale; HFS: Hypoglycemia Fear Survey; Data are estimated marginal means (SEM in parentheses) of the mean item scores for each measure adjusted for youths’ age. See text for explanation of group labels.
HbA1C
As seen in Figure 1, mean HbA1C for H-H youths (8.3%) was significantly lower than that for L-L youths (8.7%), with p < .03. No other pairwise comparisons reached significance (p > .05).
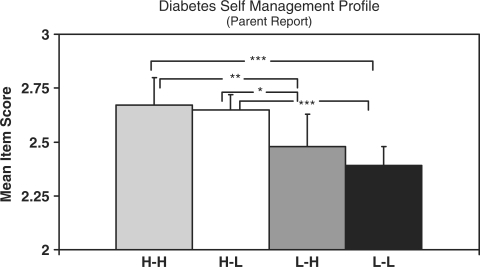
DSMP
As seen in Figure 2, both the H-H and H-L families demonstrated significantly higher treatment adherence than L-L families (p < .001 for both H-H and H-L) and the L-H families (p < .001 for H-H and p < .05 for H-L). Adherence scores did not differ significantly between H-H and H-L families or between L-H and L-L families.
Figure 2.
Summary of pairwise comparisons for diabetes self-management profile results. Data are estimated marginal means adjusted for youth age. Error bars indicate 1 SEM (standard error of measurement). Significance levels are ***p < .0001, **p < .01, *p < .05… All others are nonsignificant.
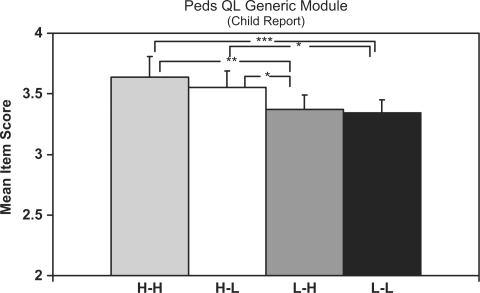
Peds QL Generic module
Figure 3 shows that scores on the Peds QL Generic module were significantly more favorable among both the H-H and H-L families compared to both the L-L and L-H families. Of note, H-L families had significantly higher Peds QL scores than did L-H families (p < .05). The comparison of H-H and H-L families was nonsignificant (p = .26).
Figure 3.
Summary of pairwise comparisons for Peds QL Core Generic module results. Data are estimated marginal means adjusted for youth age. Error bars indicate 1 SEM (standard error of measurement). Significance levels are ***p < .0001, **p < .01, *p < .05. All others are nonsignificant.
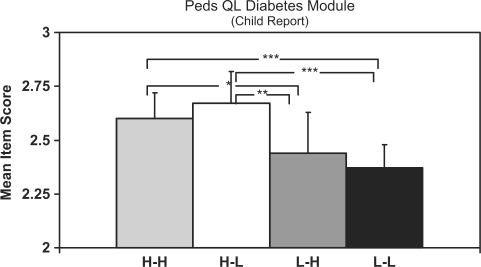
PedsQL Diabetes module
Figure 4 shows that both the H-H and H-L youths had significantly higher scores on the PedsQL diabetes module than both the L-L and L-H youths. Again, H-L youths had significantly higher Peds QL scores than did L-H youths (p < .05). The statistically significant multivariate interaction effect for this measure, as was reported above, appeared to be attributable to the fact that scores for youths from H-H families were slightly lower than those for H-L families, even though this pairwise comparison was not statistically significant.
Figure 4.
Summary of pairwise comparisons for Peds QL Diabetes module results. Data are estimated marginal means adjusted for youth age. Error bars indicate 1 SEM (standard error of measurement). Significance levels are ***p < .0001, **p < .01, *p < .05. All others are nonsignificant.
DMSES
As shown in Table IV, both H-H and H-L youths had significantly higher scores than L-L youths (p < .0001 for both H-H and H-L) and L-H youths (p < .01 for H-H and p < .05 for H-L). Also, L-H youths obtained significantly higher scores on this scale than did L-L youths (p < .01). The comparisons of means for H-H and H-L youths and for L-H and L-L youths were nonsignificant.
CDI
Table IV shows that H-H and H-L youths also had significantly fewer depressive symptoms than youths from L-L (p < .0001 for H-H and p < .01 for H-L) and L-H (p < .01 for H-H and p < .05 for H-L) families. No significant differences were found between mean scores of H-H versus H-L youths or between mean scores of L-H and L-L youths.
HFS
Table IV shows that youths from both H-H and H-L families reported significantly lower fear of hypoglycemia than L-L youths (p < .01 for both comparisons). None of the other HFS pairwise comparisons were significant. The significant interaction shown by the MANCOVA for the HFS may be attributable to the fact that scores for H-H youths were slightly higher than those for H-L youths, although this difference was not statistically significant.
DFC
Table IV shows that none of the comparisons for this scale achieved statistical significance.
DFRQ
As seen in Table IV, the only significant differences for this measure were that H-H families obtained significantly higher scores, indicating more parental responsibility for diabetes tasks, than either L-L families (p < .01) or H-L families (p < .05). There were no differences among H-L, L-H, or L-L families on this measure.
Discussion
The MANCOVA, with youth age as the covariate, and the subsequent univariate analyses and pairwise comparisons provided support for each of the stated hypotheses. Statistically significant multivariate effects were obtained for CPI-primary, CPI-secondary, and the CPI-primary × CPI-secondary interaction (Hypotheses 1 and 2). Subsequent univariate analyses further isolated the specific outcome variables that were influenced by the main and interaction effects (Hypotheses 3 and 4). Finally, pairwise comparisons identified the specific sources of these univariate effects (Hypotheses 3 and 4). These results thus affirm and extend the findings reported by other researchers who have shown that maintenance of parental involvement in T1D management is associated consistently with more favorable diabetes-related outcomes (Anderson et al., 1999; Ellis et al., 2008; Helgeson et al., 2008; Laffel et al., 2003; Wiebe et al., 2005; Wysocki et al., 2006).
Perhaps the most robust finding was that youths whose caregivers both obtained Low CPI scores (L-L) had consistently poorer outcomes than the other groups. For all but one measured outcome (DFCS), L-L youths had significantly less favorable status compared with those in which the primary caregiver had a high CPI score (H-H or H-L). The present results affirm similar previous reports (Anderson et al., 1999; Ellis et al., 2008; Helgeson et al., 2008; Laffel et al., 2003; Wiebe et al., 2005; Wysocki et al., 1996, 2006) of associations among various measures of parental supportive involvement in diabetes management and youths’ diabetes outcomes. Also, the results are consistent with the Wallander et al.'s (1989) risk and resistance model in showing that youths who do not perceive their caregivers as providing adequate collaborative involvement in diabetes management are at elevated risk of adverse diabetes outcomes.
A second interesting pattern was that CPI scores of primary caregivers were associated more strongly with T1D outcomes than were those of secondary caregivers. This conclusion is supported by the pairwise comparisons between the H-L and L-H families. For the DSMP, Peds QL Generic and Diabetes modules, DMSES, and CDI, H-L youths had significantly more favorable status than did L-H youths. If only one parent had a high CPI score, youths’ outcomes were consistently more favorable if that parent was the primary T1D caregiver. To our knowledge, this is the first exploration of how variations in families’ distributions of involvement in diabetes care between two caregivers might be associated with youths’ diabetes outcomes and the first illustration of the special importance of having a primary diabetes caregiver who the youth perceives as supportive and involved. The apparent special importance of the primary caregiver's role may indicate that the complexity of daily diabetes management requires close collaboration with one adult caregiver who is highly familiar with the nuances of the youth's diabetes management. The present study should not be interpreted as evidence that a similar approach to family management of other pediatric chronic diseases should be encouraged.
A third general conclusion is that secondary caregivers’ levels of collaborative involvement yielded only modest incremental effects beyond primary caregivers' contributions. The only pairwise comparisons that revealed an additive role of high collaborative involvement among secondary caregivers were those for HbA1C and the DFRQ. There was a significant difference in HbA1C between H-H (mean = 8.3%) and L-L (mean = 8.7%) youths, but no other pairwise comparison was significant. Thus, high collaborative involvement of both caregivers conferred a modest glycemic advantage over low collaborative involvement of both caregivers that was not enjoyed by either group (H-L or L-H) in which only one caregiver had a high CPI score. For the DFRQ, H-H youths scored significantly higher in diabetes responsibility as reported by parents compared with either H-L or L-L youths. Thus, as expected, having two caregivers with high collaborative involvement was associated with more parental responsibility for diabetes management. Some additional evidence of an incremental benefit of greater secondary caregiver involvement arose in pairwise comparisons of L-H and L-L families. For the DMSES, L-H youths scored significantly higher than L-L (p < .05). For these three comparisons (HbA1C, DFRQ, and DMSES), then, there was modest support for concluding that high collaborative involvement of secondary caregivers enhanced the influence of primary caregivers’ involvement. The somewhat weak additive benefits of high collaborative involvement from secondary caregivers could be due to ceiling effects that may have limited additional gains in certain outcomes, impeding detection of an additive effect. Also, it is possible that differing associations may emerge as this 2-year study continues.
Contributions of the Study
This article extends the existing research on the correlates of youths’ perceptions of their caregivers’ involvement in T1D care in several ways. This is the first study to explore different combinations of high and low involvement in families in which two adult caregivers play a role in childhood diabetes management. We compared a wide range of T1D-related outcomes among youths whose families had any of four patterns of higher and lower collaborative involvement by two adult caregivers. The results showed that youths who perceived both caregivers as demonstrating low collaborative involvement in diabetes management were consistently at risk of poor diabetes outcomes that high collaborative involvement of the primary caregiver appears to be especially important and modest evidence that greater involvement of the secondary caregiver may have yielded some additive benefits beyond the contributions of the primary caregiver. Second, the sample size for this study (N = 309) was quite large compared to other similar studies on T1D; the sample was diverse in race/ethnicity; participants were drawn from four geographic regions; and a concerted effort was made to enroll families who were broadly representative of the four clinic populations. Third, the study included measures of a broad array of diabetes-related outcomes and many of the variables analyzed for this article were reported by caregivers, rather than youths, providing a multimethod evaluation of associations between youth-reported CPI and these outcomes. The stability of the findings, whether a youth-reported or caregiver-reported outcome or a laboratory test was being analyzed, lends confidence to the study's conclusions. Fourth, parent–youth teamwork in T1D management is a key element of the conceptual framework underlying the intervention being tested in this ongoing randomized controlled trial (Anderson et al., 1999; Laffel et al., 2003). Consequently, the present report further justifies targeting this family process in the intervention trial. Finally, the study further confirmed the psychometric properties of the CPI (Nansel & Weissberg-Benchell, et al., 2008) with a larger sample.
Limitations
The cross-sectional analysis reported in this article cannot confirm a causal link between CPI and youths’ diabetes outcomes. As the present longitudinal study continues, it may be possible to confirm such a link. Also, because the families who agreed to participate were committing to participation in a 2-year trial of a preventive intervention, it is possible that the enrolled sample may not be representative of the broader clinical population. But the large sample size and the demographic diversity of the participants tend to diminish this concern to some extent. Although the results suggest that collaborative involvement of the primary diabetes caregiver is most robustly related to diabetes outcomes, this observation may simply reflect the status quo among the participants rather than indicating that this is an optimal strategy for family management of T1D. For example, more involvement of fathers in the management of pediatric chronic diseases has been shown to be associated cross-sectionally with more favorable marital, family, and maternal functioning (Gavin & Wysocki, 2006).
The desire to simplify the statistical analyses led to the decision to treat youth age as the only covariate in the MANCOVA's that were completed. Inclusion of other covariates or between-subject factors (e.g., gender, diabetes duration, insulin modality) could have enabled exploration of more complex interactive relationships within the data. Perhaps future research can explore such questions.
Finally, because the enrollment criteria excluded youths who had only one adult involved in their diabetes management, the results and conclusions reported here cannot be applied to the population of youths with diabetes from single-parent families, a group that is known to be at high risk of difficulties adapting to the demands of this medical condition.
Clinical and Research Implications
The most justifiable conclusion from this report is that low collaborative involvement, particularly among primary diabetes caregivers, was associated consistently with poor diabetes-related outcomes. Enabling families to understand this association and to find practical and effective ways to enhance their collaborative involvement could lead to improved diabetes-related outcomes. It is quite conceivable that such improvements could also be accompanied by improvements in marital, family, and maternal functioning, which could lead indirectly to further therapeutic benefits (Gavin & Wysocki, 2006) such as reduced diabetes burnout (Polonsky, 2000). As this ongoing 2-year study reaches its conclusion, it will become possible to examine the associations reported here with much greater depth and analytic flexibility from a longitudinal perspective, possibly affirming more strongly that CPI represents an appropriate target for family-focused interventions with this clinical population.
Funding
The following institutions and investigators comprised the steering committee of the Family Management of Diabetes multisite trial. This research was supported by the intramural research program of the National Institutes of Health, National Institute of Child Health, and Human Development. National Institute of Child Health and Human Development, Bethesda, Maryland: Tonja R. Nansel, PhD; Ronald J. Iannotti, PhD. Joslin Diabetes Center, Boston, Massachusetts: Lori Laffel, MD, MPH; Debbie Butler, MSW; Contract N01-HD-4-3361. Nemours Children's Clinic, Jacksonville, Florida: Tim Wysocki, PhD; Amanda Lochrie, PhD. Contract N01-HD-4-3362. Dr. Wysocki was also supported by an NIH Midcareer Investigator Award in Patient-Oriented Research #K24-DK-67128 during this work. Texas Children's Hospital, Houston, Texas: Barbara Anderson, PhD. Contract N01-HD-4-3363. Children's Memorial Hospital, Chicago, Illinois: Jill Weissberg-Benchell, PhD; Grayson Holmbeck, PhD. Contract N01-HD-4-3364. James Bell Associates, Arlington, Virginia; Cheryl McDonnell, PhD; MaryAnn D’Elio, Contract N01-HD-3-3360.
Conflict of interest: None declared.
References
- Amato PR. Father-child relations, mother-child relations, and offspring psychological well-being in early adulthood. Journal of Marriage and the Family. 1994;55:23–38. [Google Scholar]
- American Diabetes Association. Clinical practice recommendations, 2008: Standards of medical care in diabetes: 2008. Diabetes Care. 2008;31(Suppl. 1):S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Auslander WF, Jung KC, Miller P, Santiago JV. Assessing family sharing of diabetes responsibilities. Journal of Pediatric Psychology. 1990;15:477–492. doi: 10.1093/jpepsy/15.4.477. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: Relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. Journal of Pediatrics. 1999;130:257–265. doi: 10.1016/s0022-3476(97)70352-4. [DOI] [PubMed] [Google Scholar]
- Auslander WF, Anderson BJ, Bubb J, Jung KC. Risk factors to health in diabetic children: A prospective study from diagnosis. Health & Social Work. 1990;15:133–142. doi: 10.1093/hsw/15.2.133. [DOI] [PubMed] [Google Scholar]
- Booth J, Croutier P. Men in families. When do they get involved? What difference does it make? Mahwah, NJ: Lawrence Erlbaum; 1998. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Mahwah, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Cox DJ, Irvine A, Gonder-Frederick LA, Nowacek G, Butterfield J. Fear of hypoglycemia: Quantification, validation and utilization. Diabetes Care. 1987;23:617–621. doi: 10.2337/diacare.10.5.617. [DOI] [PubMed] [Google Scholar]
- Diabetes Research in Children Network Study Group. Diabetes Self-Management Profile for flexible insulin regimens: Cross-sectional and longitudinal analysis of psychometric properties in a pediatric sample. Diabetes Care. 2005;28:2034–2035. doi: 10.2337/diacare.28.8.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis DA, Templin T, Podolski C, Frey M, Naar-King S, Moltz K. The Parental Monitoring of Diabetes Care Scale: Development, reliability and validity of a scale to evaluate parental supervision of adolescent illness management. Journal of Adolescent Health. 2008;42:146–153. doi: 10.1016/j.jadohealth.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Gavin L, Wysocki T. Associations of paternal involvement in disease management with maternal and family outcomes in families with children with chronic illnesses. Journal of Pediatric Psychology. 2006;31:481–489. doi: 10.1093/jpepsy/jsj043. [DOI] [PubMed] [Google Scholar]
- Green LB, Wysocki T, Reineck BM. Fear of hypoglycemia in children and adolescents with diabetes mellitus. Journal of Pediatric Psychology. 1990;15:633–641. doi: 10.1093/jpepsy/15.5.633. [DOI] [PubMed] [Google Scholar]
- Hanson CL, Henggeler SW, Burghen GA. Social competence and parental support as mediators of the link between stress and diabetic control in adolescents with insulin-dependent diabetes mellitus. Journal of Consulting and Clinical Psychology. 1987;28:147–156. doi: 10.1037/0022-006X.55.4.529. [DOI] [PubMed] [Google Scholar]
- Hanson CL, Henggeler SW, Rodrigue JR, Burghen GA, Murphy WD. Father-absent adolescents with insulin-dependent diabetes mellitus: A population at special risk? Journal of Applied Developmental Psychology. 1988;9:243–252. [Google Scholar]
- Harris MA, Greco P, Wysocki T, Elder CL, White NH. Adolescents with diabetes from single parent, blended and intact families: Health-related and family functioning. Families, Systems and Health. 1999;17:181–196. [Google Scholar]
- Harris MA, Wysocki T, Sadler M, Wilkinson K, Harvey LM, Buckloh LM, et al. Validation of a structured interview for the assessment of diabetes self-management. Diabetes Care. 2000;23:1301–1304. doi: 10.2337/diacare.23.9.1301. [DOI] [PubMed] [Google Scholar]
- Helgeson VS, Reynolds KA, Siminerio L, Escobar O, Becker D. Parent and adolescent distribution of responsibility for self-care: Links to health outcome. Journal of Pediatric Psychology. 2008;33:497–508. doi: 10.1093/jpepsy/jsm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood KK, Butler DA, Anderson BJ, Laffel L. Updated and revised Diabetes Family Conflict Scale. Diabetes Care. 2007;30:1764–1769. doi: 10.2337/dc06-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti RJ, Nansel TR, Schneider S, Haynie DL, Simons-Morton B, Sobel DO, et al. Assessing regimen adherence of adolescents with type 1 diabetes. Diabetes Care. 2006;29:2263–2267. doi: 10.2337/dc06-0685. [DOI] [PubMed] [Google Scholar]
- Iannotti R, Schneider S, Nansel T, Haynie D, Simons-Morton B. Self-efficacy, outcome expectations, and metabolic control in adolescents with type 1 diabetes. Paper presented at the Society of Pediatric Psychology National Conference on Child Health Psychology, Charleston; South Carolina: 2004. [Google Scholar]
- Ingersoll GM, Orr DP, Herrold AJ, Golden MP. Cognitive maturity and self-management among adolescents with insulin-dependent diabetes mellitus. Journal of Pediatrics. 1985;108:620–623. doi: 10.1016/s0022-3476(86)80852-6. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children's Depression Inventory. Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Laffel LM, Vangsness L, Connell A, Goebel-Fabri A, Butler D, Anderson BJ. Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with type 1 diabetes. Journal of Pediatrics. 2003;142:409–416. doi: 10.1067/mpd.2003.138. [DOI] [PubMed] [Google Scholar]
- Lamb MC. The role of the father in child development. 3rd ed. New York: John Wiley; 1997. [Google Scholar]
- Lewin AB, Heidgerken AD, Geffken GR, Williams LB, Storch EA, Gelfand KM, et al. The relation between family factors and metabolic control: The role of diabetes adherence. Journal of Pediatric Psychology. 2006;31:174–183. doi: 10.1093/jpepsy/jsj004. [DOI] [PubMed] [Google Scholar]
- Nansel T, Rovner AJ, Haynie D, Iannotti RJ, Simons-Morton B, Wysocki T, et al. Development and validation of the Collaborative Parent Involvement scale. Journal of Pediatric Psychology. 2008 doi: 10.1093/jpepsy/jsn058. 10.1093/jpepsy/jsn058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nansel TR, Weissberg-Benchell J, Laffel L, Anderson B, Wysocki T. Quality of life in youth with type 1 diabetes: a comparison of general and diabetes-specific measures, and support for a unitary diabetes quality of life construct. Diabetic Medicine, 25. 2008:1316–1323. doi: 10.1111/j.1464-5491.2008.02574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares V. Psychological adjustment, maladjustment and father-child relationships. In: Lamb MC, editor. The role of the father in child development. 3rd. New York: John Wiley; 1998. pp. 261–283. [Google Scholar]
- Phares V, Compas BE. The role of fathers in child and adolescent psychopathology: Make room for daddy. Psychological Bulletin. 1992;111:387–412. doi: 10.1037/0033-2909.111.3.387. [DOI] [PubMed] [Google Scholar]
- Polonsky WH. Diabetes burnout: What to do when you can't take it anymore. Alexandria, VA: American Diabetes Association; 2000. [Google Scholar]
- Thompson SJ, Auslander WF, White NH. Comparison of single-mother and two-parent families on metabolic control of children with diabetes. Diabetes Care. 2001;24:234–238. doi: 10.2337/diacare.24.2.234. [DOI] [PubMed] [Google Scholar]
- Varni J, Burwinkle T, Jacobs J, Gottschalk M, Kaufman R, Jones K. The Peds QL in type 1 and type 2 diabetes: Reliability and validity of the Pediatric Quality of Life Inventory generic core scales and type 1 diabetes module. Diabetes Care. 2003;26:631–637. doi: 10.2337/diacare.26.3.631. [DOI] [PubMed] [Google Scholar]
- Wallander JL, Varni JW, Babani LV, Banis HT, Wilcox KT. Family resources as resistance factors for psychological maladjustment in chronically ill and handicapped children. Journal of Pediatric Psychology. 1989;14:157–173. doi: 10.1093/jpepsy/14.2.157. [DOI] [PubMed] [Google Scholar]
- Webster-Stratton C. The effects of father involvement in parent training for conduct problem children. Journal of Child Psychology and Psychiatry. 1985;26:801–810. doi: 10.1111/j.1469-7610.1985.tb00593.x. [DOI] [PubMed] [Google Scholar]
- Wiebe DJ, Berg CA, Korbel C, Palmer DL, Beveridge RM, Upchurch R, et al. Children's appraisals of maternal involvement in coping with diabetes: Enhancing our understanding of adherence, metabolic control, and quality of life across adolescence. Journal of Pediatric Psychology. 2005;30:167–178. doi: 10.1093/jpepsy/jsi004. [DOI] [PubMed] [Google Scholar]
- Winquist-Nord C. Issue brief: Students do better when their fathers are involved at school. Washington, DC: U.S. Department of Education, National Center for Education Statistics; 1998. [Google Scholar]
- Wysocki T, Buckloh LM, Lochrie A, Antal H. The psychologic context of pediatric diabetes. Pediatric Clinics of North America. 2005;52:1755–1778. doi: 10.1016/j.pcl.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Gavin L. Paternal involvement in the management of pediatric chronic diseases: Associations with adherence, quality of life and health status. Journal of Pediatric Psychology. 2006;31:501–511. doi: 10.1093/jpepsy/jsj042. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris MA, Buckloh L, Wilkinson K, Sadler M, Mauras N, White NH. Self care autonomy and outcomes of intensive therapy or usual care in youth with type 1 diabetes. Journal of Pediatric Psychology. 2006;31:1036–1045. doi: 10.1093/jpepsy/jsj017. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Iannotti R, Weissberg-Benchell J, Hood K, Laffel L, Anderson BJ, et al. Diabetes problem solving by youths with type 1 diabetes and their caregivers: Measurement, validation and longitudinal associations with glycemic control. Journal of Pediatric Psychology. 2008;33:875–884. doi: 10.1093/jpepsy/jsn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki T, Taylor A, Hough BS, Linscheid TR, Yeates KO, Naglieri JA. Deviation from developmentally appropriate self-care autonomy: Association with diabetes outcomes. Diabetes Care. 1996;19:119–125. doi: 10.2337/diacare.19.2.119. [DOI] [PubMed] [Google Scholar]