Abstract
The peptide uroguanylin regulates electrolyte transport in the intestine and kidney. Human uroguanylin has two conformations that can be stably isolated, owing to their slow interconversion rate. The A isomer potently activates the guanylate cyclase-C receptor found primarily in the intestine. The B isomer, by contrast, is a very weak agonist of this receptor, leading to a widely-held assumption that it is physiologically irrelevant. We show here, however, that human uroguanylin B has potent natriuretic activity in the kidney. Interestingly, uroguanylin A and B both induce saliuretic responses, but the activity profiles for the two peptides differ markedly. The uroguanylin B dose-response curve is sigmoidal with a threshold dose near 10 nmol/kg body weight, whereas uroguanylin A has a comparable threshold, but a bell-shaped dose-response curve. Additionally, our study indicates a unique interplay between the A and B isoforms, such that the A form at high concentrations antagonizes the natriuretic action of the B form. These data show that the kidney contains a uroguanylin receptor whose pharmacological profile does not match that of the well-defined intestinal uroguanylin receptor (guanylate cyclase-C), an observation consistent with previous studies showing that the kidney of the guanylate cyclase-C knockout mouse remains responsive to uroguanylin. The results presented here also support the unconventional notion that distinct conformations of a single endocrine peptide can elicit different responses in different tissues.
Keywords: sodium excretion, natriuretic peptide, hypertension, electrolyte homeostasis, uroguanylin, peptide isomerization
INTRODUCTION
Guanylin (Gn) 1 and uroguanylin (Ugn) 2 are closely related signaling peptides that, in the rat, are primarily produced by intestinal goblet cells and enterochromaffin cells, respectively 3,4. Both peptides function as intestinal secretagogues, acting apically on the epithelium to stimulate chloride and bicarbonate secretion into the lumen through the CFTR chloride channel 5 and to inhibit sodium absorption from the lumen by a sodium proton exchanger 6. These responses are mediated by guanylate cyclase-C (GC-C), a receptor-guanylate cyclase (rGC) located on the apical membranes of enterocytes. GC-C is also activated by bacterially-produced heat stable toxins (ST) such as STa(h), and STa(p), which are the causal agents for travelers’ diarrhea 7.
In addition to their well-described actions within the intestine, Gn and Ugn circulate in plasma 8–12 and elicit natriuretic responses from the kidneys 13–15. Thus, both peptides have been proposed as volume regulatory factors that buffer acute increases in dietary salt intake by increasing renal sodium excretion while simultaneously reducing intestinal sodium absorption 16,17. Ugn is favored over Gn in this role for several reasons. First, the enterochromaffin cells that make and secrete Ugn respond to chemical stimuli from the intestinal lumen 18,19 and release their secretory products both apically (to regulate intestinal function) and basolaterally (to regulate renal function) 20, whereas the goblet cells that make Gn release their secretory products predominantly into the intestinal lumen 21–23. Second, Ugn is a more potent natriuretic factor than Gn 14 and, unlike Gn, it is abundant in urine 24. Indeed, Gn is susceptible to proteolytic digestion by a chymotrypsin-like activity in the kidney, which limits its renal activity 25 and renders it undetectable in urine 26. Third, the Ugn knockout mouse displays mild chronic hypertension and excretes an oral salt load more slowly than its wild-type littermates 27, while the Gn knockout mouse appears normal with respect to blood pressure and salt handling capabilities 28.
Ugn and Gn are 13 – 16 amino acid peptides that share a distinctive ring structure produced by disulfide bonds which, for the human form of the peptide, occur between cysteines at positions 4 and 12 and positions 7 and 15 29. The central loop formed by amino acids 8–11 can be positioned either above or below the surface formed by the 4 cross-linked cysteines 30,31, resulting in two conformationally distinct A and B topoisomers, as shown schematically in figure 1. In the rat, mouse, and opossum, the two conformations of Gn and Ugn interconvert spontaneously at a rate of 1–2 cycles per sec at 37° C and neutral pH 32. The structure and interconversion rate of human Gn is similar to its rat counterpart, but human Ugn (huUgn) has an additional leucine residue that extends the C terminus and sterically hinders the transition between the uroguanylin A (UgnA) and uroguanylin B (UgnB) conformations, increasing the half-life of each form to about 2 days at 37° C 31,33. Because of this relative stability, huUgnA and B can be separated by HPLC and tested independently for activity. In such studies, huUgnA elicits robust cyclic GMP responses with an EC50 on the order of 10−7 M when applied to cultured T84 cells, a GC-C expressing cell line derived from human colon carcinoma, while huUgnB is approximately 100-fold less potent in the same bioassay system 33.
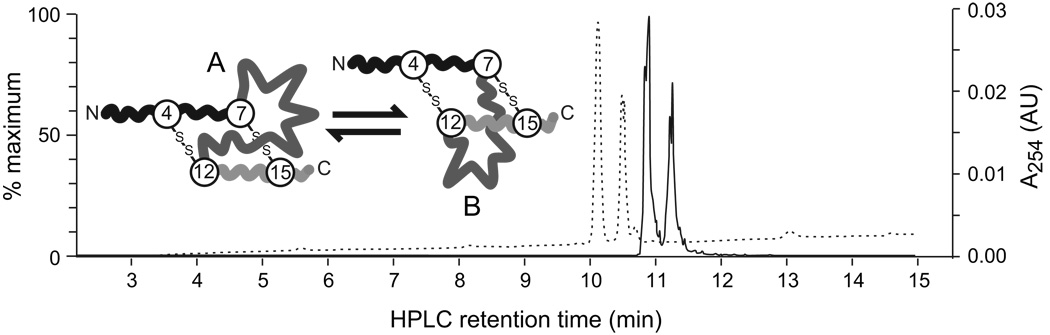
Figure 1.
The A and B isomers of huUgn are separable by HPLC and quantifiable by LC-MS. The two isomers are shown schematically in the inset (disulfide crossbridges indicated by –S-S-). LC-MS analysis was performed on a calibration sample that contained similar amounts of huUgnA and huUgnB. The dashed trace is the UV absorbance profile and the solid trace is the extracted ion chromatogram (m/z 1667–1669) from a full MS scan. In positive ion mode, the m/z ratio for huUgn is 1667.6. For both traces, the earlier-eluting peak is huUgnA and the later-eluting peak is huUgnB. The traces are offset by ~0.8 min because the UV detector is upstream of the MS detector.
The ineffectiveness of UgnB in stimulating T84 cells has led to a general consensus that this isomer has no biological relevance. However, the limited response profile available from a cell culture model does not match the complex physiologic systems that are accessible to the peptide in vivo. We therefore measured renal function in anesthetized rats that were infused with either UgnA or UgnB, to compare the effects of these peptides in a more integrative context.
METHODS
Experiments were performed on 139 male Sprague Dawley rats (Charles River Breeding Laboratories, Raleigh NC) maintained on a 12 hour light/dark cycle with free access to water and standard rat chow. Rats were not fasted prior to study. All procedures were approved by the UNC Institutional Animal Care and Use Committee, and carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Rat preparation
Animals (250 – 340 g) were anesthetized (55 mg/kg BW pentobarbital, administered intraperitoneally) and prepared for renal clearance studies as previously described 34. A jugular catheter provided constant intravenous infusion (10 µl/min/100g BW) of isotonic saline containing FITC-labeled inulin (0.4% W/V, Sigma, St Louis MO) for measurement of inulin clearance. Additional cannulae supplied supplemental anesthetic and peptides. Urine was collected via ureteric cannulae over sequential 20 minute time intervals (clearance periods), and the volume estimated by weight. A femoral artery was cannulated for continuous measurement of blood pressure, via a pressure transducer connected to a cardiovascular analyzer (Model 50110, Stoelting Instruments Wood Dale, IL), and for collecting blood samples (50 µl at the mid point of each urine collection). At least one hr was allowed for equilibration before the start of urine collections, at which point plasma inulin concentration had stabilized at an average value of 30±3 µg/ml. Left renal artery blood flow was measured in some rats with a flow probe connected to a blood flow monitor (model 1PRB probe and model T420 monitor, Transonic Systems Inc. Ithaca NY). Arterial pressure, heart rate, and renal blood flow were digitized (Keithley instruments, Cleveland, OH Model KUSB 3100) and captured (Dtx_Ez, Data Translation Inc, Marlboro, MA) for display and storage on a Windows-based personal computer. Sodium and potassium concentrations were measured by flame photometry (Model 943, Instrumentation Laboratory Co., Lexington MA). Inulin was measured by the method of Lorenz and Gruenstein 35. Glomerular filtration rate (GFR) was measured as the renal clearance rate of FITC-labeled inulin.
After post-surgical equilibration (~60 min) and three control clearance periods, a test solution [huUgnA, huUgnB, ST-core (an active core consensus sequence, CCELCCNPACTGCY, derived from the heat stable enterotoxins of E. coli and Y. enterocolitica), or isotonic saline] was infused into the jugular vein at 10 µl/min over 3 clearance periods, followed by a return to isotonic saline for the remainder of the experiment. Renal excretory responses to these infusions were slow to develop and long lasting, so clearance periods were continued for 2–3 hr after the termination of the peptide infusion. This protracted time course limited protocols to one dose of one peptide in each rat.
huUgnA and huUgnB were obtained from a commercial supplier (Bachem Americas Inc. Torrance, CA ). ST-core was provided by Ironwood Pharmaceuticals Inc (Cambridge, MA). These peptides were dissolved at a concentration of 1 µg/µl in sterile saline containing 0.1% BSA. Aliquots of these solutions were stored at −80° C, then thawed and diluted into isotonic saline to provide the desired concentration immediately prior to infusion into the animal.
HPLC analysis and mass spectroscopy of Ugn isomers
The integrity and purity of huUgnA and B isomer preparations were confirmed by liquid chromatography/mass spectroscopy (LC-MS) analysis. The two Ugn isomers have different retention times on a reverse phase HPLC column (figure 1, dashed trace; huUgnA elutes ~ 0.35 minutes before huUgnB). Separations were performed on a Hypersil Gold AQ 2.1 × 50 mm column from Thermo Fisher Scientific Inc (Waltham, MA) equilibrated in 98% buffer A (0.1% formic acid), 2% buffer B (85:10:5 Acetonitrile / Isopropyl Alcohol / 5mM NH4OAc pH 5.8) with a flow rate of 0.4 ml/min on an Acquity UPLC system. After a 2.5 minute wash with the same buffers, peptides were eluted with a linear gradient of 2% buffer B to 80% buffer B over 25 minutes and held for one minute before an increase to 90% B over 2 minutes to wash the column, followed by a return to 2% buffer B over 3 minutes.
Peptide masses were determined using a Micromass Q-Tof II instrument equipped with an electrospray ionization (ESI) source operating in positive ion mode. The instrument was programmed to scan in the mass range of m/z 100 to 1800. Molecular weight predictions and data analysis were carried out with MassLynx version 4.0 software. 20µl of urine were injected directly without any sample preparation using an Acquity UPLC system connected in line with the Q-Tof II.
Bioassay of huUgnA-like activity
The concentration of huUgnA-like bioactivity in infused peptide solutions and in urine collected during experiments was measured using a T84 cell-based bioassay, as described previously 34. Standards or urine samples were diluted into bioassay medium (1 mM 3-isobutyl-1-methylxanthine, 0.03 mM phenol red, 137 mM NaCl, 5.4 mM KCl, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 1.3 mM CaCl2, 1.0 mM MgSO4, 4.2 mM NaHCO3, 10 mM HEPES buffered at pH 7.0) and the pH was adjusted to 7.0, if required. After 30 min, the cells were lysed and cyclic GMP levels measured by radioimmunoassay (Biomedical Technologies Inc Stoughton MA). Responses evoked by unknown samples were converted to huUgnA concentrations by interpolation into the standard curve that was generated from huUgnA standards (fit using the log(agonist) vs response equation given below), and are reported as pmol UgnA-like activity per well (mean ± SEM).
Data Analysis
Results from both kidneys were averaged for each rat before calculating group means ± SEM. Sodium and potassium excretion rates (UNaV and UKV) are expressed in nmol/g kidney weight (KW)/min. UNaV and UKV are factored by filtered load to provide fractional excretion (FENa and FEK). Glomerular filtration rate (GFR) was equated with inulin clearance. To generate dose-response curves, the net natriuretic response to peptide infusions was assessed by summing total sodium excretion during infusion and post-infusion collection periods in each rat after subtraction of corresponding mean values obtained from the control group. The cumulative net excretion obtained in this way provides a measure of the natriuretic response to each dose of peptide infused.
For statistical analysis, clearance periods were binned in groups of three to provide pre-infusion data (0 – 60 min), infusion data (60 – 120 min), and two sets of post-infusion data (120 – 180 min, and 180 – 240 min). The final three collection periods (240 – 300 min) were not included in the analysis, because a few experiments were not carried out for the full 300 min. Group comparisons were made with two-way analysis of variance (ANOVA) using peptide dose as the column variable and time as the row variable. Post hoc testing used Bonferroni’s method for multiple comparisons. All graphing and statistical testing were performed with the Prism 5.01 graphing and analysis program (Graphpad Software,San Diego, CA). Dose-response curves for huUgnA- and ST-core-evoked net sodium excretion were fit using the spline algorithm. Dose-response curves for huUgnB-evoked net sodium excretion and for T84 cell bioassay responses were fitted using the log (agonist) vs response equation:
RESULTS
Characterization and stability of commercial huUgnA and B
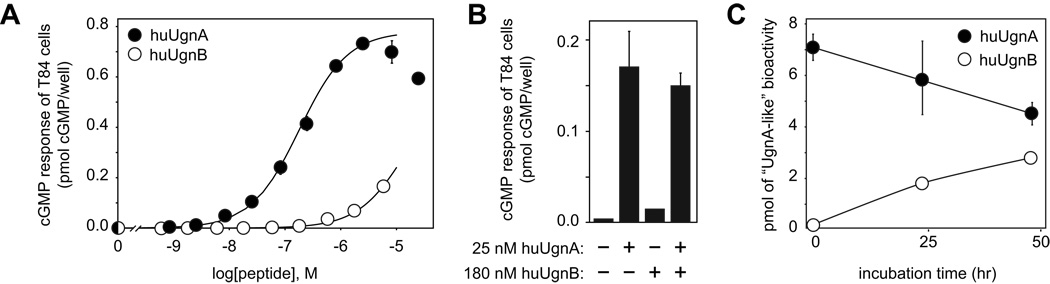
We used LC-MS to authenticate the initial identities and purities (≥94%) of commercially-obtained huUgnA and B, and to verify at periodic intervals that conversion from one isoform to the other did not occur during storage at −80° C (data not shown). We also confirmed, as expected 33, that the A isoform could activate cyclic GMP synthesis in the GC-C-expressing T84 cell line, while the B isoform was approximately 100-fold less potent (Figure 2a). Furthermore, T84 cell responses evoked by co-application of huUgnA and B were not different from those evoked by huUgnA alone (Figure 2b), indicating that the minimal responses to huUgnB reflect its intrinsically low agonist activity, rather than any antagonist properties at GC-C receptors. The decline in the responsiveness of the cells at very high huUgnA levels (Figure 2a) is likely due to agonist-mediated desensitization of GC-C, a phenomenon that has been well-documented for other rGC family members 36,37.
Figure 2.
GC-C-stimulating activities of commercially-obtained huUgnA and huUgnB. (a) Each data point represents the mean value (± SEM) for 9 experiments with huUgnA (filled symbols) and 3 experiments with huUgnB (open symbols). The curves are fit with the log (agonist) vs dose equation (see the Methods), using an EC50 of 1.8 × 10−7 M for huUgnA and an EC50 of 1.5 × 10−5 M for huUgnB. Responses to peptides were significantly different from control (p < 0.05 or lower by 2 way ANOVA) at all doses of huUgnA greater than 2.5 × 10−9 M, and all doses of huUgnB greater than 8 × 10−9 M. huUgnA responses were greater than huUgnB at all doses above 8 × 10−9 (p<0.01 or less) (b) The stimulatory effect of huUgnA on T84 cells is not antagonized by a 7-fold excess of huUgnB. The bars show (from left to right) the cyclic GMP levels in cells treated with control medium, with 25 nM huUgnA, with 180 nM huUgnB, or with a combination of the two peptides (mean ± SEM, n = 3). (c) The A and B isomers (filled and open circles, respectively) interconvert slowly in vitro. Samples were incubated at 50° C in 1 mM citrate buffer (pH 4), then diluted into bioassay medium. The pH was adjusted to 7.0, and activity was measured in the T84 cell bioassay. T84 responses to both isomers at 48 hr were significantly different from time zero (p < 0.01, two-way ANOVA), but were not different from each other.
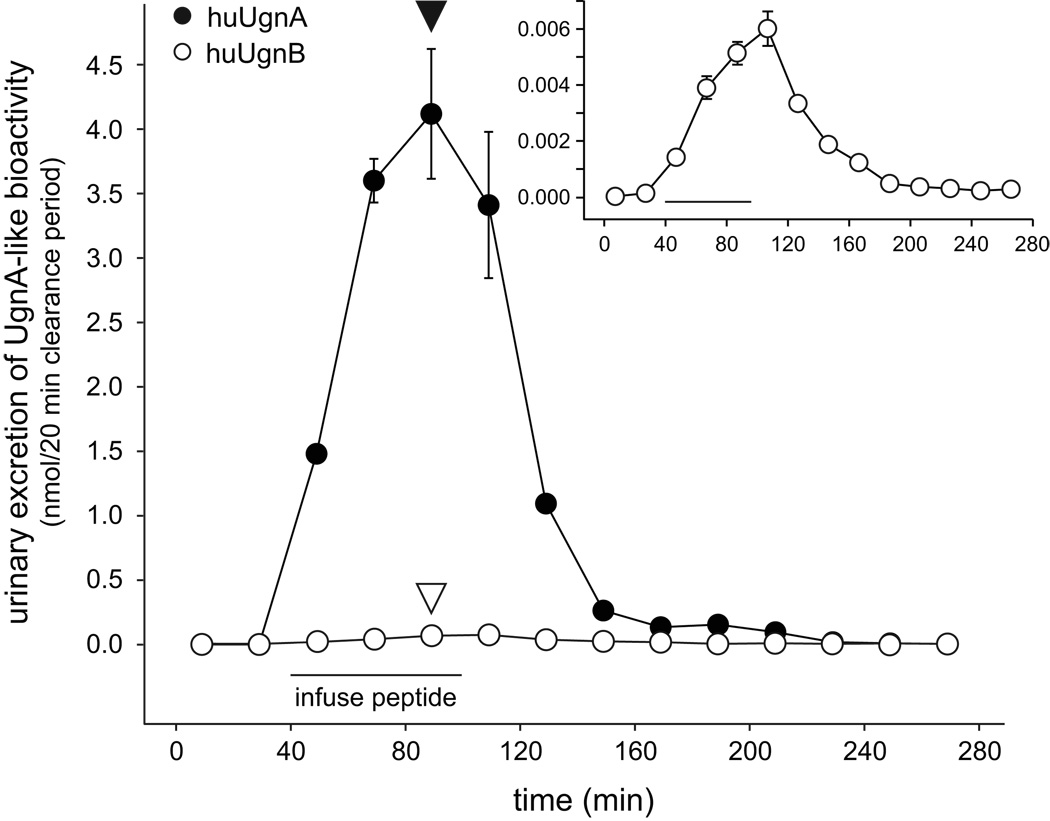
In contrast to the long-term stability of each individual isomer at −80° C, equilibration to a mixture of A and B forms occurs spontaneously when either peptide is incubated at pH 4 and 50° C (Figure 2c). However, as has been well-documented in previous studies 31, significant isomerization requires many hours in vitro. To examine the possibility that some as-yet unidentified process might catalyze a more rapid interconversion of the isomers in vivo, we infused huUgnA or B intravenously into anesthetized rats and used both the T84 bioassay and LC-MS analysis to determine which form of the peptide was excreted in the urine. When huUgnA was infused, a significant amount of GC-C-stimulating activity appeared in urine within 20 min of the start of the infusion, reached a peak by the end of the infusion, and returned to baseline within an hour after the infusion was terminated (Figure 3, black symbols; representative of 6 independent experiments). We then performed LC-MS analysis of the urine that was obtained when peptide excretion was at its peak (marked by the black arrowhead in Figure 3), and observed a clearly discernable huUgnA signal along with a barely detectible huUgnB signal (Figure 4a), indicating that essentially no isomerization had occurred within the animal.
Figure 3.
Intravenously infused huUgnA is excreted in the urine in a form that activates cGMP synthesis, while infused huUgnB is excreted in a form that fails to activate cGMP synthesis. Either huUgnA (filled symbols) or huUgnB (open symbols) was infused into an anesthetized rat during the period indicated by the horizontal bar. Urine was obtained continuously over sequential 20 min clearance periods, before, during, and after the peptide infusion. A sample of the urine acquired during each clearance period was evaluated for activity in the T84 cell bioassay. Appropriate dilutions were chosen to ensure that the activity in each sample would not saturate this assay. The inset shows the huUgnB data replotted on an expanded Y-axis. The low levels of activity observed after huUgnB infusion most likely represent weak responses to very high levels of huUgnB (see text). The black and white arrowheads mark time points that were chosen for LC-MS analysis (see Figure 4).
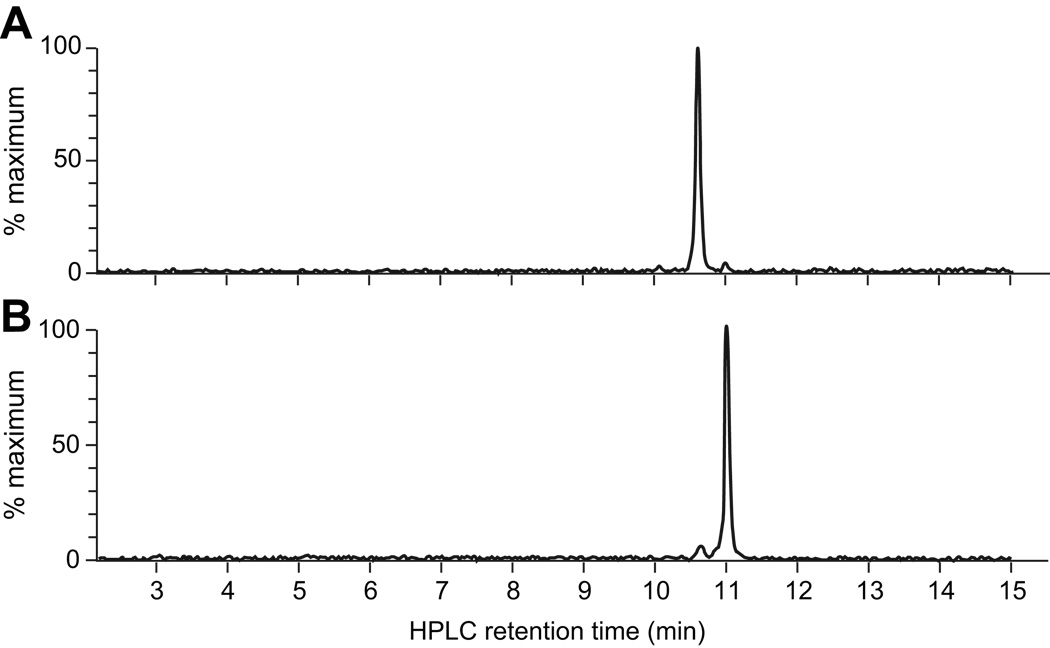
Figure 4.
Extracted ion chromatograms (m/z 1667–1669) from a full MS scan of urine that was obtained after infusion with either the A or B form of huUgn. (a) LC-MS analysis of a urine sample obtained from a huUgnA-infused animal at the end of the peptide infusion (marked by the black arrowhead in Figure 3a). The LC retention time of the prominent peak (10.61 min) corresponds to that of huUgnA, while the LC retention time of the minor peak (10.95) corresponds to that of huUgnB. (b) LC-MS analysis of a urine sample obtained from a huUgnB-infused animal at the end of the peptide infusion (marked by the white arrowhead in Figure 3a). The LC retention time of the prominent peak (10.99 min) corresponds to that of huUgnB, while the LC retention time of the minor peak (10.63) corresponds to that of huUgnA.
In contrast, when animals were infused with huUgnB, very little GC-C-stimulating activity was recovered in the urine (Figure 3, white symbols; representative of 6 independent experiments; the data are rescaled in the inset). The low levels of activity observed in the inset most likely represented weak responses to very high levels of huUgnB, as would be expected from the huUgnB dose-response curve (Figure 2a) and the well-known concentrating effect of the kidney (which generated a Ugn urine-to-plasma ratio of >500:1 in our studies). This interpretation was confirmed by the corresponding LC-MS analysis, which revealed a clearly discernable huUgnB signal in the urine along with a barely detectible huUgnA signal (Figure 4b).
We then calibrated the LC-MS procedure with a control sample that contained approximately equal amounts of the A and B peptides (Figure 1). The two isomers were detected with equal efficiency in the extracted ion chromatogram (compare peak areas in the dashed trace (the extracted chromatogram) to those in the solid trace (the UV absorbance)), arguing that the peak areas in Figures 4a and 4b provide an accurate assessment of the relative amounts of each isomer that were present in the urine. Taken together, these results indicate that infused huUgnA and huUgnB retain their identities and unique properties in vivo, and are sufficiently stable for use in acute animal studies.
Effects of huUgnA and B on renal sodium excretion
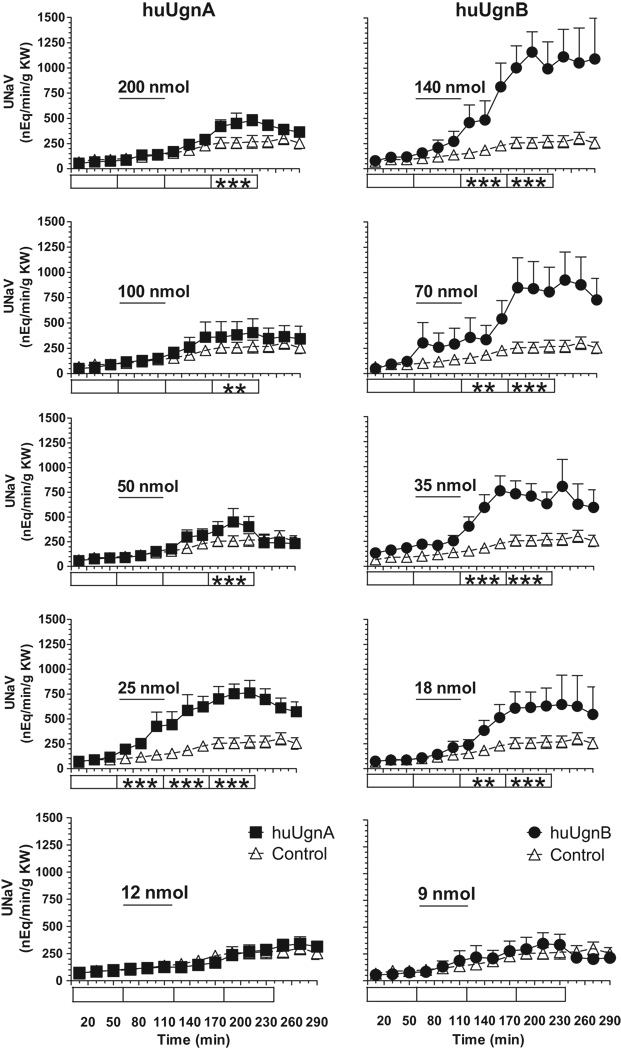
Figure 5 illustrates the effects of the two Ugn isomers on urinary sodium excretion. Prominent, dose-dependent natriuretic responses were observed, whereas the time control (saline infused) animals displayed relatively stable baseline levels of sodium excretion. Interestingly, although huUgnA and B each produced a natriuresis, the dose-dependencies of the responses were quite different. The dose-response relationship for huUgnB appeared to be conventional, with increasing doses causing a greater natriuresis, while the maximum response to huUgnA occurred at the relatively low dose of 25 nmol/kg and declined as doses increased. Two-way ANOVA revealed that highly significant responses were evoked by huUgnB at all doses greater than 9 nmol/kg, while responses to huUgnA were highly significant at 25 nmol/kg, and much smaller, though still significant, at higher doses (Figure 5).
Figure 5.
Time course of sodium excretion (UNaV, nEq/min/gKW) during infusions of huUgnA (filled squares, left column) or huUgnB (filled circles, right column) in the amounts indicated (nmol/kg BW). Peptides were infused during the time periods indicated by the horizontal bars. Rats in the control group (open triangles) received isotonic saline alone during the infusion period. Values are means ± SEM for each 20 min clearance period. Two-way ANOVA testing with post hoc comparisons was used to test significance of peptide responses against control values at various time points, as indicated in the boxes (blank = p>0.05 (not significant), * = p<0.05, ** = p<0.01, *** = p<0.001).
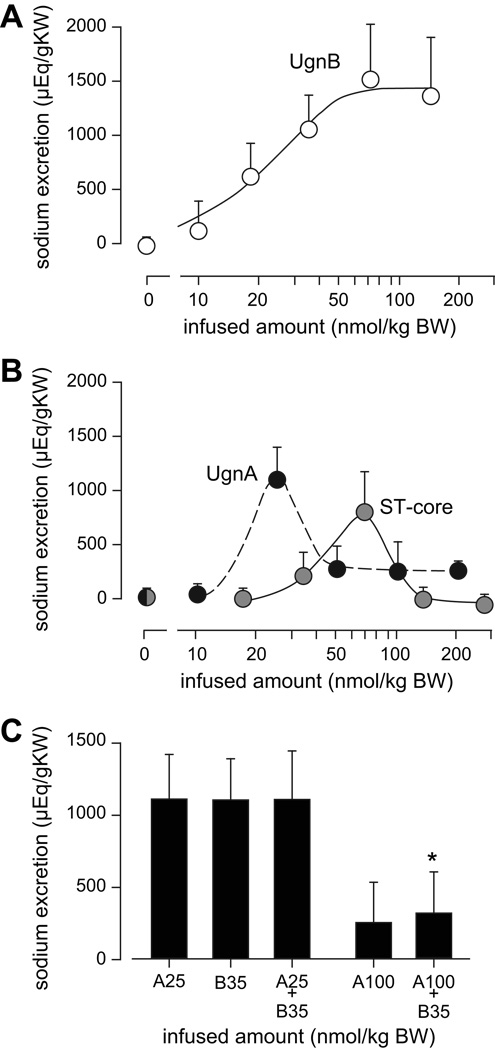
To display the dose-response relationship more conventionally, we calculated the cumulative net sodium excretion evoked by each dose of each isomer (as described in the Methods section). When these net excretory values were plotted, the resulting curve for huUgnB was well fit by the log (agonist) vs response equation (see methods; p<0.02), with an ED50 of about 20 nmol/kg BW (Figure 6a). In contrast, huUgnA produced a bell-shaped dose-response relation with a maximally effective dose at 25 nmol/kg (Figure 6b, black symbols). The striking loss of renal responsiveness to high concentrations of huUgnA is quite different from, and presumably unrelated to, the modest drop observed when extremely high doses of the peptide are applied to GC-C-expressing cells (Figure 2b).
Figure 6.
Dose-dependencies of the renal responses to huUgnA, huUgnB, and ST-core. (a) Net cumulative sodium excretion (total peptide-stimulated output minus corresponding control output) over a three hour period during and after huUgnB infusion, plotted as a function of infused dose. The curve is fitted to a log agonist response curve with an ED50 of 19 nmol/kg. (b) Net cumulative sodium excretion over a 3 hour period during and after infusions of huUgnA (open symbols) or ST-core (filled symbols). The curves were fitted by a cubic spline algorithm. (c) Net cumulative sodium excretion over a three hour period during and after combinatorial peptide infusions. Peptides were infused during the first hour in the following amounts and combinations: A25 = huUgnA 25 nmol/kg; B35 = huUgnB 35 nmol/kg; A100 + B35 = huUgnA 100 nmol/kg combined with huUgnB 35 nmol/kg; A25 + B35 = huUgnA 25 nmol/kg combined with huUgnB 35 nmol/kg. The natriuretic response to A25 + B35 was not different from the natriuresis evoked by A25 or B35 alone. The response to A100 + B35 was not different from the response to A100, but significantly less than the response to B35 alone (* p< 0.05).
The effects of the peptides on FENa were very similar to their effects on UNaV (Table 1). This, coupled with the lack of significant changes in GFR (Table 2), indicates that the peptides enhanced sodium excretion primarily by tubular transport mechanisms, rather than by hemodynamic mechanisms.
Table 1.
Effects of huUgnA and B on FENa, UKV, and FEK*
| treatment / nmol | FENa % of filtered | UKV nEq/min/g KW | FEK % of filtered | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | ||
| Pre- infuse | Infuse | Post- infuse | Pre-infuse | Infuse | Post- infuse | Pre-infuse | Infuse | Post-infuse | ||
| Time Control | 0.09±0.02 | 0.12±0.02 | 0.22±0.03 | 640±69 | 1096±64 | 1088±66 | 22.7±2.3 | 32.9±2.4 | 36.6±2.3 | 10 |
| huUgnA / 12 | 0.08±0.02 | 0.10±0.02 | 0.17±0.02 | 709±74 | 1097±54 | 1299±51‡ | 18.8±2.3 | 29.8±3.6 | 34.9±2.7 | 8 |
| huUgnA / 25 | 0.09±0.01 | 0.27±0.08§ | 0.68±0.08§ | 780±69 | 1292±80‡ | 1309±68§ | 25.6±2.4 | 40.7±3.5 | 46.5±3.8‡ | 8 |
| huUgnA / 50 | 0.06±0.01 | 0.10±0.01 | 0.28±0.05 | 722±52 | 988±66 | 1180±57 | 20.8±2.1 | 32.0±2.7 | 36.3±3.6 | 14 |
| huUgnA / 100 | 0.06±0.01 | 0.12±0.03 | 0.27±0.06 | 602±51 | 878±60¶ | 970±72 | 19.3±2.5 | 28.0±3.5 | 29.9±2.9¶ | 5 |
| huUgnA / 200 | 0.06±0.01 | 0.11±0.02 | 0.29±0.03 | 860±118 | 1203±161 | 1380±73§ | 27.7±5.9 | 40.8±7.7 | 57.5±6.7§ | 3 |
| huUgnB / 9 | 0.05±0.01 | 0.09±0.02 | 0.21±0.04 | 655±79 | 840±83¶ | 943±82 | 23.4±6.0 | 30.6±6.1 | 34.8±3.1 | 4 |
| huUgnB / 18 | 0.07±0.01 | 0.12±0.03 | 0.42±0.08‡ | 592±71 | 860±72|| | 1014±79 | 20.0±2.3 | 29.6±3.2 | 39.2±2.6 | 10 |
| huUgnB / 35 | 0.12±0.02 | 0.27±0.07 | 0.62±0.10§ | 729±79 | 837±84¶ | 998±85 | 25.7±2.4 | 32.6±3.7 | 38.9±3.0 | 10 |
| huUgnB / 70 | 0.08±0.03 | 0.24±0.14 | 0.52±0.10§ | 791±265 | 1297±209† | 1491±183§ | 20.4±5.5 | 37.7±9.8 | 47.1±5.6‡ | 8 |
| huUgnB / 140 | 0.08±0.01 | 0.18±0.05 | 0.65±0.11§ | 770±78 | 1086±124 | 1396±89§ | 28.3±0.9 | 49.2±3.6§ | 58.4±3.5§ | 3 |
Mean values ± SEM of sodium and potassium excretion in the 60 min before peptide infusion (Pre-infuse), during 60 min of huUgnA or huUgnB infusion (Infuse), and during the 120 minutes after peptide infusion (Post-infuse). The doses of peptide infused are shown in nmol, and the number of animals is given in the last column. Experimental values in each column were tested against corresponding values in the time control group by two-way ANOVA and the Bonferroni method for selected multiple comparisons.
Symbols indicate a statistically significant increase † = p<0.05, ‡ = p<0.01, § p<0.005) or decrease || = p<0.05, ¶ = p<0.01) relative to control.
Table 2.
Effects of huUgnA and B on mean arterial pressure, urine flow, and glomerular filtration rate*
| treatment / nmol | BP mmHg | V µl/min/g KW | GFR µl/min/g KW | n | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | ||
| Pre- infuse | Infuse | Post- infuse | Pre- infuse | Infuse | Post- infuse | Pre-infuse | Infuse | Post-infuse | ||
| Time Control | 123±3 | 114±3 | 109±5 | 2.1±0.2 | 2.6±0.2 | 2.8±0.2 | 814±77 | 882±71 | 901±69 | 10 |
| huUgnA / 12 | 121±4 | 110±4 | 104±3 | 2.1±0.2 | 2.3±0.1 | 2.7±0.1 | 895±51 | 869±64 | 923±66 | 8 |
| huUgnA / 25 | 119±3 | 111±4 | 105±4 | 2.1±0.2 | 3.0±0.3 | 3.5±0.3‡ | 820±40 | 886±66 | 823±49 | 8 |
| huUgnA / 50 | 117±3 | 106±3 | 101±3 | 2.0±0.1 | 2.3±0.2 | 3.1±0.2 | 858±52 | 817±53 | 869±49 | 14 |
| huUgnA / 100 | 121±4 | 115±3 | 109±4 | 1.8±0.2 | 2.5±0.3 | 2.7±0.2 | 807±62 | 858±55 | 906±38 | 5 |
| huUgnA / 200 | 121±7 | 113±5 | 112±4 | 2.1±0.4 | 2.8±0.4 | 4.1±0.4§ | 857±43 | 827±61 | 849±24 | 3 |
| huUgnB / 9 | 115±5 | 108±6 | 107±5 | 1.8±0.3 | 2.2±0.2 | 2.6±0.2 | 921±57 | 918±66 | 947±60 | 4 |
| huUgnB / 18 | 124±2 | 117±3 | 113±3 | 1.8±0.1 | 2.4±0.2 | 3.1±0.3 | 890±55 | 936±76 | 877±80 | 10 |
| huUgnB / 35 | 118±3 | 108±4 | 107±3 | 2.4±0.3 | 2.5±0.2 | 3.8±0.3§ | 866±64 | 830±48 | 870±53 | 10 |
| huUgnB / 70 | 119±3 | 107±5 | 107±4 | 2.0±0.2 | 3.1±0.7† | 3.9±0.5§ | 883±95 | 867±9 | 876±26 | 8 |
| huUgnB / 140 | 123±5 | 119±5 | 117±5 | 2.2±0.1 | 2.7±0.4 | 4.5±0.8§ | 858±86 | 788±67 | 924±46 | 3 |
Mean values ± SEM of mean arterial blood pressure (BP), urine flow rate (V), and glomerular filtration rate (GFR) in the 60 min before peptide infusion (Pre-infuse), during 60 min of huUgnA or huUgnB infusion (Infuse), and during the 120 minutes after peptide infusion (Post-infuse). The doses of peptide are shown in nmol, and the number of animals (n) is given in the last column. Experimental values in each column were tested against corresponding values in the time control group by two-way ANOVA and the Bonferroni method for selected multiple comparisons.
Daggers indicate a statistically significant increase relative to control († = p<0.05, ‡ = p<0.01, § = p<0.005).
One striking similarity between the two peptides was the relatively long latency (~ 50 min) and prolonged duration of the evoked responses, with a prominent natriuresis still recognizable at the end of the observation period. The sluggish nature of these responses cannot be accounted for by an unexpectedly slow rate of peptide delivery to the kidney, since it is apparent by comparing Figure 5 to Figure 3a that the majority of the peptide-evoked natriuresis occurred long after the infused peptides had been cleared from the animals.
The natriuretic dose-response to huUgnA is mimicked by ST-core
The unconventional natriuretic dose-response relationship for huUgnA was unexpected, as no previous study has reported this kind of response to any peptide in the Gn/Ugn family. With this in mind, the infusion protocol was repeated with ST-core, the active domain of a bacterial peptide toxin 38 that is a structural and functional analog of UgnA. The EC50 for cyclic GMP stimulation in T84 cells by ST-core is about 10 fold lower than that of huUgnA. If these relative potencies extend to the kidney, then the effects of ST-core should be similar, but slightly left-shifted, compared to those of huUgnA. This proved to be the case in a qualitative sense, as the similarity between the overall response patterns was striking (Figure 6b). However, surprisingly, ST-core appeared to be a less effective natriuretic factor than huUgnA: the maximal natriuresis elicited by ST-core was slightly less and the response was shifted to the right.
Responses to huUgnA and B are not additive
Given the distinctive nature of the response profiles obtained with the A and B isomers, it was of interest to determine how the two peptides would interact when applied simultaneously. To investigate this, animals were infused with mixed peptide solutions composed of huUgnB at 35 nmol/kg and huUgnA at either 25 or 100 nmol/kg. In this way, an effective dose of huUgnB was coupled with either an equipotent dose of huUgnA (25 nmol/kg), or a non-effective, supramaximal dose of huUgnA (100 nmol/kg). The results of these co-infusion protocols are shown in Figure 6c. Interestingly, combined maximally-active doses of huUgnA and B did not produce an additive response. Furthermore, the normal natriuretic response to 35 nmol huUgnB was almost completely suppressed when combined with the 100 nmol supramaximal dose of huUgnA, indicating a profound physiological interaction between the two peptides at high concentrations.
Effects of huUgnA and B on other physiological parameters
Blood pressure, Renal Blood Flow, and GFR
Blood pressure declined slightly over time in all animals, but did not fall below 100 mmHg in any group (Table 2). There were no significant differences between mean arterial pressures in control and experimental groups during the pre-infusion, infusion, or post-infusion time periods (Table 2)
Renal blood flow (RBF) was stable in control rats at 2.6±0.4 ml/min/g KW in the pre-infusion period and 2.5±0.5 ml/min/g KW in the post-infusion period (n=4). RBF in huUgnA and B infused rats was not different from the control group during the pre-infusion period, though small but significant increases in RBF occurred after 25 nmol of UgnA (2.7±0.3 to 3.2±0.1 ml/min/g KW, p<0.001, n=4) and after 35 nmol of huUgnB/kg (2.5±0.1 to 3.0±0.4 p<0.001 n=4). These changes corresponded to small, non-significant reductions in vascular resistance. GFR was stable in all groups (Table 2).
Diuresis
A slight increase in urine flow occurred in all groups during the first hour of the protocol (Table 2). In control rats, for example, urine flow increased from 2.1±0.2 in the pre-infusion period to 2.6±0.2 µl/min/g KW in the infusion period (Table 2). However, all experimental groups showed this same pattern, with no statistically significant differences among them. Similarly, urine flows were also not different from the control group during peptide infusions. However, urine flow was significantly higher than control in the post-infusion period at several doses of huUgnA and B, most notably at the high ends of the dose-response curves (Table 2).
Kaliuresis
The effects of huUgnA and B on potassium excretion (summarized in Table 1) were more complex, and less pronounced, than their effects on sodium excretion. Again there were distinct differences between the isomers. The kaliuretic actions of the A isomer were bimodal, with responses observed at low and high dosages and no effect at intermediate dosages, whereas the B isomer was convincingly active only at high dosages. The kaliuretic effects of these peptides at the upper ends of their dose-response curves may be related to their diuretic actions (noted above), as potassium excretion is strongly enhanced by increased tubular fluid flow through the late distal nephron and collecting ducts 39,40. Interestingly, the kaliuretic effects of huUgnA at low dosage occurred over the concentration range in which this peptide was most effective as a natriuretic (Figure 5 and Figure 6b). It is not clear, however, that a common mechanism underlies these low dose natriuretic and kaliuretic responses to the A isomer, as low doses of the B isomer were also natriuretic, but had no kaliuretic activity. Indeed, the data suggest that the B isomer may have been slightly antikaliuretic over this range (Table 1).
DISCUSSION
Members of the guanylin peptide family display an unusual form of structural isomerism that has been recognized for more than a decade 30. Until the studies reported here, however, the B conformation of these peptides has been considered physiologically inert because of its very low activity when tested on intestinal GC-C receptors. The discovery that huUgnB potently elicits renal natriuresis is, thus, a striking finding, and is the first reported physiological response specifically attributable to the B isomer in this peptide family. It is also, to our knowledge, the first time that two distinct topoisomers of a single peptide have been shown to have biological activity.
Pharmacological considerations
The pharmacological profile of the renal responses (huUgnB ≥ huUgnA ≈ ST core, with physiological antagonism by huUgnA at high concentration) does not match the well-established pharmacological profile of GC-C-mediated responses (ST core > huUgnA >> huUgnB, with all three peptides acting as pure agonists). This raises the possibility that a novel receptor, distinct from GC-C, mediates the renal natriuretic effects of huUgnB. Indeed, several other lines of evidence suggest the existence of at least one alternative Ugn receptor. The most compelling comes from the GC-C knockout mouse, in which rat Ugn and STa elicit only small, residual secretory responses from the intestine 41 but apparently full natriuretic responses from the kidneys 13. Furthermore, although GC-C mRNA expression has, in fact, been demonstrated in the kidneys of both rats and mice 13,42,43, studies on isolated principal cells from the cortical collecting duct 44 and cultures of a proximal tubule-derived human cell line 45 have shown responses to Ugn and Gn that implicate multiple receptor types which, in the case of principal cells in the cortical collecting ducts, do not appear to include GC-C. However, since huUgnB has never been tested for activity in the GC-C knockout animals, it remains possible that the responses to huUgnB in our rats is related in some way to GC-C activation, perhaps through a form of GC-C that has been post-translationally modified in the kidney to alter its ligand specificity.
The results of our experiments also do not allow us to determine whether huUgnA and huUgnB share a common receptor in the kidney. The lack of additivity when a natriuretic dose of the A peptide was paired with a natriuretic dose of the B peptide is consistent with this idea, but other possibilities can be anticipated, given the complex nature of the intact animal. Similarly, the physiological antagonism observed when a high dose of the A peptide was paired with a natriuretic dose of the B peptide could reflect interactions at a single receptor, or interactions mediated by independent receptors that have competing functional consequences. In this regard, it will be of great interest to examine whether huUgnA (especially at high doses) displays physiological antagonism with other natriuretic peptides, such as ANP. Using such an approach, one published study has already demonstrated unusual physiological interactions between Ugn and ANP (which, depending on the dose, can be either synergistic or antagonistic) 46.
As a final pharmacological consideration, the limited dose range of the natriuretic response to huUgnA seen in our studies (apparent in the bell-shaped curve presented in Figure 6b) has not been reported before. In seeking confirmatory evidence to verify this unexpected finding, we found that the renal responses evoked by ST-core (a well-established Ugn-mimetic that is locked into the A conformation by the presence of an extra disulfide bond) were right-shifted, but otherwise quite similar to those evoked by huUgnA. This validates the unusual dose-dependency of the huUgnA response, and further underscores the unique pharmacological profile of the renal responses that we report here. The failure to observe this unusual dose-dependency in previous studies is likely a reflection of differences in experimental preparations and animal species, as well as differences in the primary sequences and isomerization rates of the peptides that were employed.
Physiological considerations
The potential for differential actions of, and complex interactions between, the A and B isomers emphasizes the importance of carefully quantifying the ratio of the two peptides in physiological experiments. However, potential contributions of the B isomer have gone unrecognized in previous renal function studies. In fact, although huUgn is sold commercially as purified, semi-stable huUgnA, many laboratories use the rat or opossum forms of Ugn, which rapidly interconvert between A and B forms at physiological temperatures, as does Gn from any species. In light of our current results, it should be noted that all physiological effects on the kidney that have been reported previously using Gn or non-human Ugn raise the possibility of a contribution from the B isomer.
Despite differences in the peptides and preparations employed, a number of similarities between our current results and previous renal function studies should be emphasized. Ugn is natriuretic, kaliuretic, and diuretic in every preparation that has been examined 16. In general, these responses develop slowly and last for a long time. In addition, the natriuretic effects of Ugn are typically more pronounced than are the diuretic and kaliuretic effects (see figure 5, Table 1, and Fonteles et al. 14). However, it is not yet clear why kaliuresis and diuresis require higher doses of huUgnB than does natriuresis, nor why huUgnA is natriuretic and kaliuretic at low doses and predominantly kaliuretic at high doses. Some of this complexity may result from independent actions exerted by one or both of the isomers on distinct receptors and/or distinct cell types in the kidney, as has been suggested in previous studies 44,45. Interestingly, although we did not test Gn in our studies, this peptide was found to be more kaliuretic than Ugn by Fonteles and coworkers 14. This is in contrast to the effects of rat Gn and Ugn on GC-C receptors, where the latter is about 10-fold more potent 2, and is yet a further indication of the unusual pharmacological profile inherent to the renal responses evoked by this peptide family.
To play a physiological role in an animal, the B isomer must have biological availability as well as biological activity. Immunoassay studies by Nakazato et al 47 have identified both huUgnA and huUgnB in human plasma (at a ratio of approximately 3:1), using antibodies that were individually raised against each isomer. Similar measurements have not been reported for non-human animals, but it is likely that their A:B ratio will be close to 1:1, given the nearly-instantaneous rate of equilibration between the two isomers in these species. However, it has also been shown in rats that Ugn is stored in the intestine and circulates in plasma predominantly in the form of unprocessed prouroguanylin, and that propeptide processing is primarily a post-secretory event that is mediated locally by target organs such as the kidney 17,34. Whether a specific target organ possesses molecular machinery that could preferentially produce or stabilize a specific, locally-produced Ugn isoform remains to be determined.
Perspectives
The biological relevance of the B isomer of Ugn is firmly established by our new evidence of potent renal activity, and by previous studies that confirm its presence in human plasma and urine 47. The peptide literature contains very few descriptions of this kind of topoisomerism 48, and we believe that we are the first to demonstrate two unconventional concepts: (a) that independent topoisomers of a single peptide can both have biological activity, and (b) that target tissues can discriminate between these topoisomers (as demonstrated by the dissimilar spectra of ligand sensitivities observed in gut and kidney). The dramatic differences in the three dimensional structures of the two isomers raise interesting questions about how such divergent biological activities could have emerged evolutionarily, and whether all (or only some) species of animals will display such dual functionality. Furthermore, the availability of a semi-stable form of the B isomer provides new avenues for investigating the physiological features of the UGn-based entero-renal endocrine axis in mammals, as well as the as-yet unidentified receptor(s) that mediate(s) its actions in the kidney. Our results also have the potential to lead to novel clinical concepts and/or treatments that are applicable to hypertension, and other disorders of fluid and electrolyte homeostasis.
ACKNOWLEDGEMENTS
We thank Robert C. Fellner and Amanda L. Fitzgerald for help with T84 cultures, and Kathleen Dunlap for comments on the manuscript.
SOURCES OF FUNDING
This work was supported by the National Institutes of Health (HL078980) and the American Heart Association (0755397U).
Footnotes
CONFLICT OF INTEREST
Robert M. Solinga, Marco M. Kessler, Daniel P. Zimmer, and Mark G. Currie are employees of Ironwood Pharmaceuticals, Inc.
References
- 1.Currie MG, Fok KF, Kato J, Moore RJ, Hamra FK, Duffin KL, Smith CE. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1992;89:947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamra FK, Forte LR, Eber SL, Pidhorodeckyj NV, Krause WJ, Freeman RH, Chin DT, Tompkins JA, Fok KF, Smith CE. Uroguanylin: structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1993;90:10464–10468. doi: 10.1073/pnas.90.22.10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perkins A, Goy MF, Li Z. Uroguanylin is expressed by enterochromaffin cells in the rat gastrointestinal tract. Gastroenterology. 1997;113:1007–1014. doi: 10.1016/s0016-5085(97)70198-7. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Taylor-Blake B, Light AR, Goy MF. Guanylin, an endogenous ligand for C-type guanylate cyclase, is produced by goblet cells in the rat intestine. Gastroenterology. 1995;109:1863–1875. doi: 10.1016/0016-5085(95)90753-x. [DOI] [PubMed] [Google Scholar]
- 5.Joo NS, London RM, Kim HD, Forte LR, Clarke LL. Regulation of intestinal Cl- and HCO3-secretion by uroguanylin. Am J Physiol. 1998;274:G633–G644. doi: 10.1152/ajpgi.1998.274.4.G633. [DOI] [PubMed] [Google Scholar]
- 6.Donowitz M, Cha B, Zachos NC, Brett CL, Sharma A, Tse CM, Li X. NHERF family and NHE3 regulation. J Physiol. 2005;567:3–11. doi: 10.1113/jphysiol.2005.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulz S, Green CK, Yuen PS, Garbers DL. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990;63:941–948. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- 8.Nakazato M, Yamaguchi H, Kinoshita H, Kangawa K, Matsuo H, Chino N, Matsukura S. Identification of biologically active and inactive human uroguanylins in plasma and urine and their increases in renal insufficiency. Biochem Biophys Res Commun. 1996;220:586–593. doi: 10.1006/bbrc.1996.0447. [DOI] [PubMed] [Google Scholar]
- 9.Date Y, Nakazato M, Yamaguchi H, Miyazato M, Matsukura S. Tissue distribution and plasma concentration of human guanylin. Intern Med. 1996;35:171–175. doi: 10.2169/internalmedicine.35.171. [DOI] [PubMed] [Google Scholar]
- 10.Fan X, Hamra FK, Freeman RH, Eber SL, Krause WJ, Lim RW, Pace VM, Currie MG, Forte LR. Uroguanylin: cloning of preprouroguanylin cDNA, mRNA expression in the intestine and heart and isolation of uroguanylin and prouroguanylin from plasma. Biochem Biophys Res Commun. 1996;219:457–462. doi: 10.1006/bbrc.1996.0255. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn M, Kulaksiz H, Adermann K, Rechkemmer G, Forssmann WG. Radioimmunoassay for circulating human guanylin. FEBS Lett. 1994;341:218–222. doi: 10.1016/0014-5793(94)80460-5. [DOI] [PubMed] [Google Scholar]
- 12.Hess R, Kuhn M, Schulz-Knappe P, Raida M, Fuchs M, Klodt J, Adermann K, Kaever V, Cetin Y, Forssmann WG. GCAP-II: isolation and characterization of the circulating form of human uroguanylin. FEBS Lett. 1995;374:34–38. doi: 10.1016/0014-5793(95)01075-p. [DOI] [PubMed] [Google Scholar]
- 13.Carrithers SL, Ott CE, Hill MJ, Johnson BR, Cai W, Chang JJ, Shah RG, Sun C, Mann EA, Fonteles MC, Forte LR, Jackson BA, Giannella RA, Greenberg RN. Guanylin and uroguanylin induce natriuresis in mice lacking guanylyl cyclase-C receptor. Kidney Int. 2004;65:40–53. doi: 10.1111/j.1523-1755.2004.00375.x. [DOI] [PubMed] [Google Scholar]
- 14.Fonteles MC, Greenberg RN, Monteiro HSA, Currie MG, Forte LR. Natriuretic and kaliuretic activities of guanylin and uroguanylin in the isolated perfused rat kidney. Am J Physiol. 1998;44:F191–F197. doi: 10.1152/ajprenal.1998.275.2.F191. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg RN, Hill M, Crytzer J, Krause WJ, Eber SL, Hamra FK, Forte LR. Comparison of effects of uroguanylin, guanylin, and Escherichia coli heat-stable enterotoxin STa in mouse intestine and kidney: Evidence that uroguanylin is an intestinal natriuretic hormone. J Invest Med. 1997;45:276–283. [PubMed] [Google Scholar]
- 16.Forte LR. Uroguanylin and guanylin peptides: pharmacology and experimental therapeutics. Pharmacology & Therapeutics. 2004;104:137–162. doi: 10.1016/j.pharmthera.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Qian X, Moss NG, Fellner RC, Goy MF. Circulating prouroguanylin is processed to its active natriuretic form exclusively within the renal tubules. Endocrinology. 2008;149:4499–4509. doi: 10.1210/en.2007-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubel KA. Intestinal nerves and ion transport: stimuli, reflexes, and responses. Am J Physiol. 1985;248:G261–G271. doi: 10.1152/ajpgi.1985.248.3.G261. [DOI] [PubMed] [Google Scholar]
- 19.Braun T, Voland P, Kunz L, Prinz C, Gratzl M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology. 2007;132:1890–1901. doi: 10.1053/j.gastro.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson O, Ahlman H, Geffard M, Dahlstrom A, Ericson LE. Bipolarity of duodenal enterochromaffin cells in the rat. Cell Tissue Res. 1987;248:49–54. doi: 10.1007/BF01239961. [DOI] [PubMed] [Google Scholar]
- 21.Specian RD, Oliver MG. Functional biology of intestinal goblet cells. Am J Physiol. 1991;260:C183–C193. doi: 10.1152/ajpcell.1991.260.2.C183. [DOI] [PubMed] [Google Scholar]
- 22.Martin S, Adermann K, Forssmann WG, Kuhn M. Regulated, side-directed secretion of proguanylin from isolated rat colonic mucosa. Endocrinology. 1999;140:5022–5029. doi: 10.1210/endo.140.11.7103. [DOI] [PubMed] [Google Scholar]
- 23.Moro F, Levenez F, Nemoz-Gaillard E, Pellissier S, Plaisancie P, Cuber JC. Release of guanylin immunoreactivity from the isolated vascularly perfused rat colon. Endocrinology. 2000;141:2594–2599. doi: 10.1210/endo.141.7.7574. [DOI] [PubMed] [Google Scholar]
- 24.Kita T, Smith CE, Fok KF, Duffin KL, Moore WM, Karabatsos PJ, Kachur JF, Hamra FK, Pidhorodeckyj NV, Forte LR. Characterization of human uroguanylin: a member of the guanylin peptide family. Am J Physiol. 1994;266:F342–F348. doi: 10.1152/ajprenal.1994.266.2.F342. [DOI] [PubMed] [Google Scholar]
- 25.Santos-Neto MS, Carrithers SL, Carvalho AF, Monteiro HS, Greenberg RN, Forte LR, Fonteles MC. Guanylin and its lysine-containing analogue in the isolated perfused rat kidney: interaction with chymotrypsin inhibitor. Pharmacol Toxicol. 2003;92:114–120. doi: 10.1034/j.1600-0773.2003.920302.x. [DOI] [PubMed] [Google Scholar]
- 26.Hamra FK, Fan X, Krause WJ, Freeman RH, Chin DT, Smith CE, Currie MG, Forte LR. Prouroguanylin and proguanylin: purification from colon, structure, and modulation of bioactivity by proteases. Endocrinology. 1996;137:257–265. doi: 10.1210/endo.137.1.8536621. [DOI] [PubMed] [Google Scholar]
- 27.Lorenz JN, Nieman M, Sabo J, Sanford LP, Hawkins JA, Elitsur N, Gawenis LR, Clarke LL, Cohen MB. Uroguanylin knockout mice have increased blood pressure and impaired natriuretic response to enteral NaCl load. J Clin Invest. 2003;112:1244–1254. doi: 10.1172/JCI18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elitsur N, Lorenz JN, Hawkins JA, Rudolph JA, Witte D, Yang LE, McDonough AA, Cohen MB. The proximal convoluted tubule is a target for the uroguanylin-regulated natriuretic response. Journal of Pediatric Gastroenterology and Nutrition. 2006;43:S74–S81. doi: 10.1097/01.mpg.0000228092.36089.7c. [DOI] [PubMed] [Google Scholar]
- 29.Kita T, Smith CE, Fok KF, Duffin KL, Moore WM, Karabatsos PJ, Kachur JF, Hamra FK, Pidhorodeckyj NV, Forte LR, Currie MG. Characterization of Human Uroguanylin - A Member of the Guanylin Peptide Family. Am J Physiol. 1994;266:F342–F348. doi: 10.1152/ajprenal.1994.266.2.F342. [DOI] [PubMed] [Google Scholar]
- 30.Skelton NJ, Garcia KC, Goeddel DV, Quan C, Burnier JP. Determination of the solution structure of the peptide hormone guanylin: observation of a novel form of topological stereoisomerism. Biochemistry. 1994;33:13581–13592. doi: 10.1021/bi00250a010. [DOI] [PubMed] [Google Scholar]
- 31.Schulz A, Marx UC, Tidten N, Lauber T, Hidaka Y, Adermann K. Side chain contributions to the interconversion of the topological isomers of guanylin-like peptides. J Pept Sci. 2005;11:319–330. doi: 10.1002/psc.625. [DOI] [PubMed] [Google Scholar]
- 32.Klodt J, Kuhn M, Marx UC, Martin S, Rosch P, Forssmann WG, Adermann K. Synthesis, biological activity and isomerism of guanylate cyclase C-activating peptides guanylin and uroguanylin. J Pept Res. 1997;50:222–230. doi: 10.1111/j.1399-3011.1997.tb01188.x. [DOI] [PubMed] [Google Scholar]
- 33.Chino N, Kubo S, Kitani T, Yoshida T, Tanabe R, Kobayashi Y, Nakazato M, Kangawa K, Kimura T. Topological isomers of human uroguanylin: interconversion between biologically active and inactive isomers. FEBS Lett. 1998;421:27–31. doi: 10.1016/s0014-5793(97)01527-5. [DOI] [PubMed] [Google Scholar]
- 34.Moss NG, Fellner RC, Qian X, Yu SJ, Li Z, Nakazato M, Goy MF. Uroguanylin, an intestinal natriuretic peptide, is delivered to the kidney as an unprocessed propeptide. Endocrinology. 2008;149:4486–4498. doi: 10.1210/en.2007-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorenz JN, Gruenstein E. A simple, nonradioactive method for evaluating single-nephron filtration rate using FITC-inulin. Am J Physiol. 1999;276:F172–F177. doi: 10.1152/ajprenal.1999.276.1.F172. [DOI] [PubMed] [Google Scholar]
- 36.Potter LR, Garbers DL. Dephosphorylation of the guanylyl cyclase-A receptor causes desensitization. J Biol Chem. 1992;267:14531–14534. [PubMed] [Google Scholar]
- 37.Potter LR. Phosphorylation-dependent regulation of the guanylyl cyclase-linked natriuretic peptide receptor B: dephosphorylation is a mechanism of desensitization. Biochemistry. 1998;37:2422–2429. doi: 10.1021/bi972303k. [DOI] [PubMed] [Google Scholar]
- 38.Aimoto S, Yoshimura S, Hidaka Y, Ikemura H, Shimonishi Y. Chemical synthesis of heat-stable enterotoxins produced by enteric bacteria - structure and biological activity. In: Deber CM, Hruby VJ, Kopple KD, editors. Peptides, Structure and Function, Proceedings of the Ninth American peptide Symposium; Rockford, IL. Pierce Chemical Company; 1985. pp. 847–850. [Google Scholar]
- 39.Grimm PR, Sansom SC. BK channels in the kidney. Curr Opin Nephrol Hypertens. 2007;16:430–436. doi: 10.1097/MNH.0b013e32826fbc7d. [DOI] [PubMed] [Google Scholar]
- 40.Rieg T, Vallon V, Sausbier M, Sausbier U, Kaissling B, Ruth P, Osswald H. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int. 2007;72:566–573. doi: 10.1038/sj.ki.5002369. [DOI] [PubMed] [Google Scholar]
- 41.Sellers ZM, Mann E, Smith A, Ko KH, Giannella R, Cohen MB, Barrett KE, Dong H. Heat-stable enterotoxin of Escherichia coli (STa) can stimulate duodenal HCO3(-) secretion via a novel GC-C- and CFTR-independent pathway. FASEB J. 2008;22:1306–1316. doi: 10.1096/fj.06-7540com. [DOI] [PubMed] [Google Scholar]
- 42.Carrithers SL, Taylor B, Cai WY, Johnson BR, Ott CE, Greenberg RN, Jackson BA. Guanylyl cyclase-C receptor mRNA distribution along the rat nephron. Regul Pept. 2000;95:65–74. doi: 10.1016/s0167-0115(00)00139-7. [DOI] [PubMed] [Google Scholar]
- 43.Potthast R, Ehler E, Scheving LA, Sindic A, Schlatter E, Kuhn M. High salt intake increases uroguanylin expression in mouse kidney. Endocrinology. 2001;142:3087–3097. doi: 10.1210/endo.142.7.8274. [DOI] [PubMed] [Google Scholar]
- 44.Sindic A, Velic A, Basoglu C, Hirsch JR, Edemir B, Kuhn M, Schlatter E. Uroguanylin and guanylin regulate transport of mouse cortical collecting duct independent of guanylate cyclase C. Kidney Int. 2005;68:1008–1017. doi: 10.1111/j.1523-1755.2005.00518.x. [DOI] [PubMed] [Google Scholar]
- 45.Sindice A, Basoglu C, Cerci A, Hirsch JR, Potthast R, Kuhn M, Ghanekar Y, Visweswariah SS, Schlatter E. Guanylin, uroguanylin, and heat-stable enterotoxin activate guanylate cyclase C and/or a pertussis toxin-sensitive G protein in human proximal tubule cells. J Biol Chem. 2002;277:17758–17764. doi: 10.1074/jbc.M110627200. [DOI] [PubMed] [Google Scholar]
- 46.Santos-Neto MS, Carvalho AF, Monteiro HS, Forte LR, Fonteles MC. Interaction of atrial natriuretic peptide, urodilatin, guanylin and uroguanylin in the isolated perfused rat kidney. Regul Pept. 2006;136:14–22. doi: 10.1016/j.regpep.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 47.Nakazato M, Yamaguchi H, Kinoshita H, Kangawa K, Matsuo H, Chino N, Matsukura S. Identification of biologically active and inactive human uroguanylins in plasma and urine and their increases in renal insufficiency. Biochem and Biophy Res Commun. 1996;220:586–593. doi: 10.1006/bbrc.1996.0447. [DOI] [PubMed] [Google Scholar]
- 48.Favreau P, Krimm I, Le Gall F, Bobenrieth MJ, Lamthanh H, Bouet F, Servent D, Molgo J, Menez A, Letourneux Y, Lancelin JM. Biochemical characterization and nuclear magnetic resonance structure of novel alpha-conotoxins isolated from the venom of Conus consors. Biochemistry. 1999;38:6317–6326. doi: 10.1021/bi982817z. [DOI] [PubMed] [Google Scholar]