Abstract
A subtype of non-small cell lung cancer, bronchioalveolar adenocarcinoma (BAC), is more prevalent in Asian female non-smokers, and is more likely to respond to treatment with tyrosine kinase inhibitors such as erlotinib and gefitinib. Nuclear magnetic resonance and mass spectrometry-based metabolomic analysis of extracts from two different lung lesions and surrounding non-cancerous tissues of a BAC patient showed novel protein and phospholipid-associated metabolic differences that correlated with tumor development as well as PET and erlotinib sensitivity.
Keywords: bronchioalveolar adenocarcinoma, metabolomics, erlotinib
Introduction
Non-small cell lung cancer (NSCLC) accounts for roughly 85% of all lung cancers, most of which are tobacco-related. However, 10–15 % of lung cancer occur in nonsmokers and disproportionately in women (Wakelee et al. 2007). Among these is the slow-growing bronchioalveolar adenocarcinoma (BAC) subtype of NSCLC. Regardless of the etiology or subtypes, early stage lung cancer is generally difficult to detect and the median 5-year survival rate (ca. 15%) has barely changed in the last 20 years (Pirozynski 2006).
Current chemotherapies have not been as successful for NSCLC as for other solid tumors (Collaboration 2009). Recently, targeted therapies that inhibit tyrosine kinase receptors (including EGFR) have been developed. These agents block the signaling pathways that are important in stimulating tumor cell proliferation. Two agents in particular have gone through phase II/III clinical trials, namely gefitinib and erlotinib. Although the overall response rate in NSCLC to these agents has been disappointing, there is a sub-population that responds well to these inhibitors, namely those that carry a gain of function mutation in the EGFR. These mutations are found more frequently in females of Asian origin with the bronchioalveolar subtype of NSCLC (Ahmed et al. 2006; Giaccone et al. 2006; Kris et al. 2006; Raz et al. 2006; Riely et al. 2006).
Early and sensitive detection of lung cancer coupled with appropriate treatment may significantly improve overall survival from the disease. This may be explored through distinct metabolic activities of lung cancer cells, which are likely to precede any morphological change. The metabolism of cancer cells is altered to meet the demand for accelerated and uncontrolled growth. For example, many tumors have greatly enhanced glucose uptake and glycolysis as observed by PET detection, even under aerobic conditions (i.e. the Warburg effect (Warburg 1956;Lu et al. 2002; Altenberg et al. 2004; Garber 2004), leading to increased lactate secretion. Further, increased anabolic activity is required for the biosynthesis of macromolecules (protein, carbohydrate, lipids and nucleic acids) essential to cancer cell growth.
Although there has been extensive genetic analysis of lung tumors (Bhattacharjee et al. 2001; Brognard et al. 2001; Giaccone et al. 2006; Carretero et al. 2007; Il Na et al. 2007; Sakuma et al. 2007), the in situ metabolic differences between cancerous and normal lung tissues have not been systematically characterized. We have addressed this problem by developing NMR and mass spectrometry (MS)-based technologies to determine the profile of a wide range of metabolites and their biosynthetic pathways in lung cancer cells and tissues (Fan et al. 2005; Fan et al. 2006) and in human subjects (Lane et al. 2009) (and T. WM. Fan, A. N Lane, et al. submitted). Here we report the use of these technologies to compare the metabolic profiles of a stage I bronchioalveolar, erlotinib-sensitive carcinoma, and an erlotinib-insensitive, slower-growing (possibly earlier developmental) lesion against normal lung tissue from the same patient. Many previously unknown, drug-dependent and -independent metabolic distinctions were thereby discovered.
Results
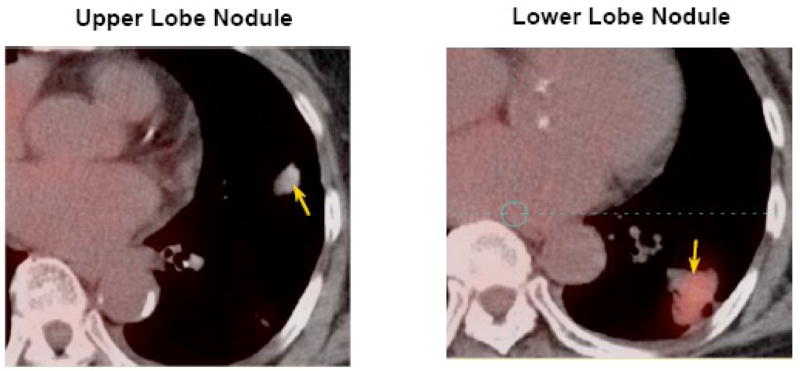
An 80-year old Asian female, never smoker was found to have two lung lesions. The PET scan (Figure 1) showed that the mass in the lower lobe (3.5×3.0 ×1.8 cm) was very active in glucose accumulation and a small nodule in the upper lobe (1.8×1.2×1.3 cm) was barely observable above background. Needle biopsy of the lower lobe lesion revealed bronchioalveolar adenocarcinoma (BAC) of pulmonary origin. After treatment with a daily dose of 150 mg of Erlotinib for one month, the lower lobe lesion was reduced by >50% in size, whereas the upper lobe lesion showed no response to the drug.
Figure 1.
PET images of upper and lower lobe lung lesions (indicated by arrows) of an 80-year old Asian female patient. Red to white colors indicates high to low density.
Lobectomy of the lower lobe (LL) and a wedge resection of the upper lobe (UL) lesion were performed and well tolerated by the patient. Pleural effusion and lymph nodes were clear of detectable cancer cells. A slice of each tumor was immediately flash frozen in liquid nitrogen (< 2 minutes after excision). A second slice was preserved in formalin for histopathology. A slice of normal tissue taken from the lobe > 4 cm from the cancerous margins was similarly treated. Blood samples were also collected pre- and post-operatively, immediately placed on ice, and centrifuged at 4 °C to separate the red cells from the plasma. Plasma was flash frozen in liquid nitrogen for storage at −80 °C with a total processing time of < 30 minutes. These protocols were developed to minimize artifactual metabolic changes during processing (Fan et al., submitted).
Pathology
Pathological examination of H&E stained tissue slices indicated both lesions to be well-differentiated adenocarcinomas with BAC-like features. The PET-positive, erlotinib-responsive LL lesion is a grade I BAC, whereas the weakly PET-positive nodule of UL was also a BAC-like tumor which may be independent of the LL lesion. Sections were also stained for EGFR using anti EGFR mAb, which showed low EGFR expression for the normal tissue, high expression in the UL lesion, and near normal expression in the LL lesion (possibly the result of Erlotinib treatment).
Metabolomics analysis
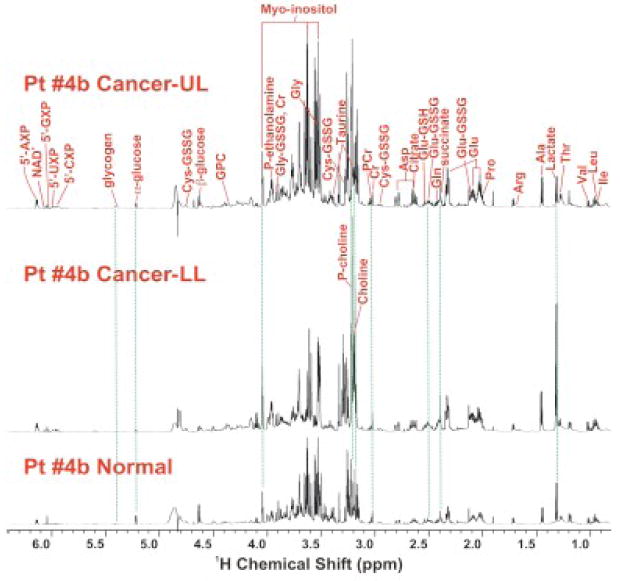
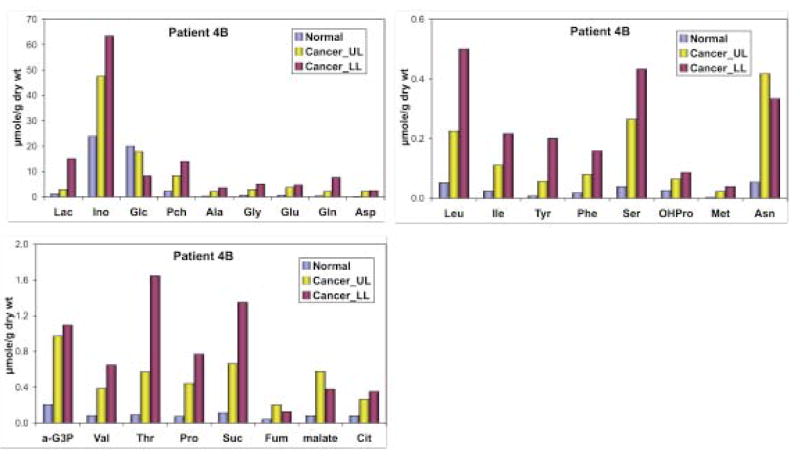
Polar metabolites from tissues and plasma were extracted according to our established protocols (Fan et al. 2005; Fan et al. 2008; Fan et al. 2008; Lane et al. 2008). Metabolites were identified and quantified using high resolution one and two-dimensional NMR spectroscopy as well as by GC-MS, as described previously (Fan et al. 1986; Fan et al. 2008; Lane et al. 2008). Figure 2 compares the one-dimensional 1H NMR spectra of normal versus lower lobe (LL) and upper lobe (UL) nodules. The three tissues showed significantly different 1H NMR profiles, particularly in terms of lactate, amino acids, phosphocholine, (P-choline), choline, and glucose. Metabolites profile had the general order of LL tumor ≥ UL lesion > normal tissue except for glucose and glycogen with a reverse order. Many of these metabolites were quantified by GC-MS and 1H NMR, as shown in Figure 3. The GC-MS analysis verified the semi-quantitative trend observed by 1H NMR in Figure 2. It is also clear that many of the metabolites (e.g. P-choline, Ser, OHPro, Asn, α-glycerol-3-phosphate or α-G3P, citrate, fumarate, malate, succinate) in the UL lesion were closer in concentration to the LL tumor than the normal lung tissue. However, lactate and glucose levels in the UL lesion exhibited more of the normal tissue type (Figure 3).
Figure 2.
1H NMR spectra of extracts of paired normal and lung lesions.
Flash frozen slices from upper lobe (UL, top panel), lower lobe (LL, middle panel) nodules, and non-cancerous tissues (bottom panel) were taken from the resected lung of the patient shown in Figure 1, and extracted with 10% TCA and 1H NMR spectra of extracts were recorded at 600 MHz. Metabolite assignments were based on our in-house database, 2-D 1H TOCSY and 1H-13C HSQC NMR spectra (not shown) (Fan et al. 2008). The three spectra were normalized in terms of tissue weight and NMR spectral parameters for direct comparison.
Figure 3.
Metabolite concentration in normal, upper and lower lobe lung nodules.
Myo-inositol (Ino), glucose (Glc), phosphocholine (PCh) were quantified from 1H NMR spectra in Fig. 1 while the other metabolites were quantified by GC-MS as described previously (Fan et al. 1986). Lac: lactate; α-G3P: α-glycerol-3-phosphate; Suc: succinate; Fum: fumarate; Cit: citrate. UL upper lobe, LL lower lobe
Discussion
Increased accumulation of lactate in the LL lesion is typical of malignant tumors and suggests the presence of accelerated glycolysis or Warburg effect. The depletion of glucose and glycogen in this lesion is presumably a result of this enhanced glycolysis, which is again linked to the much higher lactate content or accumulation of 18F-deoxyglucose-6-phosphate, as reflected by its brighter PET image density (Figure 1). By similar reasoning, the weak PET image density of the UL lesion is consistent with its near normal glucose and lactate profile. Thus, the UL lesion may not have undergone transformations into an enhanced glycolytic state, which differentiates its development from the LL lesion.
In contrast, some amino acids (e.g. Glu, Asp, Ser, OHPro, Met, Asn), P-choline, α-G3P, citrate, malate, fumarate, and succinate in the UL lesion accumulated to levels closer to the LL lesion than normal tissues. Cancer-associated accumulation of these metabolites was also observed in over 30 other non-BAC NSCLC cancer subjects (Fan et al., submitted), which suggests that metabolic transformations governing the accumulation have occurred in both UL and LL lesions. These transformations are likely to be involved in the uptake of amino acids and choline, choline phosphorylation, α-G3P production, as well as Krebs cycle function, all of which are essential to the synthesis of proteins and phospholipids. These processes appear to have preceded enhanced glucose uptake and glycolysis in the process of tumorigenesis, which is common to the LL lesion and later stage tumors from 20 other NSCLC patients. Thus, this case study suggests that deregulation of protein and phospholipid-associated metabolism could be early transforming events in BAC development, and demonstrates that metabolomic analysis may be valuable for differentiating early stage erlotinib-sensitive and insensitive NSCLC.
Acknowledgments
Supported by NIH Grant Number RR018733 from the National Center for Research Resources, National Science Foundation EPSCoR grant # EPS-0447479, NCI 1R01CA118434-01, the KY Lung Cancer Research Program, Kentucky Challenge for Excellence, and the Brown Foundation. We thank Ms. Lynn Deleeuw and Ms. Vennila Arumugum for sample processing.
Abbreviations used
- BAC
Bronchioalveolar Adenocarcinoma
- NSCLC
Non Small Cell lung Cancer
- EGFR
Epidermal Growth Factor Receptor
- MS
Mass Spectrometry
- PET
positron emission tomography
Footnotes
There were no potential conflicts of interest related to this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SM, Salgia R. Epidermal growth factor receptor mutations and susceptibility to targeted therapy in lung cancer. Respirology. 2006;11:687–692. doi: 10.1111/j.1440-1843.2006.00887.x. [DOI] [PubMed] [Google Scholar]
- Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014–1020. doi: 10.1016/j.ygeno.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Richards WG, Staunton J, Li C, Monti S, Vasa P, Ladd C, Beheshti J, Bueno R, Gillette M, Loda M, Weber G, Mark EJ, Lander ES, Wong W, Johnson BE, Golub TR, Sugarbaker DJ, Meyerson M. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13790–13795. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brognard J, Clark AS, Ni YC, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Research. 2001;61:3986–3997. [PubMed] [Google Scholar]
- Carretero J, Medina PP, Blanco R, Smit L, Tang M, Roncador G, Maestre L, Conde E, Lopez-Rios F, Clevers HC, Sanchez-Cespedes M. Dysfunctional AMPK activity, signalling through mTOR and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene. 2007;26:1616–1625. doi: 10.1038/sj.onc.1209951. [DOI] [PubMed] [Google Scholar]
- Collaboration TC. Library. John Wiley and Sons, Ltd; 2009. Chemotherapy for non-small cell lung cancer C; pp. 1–106. [Google Scholar]
- Fan T, Bandura L, Higashi R, Lane A. Metabolomics-edited transcriptomics analysis of Se anticancer action in human lung cancer cells. Metabolomics. 2005;1:1–15. [Google Scholar]
- Fan TW, Lane AN. Structure-based profiling of Metabolites and Isotopomers by NMR. Progress in NMR Spectroscopy. 2008;52:69–117. [Google Scholar]
- Fan TWM, Higashi RM, Lane AN. Integrating metabolomics and transcriptomics for probing Se anticancer mechanisms. Drug Metabolism Reviews. 2006;38:707–732. doi: 10.1080/03602530600959599. [DOI] [PubMed] [Google Scholar]
- Fan TWM, Higashi RM, Lane AN, Jardetzky O. Combined Use of H-1-NMR and GC-MS for Metabolite Monitoring and Invivo H-1-Nmr Assignments. Biochimica Et Biophysica Acta. 1986;882:154–167. doi: 10.1016/0304-4165(86)90150-9. [DOI] [PubMed] [Google Scholar]
- Garber K. Energy boost: The Warburg effect returns in a new theory of cancer. Journal of the National Cancer Institute. 2004;96:1805–1806. doi: 10.1093/jnci/96.24.1805. [DOI] [PubMed] [Google Scholar]
- Giaccone G, Ruiz MG, Le Chevalier T, Thatcher N, Smit E, Rodriguez JA, Janne P, Oulid-Aissa D, Soria JC. Erlotinib for frontline treatment of advanced non-small cell lung cancer: a phase II study. Clinical Cancer Research. 2006;12:6049–6055. doi: 10.1158/1078-0432.CCR-06-0260. [DOI] [PubMed] [Google Scholar]
- Na IlI, Rho JK, Choi YJ, Kim CH, Park JH, Koh JS, Ryoo BY, Yang SH, Lee JC. The survival outcomes of patients with resected non-small cell lung cancer differ according to EGFR mutations and the P21 expression. Lung Cancer. 2007;57:96–102. doi: 10.1016/j.lungcan.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Kris MG, Giaccone G, Davies A, Fukuoka M, Garfield DH, Jassem J, Quoix EA, Sandler AB, Scagliotti GV, Van Meerbeeck JP, West H. Systemic therapy of bronchioloalveolar carcinoma: Results of the first IASLC/ASCO consensus conference on bronchioloalveolar carcinoma. Journal of Thoracic Oncology. 2006;1:S32–S36. [PubMed] [Google Scholar]
- Lane AN, Fan TW, Higashi RM. Isotopomer-based metabolomic analysis by NMR and mass spectrometry. In: John HWD, Correia J, editors. Biophysical Tools for Biologists, Vol 1. Vol. 84. San Diego: Academic Press; 2008. pp. 541–588. [DOI] [PubMed] [Google Scholar]
- Lane AN, Fan TW, Higashi RM, Tan J, Bousamra M, Miller DM. Prospects for clinical cancer metabolomics using stable isotope tracers. J Exp Molec Pathol. 2009 doi: 10.1016/j.yexmp.2009.01.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HS, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. Journal of Biological Chemistry. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- Pirozynski M. 100 years of lung cancer. Respiratory Medicine. 2006;100:2073–2084. doi: 10.1016/j.rmed.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Raz DJ, He BA, Rosell R, Jablons DM. Bronchioloalveolar carcinoma: A review. Clinical Lung Cancer. 2006;7:313–322. doi: 10.3816/CLC.2006.n.012. [DOI] [PubMed] [Google Scholar]
- Riely GJ, Pao W, Pham DK, Li AR, Rizvi N, Venkatraman ES, Zakowski MF, Kris MG, Ladanyi M, Miller VA. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clinical Cancer Research. 2006;12:839–844. doi: 10.1158/1078-0432.CCR-05-1846. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Matsukuma S, Yoshihara M, Nakamura Y, Noda K, Nakayama H, Kameda Y, Tsuchiya E, Miyagi Y. Distinctive evaluation of nonmucinous and mucinous subtypes of bronchioloalveolar carcinomas in EGFR and K-ras gene-mutation analyses for Japanese lung adenocarcinomas - Confirmation of the correlations with histologic subtypes and gene mutations. American Journal of Clinical Pathology. 2007;128:100–108. doi: 10.1309/WVXFGAFLAUX48DU6. [DOI] [PubMed] [Google Scholar]
- Wakelee HA, Chang ET, Gomez SL, Keegan TH, Feskanich D, Clarke CA, Holmberg L, Yong LC, Kolonel LN, Gould MK, West DW. Lung cancer incidence in never smokers. Journal of Clinical Oncology. 2007;25:472–478. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]