Abstract
Introduction
Rapid growth of the elderly United States population will result in so many at risk of osteoporosis that economically efficient approaches to osteoporosis care warrant consideration.
Methods
A Markov-cohort model of annual United States age-specific incidence of clinical hip, spine, forearm, shoulder, rib, pelvis and lower leg fractures, costs (2005 US dollars), and quality-adjusted life years (QALYs) was used to assess the cost-effectiveness of osteoporosis treatment ($600/yr drug cost for 5 years with 35% fracture reduction) by gender and race/ethnicity groups. To determine the 10-year hip fracture probability at which treatment became cost-effective, average annual age-specific probabilities for all fractures were multiplied by a relative risk (RR) that was systematically varied from 0 to 10 until a cost of $60,000 per QALY gained was observed for treatment relative to no intervention.
Results
Osteoporosis treatment was cost-effective when the 10-year hip fracture probability reached approximately 3%. Although the RR at which treatment became cost-effective varied markedly between genders and by race/ethnicity, the absolute 10-year hip fracture probability at which intervention became cost-effective was similar across race/ethnicity groups, but tended to be slightly higher for men than for women.
Conclusions
Application of the WHO risk prediction algorithm to identify individuals with a 3% 10-year hip fracture probability may facilitate efficient osteoporosis treatment.
Keywords: Cost-Effectiveness, National Osteoporosis Foundation, Osteoporosis, Practice guidelines, World Health Organization
Introduction
To improve the process of identifying patients at highest risk of fracture, the World Health Organization (WHO) has sponsored development of a fracture prediction algorithm, FRAX™, designed to identify high-risk candidates for pharmacologic intervention among residents of different geographic regions, either sex, and any race (1). This endeavor is timely because the National Osteoporosis Foundation (NOF) is now in the process of updating current clinical practice guidelines to reflect the growing international consensus that intervention thresholds for osteoporosis treatment should be determined on the basis of the probability that an osteoporotic fracture will occur sometime over the next 10 years, i.e., absolute fracture risk (2). To support existing NOF guidelines, an extensive economic evaluation was undertaken in 1998 (3). A portion of that earlier evaluation has been updated to reflect present knowledge regarding fracture incidence, health consequences and costs and is described here. The objective of this analysis is to identify the level of absolute fracture risk (%) at which treatment intervention becomes cost-effective, given estimates of fracture incidence, morbidity, mortality and cost specific to the United States. Cost-effectiveness considerations are warranted because the number of fractures observed each year in the United States will increase with rapid growth of the elderly population over the next few decades (4, 5), but the potential population at risk is so large that control programs must be economically efficient as well as clinically effective.
Materials and Methods
Model structure and approach
Markov state-transition models (6), implemented in TreeAge Pro (TreeAge Software, Williamstown, MA), were used to track fracture incidence and mortality among individual cohorts defined by sex, race/ethnicity and starting age (e.g., 50, 55, …, 85 years) until age 100 years (or death). Such models allow direct modification of fracture incidence rates to reflect a patient population at increased risk of fracture due to a variety of risk factors, with treatment effects modeled by applying a relative risk reduction to the age-specific fracture incidence rates. Using tailored annual transition probabilities for each cohort, the model estimated health outcomes measured as quality-adjusted life expectancy and costs over a lifetime under two alternatives: 1) no intervention and 2) osteoporosis treatment for 5 years.
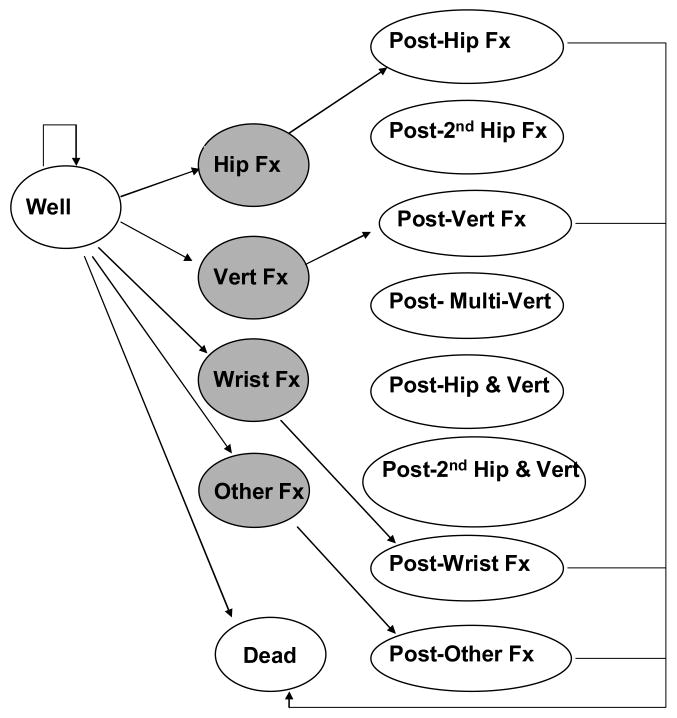
The model includes a number of discrete health states (Figure 1). Each state has an associated economic cost and health state value (i.e., utility or preference weight), which are used to track costs and quality-adjusted life years (QALYs), respectively. We modeled the incidence of proximal femur (hip), distal forearm (wrist), clinical vertebral (spine) and “other” fractures. For women, other fractures included proximal humerus, rib, pelvis, and tibia/fibula; tibia/fibula fractures were excluded for men as described elsewhere (7). Movement between health states occurs annually according to specified state-dependent transition probabilities, with first-year transitions depicted in Figure 1.
Figure 1.
Model health states and initial transitions. Shaded circles show acute events.
Early in this endeavor, two alternative approaches for modeling fracture incidence were considered as follows:
Approach 1. Model age-specific fracture incidence without attention to “first fracture” (i.e., do not alter subsequent fracture risk once a fracture has occurred). This approach mirrors models used to support UK and Swedish clinical guidelines (8, 9). The age-specific incidence rates utilized in this approach are detailed in the appendix.
Approach 2. Model the age-specific incidence of first fractures. Once a fracture has occurred, allow subsequent fractures to occur at a higher rate based on evidence from meta-analyses that have addressed this question (10, 11).
In theory, both approaches should yield approximately equivalent results. Thus, to validate the model structure, both approaches were undertaken and compared using data on sex- and age-specific fracture incidence either overall (Approach 1) or for first fracture (Approach 2) as taken from Olmsted County, MN (12), which is disproportionately white (90% in 2005) (13).
Fracture incidence rates for non-white race/ethnicity groups were based on ratios of reported incidence rates (race group/white) derived from the literature (14). Compared to white women, Black, Asian, and Hispanic women were 57%, 50%, and 47% less likely to fracture, respectively. Compared to white men, Black, Asian, and Hispanic men were 47%, 36%, and 42% less likely to fracture, respectively.
Treatment efficacy
A five-year course of treatment with a bisphosphonate-like therapy was modeled under the assumption of full persistence. Randomized clinical trial evidence for the effectiveness of treatment in reducing fracture risk varies by fracture site and the population studied (15). To maintain comparability between the current analysis and other studies that have addressed treatment intervention thresholds using economic evaluation, in baseline analyses, a fracture reduction of 35% (relative risk [RR] = 0.65) was assumed for any individual initiating therapy (8, 9, 16). The same treatment efficacy was modeled for each population group considered. Treatment effectiveness was allowed to deteriorate following discontinuation of therapy and was assumed to be fully offset 5 years after discontinuation as recommended elsewhere (17). We note that some level of treatment non-compliance is inherent in clinical trials estimates of treatment efficacy and is thus reflected in the assumed treatment efficacy. Although studies indicate that early discontinuation with treatment is common (18), the baseline assumption of 100% persistence over the 5-year treatment period reflects an optimistic assessment of the economic value of osteoporosis treatment.
Health state values
To estimate quality-adjusted life expectancy, each health state in the model has an associated value, which reflects societal preferences for that health state on a scale where 1 represents best imaginable health and 0 represents death (19). The health state values expected for each age-group and sex were derived from EuroQol EQ-5D based on United States population data (20). Mean health state values for women were 0.837 for ages 50-59, 0.811 for ages 60-69, 0.771 for ages 70-79, and 0.724 for ages 80 and older. For men, corresponding health state values were 0.861, 0.84, 0.802 and 0.782.
The loss in health-related quality of life for each type of fracture, which is referred to as a “disutility,” was limited to a 5-year time horizon with initial and second year decrements shown in Table 1. These disutilities are consistent with those used in Swedish and UK economic analyses (8, 9), however, these European studies applied disutilities beyond 5 years. In our analysis, the disutility decreased linearly for years 2 through 5 when the value of zero (i.e., no further quality of life decrement) was reached. In a sensitivity analysis, we tested the effect of both a 10-year and a lifetime period of post-fracture impairment/disutility. Although osteoporotic fractures are less common among non-white populations and men (14), it was assumed that, should a fracture occur, the associated reduction in quality of life would be the same as for a postmenopausal white woman.
Table 1.
Costs and disutilities associated with each type of fracture.
| Parameter | Hip Fracture | Vertebral Fracture | Wrist Fracture | Other Fracture* |
|---|---|---|---|---|
| Disutility in 1st yeara | 0.208 | 0.374 | 0.023 | 0.133* |
| Disutility in 2nd year | 0.187 | 0.091 | 0.001 | 0.064* |
| Cost in 1st yearb | $29,449 | $8,387 | $4,195 | $11,324* |
| Cost-subsequent years | $7,156 | n/a | n/a | n/a |
Values shown are for women and include proximal humerus, rib, pelvis and tibia/fibula. For men, tibia/fibula fractures are excluded and 1st and 2nd year disutilities are 0.071 and 0.029, respectively. First year other fracture costs for men are $6,946.
From Kanis et al (41). To model health state value following a fracture, the age-specific EQ-5D health state value was multiplied times 1 minus the disutility shown above.
From Gabriel et al (21).
Costs
All costs are represented in 2005 United States dollars ($). Fracture costs are delineated in Table 1 and are based on incremental expenditures, compared to controls, in the year following versus the year before fracture (21). It was assumed that expenditures for osteoporosis interventions and for treating fractures would be the same regardless of age, race, or gender. Annual drug costs were $600 in the base-case analysis, but were varied from $900 per year (approximating 2005 average wholesale prices for bisphosphonates) to $300, which may ultimately reflect generic bisphosphonate costs. Those receiving treatment were assumed to have an additional physician visit each year ($49 per year) and to incur the cost of a BMD test ($82) in the second year after treatment initiation (16). Therefore, treatment-related costs (drug, physician visit) in years 1 through 5 ranged from $349 to $949 depending on drug cost, with an additional BMD test cost of $82 incurred in year 2.
Mortality
Annual age, sex- and race-specific mortality rates were taken from 2001 United States lifetables (22), with 5-year mortality rates modeled for Asian and Hispanic populations. Increased mortality in the year following hip fracture was modeled, but no excess mortality risk was assumed following wrist, spine or other fractures. This is because the excess mortality following vertebral fractures is late, not early, and there is no consistent pattern of excess mortality following fractures at the other skeletal sites (23). Substantial mortality has been reported among patients in the year following hip fracture (24-31). Although hip fracture may often be the precipitating event causing death, serious underlying diseases are quite common in this group (5, 29). Thus, prevention of a hip fracture will not necessarily reduce mortality to age-specific norms.
Cost-effectiveness analysis
The cost-effectiveness of treatment relative to no intervention was estimated as the cost per QALY gained. Cost and health outcomes were tracked over a lifetime with both outcomes discounted at a rate of 3% per year. Discounting is used to value near term costs and losses in quality of life more heavily than those occurring in the future (32). Incremental cost-effectiveness ratios (ICERs) were calculated by dividing the difference in mean total discounted costs between treated and untreated cohorts by the respective difference in quality-adjusted life expectancy.
To determine the relative risk threshold at which treatment became cost-effective, we adopted a willingness-to-pay threshold of $60,000 per QALY gained as “cost-effective”. Although this is slightly higher than the $50,000 value that has become commonplace in many economic analyses, it is lower than the twice per capital gross domestic product (i.e., approximately $75,000 for the United States) that was recently advocated as a reasonable cost-effectiveness criterion (16). The $60,000 threshold had the additional advantage of being consistent with international models of treatment cost-effectiveness (8, 9, 16). In sensitivity analyses we examined the impact of alternative willingness-to-pay thresholds on results of our analysis, because many well-accepted medical practices in the United States have ratios in the $50,000 to $75,000 range (33, 34).
To identify how much higher than average risk a population must be for treatment to become cost-effective (i.e., cost per QALY gained with treatment below $60,000), all annual age-specific fracture probabilities (i.e., hip, wrist, clinical vertebral, and other) were multiplied by a relative risk (RR) that was systematically varied from 0.01 to 10 until $60,000 per QALY gained was observed for treatment relative to no intervention. As an example, consider the 60-year-old white female population. When the simulation model, which includes an average age-specific risk of hip, wrist, clinical vertebral and other fractures as detailed in the appendix, is run, the ICER for treatment is nearly $140,000 per QALY gained (Table 2). Relative to the $60,000 willingness-to-pay criterion, treatment of the average 60-year-old woman is not cost-effective. Thus, the RR applied to each type of fracture, is increased above 1 until a cost of $60,000 per QALY gained is achieved for treatment when compared with no intervention. For the 60-year-old white female population, an RR of 1.6 must be applied to each fracture type before treatment becomes cost-effective. Although all fractures are used to identify the cost-effective relative risk intervention threshold, we report intervention thresholds on the basis of absolute 10-year hip fracture risk to allow direct comparison with the WHO risk prediction algorithm as detailed in the accompanying report (35). Because the 60-year-old white female population has an average 10-year hip fracture risk of 1.79%, this translates into an absolute 10-year hip fracture probability of 3.0% (10-yr hip risk of 1.79 multiplied by relative risk of 1.6) as a cost-effective treatment intervention threshold. The impact of altering drug cost, the duration of fracture sequelae, and willingness to pay per QALY gained on the cost-effective intervention thresholds was examined for white women.
Table 2.
Cost-effectiveness analysis by age, sex and race/ethnicty for average risk individuals and relative risk and absolute risk thresholds in the United States.
| Average 10-yr Hip Fracture Probability | Mean outcomes for average-risk individual: | Thresholds for ICER to drop below $60K/QALY gained | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost | Effectiveness (QALYs)* | |||||||||
| Untreated | Treated | Δ Cost* | Untreated | Treated | Δ QALY* | ΔC / ΔQALY (ICER) |
Relative Risk† | Absolute 10-yr Hip Fracture Probability | ||
| White Female | ||||||||||
| 50 | 0.72 | 9,609 | 11,650 | $2,041 | 15.560 | 15.566 | 0.005 | $380,712 | 3.5 | 2.5 |
| 55 | 1.18 | 10,413 | 12,277 | $1,864 | 13.888 | 13.896 | 0.008 | $222,758 | 2.3 | 2.8 |
| 60 | 1.79 | 11,186 | 12,795 | $1,609 | 12.101 | 12.113 | 0.012 | $139,546 | 1.6 | 3.0 |
| 65 | 2.24 | 11,680 | 13,114 | $1,434 | 10.309 | 10.325 | 0.016 | $88,359 | 1.3 | 2.8 |
| 70 | 4.66 | 12,141 | 13,181 | $1,040 | 8.465 | 8.488 | 0.024 | $44,231 | 0.9 | 4.0 |
| 75 | 9.80 | 13,043 | 13,042 | -$1 | 6.721 | 6.766 | 0.045 | save | 0.4 | 4.4 |
| 80 | 13.52 | 12,252 | 11,517 | -$735 | 5.040 | 5.108 | 0.068 | save | 0.3 | 4.0 |
| 85 | 12.96 | 9,380 | 8,720 | -$660 | 3.671 | 3.750 | 0.079 | save | 0.3 | 3.3 |
| Black Female | ||||||||||
| 50 | 0.31 | 3,563 | 5,839 | $2,276 | 14.534 | 14.536 | 0.002 | $932,270 | 8.1 | 2.5 |
| 55 | 0.5 | 3,891 | 6,084 | $2,193 | 12.942 | 12.946 | 0.004 | $576,195 | 5.4 | 2.7 |
| 60 | 0.75 | 4,228 | 6,301 | $2,073 | 11.274 | 11.279 | 0.005 | $392,808 | 3.8 | 2.9 |
| 65 | 0.92 | 4,481 | 6,457 | $1,976 | 9.633 | 9.640 | 0.007 | $268,122 | 2.9 | 2.7 |
| 70 | 1.9 | 4,774 | 6,557 | $1,783 | 7.992 | 8.003 | 0.011 | $166,713 | 2.0 | 3.8 |
| 75 | 4.08 | 5,308 | 6,600 | $1,293 | 6.470 | 6.490 | 0.020 | $63,088 | 1.0 | 4.2 |
| 80 | 5.85 | 5,224 | 6,112 | $888 | 5.038 | 5.069 | 0.031 | $28,569 | 0.7 | 4.0 |
| 85 | 5.81 | 4,189 | 4,982 | $792 | 3.834 | 3.870 | 0.036 | $22,171 | 0.6 | 3.4 |
| Hispanic Female | ||||||||||
| 50 | 0.38 | 5,874 | 8,112 | $2,238 | 16.179 | 16.182 | 0.003 | $781,031 | 6.6 | 2.5 |
| 55 | 0.63 | 6,403 | 8,539 | $2,136 | 14.559 | 14.564 | 0.004 | $478,967 | 4.4 | 2.8 |
| 60 | 0.96 | 6,913 | 8,898 | $1,984 | 12.794 | 12.800 | 0.006 | $324,672 | 3.1 | 3.0 |
| 65 | 1.21 | 7,258 | 9,134 | $1,875 | 11.000 | 11.008 | 0.009 | $216,844 | 2.3 | 2.8 |
| 70 | 2.55 | 7,564 | 9,185 | $1,621 | 9.117 | 9.130 | 0.013 | $128,010 | 1.6 | 4.1 |
| 75 | 5.56 | 8,102 | 9,063 | $961 | 7.337 | 7.361 | 0.024 | $40,036 | 0.8 | 4.6 |
| 80 | 7.66 | 7,585 | 8,087 | $502 | 5.579 | 5.617 | 0.039 | $13,009 | 0.5 | 4.1 |
| 85 | 7.15 | 5,651 | 6,092 | $440 | 4.002 | 4.049 | 0.047 | $9,389 | 0.4 | 3.1 |
| Asian Female | ||||||||||
| 50 | 0.37 | 6,451 | 8,700 | $2,250 | 16.856 | 16.859 | 0.003 | $843,565 | 7.0 | 2.6 |
| 55 | 0.61 | 7,066 | 9,217 | $2,151 | 15.279 | 15.284 | 0.004 | $520,136 | 4.7 | 2.8 |
| 60 | 0.92 | 7,669 | 9,672 | $2,003 | 13.544 | 13.550 | 0.006 | $354,682 | 3.2 | 3.0 |
| 65 | 1.16 | 8,097 | 9,991 | $1,893 | 11.747 | 11.755 | 0.008 | $236,238 | 2.5 | 2.9 |
| 70 | 2.51 | 8,508 | 10,142 | $1,634 | 9.882 | 9.894 | 0.012 | $140,367 | 1.7 | 4.2 |
| 75 | 5.51 | 9,079 | 10,031 | $952 | 8.049 | 8.071 | 0.022 | $42,700 | 0.9 | 4.7 |
| 80 | 7.71 | 8,499 | 8,945 | $446 | 6.197 | 6.233 | 0.036 | $12,248 | 0.6 | 4.3 |
| 85 | 7.61 | 6,564 | 6,935 | $371 | 4.621 | 4.666 | 0.045 | $8,301 | 0.5 | 3.5 |
| White Male | ||||||||||
| 50 | 0.34 | 3,677 | 5,915 | $2,237 | 14.926 | 14.929 | 0.003 | $839,053 | 6.9 | 2.4 |
| 55 | 0.52 | 3,841 | 6,047 | $2,206 | 13.201 | 13.205 | 0.003 | $689,381 | 8.1 | 4.2 |
| 60 | 1.21 | 4,178 | 6,188 | $2,011 | 11.372 | 11.377 | 0.005 | $367,712 | 3.4 | 4.1 |
| 65 | 1.53 | 4,388 | 6,222 | $1,834 | 9.568 | 9.576 | 0.009 | $215,246 | 2.3 | 3.5 |
| 70 | 2.61 | 4,154 | 5,897 | $1,743 | 7.775 | 7.786 | 0.012 | $148,336 | 1.9 | 4.8 |
| 75 | 3.97 | 4,533 | 5,850 | $1,318 | 6.155 | 6.177 | 0.022 | $58,896 | 1.0 | 3.9 |
| 80 | 5.44 | 4,018 | 5,128 | $1,110 | 4.660 | 4.691 | 0.031 | $36,133 | 0.7 | 4.0 |
| 85 | 6.9 | 3,965 | 4,622 | $657 | 3.396 | 3.444 | 0.048 | $13,609 | 0.4 | 3.1 |
| Black Male | ||||||||||
| 50 | 0.18 | 1,506 | 3,824 | $2,318 | 13.314 | 13.316 | 0.001 | $1,552,303 | 13.1 | 2.4 |
| 55 | 0.26 | 1,577 | 3,863 | $2,286 | 11.707 | 11.709 | 0.002 | $1,282,841 | 11.5 | 3.0 |
| 60 | 0.59 | 1,748 | 3,919 | $2,171 | 10.073 | 10.076 | 0.003 | $700,904 | 6.5 | 3.8 |
| 65 | 0.75 | 1,878 | 3,931 | $2,053 | 8.489 | 8.494 | 0.005 | $425,717 | 4.3 | 3.3 |
| 70 | 1.25 | 1,820 | 3,786 | $1,966 | 6.959 | 6.965 | 0.006 | $306,858 | 3.5 | 4.4 |
| 75 | 1.96 | 2,096 | 3,799 | $1,703 | 5.637 | 5.650 | 0.012 | $138,522 | 1.9 | 3.6 |
| 80 | 2.72 | 1,987 | 3,503 | $1,516 | 4.444 | 4.460 | 0.016 | $92,114 | 1.4 | 3.7 |
| 85 | 3.68 | 2,117 | 3,300 | $1,183 | 3.441 | 3.468 | 0.027 | $44,552 | 0.8 | 3.0 |
| Hispanic Male | ||||||||||
| 50 | 0.2 | 2,543 | 4,871 | $2,328 | 15.583 | 15.584 | 0.002 | $1,503,710 | 11.9 | 2.4 |
| 55 | 0.3 | 2,699 | 5,003 | $2,304 | 13.931 | 13.933 | 0.002 | $1,246,620 | 10.4 | 3.1 |
| 60 | 0.71 | 2,963 | 5,137 | $2,174 | 12.150 | 12.154 | 0.003 | $691,623 | 5.8 | 4.1 |
| 65 | 0.9 | 3,148 | 5,197 | $2,049 | 10.363 | 10.367 | 0.005 | $419,975 | 3.9 | 3.5 |
| 70 | 1.59 | 3,063 | 5,028 | $1,965 | 8.573 | 8.579 | 0.007 | $284,080 | 3.1 | 4.9 |
| 75 | 2.46 | 3,348 | 4,999 | $1,651 | 6.922 | 6.935 | 0.013 | $124,935 | 1.6 | 4.0 |
| 80 | 3.49 | 3,054 | 4,530 | $1,477 | 5.394 | 5.414 | 0.020 | $74,256 | 1.2 | 4.1 |
| 85 | 4.48 | 2,986 | 4,029 | $1,043 | 4.011 | 4.043 | 0.032 | $32,341 | 0.7 | 3.0 |
| Asian Male | ||||||||||
| 50 | 0.23 | 3,353 | 5,673 | $2,321 | 16.558 | 16.559 | 0.002 | $1,396,955 | 10.9 | 2.5 |
| 55 | 0.35 | 3,573 | 5,868 | $2,295 | 14.892 | 14.894 | 0.002 | $1,157,778 | 9.5 | 3.3 |
| 60 | 0.8 | 3,924 | 6,073 | $2,149 | 13.111 | 13.114 | 0.003 | $652,588 | 5.2 | 4.2 |
| 65 | 1.03 | 4,165 | 6,181 | $2,016 | 11.278 | 11.283 | 0.005 | $396,699 | 3.5 | 3.6 |
| 70 | 1.85 | 4,054 | 5,982 | $1,928 | 9.371 | 9.379 | 0.007 | $262,471 | 2.8 | 5.1 |
| 75 | 2.87 | 4,351 | 5,926 | $1,575 | 7.576 | 7.590 | 0.014 | $111,599 | 1.5 | 4.3 |
| 80 | 4.16 | 3,909 | 5,301 | $1,392 | 5.879 | 5.901 | 0.022 | $62,595 | 1.0 | 4.3 |
| 85 | 5.29 | 3,731 | 4,660 | $929 | 4.350 | 4.386 | 0.036 | $25,759 | 0.6 | 3.2 |
Rounded values are shown for treatment minus no intervention.
This relative risk was applied to each type of fracture to achieve a cost per QALY gained of $60,000 for treatment (i.e., ΔC / ΔQALY ≤$60,000). Thus, the estimated mean costs and quality-adjusted life expectancies for treated and untreated cohorts include costs and quality of life impact associated with all fractures.
Results
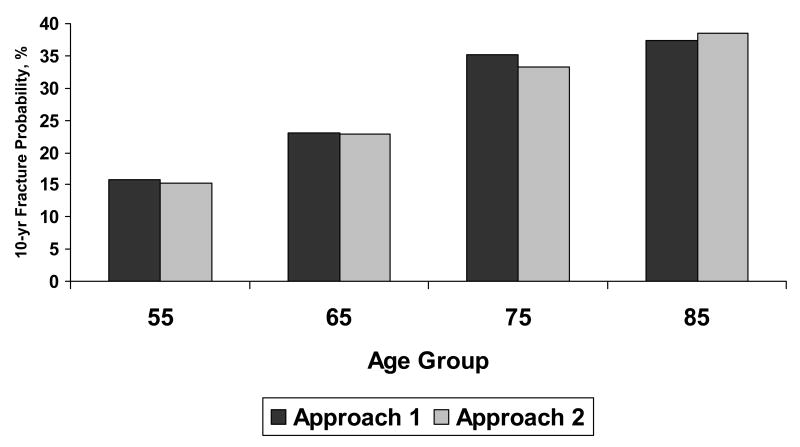
The two approaches to modeling fracture risk provided comparable results (Figure 2), and we chose the more straightforward technique of modeling fracture incidence rates (Approach 1) for the remainder of the analysis. Average absolute 10-year hip fracture probabilities as estimated using Approach 1 for populations of women and men by race/ethnicity group are shown as vertical bars in Figure 3. Although average 10-year risks differ by gender and race/ethnicity, the familiar pattern of higher fracture probabilities at older ages is evident for all groups.
Figure 2.
Model validation results comparing two modeling approaches for white women where fractures modeled include hip, wrist, spine, and other (proximal humerus, rib, pelvis, and tibia/fibula). Approach 1 modeled age-specificfracture incidence-making no distinction between first and subsequent fractures. Approach 2 modeled age-specific incidence of first fracture and increased the rate at which subsequent fractures occurred.
Figure 3.
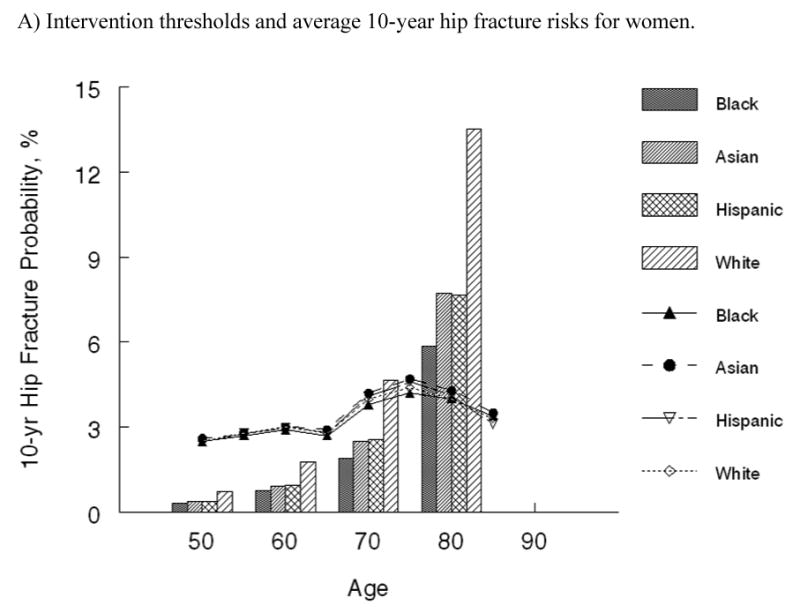
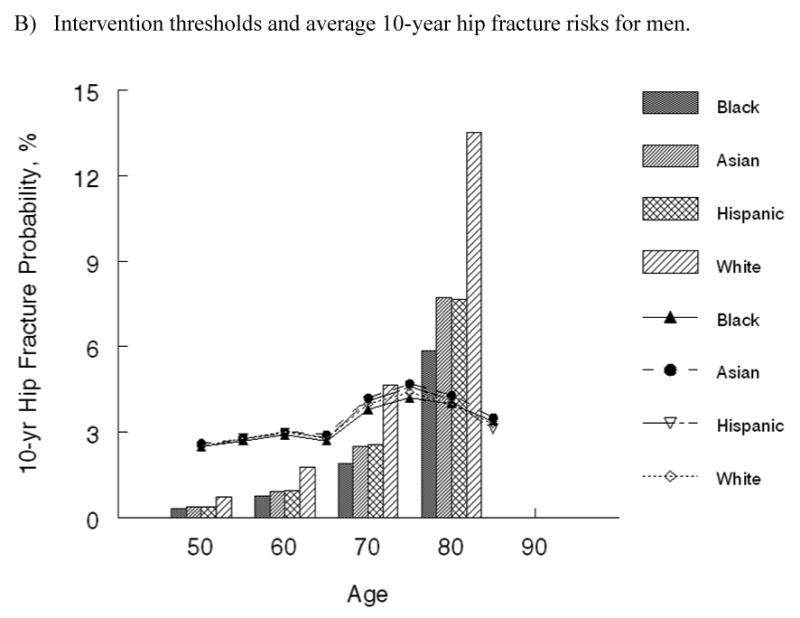
Absolute 10-year hip fracture risk by age and race at which it is cost-effective to treat (shown by lines) and average 10-year hip fracture risk by age and race (shown by vertical bars) for A) women and B) men in the United States.
The cost-effectiveness analysis of treatment relative to no intervention among average risk populations yielded ICERs that ranged from over $380,000 per QALY gained in 50-year-old white women to being cost-saving in 75-year-old white women (Table 2). At each age, the cost per QALY gained for individuals of average age-specific risk were two or more times greater for black, Asian and Hispanic women and white men than those estimated for white women. For black, Asian and Hispanic men, the cost per QALY gained was two or more times greater than those estimated for white men. Although relative risks differed markedly between race groups for each gender, the cost-effective intervention thresholds by race/ethnicity group, which are depicted as lines in Figure 3, did not. The absolute 10-year hip fracture probability at which treatment cost $60,000 per QALY gained were very comparable across race and ethnicity groups. Among women, these 10-year hip fracture probabilities ranged from 2.5% in 50-year-old women to 4.7% at age 75. Among men, intervention thresholds by age ranged from 2.4% to 4.9%. In general, the intervention thresholds are slightly higher for men compared with women.
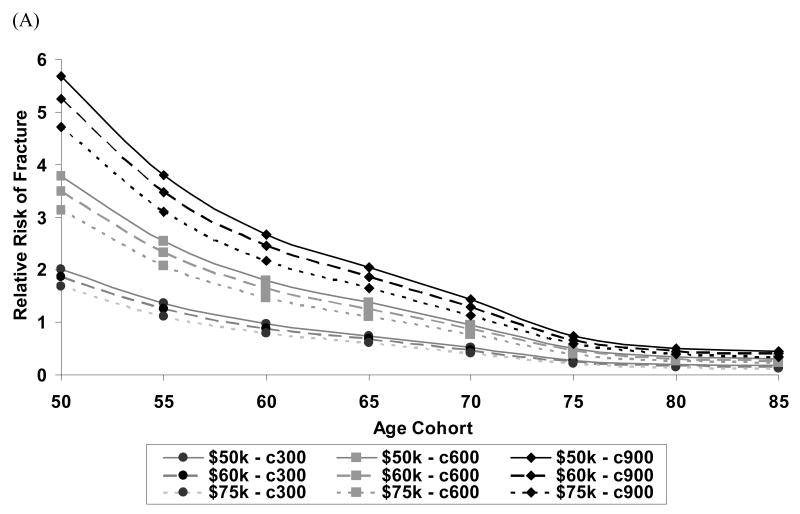
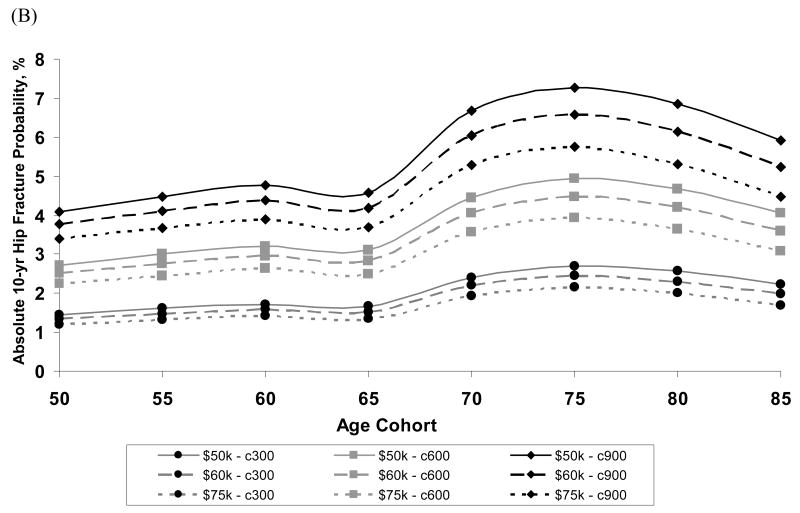
Figure 4 depicts the impact that both willingness -to-pay threshold (i.e., cost per QALY gained considered “cost-effective”) and annual drug treatment costs had on both the relative risk (Figure 4a) and absolute 10-year hip fracture probabilities (Figure 4b) at which it was cost-effective to treat white women. The annual treatment cost had more impact on the intervention threshold than the choice of a willingness-to-pay threshold.
Figure 4.
Among white women, the impact of drug treatment cost ($300, $600 or $900 per year) and willingness-to-pay threshold ($50K, $60K, or $75K per QALY gained) on (A) relative risk required to achieve cost-effectiveness where relative risks are computed using 10-year average risk of fracture for the general population at each age (B) absolute 10-year hip fracture risk at which treatment becomes cost-effective in the United States.
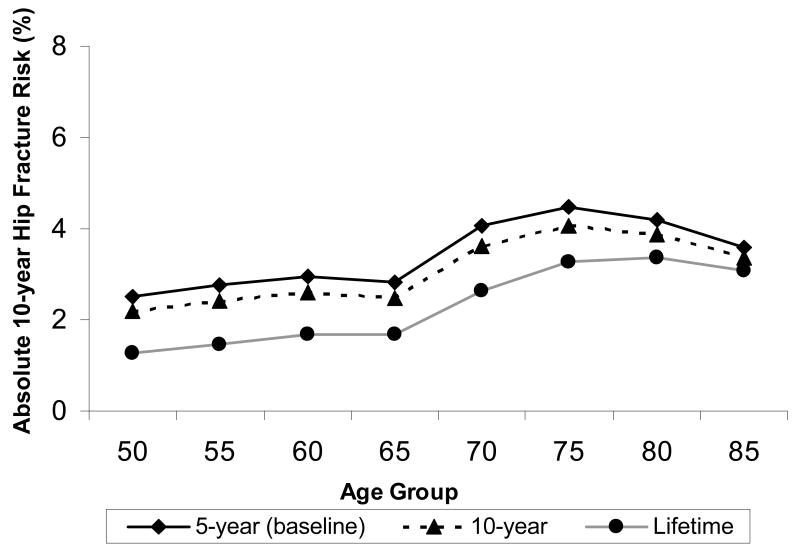
Figure 5 shows the impact that the duration that fracture adversely affects health-related quality of life has on the intervention threshold. When the duration of adverse impact was doubled to 10-years, the absolute risk at which it was cost-effective to intervene dropped by approximately 0.3%. When fracture was assumed to have a lifetime adverse impact on health-related quality of life, the intervention threshold dropped to approximately 1.5% in women under age 70.
Figure 5.
The impact that duration in fracture-related loss in quality of life (5-year, 10-year or lifetime duration) has on the absolute 10-year risk of hip fracture at which treatment becomes cost-effective is shown for white women in the United States.
Discussion
Our economic analysis employed a fracture incidence-based model to identify the absolute 10-year hip fracture risk at which osteoporosis treatment is “cost-effective.” A range of annual treatment costs ($300, $600, and $900 per year) and definitions of “cost-effective” ($50,000, $60,000 and $75,000 per QALY gained) were considered in these analyses. Overall, intervention thresholds were more markedly affected by annual treatment costs than by the cost-effectiveness definition (i.e., willingness to pay per QALY gained). With an anticipated decrease in osteoporosis treatment cost as generic bisphosphonate becomes available, the economic analyses that we report support an absolute 3% 10-year hip fracture probability among women and a slightly higher 3.5% risk among men as cost-effective treatment intervention thresholds. For those under age 65, although relative risk intervention thresholds varied markedly between race/ethnicity groups, absolute risk intervention thresholds were nearly identical. This highlights an important advantage to characterizing risks on the basis of absolute rather than relative fracture risk and the clinical scenarios that result in absolute 10-year hip fracture risks above the intervention thresholds are discussed in detail in a companion paper (35).
For older age groups, there was a tendency for absolute fracture risk intervention thresholds to increase. This is not unexpected due to the increased competing mortality risks from other diseases that individuals face as they age and has been observed in similar analyses reported for U.K. and Swedish populations (8, 9). Thus, differences between race/ethnicity groups are more apparent among those age 65 to 80 than among those under age 65. For example, average risk white women who are age 70 or older exceed the absolute risk intervention threshold while average risk black women do not exceed the threshold until they are over age 75 years.
Although intervention thresholds are presented in terms of absolute 10-year hip fracture risk, our analysis also accounted for the impact that wrist, clinical spine, proximal humerus, pelvis, rib and tibia/fibula fractures have on health-related quality of life. Their inclusion is important since judging treatment cost-effectiveness in preventing only hip fractures would require a much higher absolute fracture risk than we report here. For example, among 60-year-old white women when all fractures were included we identified a 3.0% 10-year hip fracture risk as the cost-effective intervention threshold. This threshold would more than double to 6.6% if only hip fractures were considered. Although we have addressed non-hip fractures in our analysis, the challenge in understanding the complex relationship between probability of hip fracture and other fractures requires further epidemiological investigation. As further evidence becomes available concerning risk factors for hip and non-hip fractures and their associations with mortality, more refined economic analyses will be possible.
Due to the dearth of evidence regarding the longitudinal impact of fractures on health-related quality of life, we limited the duration of adverse fracture impact to 5 years and examined 10-year and lifetime durations in sensitivity analyses. Among white women, we found that the intervention threshold would drop to below 2% if a lifetime adverse impact from incident fractures was modeled compared with the 5-year duration. Although the 5-year duration is more conservative than assumptions in other economic analyses (7-9, 16), it is important to note that our modeling of age-specific health-related quality of life already includes the adverse affects of multiple chronic conditions on health in the population. To the extent that a history of a prior fracture becomes prevalent as the population ages, chronic health effects are likely already adequately captured in the age-specific health state values that were utilized in the economic analysis.
Previous analyses that utilized similar treatment efficacy at costs of $500 per year, identified absolute 10-year hip fracture risks ranging from 2.73 to 3.18% for 60 year old women and 3.98 to 4.14% for 65 year old women as cost-effective (8, 9). With slightly higher annual treatment costs of $600 per year, we identified comparable cost-effective intervention thresholds ranging from 2.8 to 3.0% among 60 to 65 year old women across race and ethnicity groups. However, for younger women there were more marked differences in the intervention thresholds identified for United States compared with U.K. and Swedish populations, with higher intervention thresholds in the United States (e.g., 2.5 to 2.8% for United States women vs. 1.2 to 2.1% for European women ages 50 to 55 years). This difference is likely due to differing assumptions regarding the long-term adverse impact of fracture on health-related quality of life that was noted earlier. A similar pattern was noted for men, with intervention thresholds among United States men tending to be higher than those reported for Swedish men (9).
Comparison of intervention thresholds between countries is ideally made in the context of a single model, as was recently undertaken by Borgstrom et al (16). When $600 annual treatment costs were considered for 70 year old women using a $60,000 per QALY gained willingness-to-pay threshold, the absolute 10-year hip fracture risk at which intervention was cost-effective ranged from 4.97 to 5.44% between countries. Our analysis, for the same age group, treatment cost and willingness to pay criterion, identified intervention thresholds ranging from 3.8 to 4.2% for United States women. The slightly lower thresholds that we identified may be explained by our exclusion of future costs associated with prolonged survival (16).
Our analysis updates a portion of the economic underpinnings of current NOF practice guidelines that were conducted using similar analytic methods a decade ago and provides generally consistent results regarding who should be treated (3). In contrast with that earlier study, the current economic analysis focuses on when osteoporosis treatment is cost-effective relative to no treatment as the absolute risk of fracture is systematically varied. We considered neither the value of empiric treatment relative to bone density screening strategies, nor the value of screening relative to no intervention. Although one can infer that bone mineral density (BMD) testing is clinically indicated when it would change the treatment recommendation (e.g., result in crossing a threshold vs. not), it is important to recognize that the economic analysis did not explicitly consider the cost-effectiveness of BMD screening strategies. An analysis addressed the latter (universal screening vs. no intervention) in older white women in this country and highlighted the potential value of screen and treat strategies (36). A more recent analysis addressed these issues in men and found it reasonably cost-effective to screen men age 65 and older with a history of prior fracture (37). Other United States studies have shown the potential for clinical strategies involving bone densitometry to be cost-saving (38, 39).
We also did not address the value of alternative osteoporosis treatments. Instead, we follow the approach taken in European guidelines development (2) of focusing on when treatment is warranted while leaving particular treatment choices up to individual clinicians and patients. Viewed within this context, our assumption of 100% treatment persistence has little bearing on the economic analysis, because those who discontinue treatment forego both treatment costs and potential health benefits. However, as evidence regarding persistence of alternative agents becomes available, a more thorough evaluation of how differential persistence affects the economic value of competing treatments will be warranted. Meanwhile, with marked differences in the costs of alternative treatments as exemplified by the prices of parathyroid hormone analogs and bisphosphonates, there is certainly heterogeneity in the value of alternative agents. In our analyses, we adopted a 35% efficacy in fracture reduction for all types of fractures. Although this does not match the efficacy profile for any specific agent, it is well within the range of values that are reasonable for bisphosphonate treatment and has the added advantage of allowing comparison with European efforts to identify intervention thresholds (8, 9, 16).
Cost consideration in clinical guidelines development is sometimes controversial (40). Yet as health care budgets become increasingly strained by a growing elderly population, it is recognized that the value of the clinical guidelines warrants consideration. To address this, we undertook model-based cost-effectiveness analyses to identify the absolute 10-year hip fracture probability at which osteoporosis treatment becomes cost-effective. Intervention thresholds were identified separately for men and women by race and ethnicity. Application of the WHO risk prediction tool to identify individuals who meet the intervention thresholds, as detailed in the accompanying paper (35), should facilitate identification of appropriate individuals for treatment and will help optimize efficient osteoporosis care.
Acknowledgments
The authors thank Dr. John Kanis for his insightful advice regarding this analysis. We also thank David Radley and Margaret Grove for their assistance with model development and analyses.
Funding This study was supported by the National Osteoporosis Foundation. It was also supported in-part by grants from the National Institutes of Health (AR048094 and AG12262).
Appendix
Age-specific annual fracture probability by fracture type and gender for the white population*.
| Age | Hip Fracture | Clinical Vertebral Fracture | Wrist Fracture | Other Fracture** | ||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | Women | Men | |
| 50 | 0.00066 | 0.00040 | 0.00111 | 0.00053 | 0.00291 | 0.00147 | 0.00343 | 0.00400 |
| 55 | 0.00083 | 0.00032 | 0.00316 | 0.00065 | 0.00429 | 0.00064 | 0.00495 | 0.00494 |
| 60 | 0.00165 | 0.00081 | 0.00316 | 0.00065 | 0.00805 | 0.00141 | 0.00548 | 0.00503 |
| 65 | 0.00221 | 0.00189 | 0.00664 | 0.00170 | 0.00819 | 0.00095 | 0.00738 | 0.00730 |
| 70 | 0.00275 | 0.00160 | 0.00664 | 0.00170 | 0.00821 | 0.00064 | 0.01024 | 0.00669 |
| 75 | 0.00857 | 0.00533 | 0.01027 | 0.00480 | 0.00832 | 0.00045 | 0.01588 | 0.00577 |
| 80 | 0.01821 | 0.00595 | 0.01027 | 0.00480 | 0.00866 | 0.00149 | 0.02263 | 0.01409 |
| 85 + | 0.02457 | 0.01490 | 0.01257 | 0.00943 | 0.00845 | 0.00094 | 0.03396 | 0.01859 |
Fracture incidence for other race groups was estimated by applying a multiplier to white probabilities based on published literature. See text and (35) for additional details.
Other fractures include proximal humerus, pelvis, rib and tibia/fibula with tibia/fibula excluded for men.
References
- 1.Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, Oden A, Zethraeus N, Pfleger B, Khaltaev N. Assessment of fracture risk. Osteoporosis International. 2005;16:581–589. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 2.Kanis JA, Black D, Cooper C, Dargent P, Dawson-Hughes B, De Laet C, Delmas P, Eisman J, Johnell O, Jonsson B, Melton L, Oden A, Papapoulos S, Pols H, Rizzoli R, Silman A, Tenenhouse A, International Osteoporosis F. National Osteoporosis F A new approach to the development of assessment guidelines for osteoporosis. Osteoporosis International. 2002;13:527–536. doi: 10.1007/s001980200069. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. Osteoporosis: review of the evidence for prevention, diagnosis and treatment and cost-effectiveness analysis. Introduction. Osteoporosis International. 1998;8 4:S7–80. [PubMed] [Google Scholar]
- 4.Department of Health and Human Services. Bone Health and Osteoporosis A Report of the Surgeon General. Rockville, MD: 2004. [Google Scholar]
- 5.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 6.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Medical Decision Making. 1993;13:322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 7.Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporosis International. 2001;12:417–427. doi: 10.1007/s001980170112. [DOI] [PubMed] [Google Scholar]
- 8.Kanis JA, Borgstrom F, Zethraeus N, Johnell O, Oden A, Jonsson B. Intervention thresholds for osteoporosis in the UK. Bone. 2005;36:22–32. doi: 10.1016/j.bone.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Kanis JA, Johnell O, Oden A, Borgstrom F, Johansson H, De Laet C, Jonsson B. Intervention thresholds for osteoporosis in men and women: a study based on data from Sweden. Osteoporosis International. 2005;16:6–14. doi: 10.1007/s00198-004-1623-4. [DOI] [PubMed] [Google Scholar]
- 10.Haentjens P, Autier P, Collins J, Velkeniers B, Vanderschueren D, Boonen S. Colles fracture, spine fracture, and subsequent risk of hip fracture in men and women. A meta-analysis. Journal of Bone & Joint Surgery - American. 2003;85-A:1936–1943. doi: 10.2106/00004623-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Melton LJ, III, Crowson CS, O'Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporosis International. 1999;9:29–37. doi: 10.1007/s001980050113. [DOI] [PubMed] [Google Scholar]
- 13.United States Census Bureau. U S Census Bureau. 2005. State & County QuickFacts. [Google Scholar]
- 14.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 15.Cranney A, Wells G, Willan A, Griffith L, Zytaruk N, Robinson V, Black D, Adachi J, Shea B, Tugwell P, Guyatt G, Osteoporosis Methodology G. The Osteoporosis Research Advisory G Meta-analyses of therapies for postmenopausal osteoporosis. II. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocrine Reviews. 2002;23:508–516. doi: 10.1210/er.2001-2002. [DOI] [PubMed] [Google Scholar]
- 16.Borgstrom F, Johnell O, Kanis JA, Jonsson B, Rehnberg At what hip fracture risk is it cost-effective to treat? International Intervention thresholds for the treatment of osteoporosis. Osteoporosis International. 2006;17:1459–1471. doi: 10.1007/s00198-006-0107-0. [DOI] [PubMed] [Google Scholar]
- 17.Tosteson AN, Jonsson B, Grima DT, O'Brien BJ, Black DM, Adachi JD. Challenges for model-based economic evaluations of postmenopausal osteoporosis interventions. Osteoporosis International. 2001;12:849–857. doi: 10.1007/s001980170036. [DOI] [PubMed] [Google Scholar]
- 18.Badamgarav E, Fitzpatrick LA. A new look at osteoporosis outcomes: the influence of treatment, compliance, persistence, and adherence. Mayo Clinic Proceedings. 2006;81:1009–1012. doi: 10.4065/81.8.1009. comment. [DOI] [PubMed] [Google Scholar]
- 19.Tosteson AN. Characterizing preferences for health outcomes in economic evaluations. Journal of Rheumatology. 2003 68:15–18. [PubMed] [Google Scholar]
- 20.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006;26:391–400. doi: 10.1177/0272989X06290497. [DOI] [PubMed] [Google Scholar]
- 21.Gabriel SE, Tosteson AN, Leibson CL, Crowson CS, Pond GR, Hammond CS, Melton LJ., 3rd Direct medical costs attributable to osteoporotic fractures. Osteoporosis International. 2002;13:323–330. doi: 10.1007/s001980200033. [DOI] [PubMed] [Google Scholar]
- 22.Arias E, Anderson RN, Kung HC, Murphy SL, Kochanek KD. Deaths: final data for 2001. National Vital Statistics Reports. 2003;52:1–115. [PubMed] [Google Scholar]
- 23.Melton LJ., III Adverse outcomes of osteoporotic fractures in the general population. Journal of Bone & Mineral Research. 2003;18:1139–1141. doi: 10.1359/jbmr.2003.18.6.1139. [DOI] [PubMed] [Google Scholar]
- 24.Fisher ES, Baron JA, Malenka DJ, Barrett JA, Kniffin WD, Whaley FS, Bubolz TA. Hip fracture incidence and mortality in New England. Epidemiology. 1991;2:116–122. doi: 10.1097/00001648-199103000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Miller CW. Survival and ambulation following hip fracture. Journal of Bone & Joint Surgery - American. 1978;60:930–934. [PubMed] [Google Scholar]
- 26.Cooper C, Atkinson EJ, Jacobsen SJ, O'Fallon WM, Melton LJ., 3rd Population-based study of survival after osteoporotic fractures. American Journal of Epidemiology. 1993;137:1001–1005. doi: 10.1093/oxfordjournals.aje.a116756. [DOI] [PubMed] [Google Scholar]
- 27.Katelaris AG, Cumming RG. Health status before and mortality after hip fracture. American Journal of Public Health. 1996;86:557–560. doi: 10.2105/ajph.86.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. BMJ. 1993;307:1248–1250. doi: 10.1136/bmj.307.6914.1248. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poor G, Atkinson EJ, O'Fallon WM, Melton LJ., III Determinants of reduced survival following hip fractures in men. Clinical Orthopaedics & Related Research. 1995:260–265. [PubMed] [Google Scholar]
- 30.Magaziner J, Lydick E, Hawkes W, Fox KM, Zimmerman SI, Epstein RS, Hebel JR. Excess mortality attributable to hip fracture in white women aged 70 years and older. American Journal of Public Health. 1997;87:1630–1636. doi: 10.2105/ajph.87.10.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melton LJ, III, Therneau TM, Larson DR. Long-term trends in hip fracture prevalence: the influence of hip fracture incidence and survival. Osteoporosis International. 1998;8:68–74. doi: 10.1007/s001980050050. [DOI] [PubMed] [Google Scholar]
- 32.Gold M, Siegel J, Russell L, Weinstein M. Cost-Effectiveness in Health and Medicine. Oxford University Press; New York: 1996. [Google Scholar]
- 33.Center for the Evaluation of Value and Risk in Health. Institute of Clinical Research and Health Policy Studies, Tufts New England Medical Center.; 2007. CEA Registry. http://www.tufts-nemc.org/cearegistry/ [Google Scholar]
- 34.Stone PW, Teutsch S, Chapman RH, Bell C, Goldie SJ, Neumann PJ. Cost-utility analyses of clinical preventive services: published ratios, 1976-1997. American journal of preventive medicine. 2000;19:15–23. doi: 10.1016/s0749-3797(00)00151-3. [DOI] [PubMed] [Google Scholar]
- 35.National Osteoporosis Foundation Guide Committe. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the U S. submitted. [DOI] [PubMed] [Google Scholar]
- 36.Schousboe JT, Ensrud KE, Nyman JA, Melton LJ, 3rd, Kane RL. Universal bone densitometry screening combined with alendronate therapy for those diagnosed with osteoporosis is highly cost-effective for elderly women. Journal of the American Geriatrics Society. 2005;53:1697–1704. doi: 10.1111/j.1532-5415.2005.53504.x. [DOI] [PubMed] [Google Scholar]
- 37.Schousboe JT, Taylor BC, Fink HA, Kane RL, Cummings SR, Orwoll ES, Melton LJ, 3rd, Bauer DC, Ensrud KE. Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men. JAMA. 2007;298:629–637. doi: 10.1001/jama.298.6.629. [DOI] [PubMed] [Google Scholar]
- 38.King AB, Saag KG, Burge RT, Pisu M, Goel N. Fracture Reduction Affects Medicare Economics (FRAME): impact of increased osteoporosis diagnosis and treatment. Osteoporos Int. 2005;16:1545–1557. doi: 10.1007/s00198-005-1869-5. [DOI] [PubMed] [Google Scholar]
- 39.Kraemer DF, Nelson HD, Bauer DC, Helfand M. Economic comparison of diagnostic approaches for evaluating osteoporosis in older women. Osteoporosis International. 2006;17:68–76. doi: 10.1007/s00198-005-1922-4. [DOI] [PubMed] [Google Scholar]
- 40.Guyatt G, Baumann M, Pauker S, Halperin J, Maurer J, Owens DK, Tosteson AN, Carlin B, Gutterman D, Prins M, Lewis SZ, Schunemann H. Addressing resource allocation issues in recommendations from clinical practice guideline panels: suggestions from an American College of Chest Physicians task force. Chest. 2006;129:182–187. doi: 10.1378/chest.129.1.182. [DOI] [PubMed] [Google Scholar]
- 41.Kanis JA, Johnell O, Oden A, Borgstrom F, Zethraeus N, De Laet C, Jonsson B. The risk and burden of vertebral fractures in Sweden. Osteoporos Int. 2004;15:20–26. doi: 10.1007/s00198-003-1463-7. [DOI] [PubMed] [Google Scholar]