Abstract
PorT is a membrane-associated protein shown to be essential for the maturation and secretion of a class of cysteine proteinases, the gingipains, from the periodontal pathogen Porphyromonas gingivalis. It was previously reported that PorT is located on the periplasmic surface of the inner membrane to function as a chaperone for the maturing proteinases. Our modeling suggested it to be an integral outer membrane protein with eight anti-parallel, membrane-traversing β-strands. In this report, the outer membrane localization model was confirmed by the structural and functional tolerance of PorT to hexa-histidine (6×His) tag insertions at selected locations within the protein using site-directed mutagenesis. Interestingly, those PorT mutations adversely affecting gingipain secretion enhanced expression of the porT gene but at the same time suppressed the transcription of the gingipain rgpB gene. Further, PorT mutants deficient in gingipain activities produced significantly more di- and tri-aminopeptidase activities. PorT homologues have been found in restricted members of the Bacteroidetes phylum where there is potential for PorT to participate in the maturation and secretion of proteins with characteristic C-terminal domains (CTD). Knowledge of the cellular localisation of PorT will enable analysis of the role of this protein in a new secretory pathway for the export of gingipains and other CTD-class proteins.
Keywords: PorT, outer membrane, gingipains, secretion, Porphyromonas gingivalis
Introduction
Gingipains comprise an important class of cysteine proteinases from the Gram-negative pathogen Porphyromonas gingivalis important in the pathogenesis of periodontal disease or “gum disease”. Accounting for 85% of the general proteolytic activity of the organism, the gingipains provide an important proteolytic tool for the generation of proteinaceous nutrients essential for growth (Potempa et al., 1995). There are three members of the gingipain family with Kgp having specificity for cleavage after lysine residues, and two highly homologous enzymes, RgpA and RgpB, having specificity for arginine residues (Potempa et al., 2003). As indispensable virulence factors, gingipains play a critical role in the colonization and survival of P. gingivalis in the human host by facilitating bacterial attachment (Kamaguchi et al., 2001; Weinberg et al., 1997), by dysregulating the local host immune response through cytokine and receptor cleavage (Imamura et al., 2003) and by disruption of the coagulation and fibrinolytic pathways to promote the release of erythrocytes and plasma proteins to obtain essential nutrients for growth of the organism (Imamura, 2003). In keeping, gingipain isogenic mutants are attenuated in a murine virulence model (O’Brien-Simpson et al., 2001), confirming the importance of this class of proteinases in pathogenicity.
Recently, evidence has emerged that gingipains together with a number of other surface proteins from distinct members of the Bacteriodetes (formerly Cytophaga-Flavobacterium-Bacteroidetes) phylum of Gram-negative bacteria sharing a characteristic C-terminal domain (CTD), are exported through the outer membrane (OM) by a novel secretory pathway (Nguyen et al., 2007; Seers et al., 2006). The exclusive presence of two novel proteins, PorT and Sov, in organisms with CTD proteins suggested a functional relationship that was supported by the failure of gingipain maturation and export in P. gingivalis strains bearing inactivation mutants of porT and sov (Nguyen et al., 2007; Saiki & Konishi, 2007; Sato et al., 2005). It is unclear, however, how these proteins participate in the maturation and secretion process. Although the localization of Sov is yet to be determined, PorT was reported to be located on the periplasmic surface of the inner membrane (IM) in P. gingivalis (Sato et al., 2005). Due to the perceived location of the protein and its effect on gingipain maturation, it was suggested that PorT may function as a periplasmic chaperone. In the present study, structural modeling for PorT indicated an integral outer membrane protein comprising of 8 anti-parallel, amphipathic membrane-traversing β-strands. Evidence to validate this prediction is presented along with characterization of the response by the organism in coping with deficiencies imposed by mutations of PorT.
Methods
Materials and reagents
All bacterial media were sourced from Oxoid (Adelaide, Australia) while enzymes for molecular biology work were from Promega Inc. (Wisconsin, USA) unless stated otherwise. Qiagen (Valencia, CA) kits were used in DNA purifications including the QIAprep Spin Miniprep kit (for plasmid extraction), DNeasy Tissue kit (for genomic DNA purification) and the Ambion (Austin, TX) RNAqueous kit was used for RNA purification. All general chemicals were bought from Sigma-Aldrich Inc. (Sydney, Australia) unless noted otherwise. DNA oligonucleotide primers were synthesized either by IDT Inc. (Iowa, USA) or Sigma-Genosys Pty. Ltd. (Sydney, Australia) and ethidium bromide was obtained from Bio-Rad Lab. (Hercules, CA). Anti-RgpB (18E6) mAb was produced on-site in a monoclonal facility at the University of Georgia and the P. gingivalis anti-glycan (1B5) mAb was a kind gift from Dr. Mike Curtis. Alkaline phosphatase-conjugated secondary antibodies and streptavidin were purchased from DakoCytomation (Denmark).
Bacterial strains and general growth conditions
Porphyromonas gingivalis strains and Escherichia coli DH5α (used for all plasmid construction work) were grown as described previously (Nguyen et al., 2007). Ampicillin was used at 100 μg/mL for plasmid selection and tetracycline was used at 1 μg/mL for P. gingivalis mutant selection. For growth curve experiments, initial starter cultures were grown under antibiotic selection as appropriate but subsequent passage cultures for growth kinetics were carried out without antibiotic supplementation as described previously (Nguyen et al., 2007). Cells collected at OD600 0.8–0.9 were stabilized in RNAProtect Bacteria reagent (Qiagen, Germany) and stored at −80°C to preserve mRNA for gene expression studies.
Cell fractionation procedures
Cell fractionation procedures and separation of IM and OM membrane fractions by Sarkosyl treatment were essentially the same as previously published but with the addition of 2 mM tosyl-L-lysine chloromethyl ketone (TLCK) protease inhibitor at all stages of purification (Nguyen et al., 2007). Purity of the OM fraction was confirmed by the exclusive presence of lipopolysaccharide (LPS) as detected by Western blot using anti-LPS 1B5 mAb. Purify of the IM fraction was confirmed by the exclusive presence of a biotin-containing protein as detected by Western blot using alkaline-phosphatase (AP)-conjugated streptavidin. N-terminal sequencing of the protein purified over streptavidin-agarose revealed the protein to be an oxaloacetate decarboxylase (oadA gene; TIGR ID: PG1609). This protein has been predicted to be on the inner membrane and as biotin is an essential co-enzyme for carboxylases, the biotin-association with the oadA gene product on the inner membrane was as expected.
Western blot analysis
Western blot analysis was carried out using essentially the same method as previously published (Nguyen et al., 2007). Anti-PorT polyclonal antibodies were produced through a subcontractor by immunizing rabbits with a synthetic peptide ERPDLLDDYKLIYTQSISRA present on the putative fourth external loop of PorT. Alkaline phosphatase (AP)-conjugated goat anti-rabbit pAb was used as the secondary antibody and chromogenic development was carried out using AP Conjugate Substrate Kit (Bio-Rad Inc., CA).
Heat-modifiability assay of PorT
Outer membrane fraction of the wild-type W83 P. gingivalis was pre-reduced with 0.5% β-mercaptoethanol and 4mM TLCK for 10 minutes at 37°C for complete inactivation of gingipain proteases before being mixed 1:1 with SDS-PAGE sample buffer. Samples were incubated at various temperatures for 15 minutes before being electrophoresed on SDS-PAGE and transferred onto 0.2 μm nitrocellulose membranes for Western blot procedures.
Plasmid construction for the PorT+ mutant
Using a similar strategy to that as described previously to create the RgpB+ construct (Nguyen et al., 2007), a 1.4-kb segment comprising the porT gene (TIGR accession no.: PG0751) and a region 5′ to the gene was amplified by PCR using Accuprime™ Pfx DNA Polymerase (Invitrogen Inc., USA) and inserted into pUC19 plasmid (New England Biolabs Inc., USA). All primers used in this paper are listed in Table S1. An intervening tetracycline resistance cassette (tetQ) from the plasmid pNFD13-2 (Nikolich et al., 1992) was amplified and inserted 3′ to the porT gene on the modified pUC19. The resultant plasmid was further modified by incorporation of a 1-kb 3′ flanking region to the porT gene to create the final master plasmid pPorTAtB-C. Correct placement and orientation of the DNA segments were confirmed by sequencing.
Creation of deletional and insertional mutants of PorT
Using the pPorTAtB-C master plasmid as template, a modified SLIM mutagenesis method (Chiu et al., 2004; Nguyen et al., 2007) was used to create various plasmids with porT excised or affinity-tag insertions into selected regions of porT gene (Table 1). Primer sets used for mutagenesis are listed in Table S1. All resultant plasmids were screened for the correct mutation by DNA sequencing of the pertinent region. The purified plasmids were electroporated into competent P. gingivalis W83 cells as described previously (Nguyen et al., 2007) and integration of the modified genes into the P. gingivalis genome by a double crossover recombination event was confirmed by PCR and DNA sequencing. Southern blots with DIG-labelled tetQ probes were used to confirm the presence of only one crossover event in the genome of each mutant.
Table 1.
P. gingivalis strains and mutants used in this study.
| Strain | Relevant genotype | Source |
|---|---|---|
| W83 | Wild-type | Reference strain (Nelson et al., 2003) |
| PorT+ | porT+ (Tcr) | This study |
| ΔPorT | porT (Tcr) | This study |
| 6H33 | porT100::6xHis * (Tcr) | This study |
| 6H37 | porT112::6xHis (Tcr) | This study |
| 6H67 | porT202::6xHis (Tcr) | This study |
| 6H97 | porT292::6xHis (Tcr) | This study |
| 6H121 | porT364::6xHis (Tcr) | This study |
| 6H135 | porT406::6xHis (Tcr) | This study |
| 6H147 | porT442::6xHis (Tcr) | This study |
| 6H169 | porT508::6xHis (Tcr) | This study |
| 6H191 | porT574::6xHis (Tcr) | This study |
| 6H197 | porT592::6xHis (Tcr) | This study |
| 6H213 | porT640::6xHis (Tcr) | This study |
| 6H244 | porT733::6xHis (Tcr) | This study |
| Str213 | porT640::Strep-tag† (Tcr) | This study |
Sequence for 6×His tag is 5′-CATCACCATCACCATCAC
Sequence for Strep-tag is 5′-TGGTCTCATCCTCAGTTCGAAAAG
Enzyme activity assay
Early stationary phase cultures of mutants were adjusted to OD600 1.5 and 10 μL or 20 μL were assayed for Rgp or Kgp activity, respectively, using the chromogenic substrates Benzoyl-L-Arg-p-nitroanilide (BApNA) or acetyl-L-Lys-p-nitroanilide (AcKpNA; Bachem, Germany). Briefly, in a 96-well format, samples were preincubated in assay buffer (200 mM Tris-HCl, 100 mM NaCl, 5 mM CaCl2, pH 7.6, supplemented with fresh L-cysteine to 10 mM) for 2 mins prior to the addition of 0.5 mM substrate in a total volume of 200 μL. Likewise, 50 μL aliquots of the adjusted cultures were assayed for dipeptidyl peptidase IV (DPPIV) and prolyl tripeptidyl peptidase (PTP) activity in 200 mM HEPES, 100 mM NaCl, pH 7.5 using the substrates H-Ala-Pro-p-nitroanilide (APpNA; Bachem, Germany) and H-Ala-Phe-Pro-p-nitroanilide (AFPpNA; Bachem, Germany), respectively. The rate of formation of p-nitroanilide was measured at 405 nm using the kinetics mode over 5 mins on a Benchmark Microplate Reader (Bio-Rad Corp., USA). For ease of comparison between mutants and statistical analyses of independent repetitions, activity units were defined as the total activity present in the wild-type W83 culture equaling 100U.
Real-time PCR procedure
Total RNA from mid-log growth cells at OD600 0.8–0.9 were stabilized with RNAprotect Bacteria Reagent (Qiagen, Germany) before being extracted with the RNAqueous kit (Ambion; Austin, TX). Reverse transcription was carried out on 4 μL of total RNA using the Superscript III First-Strand Synthesis kit (Invitrogen Corp., Carlsbad, CA) in a volume of 10 μL as per the manufacturer’s instructions. Singleplex real-time PCR for rgpB was carried out in using 2μL of 1:200 dilution of the cDNA in triplicate on a Strategene Mx3005P Real-Time PCR System® using the Power SYBR® Green PCR Master Mix (Applied Biosystems, CA, USA) as published previously (Nguyen et al., 2007). For porT expression, singleplex real-time PCR using Platinum Quantitative PCR Supermix (Invitrogen Corp.) and 5μL of 1:35 dilution of the cDNA along with TaqMan probes against porT and a housekeeping DNA gyrase gyrA gene (PG1386 TIGR database; see primer sequences in Table S1). DNA gyrase is an essential protein to unwind genomic DNA for replication, hence, it is commonly used as a housekeeping gene or a “calibrator” for standardization of gene expression data (Eleaume & Jabbouri, 2004; Nollmann et al., 2007). Fluorescence intensities were normalised against a passive fluorophore (ROX) present in the Mastermix and converted to absolute quantities using standard curves. To control for variability of cDNA in the samples, the expression of rgpB or porT were normalised to the constant housekeeping gene gyrA by expressing the data as the ratio between the average rgpB or porT CT value divided by the average gyrA CT value for each sample and the average ratio in the wild-type W83 strain was set at 100 for ease of comparison between independent runs. Real-time PCR data were obtained from two separate experiments.
Statistical analysis
Prism v3.03 software (GraphPad Software Inc., CA, USA) were used for all statistical analyses. Enzymatic activities and real-time PCR data were tested for normality distribution and differences were analysed using repeated measures and one-way ANOVA, respectively, with Bonferroni’s correction and 95% confidence intervals. P values below 0.05 were considered significant.
Results
Cellular localisation of PorT
Using established cellular fractionation techniques including Sarkosyl solubilisation of the inner membrane (IM) and loosely bound peripheral membrane proteins (Filip et al., 1973; Nguyen et al., 2007; Nikaido, 1994), PorT was detected only in the outer membrane (OM) fraction of wild-type P. gingivalis cells by Western blot (Fig. 1A). Successful fractionation of the IM and OM was confirmed by the exclusive presence of a biotin-containing protein in the IM fraction – an oxaloacetate decarboxylase as determined by N-terminal sequencing (oadA gene; TIGR ID: PG1609) – detected by alkaline phosphatase (AP)-conjugated streptavidin (Fig. 1B) and the exclusive presence of lipopolysaccharide (LPS) in the OM fraction as detected by anti-LPS mAb 1B5 (Fig. 1C). Moreover, due to the extraordinary stability of outer membrane proteins, they have been reported to exhibit heat-modifiability characteristics whereby SDS detergent cannot denature the protein unless heated above a threshold temperature (Barnard et al., 2007; Freudl et al., 1986). By Western blot, PorT does have heat-modifiability characteristics with denaturation in the presence of SDS occurring between 50 and 60°C (Fig. 1D).
Figure 1.
Localisation and heat-modifiability of PorT. Wild-type P. gingivalis was fractionated as: whole cell (1), total membrane (2), inner membrane (3), outer membrane (4), cytoplasm (5) and periplasm (6) as per Methods. Samples were subjected to SDS-PAGE, transferred onto nitrocellulose membranes and probed with anti-PorT pAb (panels A and D). Purity of the membrane fractions was confirmed by the exclusive presence of a biotin-containing protein oxaloacetate decarboxylase in the IM as detected with alkaline phosphatase-conjugated streptavidin (B) as per Methods and the exclusive presence of LPS in the OM as detected with anti-LPS 1B5 mAb (C). Heat-modifiability of PorT (D) in the outer membrane fraction was evident when heated to various temperatures for 15 minutes in SDS-PAGE sample buffer with reducing agent.
Predicted topology of PorT
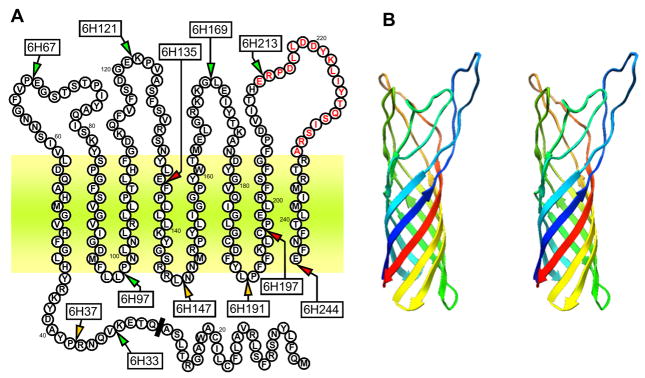
While web-based functional prediction server ProFunc (Laskowski et al., 2005a; Laskowski et al., 2005b) identified PorT as an integral OM protein, the structure is not yet known. Prediction of the secondary structure using PSI-PRED (Jones, 1999) yielded eight beta-strands, suggesting a beta-barrel structure. This interpretation was subsequently confirmed using programs designed to predict beta-barrels: ProfTMB (Bigelow et al., 2004) and HHomp (http://toolkit.tuebingen.mpg.de/hhomp/). A surface protein A (PDB code: 1P4T) from Neisseria meningitidis was identified by HHpred (Soding et al., 2005) as the best structural template for PorT and the final model was built using Modeller (Sali & Blundell, 1993) on the basis of the manually edited alignment (data not shown). The predicted structure comprising of 8 membrane spanning β-sheets with 4 extensive extracellular loops and 3 short periplasmic turns along with a periplasmic N-terminal extension is depicted in Figure 2.
Figure 2.
Membrane spanning regions of PorT as predicted by the HHPred program. Panel A: Sites of 6×His insertions are indicated by arrows along with the mutant name (boxed). Mutants with fully functional PorT are represented in green, those with partially defective PorT in orange and non-functional PorT in red. A synthetic peptide corresponding to the residues in red was used to immunised rabbits for anti-PorT antibody production. Predicted cleavage site of the signal peptide is indicated by a black bar and the outer membrane bilayer is depicted by the horizontal coloured zone. Panel B: The modeled stereo 3D structure of PorT without the N-terminal extension.
Generation of PorT mutants
Integral OM proteins can tolerate insertion of peptides into the extracellular-exposed loops without significant loss of function whereas disruption within the short periplasmic turns is less tolerated and disruption within the transmembrane β-strands leads to the failure to insert into the membrane resulting in a complete loss of function (Freudl, 1989; Koebnik et al., 2000). In order to verify the predicted model of PorT, site-directed mutagenesis was used to insert hexa-histidine (6×His) residues at 12 selected locations within the protein to investigate its functional tolerance to the insertions (Fig. 2, Table 1). As PorT has been shown to be essential for gingipain maturation (Sato et al., 2005), the level of gingipain activity as compared to wild-type strain was employed to assess the degree of impairment of PorT function for each insertional mutation.
The strategy to create the 6×His insertional PorT mutants involved the initial assembly of a master plasmid, pPorTAtB-C, whereby a 2.4 kb region containing the porT gene was amplified by PCR and ligated into pUC19 plasmid along with an intervening tetracycline-resistance gene tetQ, placed 3′ to the porT gene. After introduction into P. gingivalis W83 strain by electroporation, homologous recombination of this construct into the genome results in a fully functional PorT+ mutant to serve as a control for possible polar effects from genetic manipulations. By using the SLIM method of mutagenesis (Chiu et al., 2004), DNA encoding for 6×His tags was introduced by insertional mutagenesis at twelve selected sites within the porT gene in the master plasmid pPorTAtB-C and deletional mutagenesis was used to remove the porT gene in one plasmid construct. After electroporation of the plasmids into P. gingivalis for homologous recombination, the corresponding His-tagged PorT mutants and a PorT deletional inactivation mutant were created respectively (Table 1). As a further control against the 6×His tag causing a phenotypic change, an additional construct using a Strep tag (Schmidt & Skerra, 2007) was inserted at one location to verify that the observed effects were tag-independent (Table 1).
Production of PorT in the mutants
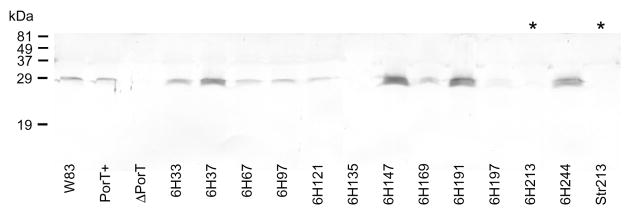
By semi-quantitative Western blot, PorT was found to be expressed naturally at low levels in the wild-type and PorT+ control mutant. Tag insertions into the first three extracellular loops (mutants 6H67, 6H121, 6H169) and into the first periplasmic turn (6H97) did not significantly affect the expression of PorT in the OM fraction as compared to wild-type and the PorT+ control (Fig. 3). Unfortunately, due to the immunizing epitope for antibody production being located in the fourth extracellular loop (Fig. 2), tag insertions into the fourth extracellular loop (6H213 and Str213) disrupted the antigenic site resulting in failure of the antibody to recognize PorT in the these mutants (Fig. 3). PorT production seems to increase slightly with tag insertion at the far N-terminal extension (6H33), moderate increase was seen with insertions into the mid-N-terminal extension (6H37) and at the C-terminus (6H244) and the greatest increase being detected in mutants with insertions into the last two periplasmic turns (6H147 and 6H191) (Fig. 3). Tag insertion into the membrane spanning regions resulted in a trace amount of PorT in one mutant (6H197) and a total loss in another (6H135). Presumably, the presence of the tag in the transmembrane regions interfered with membrane insertion, hence, PorT was poorly detected in the OM fraction. PorT was undetectable in the PorT deletional mutant (ΔPorT) as expected.
Figure 3.
Presence of PorT in the outer membrane (OM) fraction of PorT mutants. Early stationary phase cultures were adjusted to OD600 1.5 and the OM were prepared as per Methods. 50 μL samples were subjected to SDS-PAGE, transferred onto nitrocellulose membranes and probed with anti-PorT polyclonal antibodies prior to detection by alkaline phosphatase-conjugated secondary antibodies. *, the insertion of the 6×His and Strep-tag into these mutants disrupted the epitope as recognised by the anti-PorT antibody.
Effects of PorT mutation on the CTD-dependent secretion
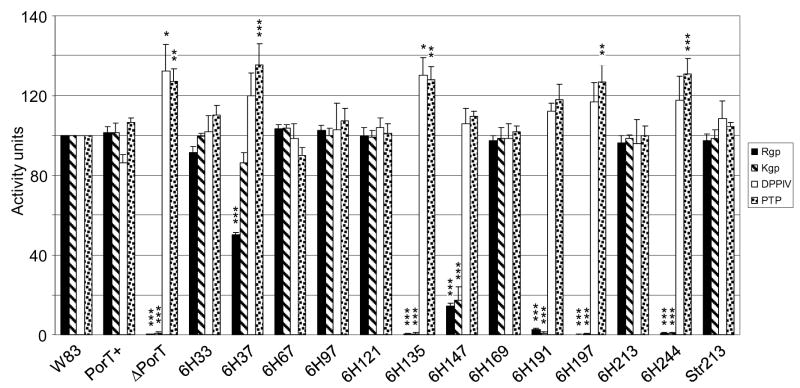
PorT has been reported to be essential for gingipain processing and transport across the OM (Sato et al., 2005), and was suggested to be involved in the maturation and export of a class of proteins with a characteristic C-terminal domain (CTD) (Nguyen et al., 2007). To assess the effect of insertional mutation of PorT on the CTD-dependent secretory pathway, a number of P. gingivalis cell surface enzymes with or without the CTD domain were assayed (Fig. 4). Of the PorT mutants, CTD-dependent gingipain production, specifically, surface-bound Rgp and Kgp activities as measured using synthetic substrates, were essentially abolished in the PorT deletional mutant along with mutants having 6×His insertion into the transmembrane regions (mutants 6H135 and 6H197) and insertion at the C-terminus (6H244). Insertions in the periplasmic turns caused a range of effects from no effect in disruption to the first turn (T1; mutant 6H97), 80% reduction at the second turn (T2; mutant 6H147) and a complete loss of gingipain activity at the third turn (T3; mutant 6H191). Interestingly, one insertion within the N-terminal extension (mutant 6H37) also caused a 50% reduction in Rgp production (Fig. 4). The insertion of a different peptide (Strep tag) in mutant Str213 gave the same result as the insertion of a 6×His tag at the same location in mutant 6H213 (Fig. 4). Of note, active gingipains were not detected in the media fractions from any of the mutants and lysis of the cells by sonication to release internal partially processed gingipains did not significantly alter the level of active gingipains assayed (data not shown). Further, since Kgp production has been linked to colony pigmentation on blood agar (Okamoto et al, 1998), the degree of pigmentation displayed by the PorT mutants directly correlates to the amount of gingipain production as predicted (Fig. S1).
Figure 4.
Extracellular enzymatic activity in PorT mutants. Four independent early stationary phase cultures were adjusted to OD600 1.5 and each whole bacterial culture was assayed for Rgp, Kgp, dipeptidyl peptidase IV (DPPIV) and prolyl tripeptidase (PTP) activities as described in Methods. Data were normalised to the wild-type W83 in each culture set (W83 activity = 100 units) for statistical comparison. Significant differences from the wild-type activity levels were analysed by repeated measures ANOVA with Bonferroni’s correction at 95% confidence intervals. Significance: *, p<0.05; **, p<0.01; ***, p<0.001; error bars denoting the SEM.
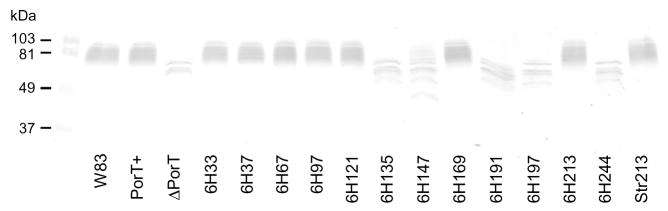
By Western blot against a member of the gingipain family, RgpB, using anti-RgpB mAb 18E6, (Nguyen et al., 2007), the characteristic smear of mature, glycosylated RgpB in the wild-type was replaced by partially processed RgpB bands in the PorT defective and deletional mutants (Fig. 5), compatible with partially processed RgpB observed when the C-terminus of the CTD domain of RgpB was truncated (Nguyen et al., 2007). The residual gingipain activity in 6H147 (Fig. 4) correlated well to the faint glycosylated RgpB smear present on the Western blot (Fig. 5).
Figure 5.
Maturation of RgpB in PorT mutants. Fifty-microliter samples of whole cultures of various mutants at early stationary growth phase were subjected to SDS-PAGE, transferred onto nitrocellulose membranes and probed with anti-RgpB mAb 18E6 (Nguyen et al., 2007).
In contrast, the cell surface enzymes dipeptidyl peptidase IV (DPPIV) and prolyl tripeptidase (PTP) do not contain a CTD domain and are presumably exported by a different pathway (Banbula et al., 1999; Nakamura et al., 1992). As predicted, the production of these enzymes was not reduced in PorT mutants, but interestingly, they are significantly upregulated in those strains with defective gingipain production, presumably, in an attempt to compensate for the loss of general proteolytic activity in the absence of gingipain activity (Fig. 4). The absence of functional gingipains in a number of mutants did not reduce growth rate in the complex media used but instead, the PorT defective mutants mostly grew more rapidly and reached a higher cell density in culture than for the wild-type and functional PorT mutants (Fig. S2).
Analysis of mutants for transcriptional activity of porT and rgpB
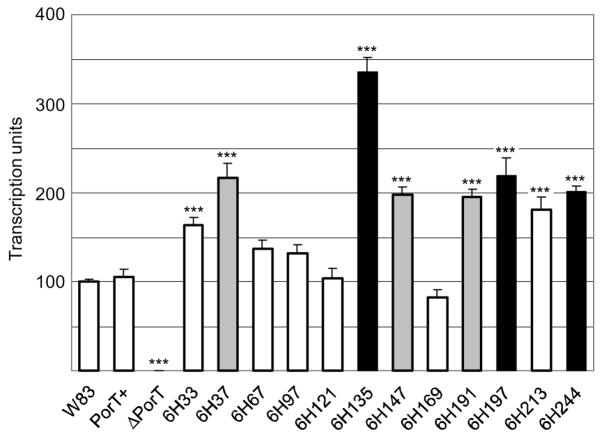
For accurate comparison of porT and rgpB expression levels between each mutant as quantified by real-time PCR, the housekeeping DNA gyrase (gyrA) gene was used as an internal standard or a “calibrator”, to control for varying levels of mRNA in each sample. The level of porT transcription normalised to the gyrA gene at mid-logarithmic growth of each mutant (Fig. 6) was found to correlate well with the PorT Western blot (Fig. 3). As predicted, no significant change in porT transcription was detected in mutants with similar PorT production to the wild-type (Fig. 6). In mutants with elevated PorT production such as those with insertions into the periplasmic turns (6H147 and 6H191), into the N-terminal extension (6H33 and 6H37) or into the fourth extracellular loop (6H213), porT transcription was also elevated. However, the highest level of porT transcription was detected in mutants with insertions into the transmembrane regions (6H135 and 6H197) and at the C-terminus (6H244). This data suggests that a negative feedback mechanism operates to regulate porT transcription in response to a perceived functional deficiency of PorT. No transcripts were detected in the PorT deletional mutant, as expected.
Figure 6.
porT transcription level as measured by real-time PCR. Total RNA was extracted from mid-log growing cultures and a two-step reverse transcription and quantitative real-time PCR were carried out as per Methods. porT transcription levels were normalised against a housekeeping DNA gyrase (gyrA) gene in each sample and the average wild-type (W83) expression level was set as 100 transcription units. Data were obtained from triplicate measurements from two independently grown cultures for each strain with error bars denoting the SEM. Differences in each strain against the wild-type were statistically analysed using one-way ANOVA with Bonferroni’s correction. Mutants with fully functional PorT are represented in white, those with partially defective PorT in grey and non-functional PorT in black. Significance: ***, p<0.001
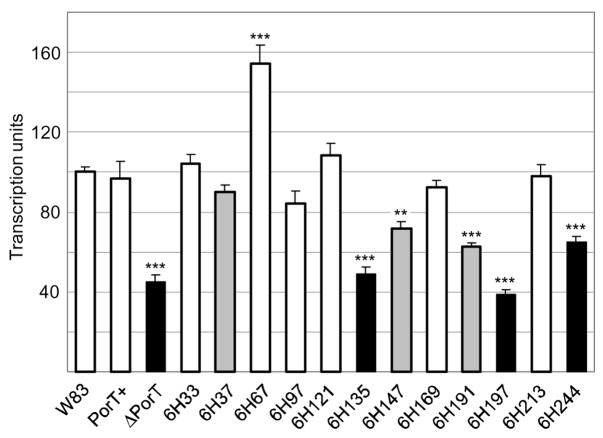
On the contrary, transcription of rgpB was significantly downregulated in the PorT defective and deletional mutants and the degree of repression correlated to the severity of the defect; being as low as 40% of wild-type levels in ΔPorT and 6H197 mutants (Fig. 7).
Figure 7.
rgpB transcription level as measured by real-time PCR. Total RNA was extracted from mid-log growing cultures and a two-step reverse transcription and quantitative real-time PCR were carried out as per Methods. rgpB transcription levels were normalised against a housekeeping DNA gyrase (gyrA) gene in each sample and the average wild-type (W83) expression level was set as 100 transcription units. Data were obtained from triplicate measurements from two independently grown cultures for each strain with error bars denoting the SEM. Differences in each strain against the wild-type were statistically analysed using one-way ANOVA with Bonferroni’s correction. Mutants with fully functional PorT are represented in white, those with partially defective PorT in grey and non-functional PorT in black. Significance: **, p<0.01; ***, p<0.001
Discussion
The export of proteins through the two membrane structure of the cell envelope in Gram-negative bacteria is a complicated endeavor. To date, six types of secretion pathways through the OM of Gram negative bacteria have been described and many of these pathways have been characterized as virulence traits in a number of species (Ghosh, 2004; Mougous et al., 2006). The secretion pathways are divided into two categories: Sec-independent and Sec-dependent systems, depending on whether the effector proteins are exported out of the double membrane structure through one or two steps, respectively. Types I, III, IV and VI secretion systems export the target protein through both inner and outer membranes in a single step (Gerlach & Hensel, 2007; Pukatzki et al., 2006). In contrast, proteins exported by type II and V secretion systems are first translocated in an unfolded state through the IM by an N-terminal signal peptide and the Sec apparatus. They achieve an intermediate folded state within the periplasm before being exported through the OM via various portals (Cianciotto, 2005; Henderson et al., 2004). In the case of P. gingivalis, types II, III and VI secretion apparatus are not present in the genome and the primary sequences of the gingipains are incompatible with the remaining known secretory systems (Nguyen et al., 2007; Sato et al., 2005). As a consequence, there has been considerable interest in uncovering a new secretory apparatus operating in P. gingivalis to export a number of virulence factors, including the gingipains.
Recent discovery of two novel proteins, PorT and Sov, which are essential for the maturation and export of the gingipains and possibly other surface proteins carrying the CTD motif in a restricted group of bacteria, has suggested that these three elements play a key role in a new secretory pathway (Nguyen et al., 2007; Saiki & Konishi, 2007; Sato et al., 2005). In order to assign possible functions to the two known protein components of this pathway, knowledge of their cellular localization is essential. Previously, Sato et al. (2005) interpreted PorT localization to be on the periplasmic surface of the inner membrane by using selective solubilisation of the inner membrane with the detergent Triton X-100 and using a pattern of Proteinase K digestion of spheroplast preparations in the presence or absence of the same detergent. Although Triton X-100 has been used successfully to fractionate E. coli membranes under specific conditions and detergent concentrations (Schnaitman, 1971), the technique has not been thoroughly validated for P. gingivalis. Specific markers for OM and IM were not used to validate the fractionation of PorT, therefore, its cellular localization could not be confirmed (Sato et al., 2005). Further, the relative resistance of PorT in P. gingivalis spheroplasts to Proteinase K digestion without Triton X-100 as compared to Kgp high molecular weight precursor proteins as demonstrated by these authors, suggests that PorT is intrinsically more stable (also a feature of integral OM proteins) requiring solubilisation by Triton X-100 before becoming susceptible to Proteinase K cleavage.
On the other hand, the detergent Sarkosyl has been used extensively in membrane fractionation of E. coli (Filip et al., 1973; Nikaido, 1994) and we have used Sarkosyl to successfully fractionate IM and OM proteins in P. gingivalis previously (Nguyen et al., 2007). In this report, we found that PorT partitioned to the OM fraction by Western blot analysis and displayed heat-modifiability characteristics of OM proteins (Fig. 1). Further, due to low abundance of the protein in the wild-type strain (Fig. 3), attempts to verify PorT localization to the OM by immunofluorescence, flow cytometry and ultrastructural studies were unsuccessful. Although additional confirmation of the OM localization could have been possible by using a detergent-independent membrane fractionation procedure such as sucrose gradient fractionation, we have gone a step further and modelled the OM topology of the protein and to verify this model by studying the functional tolerance of PorT to peptide insertions at various positions within the predicted structure (Fig. 2). Web-based structure prediction programs have predicted PorT to be an 8-stranded OM β-barrel with characteristic OM protein features such as an even number of anti-parallel β-strands, long extracellular loops, short periplasmic turns and both termini facing the periplasmic side (Galdiero et al., 2007; Schulz, 2002). Functional tolerance to disruption by peptide insertion at selected sites within PorT accurately correlated with the model: all insertions into the extracellular loops being tolerated well, insertions into the short periplasmic turns were tolerated to a variable degree while insertions into the transmembrane β-strands interfered with membrane insertion and hence, were not tolerated. Interestingly, peptide insertion into the periplasmic N-terminal extension at position 37 also reduced gingipain maturation to some degree suggesting that this region may be important in binding to its substrate or to other subunit component(s) of the secretory pathway. Further, insertion at the C-terminus totally inhibits maturation of the gingipains but did not interfere with membrane insertion as PorT was readily detected in the OM fraction of this mutant (6H244; Fig. 3). This may indicate that the ultimate glutamate residue is either critical for the function of the protein or is essential for the correct folding of the protein within the OM. Outer membrane porins have previously been shown to possess distinct C-terminal motifs for binding to OM assembly factor proteins, such as Omp85, for proper assembly into the OM (Robert et al., 2006; Voulhoux et al., 2003). Although P. gingivalis genome has an Omp85 analogue and predicted OM proteins carrying an Omp85 binding motif such as a terminal aromatic residue followed by hydrophobic residues at positions 5, 7 and 9 from the C-terminus (Robert et al., 2006), PorT C-terminus does not have this signature. However, it is possible that the PorT C-terminus may possess a recognition motif for an unknown OM assembly factor. Indeed, an alignment of 23 PorT homologues from other members of the Bacteroidetes phylum shows not only a high conservation of a terminal glutamate or glutamine residue but a strict conservation of a phenylalanine at positions 2 and 4 from the C-terminus. The presence of hydrophobic residues at positions 6 and 8 as well as a highly conserved arginine at position 10, suggests that this region may similarly act as a recognition motif for an unknown OM assembly apparatus (Fig. S3). Also of note in the multiple alignment is the high conservation of residues within the predicted transmembrane β-strands but a considerable heterogeneity of the extracellular loops in PorT analogues is present (Fig. S3) – a feature that is characteristic of many families of OM proteins (Galdiero et al., 2007; Schulz, 2002).
Apparent negative feedback operating to regulate PorT expression was indicated by significant up-regulation of transcriptional activity for this gene in mutants with defective PorT, with the highest number of transcripts being found in mutants with non-functional PorT (Fig. 6). Moreover, porT transcription was also up-regulated in two fully functional PorT mutants, 6H33 and 6H213, with His-peptides inserted nearing proximity to the N-terminus and within the L4 loop, respectively. Whether these areas are important in the regulatory signaling pathway and their disruption causing an elevated negative feedback response requires further investigation. In contrast, transcription for the gingipain rgpB gene was significantly down-regulated in mutants with defective PorT with the most severe suppression being found in non functional PorT mutants (Fig. 7). Despite the indication that these genes could be coordinately regulated, the absence of detectable suppression of rgpB transcription in the functional PorT mutants 6H33 and 6H213 (which have elevated porT transcription) argues against this possibility. It is more likely that an excessive accumulation of partially processed RgpB within the defective PorT mutants (Fig. 5) is the stimulus for an unknown negative feedback mechanism to slow down rgpB transcription until the bottleneck in RgpB maturation and export has been cleared. These data seems to indicate that the expression and maturation of gingipains are regulated on many levels and its dissection in future studies should be a challenging endeavour.
In conclusion, PorT – an essential component in a new CTD-dependent secretion pathway – has been shown to be an integral outer membrane protein. Knowledge of location and structure of PorT will provide valuable insight into its possible role in the pathway for the export of a number of surface proteins, including virulence factors such as proteases and adhesins from three periodontal pathogens P. gingivalis, Prevotella intermedia and Tannerellaforsythia. An understanding of this secretion pathway could provide an important strategy for the simultaneous control of a number of virulence factors in the prevention and treatment of periodontal disease.
Supplementary Material
Acknowledgments
We would like to thank Dr. M. Curtis for the 1B5 mAb. This work was supported by an NIH grant (DE 09761) and a grant 1642/B/P01/2008/35 from the MNiSW (Warsaw, Poland) to JP and a Ramaciotti Grant RN38/08 to KAN.
References
- Banbula A, Mak P, Bugno M, Silberring J, Dubin A, Nelson D, Travis J, Potempa J. Prolyl tripeptidyl peptidase from Porphyromonas gingivalis. A novel enzyme with possible pathological implications for the development of periodontitis. J Biol Chem. 1999;274:9246–9252. doi: 10.1074/jbc.274.14.9246. [DOI] [PubMed] [Google Scholar]
- Barnard TJ, Dautin N, Lukacik P, Bernstein HD, Buchanan SK. Autotransporter structure reveals intra-barrel cleavage followed by conformational changes. Nat Struct Mol Biol. 2007;14:1214–1220. doi: 10.1038/nsmb1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow HR, Petrey DS, Liu J, Przybylski D, Rost B. Predicting transmembrane beta-barrels in proteomes. Nucleic Acids Res. 2004;32:2566–2577. doi: 10.1093/nar/gkh580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu J, March PE, Lee R, Tillett D. Site-directed, Ligase Independent Mutagenesis (SLIM): a single-tube methodology approaching 100% efficiency in 4 h. Nucleic Acids Res. 2004;32:el74. doi: 10.1093/nar/gnh172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciotto NP. Type II secretion: a protein secretion system for all seasons. Trends Microbiol. 2005;13:581–588. doi: 10.1016/j.tim.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Eleaume H, Jabbouri S. Comparison of two standardisation methods in real-time quantitative RT-PCR to follow Staphylococcus aureus genes expression during in vitro growth. J Microbiol Methods. 2004;59:363–370. doi: 10.1016/j.mimet.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Filip C, Fletcher G, Wulff JL, Earhart CF. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudl R, Schwarz H, Stierhof YD, Gamon K, Hindennach I, Henning U. An outer membrane protein (OmpA) of Escherichia coli K-12 undergoes a conformational change during export. J Biol Chem. 1986;261:11355–11361. [PubMed] [Google Scholar]
- Freudl R. Insertion of peptides into cell-surface-exposed areas of the Escherichia coli OmpA protein does not interfere with export and membrane assembly. Gene. 1989;82:229–236. doi: 10.1016/0378-1119(89)90048-6. [DOI] [PubMed] [Google Scholar]
- Galdiero S, Galdiero M, Pedone C. beta-Barrel membrane bacterial proteins: structure, function, assembly and interaction with lipids. Curr Protein Pept Sci. 2007;8:63–82. doi: 10.2174/138920307779941541. [DOI] [PubMed] [Google Scholar]
- Gerlach RG, Hensel M. Protein secretion systems and adhesins: The molecular armory of Gram-negative pathogens. Int J Med Microbiol. 2007;297:401–415. doi: 10.1016/j.ijmm.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Ghosh P. Process of protein transport by the type III secretion system. Microbiol Mol Biol Rev. 2004;68:771–795. doi: 10.1128/MMBR.68.4.771-795.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson IR, Navarro-Garcia F, Desvaux M, Fernandez RC, Ala’Aldeen D. Type V protein secretion pathway: the autotransporter story. Microbiol Mol Biol Rev. 2004;68:692–744. doi: 10.1128/MMBR.68.4.692-744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura T. The role of gingipains in the pathogenesis of periodontal disease. J Periodontol. 2003;74:111–118. doi: 10.1902/jop.2003.74.1.111. [DOI] [PubMed] [Google Scholar]
- Imamura T, Travis J, Potempa J. The biphasic virulence activities of gingipains: activation and inactivation of host proteins. Curr Protein Pept Sci. 2003;4:443–450. doi: 10.2174/1389203033487027. [DOI] [PubMed] [Google Scholar]
- Jones DT. Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol. 1999;292:195–202. doi: 10.1006/jmbi.1999.3091. [DOI] [PubMed] [Google Scholar]
- Kamaguchi A, Nakayama K, Ohyama T, Watanabe T, Okamoto M, Baba H. Coaggregation of Porphyromonas gingivalis and Prevotella intermedia. Microbiol Immunol. 2001;45:649–656. doi: 10.1111/j.1348-0421.2001.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Koebnik R, Locher KP, Van Gelder P. Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol Microbiol. 2000;37:239–253. doi: 10.1046/j.1365-2958.2000.01983.x. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, Watson JD, Thornton JM. ProFunc: a server for predicting protein function from 3D structure. Nucleic Acids Res. 2005a;33:W89–93. doi: 10.1093/nar/gki414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski RA, Watson JD, Thornton JM. Protein function prediction using local 3D templates. J Mol Biol. 2005b;351:614–626. doi: 10.1016/j.jmb.2005.05.067. [DOI] [PubMed] [Google Scholar]
- Mougous JD, Cuff ME, Raunser S, et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science. 2006;312:1526–1530. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Takeuchi A, Masamoto Y, Abiko Y, Hayakawa M, Takiguchi H. Cloning of the gene encoding a glycylprolyl aminopeptidase from Porphyromonas gingivalis. Arch Oral Biol. 1992;37:807–812. doi: 10.1016/0003-9969(92)90114-n. [DOI] [PubMed] [Google Scholar]
- Nelson KE, Fleischmann RD, DeBoy RT, et al. Complete genome sequence of the oral pathogenic bacterium Porphyromonas gingivalis strain W83. J Bacteriol. 2003;185:5591–5601. doi: 10.1128/JB.185.18.5591-5601.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KA, Travis J, Potempa J. Does the importance of the C terminal residues in the maturation of RgpB from Porphyromonas gingivalis reveal a novel mechanism for protein export in a subgroup of Gram-negative bacteria? J Bacteriol. 2007;189:833–843. doi: 10.1128/JB.01530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Isolation of outer membranes. Methods Enzymol. 1994;235:225–234. doi: 10.1016/0076-6879(94)35143-0. [DOI] [PubMed] [Google Scholar]
- Nikolich MP, Shoemaker NB, Salyers AA. A Bacteroides tetracycline resistance gene represents a new class of ribosome protection tetracycline resistance. Antimicrob Agents Chemother. 1992;36:1005–1012. doi: 10.1128/aac.36.5.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollmann M, Crisona NJ, Arimondo PB. Thirty years of Escherichia coli DNA gyrase: from in vivo function to single-molecule mechanism. Biochimie. 2007;89:490–499. doi: 10.1016/j.biochi.2007.02.012. [DOI] [PubMed] [Google Scholar]
- O’Brien-Simpson NM, Paolini RA, Hoffmann B, Slakeski N, Dashper SG, Reynolds EC. Role of RgpA, RgpB, and Kgp proteinases in virulence of Porphyromonas gingivalis W50 in a murine lesion model. Infect Immun. 2001;69:7527–7534. doi: 10.1128/IAI.69.12.7527-7534.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Nakayama K, Kadowaki T, Abe N, Ratnayake DB, Yamamoto K. Involvement of a lysine-specific cysteine proteinase in hemoglobin adsorption and heme accumulation by Porphyromonas gingivalis. J Biol Chem. 1998;273:21225–21231. doi: 10.1074/jbc.273.33.21225. [DOI] [PubMed] [Google Scholar]
- Potempa J, Pike R, Travis J. The multiple forms of trypsin-like activity present in various strains of Porphyromonas gingivalis are due to the presence of either Arg-gingipain or Lys-gingipain. Infect Immun. 1995;63:1176–1182. doi: 10.1128/iai.63.4.1176-1182.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa J, Sroka A, Imamura T, Travis J. Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis’. structure, function and assembly of multidomain protein complexes. Curr Protein Pept Sci. 2003;4:397–407. doi: 10.2174/1389203033487036. [DOI] [PubMed] [Google Scholar]
- Pukatzki S, Ma AT, Sturtevant D, Krastins B, Sarracino D, Nelson WC, Heidelberg JF, Mekalanos JJ. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci USA. 2006;103:1528–1533. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert V, Volokhina EB, Senf F, Bos MP, Van Gelder P, Tommassen J. Assembly factor Omp85 recognizes its outer membrane protein substrates by a species-specific C-terminal motif. PLoS Biol. 2006;4:e377. doi: 10.1371/journal.pbio.0040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki K, Konishi K. Identification of a Porphyromonas gingivalis Novel Protein Sov Required for the Secretion of Gingipains. Microbiol Immunol. 2007;51:483–491. doi: 10.1111/j.1348-0421.2007.tb03936.x. [DOI] [PubMed] [Google Scholar]
- Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Sato K, Sakai E, Veith PD, et al. Identification of a new membrane-associated protein that influences transport/maturation of gingipains and adhesins of Porphyromonas gingivalis. J Biol Chem. 2005;280:8668–8677. doi: 10.1074/jbc.M413544200. [DOI] [PubMed] [Google Scholar]
- Schmidt TG, Skerra A. The Strep-tag system for one-step purification and high-affinity detection or capturing of proteins. Nat Protoc. 2007;2:1528–1535. doi: 10.1038/nprot.2007.209. [DOI] [PubMed] [Google Scholar]
- Schnaitman CA. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971;108:545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz GE. The structure of bacterial outer membrane proteins. Biochim Biophys Acta. 2002;1565:308–317. doi: 10.1016/s0005-2736(02)00577-1. [DOI] [PubMed] [Google Scholar]
- Seers CA, Slakeski N, Veith PD, Nikolof T, Chen YY, Dashper SG, Reynolds EC. The RgpB C-terminal domain has a role in attachment of RgpB to the outer membrane and belongs to a novel C-terminal-domain family found in Porphyromonas gingivalis. J Bacteriol. 2006;188:6376–6386. doi: 10.1128/JB.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soding J, Biegert A, Lupas AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005;33:W244–248. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science. 2003;299:262–265. doi: 10.1126/science.1078973. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Belton CM, Park Y, Lamont RJ. Role of fimbriae in Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1997;65:313–316. doi: 10.1128/iai.65.1.313-316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.