Abstract
Hypertension develops in many patients receiving the immunosuppressive drug tacrolimus (FK506). One possible mechanism for hypertension is a reduction in vasodilatory nitric oxide. We found that tacrolimus and a calcineurin autoinhibitory peptide significantly decreased vascular calcineurin activity; however, only tacrolimus altered intracellular calcium release in mouse aortic endothelial cells. In mouse aortas, incubation with tacrolimus increased protein kinase C activity and basal endothelial nitric oxide synthase phosphorylation at threonine 495 but reduced basal and agonist-induced endothelial nitric oxide synthase phosphorylation at serine 1177, a mechanism known to inhibit synthase activity. While this decreased nitric oxide production and endothelial function, the calcineurin autoinhibitory peptide had no such effects. Inhibition of ryanodine receptor opening or protein kinase C blocked the effects of tacrolimus. Since it is known that the FK506 binding protein (FKBP12/12.6) interacts with the ryanodine receptor to regulate calcium release, we propose this as the mechanism by which tacrolimus alters intracellular calcium and endothelial nitric oxide synthase rather than by its effect on calcineurin. Our study shows that prevention of the tacrolimus-induced intracellular calcium leak may attenuate endothelial dysfunction and the consequent hypertension.
Keywords: endothelium, hypertension, nitric oxide synthase, protein kinase C
INTRODUCTION
The immunosuppressive drug tacrolimus (FK506) is used clinically to reduce rejection in renal transplant recipients, however hypertension develops in many of these patients (1). Post-transplant hypertension is a significant risk factor for allograft vasculopathy (an accelerated form of atherosclerosis), peripheral artery disease, and decreased allograft and patient survival (2–4). The mechanisms by which tacrolimus elicits hypertension are not completely understood but are believed to originate in the vasculature (5). A likely pathogenetic candidate is endothelial dysfunction and more specifically a decrease in production of the vasodilator nitric oxide (NO) by endothelial NO synthase (eNOS). A limited number of animal studies have examined the role of endothelial dysfunction and NO bioavailability in tacrolimus-induced hypertension. Treatment of rats with tacrolimus for 2–3 weeks increases systolic blood pressure and decreases mesenteric endothelium-dependent relaxation responses as well as aortic eNOS activity (6,7). Additionally, acute treatment of human and rat resistance vessels with pharmacological concentrations of tacrolimus decreases endothelium-dependent dilation (6). We reported previously that acute treatment of mouse aortas with tacrolimus attenuates peak NO production, NO release, and endothelium-dependent relaxation responses in a concentration-dependent manner (8). These findings suggest that tacrolimus directly impairs vascular NO biosynthesis however the mechanisms remain unknown.
The decrease in aortic eNOS activity and vasodilator function by tacrolimus may be due to several factors. The formation of NO from eNOS is dependent on Ca2+/calmodulin and is regulated by numerous factors including changes in phosphorylation status (9). Tacrolimus binds its intracellular target FK506 Binding Proteins 12 and 12.6 (FKBP12/12.6) and this complex then binds to and inhibits the Ca2+/calmodulin-dependent phosphatase calcineurin (protein phosphatase 2B). The inhibition of calcineurin in lymphocytes prevents NF-AT-mediated proliferation leading to immune suppression. However, the binding of FKBP12/12.6 and inhibition of calcineurin also occurs in non-immune cells such as endothelial cells and elicits numerous effects, many of which are unknown. One known effect is the displacement of FKBP12/12.6 by tacrolimus from intracellular Ca2+ release channels (ryanodine receptors, RyRs) leading to an intracellular Ca2+ leak mediated by increased probability and duration of RyR opening (8,10–12). We reported previously that tacrolimus-mediated removal of FKBP12/12.6 from RyRs in mouse aortic endothelial cells causes a concentration-dependent Ca2+ leak and decreases agonist-induced intracellular Ca2+ release (8). However, it remains unknown whether this alteration in intracellular Ca2+ handling alters eNOS phosphorylation and contributes to the decrease in eNOS activity by tacrolimus.
Earlier studies suggested that tacrolimus-induced hypertension was caused by the inhibition of calcineurin in the peripheral vasculature since another immunosuppressive drug that inhibits calcineurin, ciclosporin, also causes hypertension (13,14). Calcineurin has been reported to decrease phosphorylation of eNOS at the inhibitory site threonine 495 (Thr495) in response to bradykinin, however other studies reported PP1 and PP2A as the phosphatases that dephosphorylate Thr495 (15–17). Another possibility is that the binding of these drugs to their immunophilins (FKBP12/12.6 for tacrolimus and cyclophilin A for ciclosporin) may mediate the detrimental vascular and blood pressure effects. Sirolimus, an immunosuppressive drug structurally similar to tacrolimus which binds FKBP12/12.6 but has no effect on calcineurin, is also associated with endothelial dysfunction and hypertension. We reported previously that vascular depletion of FKBP12/12.6 by genetic deletion of FKBP12.6 or sirolimus increases conventional protein kinase C (cPKC)-mediated eNOS phosphorylation at Thr495 and decreases NO production and endothelium-dependent dilation (12). Whether tacrolimus decreases eNOS activity by inhibiting calcineurin-mediated dephosphorylation or by increasing cPKC-mediated phosphorylation of eNOS Thr495 has not been examined.
Based on our previous data and others, we hypothesized that tacrolimus decreases vascular eNOS activity by reducing agonist-induced intracellular Ca2+ levels and preventing stimulatory changes in eNOS phosphorylation, but not through its effects on calcineurin. To test this we measured intracellular Ca2+ levels in primary mouse aortic endothelial cells (MAECs) treated acutely with tacrolimus or a specific calcineurin inhibitor, calcineurin autoinhibitory peptide (CAIP). We also measured aortic PKC activity, eNOS Thr495 and Ser1177 phosphorylation, NO production, and endothelium-dependent dilation in mouse aortas treated acutely with tacrolimus or CAIP to examine the direct vascular effects.
RESULTS
Effect of Tacrolimus and CAIP on Intracellular Calcineurin Activity and Ca2+ Levels
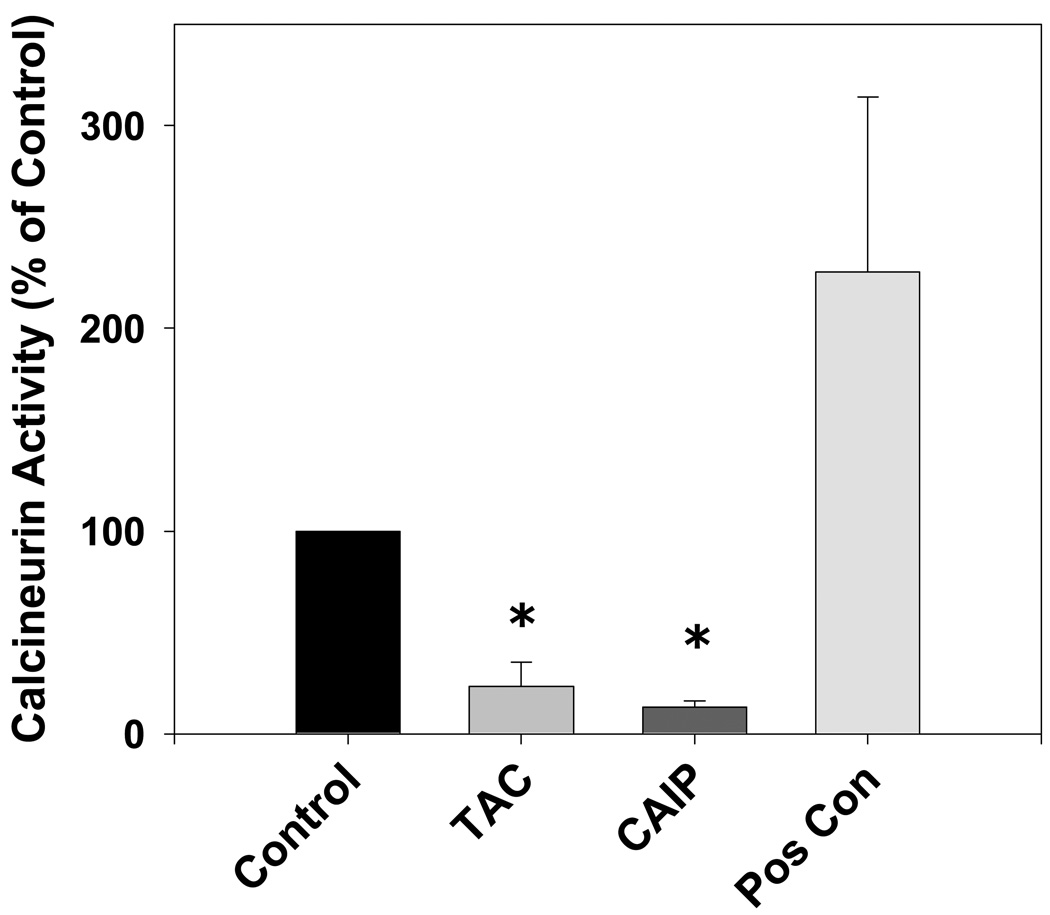
To verify the calcineurin-inhibiting effects of both tacrolimus and CAIP, we measured aortic calcineurin activity following in vitro treatment with either tacrolimus (1 µmol/L) or CAIP (10 µmol/L) for 20 minutes. Figure 1 demonstrates that both tacrolimus and CAIP at the concentrations and durations used throughout the study significantly inhibited aortic calcineurin activity 77% and 87%, respectively.
Figure 1.
Effect of tacrolimus and calcineurin autoinhibitory peptide on vascular calcineurin activity. Both tacrolimus (TAC; 1 µmol/L) and calcineurin autoinhibitory peptide (CAIP; 10 µmol/L) significantly decreased aortic calcineurin activity. Human recombinant calcineurin was used as a positive control (Pos Con). Results are expressed as mean ± SEM (n = 2–3 pooled aortas for each group and 3 independent experiments). *p<0.05 vs control.
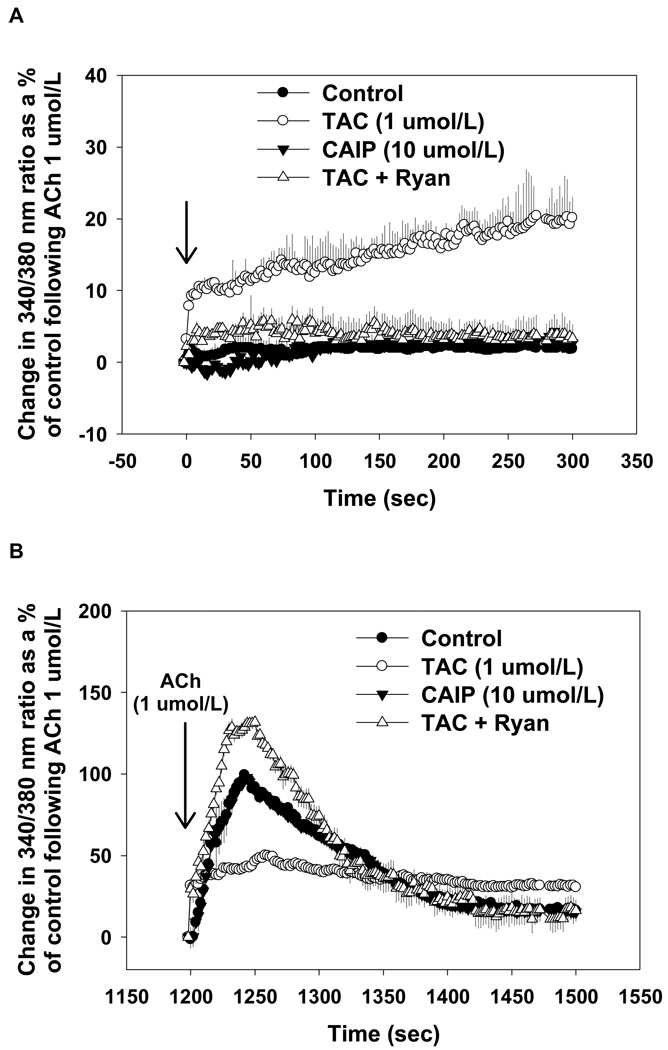
To determine the effects of tacrolimus and CAIP on endothelial intracellular Ca2+ homeostasis, we performed ratiometric Ca2+ imaging in primary MAECs. Tacrolimus (1 µmol/L) induced an intracellular Ca2+ leak of ∼20% of the maximal ACh-induced intracellular Ca2+ release in non-treated control MAECs (Figure 2A). Inhibition of RyR opening with ryanodine abolished the intracellular Ca2+ leak following tacrolimus which supports previous findings that FKBP12/12.6 removal from RyRs mediates the intracellular Ca2+ leak. In contrast, CAIP (10 µmol/L) had no effect on basal intracellular Ca2+ levels. In response to acetylcholine (ACh), peak Ca2+ release was markedly decreased in tacrolimus-treated MAECs (50 ± 5%; p < 0.05 versus controls) followed by a sustained release of 30–35% (Figure 2B). These responses were normalized by ryanodine. CAIP had no effect on ACh-induced intracellular Ca2+ release (Figure 2B).
Figure 2.
Effect of tacrolimus and calcineurin autoinhibitory peptide on endothelial intracellular Ca2+ levels. Experiments were performed in aortic endothelial cells from control mice in the absence of extracellular Ca2+. (A) Tacrolimus (TAC; 1 µmol/L) caused an intracellular Ca2+ leak, which was prevented by ryanodine (Ryan; 50 µmol/L). Calcineurin autoinhibitory peptide (CAIP; 10 µmol/L) had no effect endothelial intracellular Ca2+ levels. (B) Tacrolimus-treated control aortic endothelial cells exhibited decreased acetylcholine-induced (ACh; 1 µmol/L) intracellular Ca2+ release which was augmented by Ryan (50 µmol/L). CAIP (10 µmol/L) had no effect. Results are expressed as the mean change in 340/380 nm ratio as a % of the peak response to ACh in untreated controls (n>10 cells from 3–5 mice for each group).
Effect of Tacrolimus and CAIP on eNOS Phosphorylation
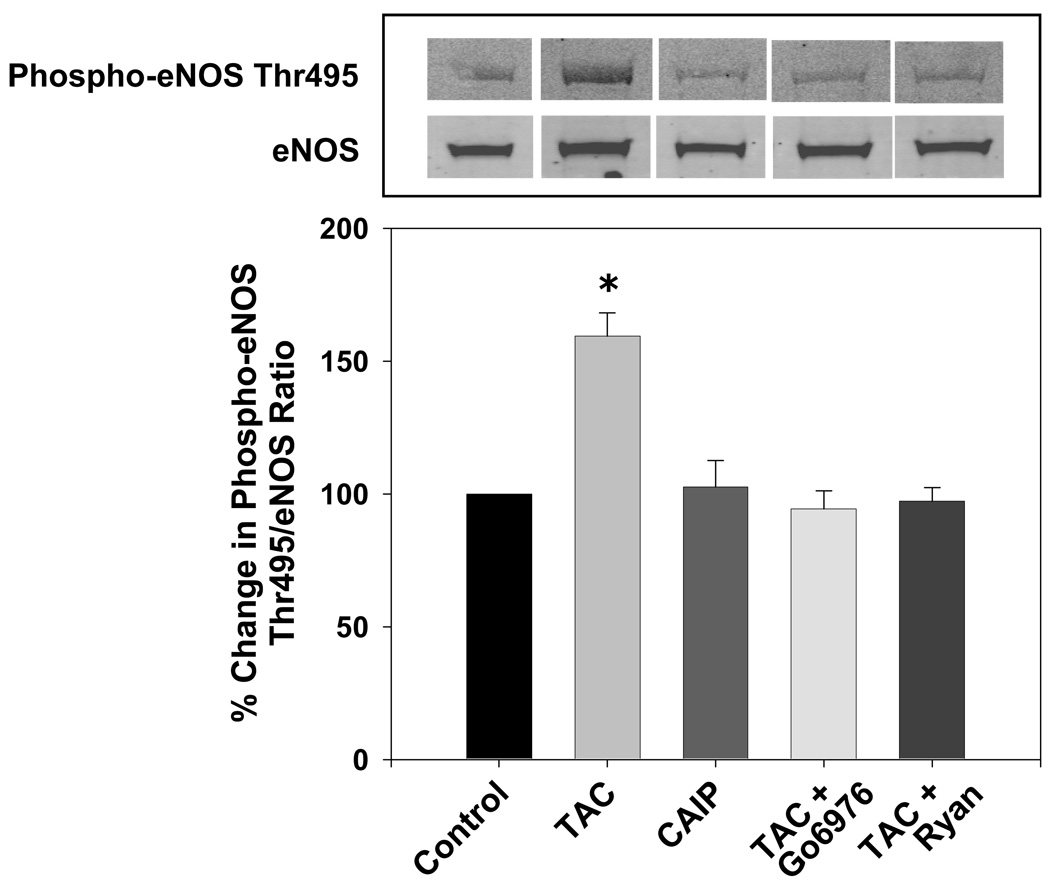
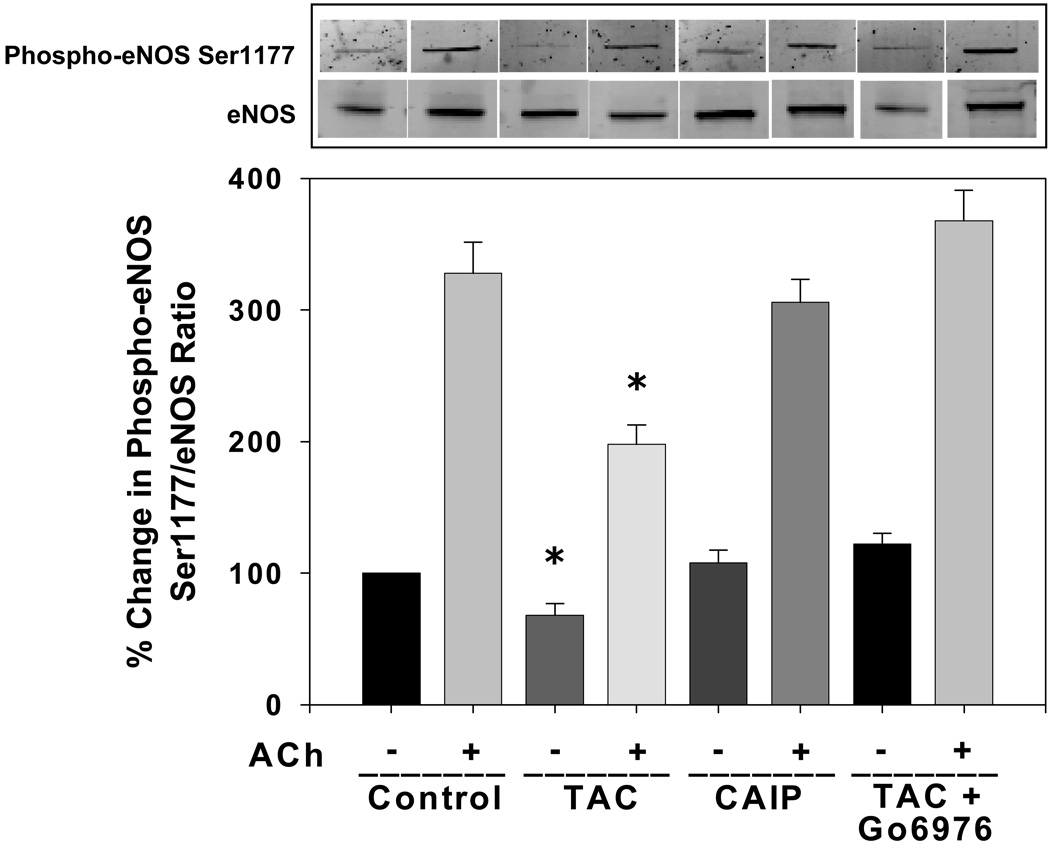
To determine whether inhibition of FKBP12/12.6 or calcineurin may contribute to altered eNOS phosphorylation, we measured aortic eNOS phosphorylation at an inhibitory site, Thr495, and at a stimulatory site, Ser1177, following in vitro treatment with either tacrolimus or CAIP. Tacrolimus significantly increased basal phosphorylation of eNOS at Thr495 (Figure 3) and decreased basal phosphorylation of eNOS at the stimulatory site Ser1177 (Figure 4) compared to controls, however CAIP had no effect on either phosphorylation site. Agonist-induced increases in eNOS Ser1177 phosphorylation were evident in non-treated control aortas (Figure 4). However, these changes were attenuated by tacrolimus but not by CAIP (Figure 4). Since PKC is known to phosphorylate eNOS at Thr495 and decrease eNOS Ser1177 phosphorylation and activity, we tested whether inhibition of the Ca2+-dependent PKC isoforms (cPKC) could prevent the tacrolimus-induced changes in eNOS phosphorylation. Co-incubation of aortas with the cPKC inhibitor Gö6976 prevented the increase in eNOS Thr495 phosphorylation and the basal and agonist-induced decrease in eNOS Ser1177 phosphorylation by tacrolimus (Figure 3 and Figure 4). Additionally, inhibition of the intracellular Ca2+ leak with ryanodine prevented the increase in eNOS Thr495 phosphorylation induced by tacrolimus (Figure 3). These findings support our hypothesis that altered endothelial intracellular Ca2+ levels via tacrolimus/FKBP12/RyR interaction negatively affects eNOS phosphorylation, but acute, direct inhibition of calcineurin does not affect basal or agonist-induced eNOS phosphorylation.
Figure 3.
Effect of tacrolimus and calcineurin autoinhibitory peptide on eNOS Thr495 phosphorylation. Tacrolimus (TAC; 1 µmol/L) increased eNOS Thr495 phosphorylation which was reversed by the cPKC inhibitor Gö6976 (1 µmol/L) or the RyR inhibitor ryanodine (Ryan; 50 µmol/L). Calcineurin autoinhibitory peptide (CAIP; 10 µmol/L) had no effect on eNOS Thr495 phosphorylation. A, Representative Western blots showing aortic eNOS Thr495 phosphorylation and eNOS expression. B, Densitometry for ratio of eNOS Thr495 phosphorylation to eNOS expression as a percent of control. Results are expressed as mean ± SEM (n = 3 pooled aortas for each group and 3 independent experiments). *p<0.05 vs control.
Figure 4.
Effect of tacrolimus and calcineurin autoinhibitory peptide on eNOS Ser1177 phosphorylation. Tacrolimus (TAC; 1 µmol/L) decreased both basal and acetylcholine-induced eNOS Ser1177 phosphorylation which was reversed by the cPKC inhibitor Gö6976 (1 µmol/L). Calcineurin autoinhibitory peptide (CAIP; 10 µmol/L) had no effect on eNOS Ser1177 phosphorylation. A, Representative Western blots showing basal and acetylcholine-induced aortic eNOS Ser1177 phosphorylation and eNOS expression. B, Densitometry for ratio of eNOS Ser1177 phosphorylation to eNOS expression as a percent of control. Results are expressed as mean ± SEM (n = 3 pooled aortas for each group and 3 independent experiments). *p<0.05 vs control.
Effect of Tacrolimus and CAIP on Vascular PKC Activity
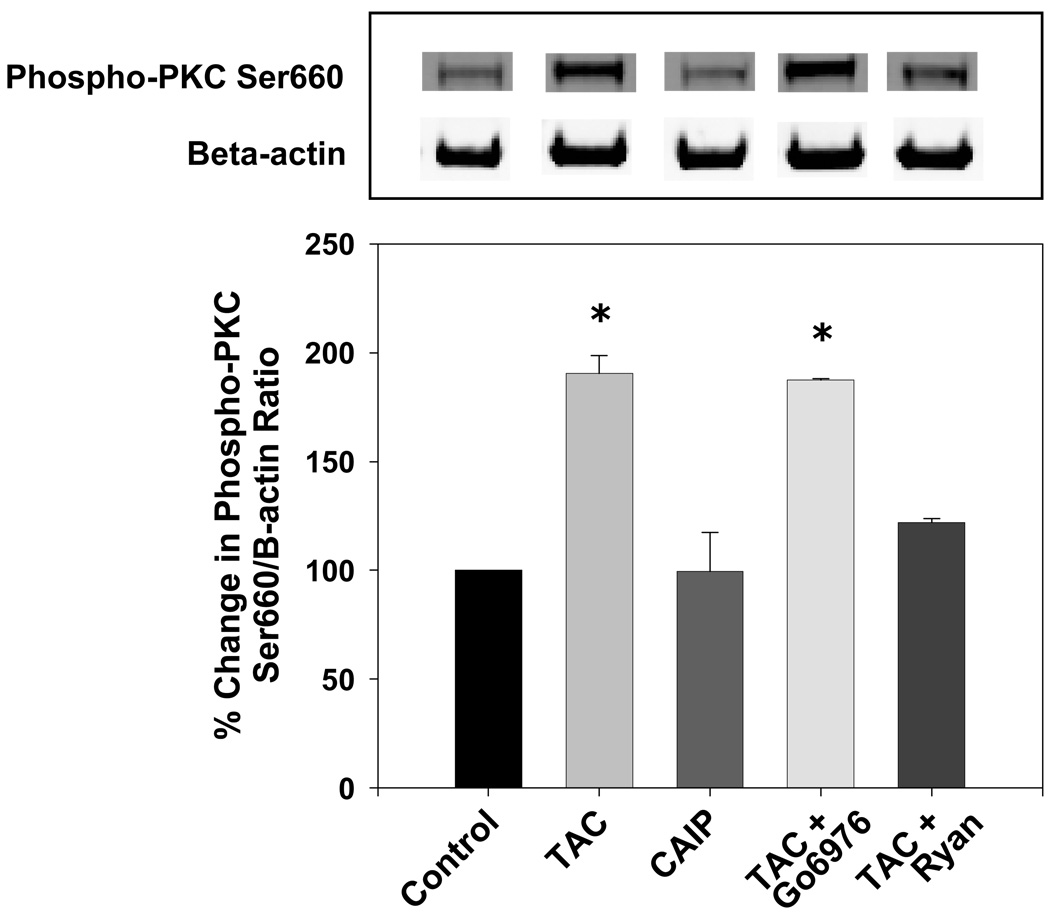
Since cPKC inhibition prevented the detrimental effects of tacrolimus on eNOS phosphorylation, we tested whether tacrolimus increased PKC activity and whether this was due to the intracellular Ca2+ leak by measuring PKC Ser660 phosphorylation (18). Tacrolimus significantly increased PKC Ser660 phosphorylation compared to controls (Figure 5), however CAIP had no effect. The tacrolimus-induced increase in PKC Ser660 phosphorylation was not blocked by Gö6976, however this was expected since Gö6976 inhibits the ATP binding site on cPKC and its catalytic activity but has no effect on PKC autophosphorylation and activation (19). However, the increase in PKC Ser660 phosphorylation by tacrolimus was blocked by ryanodine suggesting that a RyR-mediated Ca2+ leak contributes to PKC activation and eNOS Thr495 phosphorylation.
Figure 5.
Effect of tacrolimus and calcineurin autoinhibitory peptide on PKC Ser660 phosphorylation. Tacrolimus (TAC; 1 µmol/L) increased PKC Ser660 phosphorylation which was reversed by the RyR inhibitor ryanodine (Ryan; 50 µmol/L). The cPKC inhibitor Gö6976 (1 µmol/L) or calcineurin autoinhibitory peptide (CAIP; 10 µmol/L) had no effect. A, Representative Western blots showing aortic PKC Ser660 phosphorylation. B, Densitometry for ratio of PKC Ser660 phosphorylation to β-actin as a percent of control. Results are expressed as mean ± SEM (n = 3 pooled aortas for each group and 3 independent experiments). *p<0.05 vs control.
Effect of Tacrolimus and CAIP on Aortic NO Production
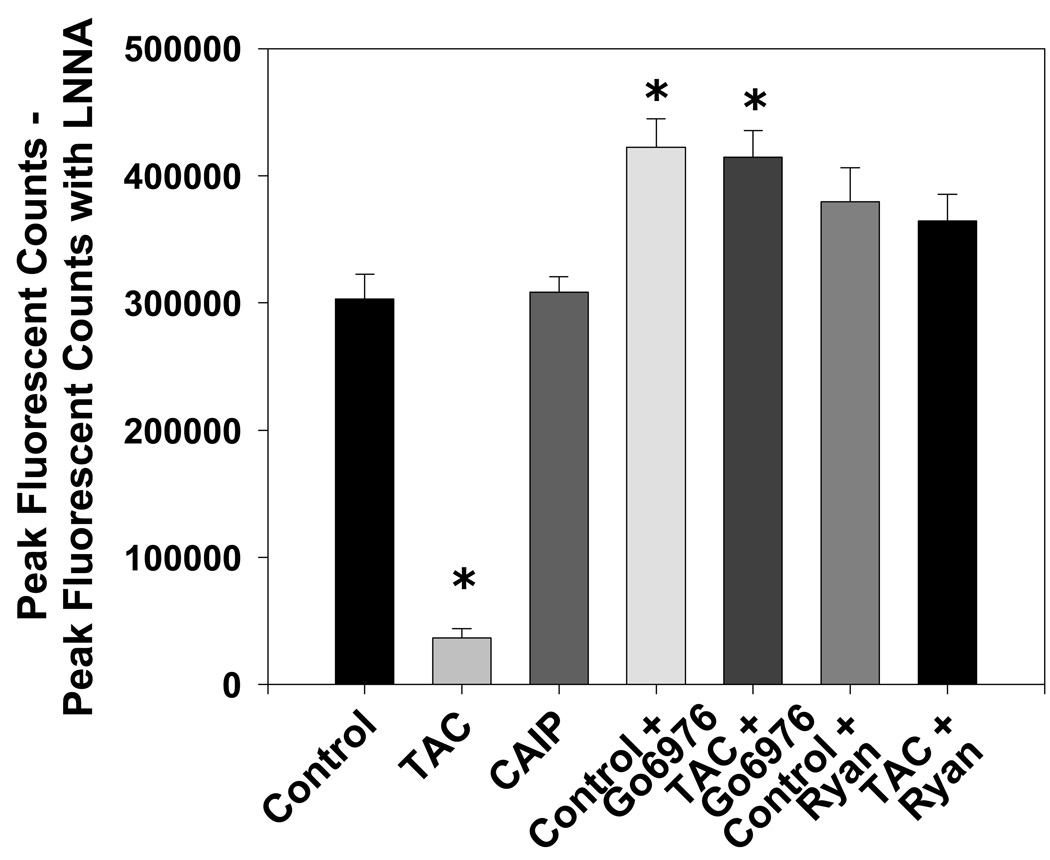
To verify that tacrolimus decreases vascular eNOS activity as previously reported (7), we measured NO production in aortic homogenates using 4-amino-5-methylamino-2’,7’-difluorofluorescein (DAF-FM) diacetate. Tacrolimus decreased peak aortic NO production 88% compared to untreated controls [Nω-nitro-L-arginine (L-NNA)-sensitive peak DAF-FM fluorescent counts: tacrolimus = 36456 ± 7548 and controls = 303128 ± 19457, p < 0.05 versus controls; Figure 6]. However, acute treatment with CAIP did not affect peak aortic NO production when compared to controls (Figure 6). Inhibition of cPKC activity with Gö6976 increased peak NO production significantly in tacrolimus-treated aortas and untreated control aortas (L-NNA-sensitive peak DAF-FM fluorescent counts: tacrolimus + Gö6976 = 414697 ± 20730 and control + Gö6976 = 422284 ± 22602, p > 0.05 versus non-treated controls; Figure 6). Additionally, blockade of the tacrolimus-induced Ca2+ leak with ryanodine restored peak NO production to control levels and had no significant effect in untreated aortas (Figure 6).
Figure 6.
Effect of tacrolimus and calcineurin autoinhibitory peptide on peak aortic NO production. Tacrolimus (TAC; 1 µmol/L) decreased Nω-nitro-L-arginine (LNNA)-sensitive DAF-FM fluorescence which was increased significantly by the cPKC inhibitor Gö6976 (1 µmol/L) or normalized by the RyR inhibitor ryanodine (Ryan; 50 µmol/L). Calcineurin autoinhibitory peptide (CAIP; 10 µmol/L) had no effect on aortic Nω-nitro-L-arginine (LNNA)-sensitive DAF-FM fluorescence. Results are expressed as mean ± SEM (n = 5 mice for each group). *p<0.05 vs control.
Effect of Tacrolimus and CAIP on Endothelium-Dependent and Endothelium-Independent Relaxation
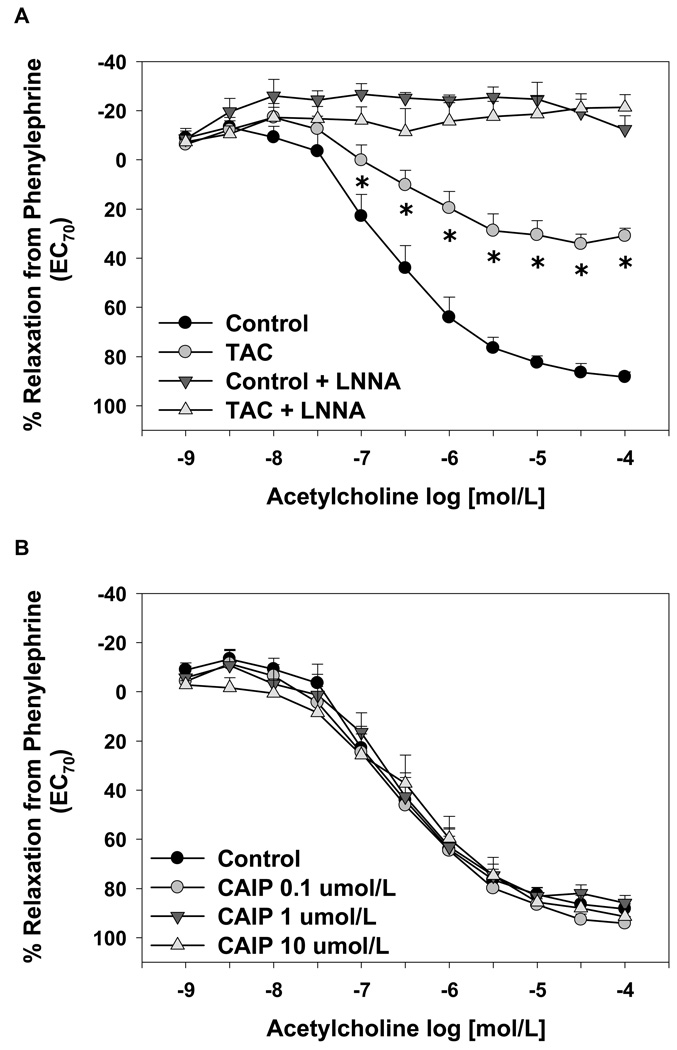
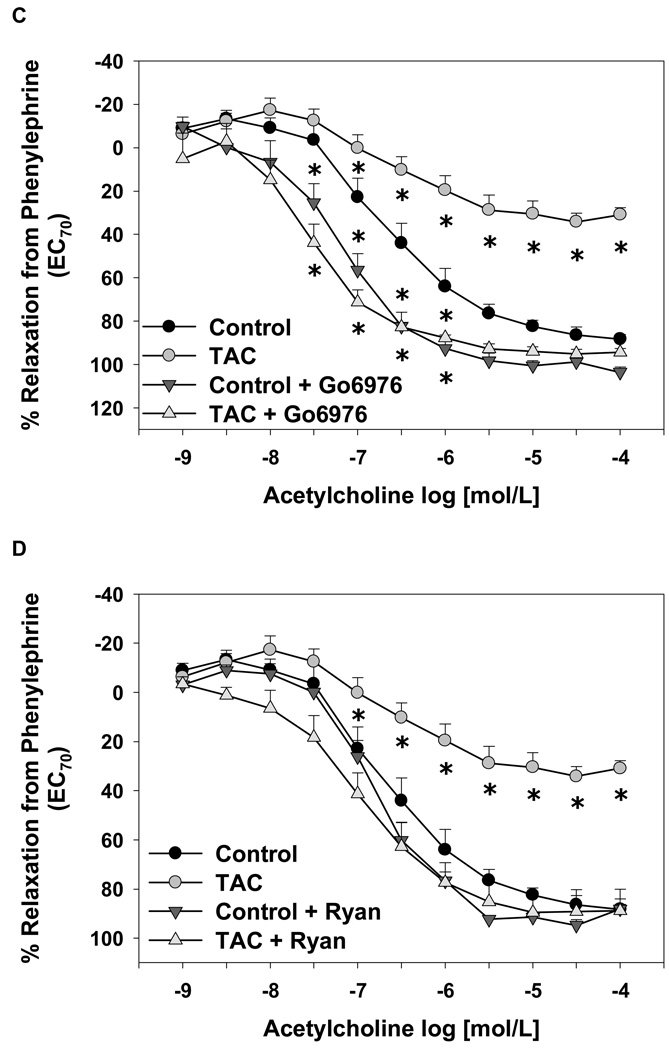
To test whether acute inhibition of FKBP12/12.6 or calcineurin had functional vascular effects, we measured aortic endothelium-dependent and endothelium–independent relaxation responses in isolated mouse aortas treated with tacrolimus or CAIP. Tacrolimus significantly decreased maximal ACh-induced relaxation responses compared to non-treated controls (relaxation from PE-induced contraction: tacrolimus = 31 ± 3% and controls = 80 ± 4%; p < 0.05 versus controls, Figure 7A). In both groups, the NOS inhibitor L-NNA (10 µmol/L) abolished relaxation responses to ACh (Figure 7A). However, CAIP, at concentrations ranging from 0.1 to 10 µmol/L, had no effect on endothelium-dependent relaxation responses (Figure 7B). Inhibition of cPKC isoforms specifically with Gö6976 (1 µmol/L) increased the sensitivity to ACh significantly in tacrolimus-treated and control aortas (Figure 7C). Ryanodine (50 µmol/L) also restored relaxation responses in tacrolimus-treated aortas to control levels, but had no significant effect on relaxation responses in untreated aortas (Figure 7D). The restoration of relaxation responses by Gö6976 or ryanodine were blocked by L-NNA (data not shown) supporting the findings in Figure 6 that inhibition of RyR opening or cPKC activity increased NO production in tacrolimus-treated aortas. Neither tacrolimus nor CAIP had an effect on maximal endothelium-independent relaxation responses or sensitivity to the NO donor sodium nitroprusside compared to controls (data not shown).
Figure 7.
Effect of tacrolimus and calcineurin autoinhibitory peptide on endothelium-dependent aortic relaxation responses. (A) Tacrolimus (TAC; 1 µmol/L) decreased acetylcholine-induced relaxation responses and Nω-nitro-L-arginine (LNNA) abolished all relaxation responses. (B) Calcineurin autoinhibitory peptide (CAIP; 0.1–10 µmol/L) had no effect on acetylcholine-induced aortic relaxation responses. Acetylcholine-induced relaxation responses were increased significantly by the cPKC inhibitor Gö6976 (1 µmol/L) (C) or normalized by the RyR inhibitor ryanodine (Ryan; 50 µmol/L) (D). Results are expressed as mean ± SEM (n = 4–7 mice for each group). *p<0.05 vs control.
DISCUSSION
The mechanisms by which tacrolimus decreases eNOS activity and causes endothelial dysfunction and hypertension are largely unknown. The aim of this paper was to address three questions: 1) Does tacrolimus decrease vascular eNOS activity and NO production by altering eNOS phosphorylation, 2) Is the altered eNOS phosphorylation due to changes in intracellular Ca2+ levels, and 3) Does calcineurin inhibition by tacrolimus play a role. Our findings demonstrate that a pharmacological concentration of tacrolimus causes an endothelial intracellular Ca2+ leak and decreases agonist-induced intracellular Ca2+ release which negatively affects eNOS phosphorylation, NO production, and endothelium-dependent dilation. This decrease in vasodilator function by tacrolimus may be due to an increase in cPKC-mediated phosphorylation of eNOS at Thr495 and result from the displacement of FKBP12/12.6 from endothelial RyRs and altered intracellular Ca2+ release. Our data do not support a role for acute calcineurin inhibition in the decreased eNOS activity caused by tacrolimus.
We previously reported that tacrolimus and sirolimus exert detrimental effects on NO production and vasodilation through FKBP12/12.6 displacement from endothelial RyRs and the resulting intracellular Ca2+ leak (8). Furthermore, we have demonstrated that the endothelial intracellular Ca2+ leak induced by sirolimus leads to increased cPKC-mediated eNOS Thr495 phosphorylation, which blocks calmodulin binding to eNOS and stimulation of eNOS activity (12). However, it remained unknown whether tacrolimus alters eNOS phosphorylation and the mechanisms involved (i.e., FKBP12/12.6 versus calcineurin). In the current study, tacrolimus, like sirolimus, increased phosphorylation of eNOS Thr495, and decreased eNOS Ser1177 phosphorylation, peak NO production, and endothelium-dependent relaxation responses in mouse aortas. Additionally, agonist-induced intracellular Ca2+ release was markedly attenuated in mouse aortic endothelial cells treated with tacrolimus confirming our previous study (8). The basal Ca2+ leak and reduced agonist-induced Ca2+ release likely prevents the achievement of the intracellular Ca2+ threshold needed for eNOS activation. The basal RyR-mediated Ca2+ leak increases cPKC-mediated eNOS Thr495 phosphorylation and the reduced agonist-induced intracellular Ca2+ levels prevent eNOS Thr495 dephosphorylation, which is necessary for calmodulin binding, eNOS Ser1177 phosphorylation, and eNOS activity. Evidence to support this comes from the findings that restoration of agonist-induced intracellular Ca2+ release via ryanodine in tacrolimus-treated endothelial cells was associated with decreased aortic eNOS Thr495 and PKC activation and increased aortic eNOS Ser1177 phosphorylation, NO production, and endothelium-dependent dilation. Although ryanodine can open RyRs at low concentrations, micromolar concentrations, such as that used here, lock the channel in a closed state supporting our hypothesis that a tacrolimus-induced, RyR-mediated Ca2+ leak initiates this pathway. These data confirm and extend our previous findings that displacement of FKBP12/12.6, either pharmacologically or genetically, from endothelial RyRs alters intracellular Ca2+, which affects numerous endothelial cell signaling pathways that modulate vascular tone including eNOS and cPKC activation. Furthermore, the current findings support the importance of eNOS Thr495 (de)phosphorylation in endothelial function and adds tacrolimus to the growing list of molecules that increase eNOS Thr495 phosphorylation and cause endothelial dysfunction including sirolimus, β-amyloid peptide, and homocysteine (12,18,20).
Since both calcineurin inhibitors ciclosporin and tacrolimus cause endothelial dysfunction and hypertension, earlier studies attributed these side effects to the inhibition of the Ca2+/calmodulin-dependent phosphatase (13,14). However, the lack of studies using a direct, specific calcineurin inhibitor has made it difficult to interpret previous results. Our data demonstrate that acute treatment with the calcineurin inhibitor CAIP, at a concentration that inhibits 87% of calcineurin activity and is similar to that achieved in patients treated with tacrolimus (21), had no direct effect on eNOS phosphorylation, NO production, and endothelium-dependent relaxation responses in control mouse aortas suggesting that the immunophilins cyclophilin A and FKBP12/12.6 mediate the detrimental effects of ciclosporin and tacrolimus, respectively, on endothelial function. Cyclophilin A has been shown to mediate several cellular processes including cholesterol trafficking to caveolae, proinflammatory responses, cell proliferation and apoptosis, and protein folding and isomerization (22–25). With respect to endothelial function, ciclosporin analogs that bind cyclophilin A but do not inhibit calcineurin were reported to have a dose-dependent cytotoxic/proapoptotic effect on cultured endothelial cells; an effect not duplicated by CAIP (22). Additionally, cyclophilin A interacts with caveolin-1 in caveolae, and ciclosporin disrupts this complex, the cholesterol content of caveolae, and the plasmalemmal localization of eNOS (26). Further supportive evidence for immunophilin involvement in vascular function comes from the findings that calcineurin knockout mice do not exhibit systolic blood pressures different from control mice either at rest or when challenged with angiotensin II, whereas FKBP12.6 knockout mice exhibit hypertension (27,28). Furthermore, sirolimus, which binds FKBP12/12.6 but inhibits mTOR, also leads to altered eNOS phosphorylation, endothelial dysfunction, and hypertension in animals and the reported incidence of hypertension in sirolimus-treated patients is increasing. Taken together, the immunophilins that bind tacrolimus, sirolimus, and ciclosporin may be the primary mediators affecting vasodilator function and blood pressure regulation, however more studies are needed to determine the role of cyclophilin A in ciclosporin-induced hypertension to confirm this hypothesis.
We cannot rule out the long term effects of calcineurin inhibition on NO production and endothelial function. One of the downstream targets of calcineurin is the transcription factor NF-AT, which translocates to the nucleus upon dephosphorylation by calcineurin. Ritter and colleagues elegantly showed that two consensus NF-AT binding sites exist on the eNOS promoter (with one being a typical NF-AT/AP-1 binding site) and direct calcineurin inhibition decreased angiotensin II AT2-receptor-induced eNOS promoter activity and eNOS expression in myocardium (29). However, studies performed in bovine aortic endothelial cells reported that ciclosporin and tacrolimus at doses similar to that used in this study increases eNOS transcription via AP-1 (30,31). Thus it is unknown whether chronic, direct calcineurin inhibition alters vascular eNOS expression. Another phosphorylation site on eNOS, Ser116, decreases agonist-induced NO production and may also contribute to the tacrolimus-induced decrease in eNOS activity and endothelial dysfunction. Kou and colleagues reported that in response to the eNOS agonist vascular endothelial growth factor (VEGF), eNOS Ser116 may be dephosphorylated by calcineurin and phosphorylated by PKC as these were blocked by ciclosporin and the PKC inhibitor calphostin, respectively (32). Based on the current findings, it is plausible that the binding of ciclosporin to its immunophilin leads to increased PKC-mediated eNOS Ser116 phosphorylation and this may explain their results. Studies are underway to determine the role of eNOS Ser116 phosphorylation in tacrolimus- and sirolimus-induced endothelial dysfunction and which PKC isoforms are responsible for phosphorylating the eNOS inhibitory sites.
In conclusion, these data demonstrate that tacrolimus abrogates NO production and endothelial vasodilator function by negatively altering endothelial intracellular Ca2+ and eNOS phosphorylation. These acute, direct effects are mediated by FKBP12/12.6 and not through the inhibition of calcineurin. The development and implementation of tacrolimus derivatives that inhibit calcineurin directly in lymphocytes but do not bind FKBP12/12.6 or alter eNOS phosphorylation in endothelial cells may markedly reduce the incidence of tacrolimus-induced hypertension.
METHODS
Animals
Male C57Bl/6 mice (Harlan; Indianapolis, IN) aged 10–18 weeks were used in all experiments. Mice were maintained on a 12:12 light/dark cycle and had access to standard chow ad libitum. All procedures were approved by the respective Institutional Animal Care and Use Committees.
Calcineurin Activity Assay
Endothelium-intact aortas from control mice were isolated, cleaned in fresh physiological salt solution (PSS – 119.0 mmol/L NaCl, 4.7 mmol/L KCl, 1.18 mmol/L KH2PO4, 1.17 mmol/L MgSO4-7H2O, 25 mmol/L NaHCO3, 11.1 mmol/L dextrose, and 2.5 mmol/L CaCl2), and then treated with tacrolimus (1 µmol/L, 20 minutes), CAIP (10 µmol/L, 20 minutes), or vehicle (DMSO <0.01% for tacrolimus). Two to three aortas from each group were pooled together and then homogenized in the presence of fresh protease inhibitors. Calcineurin activity was measured using the Colorimetric Calcineurin Cellular Activity assay from Calbiochem (San Diego, CA) per the manufacturer’s protocol. In brief, dephosphorylation of the RII peptide in the absence and presence of EGTA was measured using malachite green and the amount of phosphate released was read at 620 nm using a spectrophotometer. Recombinant human calcineurin was used as a positive control and the results are presented as percent of control for each independent experiment.
Intracellular Ca2+ Imaging in Mouse Aortic Endothelial Cells
Primary MAECs were isolated using Matrigel (BD Biosciences Discovery Labware; Franklin Lakes, NJ) and intracellular Ca2+ levels were measured as described previously (12). MAECs were loaded with Fura-2 AM (10 µmol/L) diluted in PSS and were imaged on a Nikon Eclipse E600FN equipped with a Cascade 512B CCD camera (Photometrics; Tucson, AZ). Cells were placed in zero-Ca2+ PSS (no CaCl2, 100 µmol/L EGTA) and alternately excited at 340 nm and 380 nm. After a 5 minute baseline, cells were treated with either tacrolimus (1 µmol/L), CAIP (10 µmol/L), or vehicle (DMSO <0.01% for tacrolimus) for 15 minutes. To increase Ca2+ mobilization from intracellular stores, cells were treated with ACh (1 µmol/L) and monitored for 5 minutes. Sixteen-bit time-lapsed fluorescent images (∼30 Hz) were collected at an emission wavelength of 510 nm throughout. In parallel experiments, endothelial cells were pre-treated with ryanodine (50 µmol/L, 60 minutes) to inhibit RyR opening.
Immunoblotting
Endothelium-intact aortas were treated with tacrolimus (1 µmol/L, 20 minutes), with or without the cPKC-specific inhibitor Gö6976 (1 µmol/L, 20 minutes) or ryanodine (50 µmol/L, 60 minutes), CAIP (10 µmol/L, 20 minutes), or vehicle (DMSO <0.01% for tacrolimus) and then homogenized in the presence of fresh protease inhibitors. The homogenate was centrifuged at 11,000 rpm for 10 minutes at 4°C. Protein concentration was determined by Lowry assay using bovine serum albumin as the standard (33). Vascular homogenates (30 µg for phospho-PKC Ser660 and 60 µg for phospho-eNOS) were separated by electrophoresis on 4–12% gradient SDS polyacrylamide gels and then transferred to Immobilon-FL PVDF Membranes (Millipore; Billerica, MA) as described previously (12). Western blot analyses were performed using the following primary antibodies: eNOS 1:2500 (BD Biosciences Transduction Labs; Franklin Lakes, NJ), phospho-eNOS Thr495 1:1000 (Cell Signaling; Danvers, MA), phospho-eNOS Ser1177 1:1000 (Cell Signaling; Danvers, MA), phospho-PKC Ser660 1:1000 (Cell Signaling; Danvers, MA), and beta-actin 1:5000 (Sigma; St. Louis, MO). Secondary antibodies consisted of anti-mouse and anti-rabbit IgGs conjugated to Alexa-Fluor 680 and IR800Dye (LI-COR Biosciences; Lincoln, NE), respectively. The bands for phospho-eNOS Thr495/eNOS, phospho-eNOS Ser1177/eNOS, and phospho-PKC Ser660/beta-actin were identified simultaneously (800 nm and 700 nm wavelengths, respectively) using infrared visualization (Odyssey System, LI-COR Biosciences; Lincoln, NE) and densitometry was performed using the Odyssey software.
NO Production
NO production was measured using the cell-permeable dye DAF-FM diacetate (Invitrogen Molecular Probes; Carlsbad, CA) as described previously (12). In brief, 20 µg of protein, 12 µmol/L DAF-FM diacetate, and water were added to the assay buffer to a final volume of 1 mL and the sample was stirred continuously and warmed to 37°C. Fluorescence was recorded for 20 minutes using a spectrofluorometer with an excitation wavelength of 510 nm, an emission wavelength of 530 nm, a bandwidth of 4 nm, and a count rate of 1 per second. Fluorescence was also measured following NOS inhibition with L-NNA (100 µmol/L, 20 minutes). NO production was determined by measuring peak fluorescent counts in the absence of L-NNA minus peak fluorescent counts in the presence of L-NNA.
Organ Chamber Experiments
Vascular reactivity was measured as described previously (12). Mice were anesthetized with isoflurane and euthanized by cervical dislocation. Indomethacin (10 µmol/L, 45 minutes) was present for all experiments to inhibit cyclooxygenase. Endothelium-intact aortic rings were incubated with or without tacrolimus (1 µmol/L, 20 minutes), L-NNA (10 µmol/L, 20 minutes), ryanodine (50 µmol/L, 60 minutes), Gö6976 (1 µmol/L, 20 minutes), CAIP (0.1–10 µmol/L, 20 minutes), or vehicle (DMSO <0.01% for tacrolimus). Concentration-force curves were obtained in a half-log, cumulative fashion to the endothelium-dependent dilator ACh and the endothelium-independent dilator sodium nitroprusside following contraction to an EC70 concentration of phenylephrine (PE).
Statistical Analyses
Results are presented as mean ± SEM. The two-tailed Student’s t-test was used to compare variables between 2 groups. An analysis of variance was used for multiple comparisons followed by the Student's-Newman-Keuls post hoc test when necessary. The significance level was 0.05.
ACKNOWLEDGMENTS
This work was supported by NIH grant HL084299 (BMM) and an American Heart Association Scientist Development Grant (BMM).
Footnotes
DISCLOSURE
There are no conflicts of interest to disclose.
REFERENCES
- 1.Morales JM, Dominguez-Gil B. Impact of tacrolimus and mycophenolate mofetil combination on cardiovascular risk profile after kidney transplantation. J Am Soc Nephrol. 2006;17:S296–S303. doi: 10.1681/ASN.2006080930. [DOI] [PubMed] [Google Scholar]
- 2.Opelz G, Wujciak T, Ritz E. Association of chronic kidney graft failure with recipient blood pressure. Collaborative Transplant Study. Kidney Int. 1998;53:217–222. doi: 10.1046/j.1523-1755.1998.00744.x. [DOI] [PubMed] [Google Scholar]
- 3.Mange KC, Cizman B, Joffe M, et al. Arterial hypertension and renal allograft survival. J Am Med Assoc. 2000;283:633–638. doi: 10.1001/jama.283.5.633. [DOI] [PubMed] [Google Scholar]
- 4.The US Multicenter FK506 Liver Study Group. A comparison of tacrolimus for immunosuppression in liver transplantation. N Engl J Med. 1994;331:1110–1115. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 5.Textor SC, Russell R, Wilson DJ, et al. Systemic and renal hemodynamic differences between FK506 and cyclosporine in liver transplant recipients. Transplantation. 1993;55:1332–1339. doi: 10.1097/00007890-199306000-00023. [DOI] [PubMed] [Google Scholar]
- 6.De Lima JJG, Xue H, Coburn L, et al. Effects of FK506 in rat and human resistance arteries. Kidney Int. 1999;55:1518–1527. doi: 10.1046/j.1523-1755.1999.00366.x. [DOI] [PubMed] [Google Scholar]
- 7.Takeda Y, Miyamori I, Furukawa K, et al. Mechanisms of FK 506-induced hypertension in the rat. Hypertension. 1999;33:130–136. doi: 10.1161/01.hyp.33.1.130. [DOI] [PubMed] [Google Scholar]
- 8.Long C, Cook LG, Wu GY, et al. Removal of FKBP12/12.6 from endothelial ryanodine receptors leads to an intracellular calcium leak and endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2007;27:1580–1586. doi: 10.1161/ATVBAHA.107.144808. [DOI] [PubMed] [Google Scholar]
- 9.Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn’t calcium/calmodulin enough? J Pharmacol Exp Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 10.Ahern GP, Junankar PR, Dulhunty AF. Single channel activity of the ryanodine receptor calcium release channel is modulated by FK-506. FEBS Lett. 1994;352:369–374. doi: 10.1016/0014-5793(94)01001-3. [DOI] [PubMed] [Google Scholar]
- 11.Brillantes AB, Ondrias K, Scott A, et al. Stabilization of calcium release channel (ryanodine receptor) function by FK-506 binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 12.Long C, Cook LG, Hamilton SL, et al. FK506 binding protein 12/12.6 depletion increases endothelial nitric oxide synthase threonine 495 phosphorylation and blood pressure. Hypertension. 2007;49:569–576. doi: 10.1161/01.HYP.0000257914.80918.72. [DOI] [PubMed] [Google Scholar]
- 13.Miller LW. Cardiovascular toxicities of immunosuppressive agents. Am J Transplant. 2002;2:807–818. doi: 10.1034/j.1600-6143.2002.20902.x. [DOI] [PubMed] [Google Scholar]
- 14.Sander M, Lyson T, Thomas GD, et al. Sympathetic neural mechanisms of cyclosporine-induced hypertension. Am J Hypertens. 1996;9:121S–138S. doi: 10.1016/0895-7061(96)00288-9. [DOI] [PubMed] [Google Scholar]
- 15.Greif DM, Kou R, Michel T. Site-specific dephosphorylation of endothelial nitric oxide synthase by protein phosphatase 2A: evidence for crosstalk between phosphorylation sites. Biochemistry. 2002;41:15842–15853. doi: 10.1021/bi026732g. [DOI] [PubMed] [Google Scholar]
- 16.Harris MB, Ju H, Venema VJ, et al. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem. 2001;276:16587–16591. doi: 10.1074/jbc.M100229200. [DOI] [PubMed] [Google Scholar]
- 17.Michell BJ, Chen ZP, Tiganis T, et al. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 18.Gentile MT, Vecchione C, Maffei A, et al. Mechanisms of soluble β-amyloid impairment of endothelial function. J Biol Chem. 2004;279:48135–48142. doi: 10.1074/jbc.M407358200. [DOI] [PubMed] [Google Scholar]
- 19.Martiny-Baron G, Kazanietz MG, Mischak H, et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 20.Jiang XH, Yang F, Tan HM, et al. Hyperhomocysteinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscler Thromb Vasc Biol. 2005;25:2515–2521. doi: 10.1161/01.ATV.0000189559.87328.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrino BA. Regulation of calcineurin phosphatase activity by its autoinhibitory domain. Arch Biochem Biophys. 1999;372:159–165. doi: 10.1006/abbi.1999.1485. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez-Arroyo MV, Yague S, Wenger RM, et al. Cyclophilin-mediated pathways in the effect of cyclosporin A on endothelial cells: role of vascular endothelial growth factor. Circ Res. 2002;91:202–209. doi: 10.1161/01.res.0000027562.91075.56. [DOI] [PubMed] [Google Scholar]
- 23.Jin ZG, Lungu AO, Xie L, et al. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:1186–1191. doi: 10.1161/01.ATV.0000130664.51010.28. [DOI] [PubMed] [Google Scholar]
- 24.Uittenbogaard A, Ying YS, Smart EJ. Characterization of a cytosilic heat-shock protein-caveolin chaperone complex: involvement in cholesterol trafficking. J Biol Chem. 1998;273:6525–6532. doi: 10.1074/jbc.273.11.6525. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Li M, Chai H, et al. Effects of cyclophilin A on cell proliferation and gene expression in human vascular smooth muscle cells and endothelial cells. J Surg Res. 2005;123:312–319. doi: 10.1016/j.jss.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Lungu AO, Jin ZG, Yamawaki H, et al. Cyclosporin A inhibits flow-mediated activation of endothelial nitric-oxide synthase by altering cholesterol content in caveolae. J Biol Chem. 2004;279:48794–48800. doi: 10.1074/jbc.M313897200. [DOI] [PubMed] [Google Scholar]
- 27.Bueno OF, Wilkins BJ, Tymitz KM, et al. Impaired cardiac hypertrophic response in calcineurin Aβ-deficient mice. Proc Natl Acad Sci. 2002;99:4586–4591. doi: 10.1073/pnas.072647999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xin HB, Senbonmatsu T, Cheng DS, et al. Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature. 2002;416:334–337. doi: 10.1038/416334a. [DOI] [PubMed] [Google Scholar]
- 29.Ritter O, Schuh K, Brede M, et al. AT2-Receptor activation regulates myocardial eNOS expression via the calcineurin-NF-AT pathway. FASEB J. 2003;17:283–285. doi: 10.1096/fj.02-0321fje. [DOI] [PubMed] [Google Scholar]
- 30.Navarro-Antolin J, Hernandez-Perera O, Lopez-Ongil S, et al. CsA and FK506 up-regulate eNOS expression: role of reactive oxygen species and AP-1. Kidney Int Suppl. 1998;68:S20–S24. doi: 10.1046/j.1523-1755.1998.06807.x. [DOI] [PubMed] [Google Scholar]
- 31.Navarro-Antolin J, Rey-Campos J, Lamas S. Transcriptional induction of endothelial nitric oxide gene by cyclosporine A: a role for activator protein-1. J Biol Chem. 2000;275:3075–3080. doi: 10.1074/jbc.275.5.3075. [DOI] [PubMed] [Google Scholar]
- 32.Kou R, Grief D, Michel T. Dephosphorylation of endothelial nitric-oxide synthase by vascular endothelial growth factor: implications for the vascular responses to cyclosporin A. J Biol Chem. 2002;277:29669–29673. doi: 10.1074/jbc.M204519200. [DOI] [PubMed] [Google Scholar]
- 33.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]