Abstract
Objective
We conducted a population-based case-control study to investigate the association between hormone therapy (HT) and ovarian cancer incidence, and followed all these cancer cases to determine the association of HT use with ovarian cancer mortality.
Methods
Seven hundred fifty-one incident cases of invasive epithelial ovarian cancer aged 40–79 years were diagnosed in Wisconsin and Massachusetts between 1993–1995 and 1998–2001 and matched to similarly-aged controls (n=5808). Study subjects were interviewed by telephone, which ascertained information on HT use and specific preparation, estrogen alone (E-alone) or estrogen plus progestin (EP). Ovarian cancer cases were followed-up for mortality through December 2005. Multivariate logistic regression was used to estimate odds ratios and 95% confidence intervals (CI) for ovarian cancer incidence, and Cox proportional hazards modeling was used to estimate hazard ratios and corresponding confidence intervals for ovarian cancer mortality.
Results
Ever use of HT was significantly associated with an increased risk of ovarian cancer (odds ratio 1.57, 95% CI 1.31–1.87). The excess risk was confined to women who used E-alone preparations (OR 2.33, 95% CI 1.85–2.95). No significant associations were detected between pre-diagnosis HT use and ovarian cancer survival.
Conclusions
Hormone therapy increases risk of ovarian cancer among E-alone users, but there is no substantial impact on survival after diagnosis.
Keywords: incidence, hormone therapy, ovarian cancer, survival
INTRODUCTION
Approximately 10 million women in the United States continue to use hormone therapy (HT) for treatment of menopausal symptoms and despite clinical guidelines, for disease prevention in the era following the 2002 Women’s Health Initiative (WHI) findings [1, 2]. While positive associations between HT and endometrial and breast cancer incidence are well-established [3, 4], the relationship with ovarian cancer is less clear. Some studies have shown a modestly increased risk of ovarian cancer associated with HT [5–7], but other studies have found no association [8–11]. A meta-analysis conducted nearly 10 years ago suggested a 15% increased risk of ovarian cancer associated with ever use of HT [12]. More recently, studies have investigated the type of hormone preparation used [5, 7, 13–15]. The WHI trial detected an elevated, but not statistically significant, association between estrogen plus progestin therapy (EP) and ovarian cancer [hazard ratio (HR) 1.58, 95% CI 0.77–3.24] [13]; however, during the 5.6-year follow-up period, there were only 32 women diagnosed with ovarian cancer.
A number of studies have investigated use of HT after ovarian cancer diagnosis in relation to mortality [16–19] or the association between users and non-users of HT with fatal ovarian cancer [7, 20, 21]. The latter analyses suggest that HT may be etiologically relevant in ovarian cancer incidence and may also affect mortality. Only one study to date has investigated pre-diagnosis use of HT in relation to ovarian cancer mortality among ovarian cancer cases and detected no significant association with ever versus never use of HT (HR 0.83, 95% CI 0.65–1.08) [22].
Since preparation specific information was limited, we investigated the association between type and duration of HT use in relation to ovarian cancer in a population-based case-control study among women living in Massachusetts and Wisconsin. Following the ovarian cancer cases from the case-control study for mortality outcomes, we further evaluated ovarian cancer mortality in relation to specific HT use prior to diagnosis.
MATERIALS AND METHODS
Case Population
Eligible women aged 20–79 years were diagnosed with incident invasive epithelial ovarian cancer (ICDO-183.0) between 1993–1995 and 1998–2001 and were residents of Massachusetts and Wisconsin. Eligible case subjects must have had a publicly-available telephone number and been a licensed driver verified by self-report (if <65 years) or a Medicare beneficiary (if ≥65 years). According to the protocol approved by the Institutional Review Board (IRB) at the participating centers (University of Wisconsin Comprehensive Cancer Center and the Harvard School of Public Health), the physician of each case subject was contacted to obtain permission to interview the patient. A total of 1,262 incident ovarian cancer cases were reported to the state registries during the study periods. Reasons for nonparticipation included refusal by the physician (n=33), unable to locate (n=23), subject refusal (n=139), and subject death (n=234). A total of 833 women were interviewed (overall response rate 66%). We restricted the sample to women aged 40–79 years, who were most likely to be perimenopausal or post-menopausal, resulting in a final sample of 751 case women. According to registry reports, 96% of cases’ diagnoses were histologically confirmed.
Selection of Controls
Controls were randomly selected in each state from the community using two sampling frames: 1) for women <65 years, a list of licensed drivers; or 2) for women ≥65 years, rosters of Medicare beneficiaries compiled by the Centers for Medicare and Medicaid Services (formerly known as the Health Care Financing Administration). All eligible controls had a publicly available telephone number. Controls were frequency matched within 5-year stratum to the age distribution of breast cancer cases enrolled in a concurrent study [23, 24]. Of the 11,683 potential controls, interviews were obtained from 9,420 women (overall response 80.6%). Women were excluded from the analysis if they reported a bilateral oophorectomy (n=1684) or their interviews were deemed unreliable (n=27). To match the age distribution of the ovarian cases, the control sample was also restricted to women aged 40–79 years, which resulted in a final sample of 5808 control women.
Data collection
All potential study participants were mailed a letter introducing the study before they were contacted on the telephone. A trained interviewer administered a 45-minute telephone survey which elicited information on current and past use of specific HT use. Questions ascertained use of any kind of HT for menopausal symptoms or osteoporosis including pills, patches, injections, or creams. Women were asked how frequently they took the medication, the start and stop dates for each episode of use, and how long in total they took each medication. Women were also asked about demographic characteristics, reproductive experiences, personal and family history of cancer, physical activity, smoking, and alcohol consumption.
During the interview, all women were asked about exposures occurring before a reference date. The date of diagnosis was the reference date for case women. Cases were interviewed on average one year after their diagnosis date. For the controls, the reference date was the one year prior to the interview date to reflect the average time between diagnosis and interview among the cases. Interviewers were blinded to case-control status until the end of the study interview.
Identification of Ovarian Cancer Case Deaths
Ovarian cancer cases were followed for subsequent death, through automated matches of study files to the National Death Index (NDI) [25]. Follow-up was completed through December 31, 2005. We considered cause-specific ovarian cancer mortality (ICD-9 code 183.0 and ICD-10 code C56) as the primary outcome.
Statistical Analysis
Exposure classification
Ever use of HT was defined as the use of oral, injectable, or transdermal noncontraceptive hormones for six months or more. A woman was defined as a current user of HT if she reported use within 12 months of the reference date including an episode lasting at least six months in duration. Former use was defined as use for at least six months duration, but prior to the 12 months preceding the reference date. A woman was defined as a never user if she responded that she had used HT for less than 6 months. Duration of use and recency (use within years prior to the reference date) was categorized as never, <1, 1–4, and ≥5 years.
We calculated risks associated with ever use of HT as well as specific preparations, including estrogen alone (E-alone) and estrogen plus progestin (EP). To minimize misclassification of exposure categories, women who had used both E-alone and EP or who could not remember the types of preparations used were categorized as ever exposed to HT, but were excluded from type-specific analysis. The majority of hormone therapy users had used conjugated equine estrogen (CEE) if E-alone users or CEE with medroxyprogesterone acetate if EP users. Although data were available, sample sizes were too limited to evaluate days per month of progesterone.
Women were classified as postmenopausal if they reported natural menopausal in the interview prior to their reference date. Women who had at least one ovary and had a hysterectomy were classified as premenopausal if their reference age was in the first decile of age (≤43 years) at natural menopause among the controls, and as postmenopausal if their reference age was in the highest decile for age (≥55 years) at natural menopause in the control group. A woman’s menopausal status was considered unknown if she had undergone a hysterectomy without bilateral oophorectomy at an intermediate age (second to ninth decile).
Case-control analysis
Logistic regression analysis was used to calculate odds ratios (OR) and 95 percent confidence intervals (CI) from Stata 9 (Stata Corp, College Station, TX) [26]. Models were adjusted for age at interview, state, study year, parity (0, 1, 2, 3, 4, ≥5), oral contraceptive use (never use, <5 year and ≥5 year), menopausal status (premenopausal, postmenopausal, unknown), body mass index (<18.5, 18.5–24.9, 25.0–29.9, ≥30 kg/m2), history of tubal ligation, hysterectomy status, and family history of ovarian cancer. Tests for trend with 2-sided p-values in categorical variables were evaluated by using the continuous term in the model.
Cohort analysis
Cox proportional hazards modeling was used to calculate hazard ratios (HR) and 95% confidence intervals (CI) from SAS version 9.1 (SAS Institute, Inc, Carey, N.C.) [27]. Person-time was calculated from the date of diagnosis until the date of death or end of follow-up, December 31, 2005. Models were stratified by age at interview, state, and study year, and adjusted for parity (0, 1, 2, 3, 4, ≥5), oral contraceptive use (never use, <5 year and ≥5 year), menopausal status (premenopausal, postmenopausal, unknown), body mass index (<18.5, 18.5–24.9, 25.0–29.9, ≥30 kg/m2), and tumor stage (local, regional, distant, unknown). Tests for trend with 2-sided p-values in categorical variables were evaluated by using the continuous term in the model. The Kaplan-Meier method was used to plot cause-specific ovarian cancer mortality according to HT preparation.
Reliability
To assess the reliability of the questionnaire, a sequential sample of 184 cases (both breast and ovarian cancer cases) and 188 control subjects were re-interviewed an average of 3.4 months (range 2–6 months) after the initial interview (71% response rate). Cohen’s kappa statistic showed good reliability for ever versus never use of HT for both case [κ=0.83, 95% CI 0.75–0.91] and control women (κ=0.87, 95% CI 0.79–0.95). The intraclass correlation coefficient (ICC) for the reproducibility of reported duration (in months of use) was also good for both case (ICC=0.89, 95% CI 0.83–0.93) and control women (ICC=0.83, 95% CI 0.74–0.90).
RESULTS
Incidence
Compared to controls, women with ovarian cancer had fewer children and were more likely to have a family history of ovarian cancer (Table 1). A larger proportion of cases than controls had ever had a hysterectomy. There were few differences between the two groups on education, BMI, oral contraceptive use, age at first birth, smoking, or menopausal status. The majority of women (>97%) in the study were Caucasian.
TABLE 1.
Demographic characteristics of ovarian cancer cases and population controls, Wisconsin and Massachusetts, 1993–1995 and 1998–2001.
| Controls (N=5808) | Cases (N=751) | |
|---|---|---|
| Demographic Characteristic | N (%) | N (%) |
| Age (years) | ||
| 40–49 | 904 (15.6) | 165 (22.1) |
| 50–59 | 1718 (29.6) | 204 (27.3) |
| 60–69 | 2040 (35.2) | 266 (35.6) |
| 70–79 | 1138 (19.6) | 113 (15.1) |
| Education (years) | ||
| < 12 | 299 (5.6) | 29 (4.2) |
| 12 | 2530 (47.5) | 340 (48.9) |
| 13–15 | 1353 (25.4) | 164 (23.6) |
| ≥16 | 1144 (21.5) | 163 (23.4) |
| Body mass index categories | ||
| <18.5 | 121 (2.1) | 12 (1.6) |
| 18.5–24.9 | 2639 (46.1) | 321 (43.3) |
| 25–29.9 | 1873 (32.7) | 247 (33.3) |
| ≥30 | 1088 (19.0) | 162 (21.8) |
| Duration of oral contraceptive use (years) | ||
| Never | 3570 (62.1) | 451 (60.6) |
| <5 | 1165 (20.3) | 172 (23.1) |
| ≥5 | 1015 (17.7) | 121 (16.3) |
| Age at first birth (years) | ||
| <20 | 887 (17.1) | 109 (17.4) |
| 20–24 | 2513 (48.5) | 325 (51.9) |
| 25–29 | 1263 (24.4) | 141 (22.5) |
| ≥30 | 514 (9.9) | 51 (8.1) |
| Parity | ||
| 0 | 616 (10.6) | 124 (16.5) |
| 1 | 540 (9.3) | 100 (13.3) |
| 2 | 1527 (26.3) | 191 (25.5) |
| 3 | 1323 (22.8) | 162 (21.6) |
| 4 | 861 (14.8) | 86 (11.5) |
| ≥5 | 938 (16.2) | 87 (11.6) |
| Menopausal status | ||
| Premenopausal | 1211 (20.9) | 177 (23.6) |
| Postmenopausal | 4335 (74.6) | 549 (73.1) |
| Unknown | 262 (4.5) | 25 (3.3) |
| Age at menopause (years)a | ||
| ≤43 | 1231 (28.3) | 180 (32.8) |
| 44–49 | 1002 (23.1) | 127 (23.1) |
| 50–54 | 1529 (35.3) | 179 (32.6) |
| ≥55 | 573 (13.2) | 61 (11.1) |
| Ever smoker | 2954 (50.9) | 389 (51.8) |
| Tubal ligation | 938 (16.2) | 108 (14.4) |
| Hysterectomy | 910 (15.7) | 162 (21.6) |
| Family history of ovarian cancer | 131 (2.3) | 36 (4.8) |
Among postmenopausal women
Approximately 34% of cases and 23% of controls reported ever use of HT in their lifetime.
Compared to women who had never used HT, risk of ovarian cancer was increased 57% among ever HT users (OR 1.57, 95% CI 1.31–1.87) adjusting for age and other covariates (Table 2). Risk was significantly increased among current users, the largest user group, though not among former users which comprised only 39 cases. There was an overall increased risk associated with ever use of HT regardless of the duration; no consistent dose-response relationship was observed. Risk associated with HT use remained elevated among women who had used hormones within 1 year (OR 1.65, 95% CI 1.34–1.92) and 1–4 years (OR 1.85, 95% CI 1.09–3.14) compared to non-users, but there was no significant relationship with use five years ago or more (OR 1.12, 95% CI 0.74–1.74) (p-trend < 0.002 comparing <1 to ≥5 years).
TABLE 2.
Odds ratios for ovarian cancer by hormone regimen, recency and duration of use.
| Any Hormone Therapy | Estrogen alone | Estrogen + Progestin only | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls (n=5808) |
Cases (n=751) |
Controls (n=5025) |
Cases (n=647) |
Controls (n=5094) |
Cases (n=566) |
|||||
| N (%) | N (%) | OR (95% CI)a | OR (95% CI)b | N (%) | N (%) | OR (95% CI)b | N (%) | N (%) | OR (95% CI)b | |
| Never use | 4463 (76.8) | 497 (66.2) | 1.00 (Referent) | 1.00 (Referent) | 4463 (88.8) | 497 (76.8) | 1.00 (Referent) | 4463 (87.6) | 497 (87.8) | 1.00 (Referent) |
| Ever use | 1345 (23.2) | 254 (33.8) | 1.55 (1.31–1.83) | 1.57 (1.31–1.87) | 562 (11.2) | 150 (23.2) | 2.33 (1.85–2.95) | 633 (12.4) | 69 (12.2) | 0.94 (0.70–1.26) |
| Current | 1039 (17.9) | 215 (28.6) | 1.63 (1.37–1.95) | 1.69 (1.39–2.05) | 347 (6.9) | 127 (19.6) | 3.19 (2.43–4.17) | 564 (11.1) | 56 (9.9) | 0.85 (0.62–1.17) |
| Former | 305 (5.3) | 39 (5.2) | 1.23 (0.86–1.74) | 1.17 (0.82–1.67) | 215 (4.3) | 23 (3.6) | 1.09 (0.69–1.71) | 66 (1.3) | 13 (2.3) | 1.62 (0.87–3.01) |
| Duration of use (years) | ||||||||||
| <1 | 138 (2.4) | 23 (3.1) | 1.52 (0.96–2.39) | 1.50 (0.94–2.38) | 77 (1.5) | 14 (2.2) | 1.82 (1.00–3.32) | 51 (1.0) | 7 (1.2) | 1.19 (0.53–2.68) |
| 1–4 | 558 (9.6) | 126 (16.8) | 1.84 (1.48–2.29) | 1.94 (1.54–2.43) | 198 (3.9) | 82 (12.7) | 3.68 (2.74–4.94) | 303 (6.0) | 30 (5.3) | 0.85 (0.56–1.28) |
| >5 | 648 (11.2) | 105 (14.0) | 1.30 (1.03–1.64) | 1.24 (0.97–1.60) | 287 (5.7) | 54 (8.3) | 1.44 (1.01–2.03) | 276 (5.4) | 32 (5.7) | 0.99 (0.66–1.49) |
| Recency (years) | ||||||||||
| <1 | 1024 (17.6) | 208 (27.7) | 1.60 (1.34–1.92) | 1.65 (1.36–2.01) | 344 (6.8) | 126 (19.5) | 3.17 (2.42–4.16) | 555 (10.9) | 52 (9.2) | 0.80 (0.57–1.11) |
| 1–4 | 87 (1.5) | 18 (2.4) | 1.76 (1.05–2.97) | 1.85 (1.09–3.14) | 31 (0.6) | 5 (0.8) | 1.55 (0.59–4.08) | 45 (0.9) | 10 (1.8) | 1.94 (0.95–3.94) |
| >5 | 230 (4.0) | 28 (3.7) | 1.21 (0.80–1.82) | 1.12 (0.74–1.70) | 185 (3.7) | 19 (2.9) | 1.06 (0.64–1.74) | 30 ( 0.6) | 7 (1.2) | 1.82 (0.78–4.28) |
Adjusted for age at diagnosis, study state, and year of interview.
Additionally adjusted for body mass index, oral contraceptive use, tubal ligation, parity, family history of ovarian cancer, hysterectomy, and menopausal status.
Approximately 23% of cases and 11.2% of controls had ever used E-alone. The results for women who only used E-alone preparations were similar to the overall association for HT use (Table 2). Ever use of E-alone was associated with a 2.3-fold increased risk of ovarian cancer (95% CI 1.85–2.95), and risk was increased among current users but not among former users. Elevated risks were detected for all duration categories, but there was no evidence of greater risk with increasing duration of use. The association was limited to recent use, especially use occurring within 1 year prior to the referent date (OR 3.17, 95% CI 2.42–4.16), with the excess risk diminishing with increasing time since last use (p-trend < 0.0001 comparing <1 to ≥5 years).
Use of EP was less common than E-alone in this study population; about 12% of cases and controls reported ever having used EP prior to the reference date. No significant associations were observed with these preparations, regardless of currency, duration or recency of use (Table 2).
Women who had used both E-alone and EP were at similar risk as women who had used E-alone preparations exclusively (data not shown). Further we detected no differences in the odds ratios when we stratified the analysis by extent of disease (i.e., local, regional and distant). When we restricted the analysis to women without a hysterectomy, we also detected no differences in the odds ratios (data not shown) among cases.
Survival
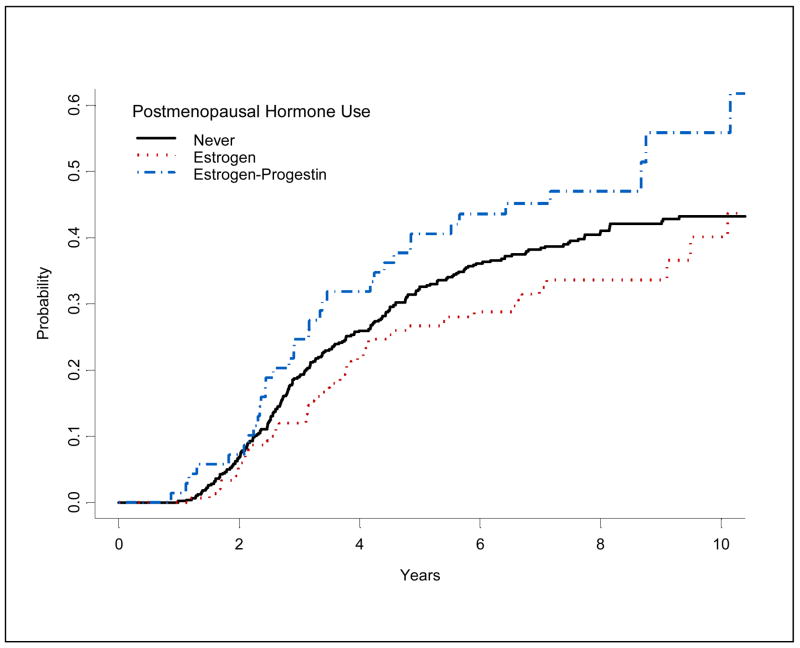
A total of 305 deaths due to ovarian cancer among 751 cases were ascertained during an average of seven years of follow-up. Mortality rates varied according to HT type in univariate Kaplan-Meier analysis (Figure 1), with higher mortality observed in EP users, and lower mortality in E-alone users when compared to never users. In multivariate analysis adjusted for differences in usage by stage and other factors, history of any HT was not associated with ovarian cancer mortality (HR=1.09, 95% CI: 0.84–1.43 for ever use), regardless of currency or total duration of use (Table 3). Similarly, no associations were evident for E-only. Mortality was modestly elevated, but not statistically significant among current and long-term users of EP.
Figure 1.
Kaplan-Meier cumulative cause-specific ovarian cancer mortality by type of hormone used.
TABLE 3.
Hazard ratios and 95% confidence intervals for cause-specific ovarian cancer mortality by hormone regimen and duration of use among women with ovarian cancer.
| Any Hormone Therapy | Estrogen alone | Estrogen+Progestin only | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. Censored |
No. of Deaths |
HR (95% CI)a | HR (95% CI)b | No. Censored |
No. of Deaths |
HR (95% CI)b | No. Censored |
No. of Deaths |
HR (95% CI)b | |
| Never use | 291 | 206 | 1.00 (Referent) | 1.00 (Referent) | 291 | 206 | 1.00 (Referent) | 291 | 206 | 1.00 (Referent) |
| Ever use | 155 | 99 | 1.03 (0.80–1.34) | 1.10 (0.85–1.43) | 99 | 51 | 0.99 (0.71–1.37) | 34 | 35 | 1.25 (0.85–1.86) |
| Current | 135 | 80 | 1.04 (0.78–1.37) | 1.09 (0.82–1.45) | 86 | 41 | 0.96 (0.67–1.39) | 28 | 28 | 1.28 (0.82–1.99) |
| Former | 20 | 19 | 1.03 (0.64–1.67) | 1.15 (0.70–1.89) | 13 | 10 | 1.17 (0.77–1.78) | 6 | 7 | 1.18 (0.52–2.65) |
| Duration of use (years) | ||||||||||
| <5 | 96 | 53 | 0.99 (0.72–1.36) | 1.12 (0.81–1.55) | 67 | 29 | 1.17 (0.77–1.78) | 18 | 19 | 1.13 (0.68–1.88) |
| ≥5 | 59 | 46 | 1.09 (0.78–1.53) | 1.08 (0.76–1.53) | 32 | 22 | 0.80 (0.50–1.28) | 16 | 16 | 1.40 (0.81–2.43) |
Adjusted for age at diagnosis, study state, and year of interview.
Additionally adjusted for body mass index, oral contraceptive use, parity, menopausal status, and stage.
DISCUSSION
In this case-control study, ever use of HT was associated with a 57% increased risk of ovarian cancer. Risk was confined to E-alone therapy with 2.3-fold increased risk of ovarian cancer, whereas no association was detected for EP. Duration and recency of use of E-alone preparations was significantly associated with ovarian cancer risk in these data.
A meta-analysis of 15 studies conducted between 1969–1997 suggested no association of E-alone use with ovarian cancer [28]. In contrast, several studies published since this meta-analysis have suggested that long-term use of E-alone may increase risk [5, 7, 15, 29, 30]. In a cohort study, Lacey et al. detected a significantly increased risk of ovarian cancer in women who had used estrogen therapy for 10–19 years (HR=1.8, 95% CI: 1.1–3.0) or more than 20 years (HR=3.2, 95% CI: 1.7–5.7) compared to never users [15]. Danforth and colleagues detected a two-fold increased risk among users of 5 or more years duration [5], and the recent findings from the Million Women study also suggested an elevated risk with long-duration (≥5 years) of use (relative risk-1.53, 95% CI 1.27–1.84). In the current data, we detected a statistically significant increased risk of ovarian cancer associated with E-alone use, though we could demonstrate no clear dose-response relationship with increasing duration. The study thus adds to the evidence that HT preparations containing estrogen alone contributes to ovarian cancer risk
Results from studies conducted in the U.S. [5, 11, 13–15, 31, 32], Australia [33], Norway [8], Sweden [29], and U.K [7] have been inconclusive regarding the relationship between EP and ovarian cancer. Overall, we detected no associations with EP use. Likewise, many observational studies have not supported an association [5, 11, 15, 29, 31, 33]. Two large cohort studies and one randomized controlled trial have suggested an increased risk of 50% in association with EP use [8, 13, 14]. In comparison, the most recent cohort study, the Million Women study, detected a statistically significant 10% increased risk [7]. These studies tend to suggest that there is no association or perhaps a modest increased risk of ovarian cancer associated with EP use. Nearly all studies have been conducted in women born between 1910–1960, and most studies were conducted in the 1990s. European countries tend to prescribe EP preparations over E-alone, though this might also be a reflection of lower hysterectomy rates than in the US [34–36].
Risch has suggested that androgens and progesterone might play an etiologic role in ovarian carcinogenesis [37]. Pregnancy results in increases in maternal circulating progesterone due to production from the placenta. Among cell lines of normal human ovarian surface epithelium and ovarian cancer epithelium, low doses of progesterone were shown to stimulate cell growth in both cell lines whereas high doses resulted in cell growth inhibition [38]. In contrast, among the same cell lines exposed to estrone and 17-β estradiol, there was continued cell growth stimulation with increasing levels of hormone [38]. While it has been suggested that estrone exerts limited effect on ovarian surface epithelium cells, the link between HT and ovarian cancer risk suggested in these and other data suggest that estrone, and perhaps synthetic components of HT, might enhance neoplastic changes in ovarian epithelium.
Few previous studies have examined the relationship between HT use and mortality after ovarian cancer diagnosis. We detected no association between E-alone use and mortality due to ovarian cancer, although we could not exclude the possibility of a slightly increased mortality among EP users. In the American Cancer Society Prevention Study Cohort, Rodriguez and colleagues reported that women who used ET at baseline had higher death rates from ovarian cancer compared to never users [21]. However, the finding could reflect an etiologic role of ET in ovarian cancer, a survival disadvantage associated with its use, or both. Mascarenhas and colleagues assessed hormone use both before and after ovarian cancer diagnosis in relation to mortality from ovarian cancer in a Swedish population [22]. Similar to the present data, the authors detected no association between HT use and ovarian cancer mortality regardless of the type of hormone used (E-alone or EP). One difference, though, between our study and Mascarenhas’ study is that theirs included both exclusive and non-exclusive users of EP.
There are several strengths to this study. The analysis is based on a large, homogenous population from whom information on HT and other risk factors was uniformly collected in a structured telephone interview. Accuracy of information on HT use and potential for recall bias was minimized by the use of photographic aids of specific HT preparations for women in the early recruitment period of the study. Reliability studies indicate that women accurately reported HT use. We assessed use of E and EP in exclusive users of these hormones, so their risk is not influenced by prior use of a different hormone preparation. There is also complete follow-up on mortality for all the ovarian cancer cases for the cohort analysis.
Several limitations of this study should also be considered. We were unable to interview about 35% and 25% of cases and controls, respectively. If there are differences in postmenopausal hormone use between the women who participated and women who did not, our risk estimates could be biased. In particular, if case or control women who agreed to our interview were more likely to use hormones, this may have attenuated the apparent relation between postmenopausal hormones and ovarian cancer. In a sensitivity analysis assuming that all non-responders were not HT users, we estimate the crude OR would 1.61 (95% CI 1.37–1.89) It seems unlikely that women who used one type of HT (e.g., EP) would preferentially participate in a research study compared to women who used a different HT (e.g., E-alone). However, the consistency of the association with E-only therapy compared with other studies suggests that such bias did not materially affect our results. Since registry data was used to identify the ovarian cancer cases, reporting delays of approximately one year were unavoidable (the majority of women were interviewed within 18 months of diagnosis) and approximately 20% of cases died before they could be interviewed. Thus, our results for incidence and survival are applicable to women who survive approximately the first year after ovarian cancer diagnosis. One-year overall survival rate for ovarian cancer is 76.4% [39]. Given the poor prognosis with this disease, it is likely that ovarian cancer cases in the study had less aggressive disease on average when compared to all eligible ovarian cancer cases. We detected no association between use of HT and extent of disease or time to interview. However, regardless of time to interview from diagnosis, women who used E-alone had an elevated risk of ovarian cancer compared to EP users (not shown), suggesting that survival bias does not account for the observed associations.
Another potential limitation of this study is the potential for misclassification of the timing of exposure among ovarian cancer survivors, specifically the possibility that cases may have initiated use of HT after their diagnosis. We did not collect data on post-diagnosis HT use in the ovarian cancer cases. Some premenopausal women, as a result of oophorectomy and hysterectomy for treatment of ovarian cancer, might initiate the use of hormone therapy to mitigate the menopausal symptoms experienced after surgery. Mascarenhas’ study suggests that 15.8% of ovarian cancer cases who had not previously used HT will initiate use and 44.5% of previous users will continue to use HT post-diagnosis. Observational studies have suggested either no association [16, 18] or decreased risk [22] in the relationship between HT use after diagnosis and ovarian cancer mortality or recurrence. A randomized-controlled trial of 130 women diagnosed with ovarian cancer aged less than 59 years suggested no association between estrogen therapy and overall mortality or ovarian cancer recurrence [19]. We attempted to limit the influence of this misclassification by restricting the study population to older, mainly postmenopausal women. However, the excess risk associated with currency of use in these data may reflect in part post-diagnosis use of HT.
We did not have available information on treatment or ovarian cancer recurrence. Further studies should evaluate whether these relationships are modified by treatment choice.
In summary, our study suggests that the use of HT, specifically E-alone, is associated with an increased risk of ovarian cancer, but has no association with death from ovarian cancer. Women should continue to discuss the risks, including ovarian cancer, and benefits of HT use with their physicians in making decisions about initiating and continuing HT use.
Acknowledgments
We are indebted to Drs. Linda Titus-Ernstoff, John Baron, Patrick Remington, Meir Stampfer, and Walter Willett for advice regarding the conduct of this study and Deanne Young for editorial assistance.
Acknowledgement of financial support: This research was funded by National Institute of Health grants R01 CA47147 and CA47305.
References
- 1.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 2.Writing Group for the Women’s Health Initiative I. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 3.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Combined estrogen-progestogen menopausal therapy. Lyon: IARC; In press. [PMC free article] [PubMed] [Google Scholar]
- 4.Cook LS, Weiss NS, Doherty JA, Chen C. Endometrial Cancer. In: Schottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. Oxford: Oxford Press; 2006. [Google Scholar]
- 5.Danforth KN, Tworoger SS, Hecht JL, Rosner BA, Colditz GA, Hankinson SE. A prospective study of postmenopausal hormone use and ovarian cancer risk. Br J Cancer. 2007;96:151–156. doi: 10.1038/sj.bjc.6603527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills PK, Riordan DG, Cress RD, Goldsmith DF. Hormone replacement therapy and invasive and borderline epithelial ovarian cancer risk. Cancer Detect Prev. 2005;29:124–132. doi: 10.1016/j.cdp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Beral V, Bull D, Green J, Reeves G. Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet. 2007;369:1703–1710. doi: 10.1016/S0140-6736(07)60534-0. [DOI] [PubMed] [Google Scholar]
- 8.Bakken K, Alsaker E, Eggen AE, Lund E. Hormone replacement therapy and incidence of hormone-dependent cancers in the Norwegian Women and Cancer study. Int J Cancer. 2004;112:130–134. doi: 10.1002/ijc.20389. [DOI] [PubMed] [Google Scholar]
- 9.Hempling RE, Wong C, Piver MS, Natarajan N, Mettlin CJ. Hormone replacement therapy as a risk factor for epithelial ovarian cancer: results of a case-control study. Obstet Gynecol. 1997;89:1012–1016. doi: 10.1016/s0029-7844(97)00118-x. [DOI] [PubMed] [Google Scholar]
- 10.Risch HA, Marrett LD, Jain M, Howe GR. Differences in risk factors for epithelial ovarian cancer by histologic type. Results of a case-control study. Am J Epidemiol. 1996;144:363–372. doi: 10.1093/oxfordjournals.aje.a008937. [DOI] [PubMed] [Google Scholar]
- 11.Sit AS, Modugno F, Weissfeld JL, Berga SL, Ness RB. Hormone replacement therapy formulations and risk of epithelial ovarian carcinoma. Gynecol Oncol. 2002;86:118–123. doi: 10.1006/gyno.2002.6746. [DOI] [PubMed] [Google Scholar]
- 12.Garg PP, Kerlikowske K, Subak L, Grady D. Hormone replacement therapy and the risk of epithelial ovarian carcinoma: a meta-analysis. Obstet Gynecol. 1998;92:472–479. doi: 10.1016/s0029-7844(98)00139-2. [DOI] [PubMed] [Google Scholar]
- 13.Anderson GL, Judd HL, Kaunitz AM, et al. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women’s Health Initiative randomized trial. JAMA. 2003;290:1739–1748. doi: 10.1001/jama.290.13.1739. [DOI] [PubMed] [Google Scholar]
- 14.Lacey JV, Jr, Brinton LA, Leitzmann MF, et al. Menopausal hormone therapy and ovarian cancer risk in the National Institutes of Health-AARP Diet and Health Study Cohort. J Natl Cancer Inst. 2006;98:1397–1405. doi: 10.1093/jnci/djj375. [DOI] [PubMed] [Google Scholar]
- 15.Lacey JV, Jr, Mink PJ, Lubin JH, et al. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002;288:334–341. doi: 10.1001/jama.288.3.334. [DOI] [PubMed] [Google Scholar]
- 16.Eeles RA, Tan S, Wiltshaw E, et al. Hormone replacement therapy and survival after surgery for ovarian cancer. BMJ. 1991;302:259–262. doi: 10.1136/bmj.302.6771.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bebar S, Ursic-Vrscaj M. Hormone replacement therapy after epithelial ovarian cancer treatment. Eur J Gynaecol Oncol. 2000;21:192–196. [PubMed] [Google Scholar]
- 18.Ursic-Vrscaj M, Bebar S, Zakelj MP. Hormone replacement therapy after invasive ovarian serous cystadenocarcinoma treatment: the effect on survival. Menopause. 2001;8:70–75. doi: 10.1097/00042192-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Guidozzi F, Daponte A. Estrogen replacement therapy for ovarian carcinoma survivors: A randomized controlled trial. Cancer. 1999;86:1013–1018. doi: 10.1002/(sici)1097-0142(19990915)86:6<1013::aid-cncr17>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez C, Calle EE, Coates RJ, Miracle-McMahill HL, Thun MJ, Heath CW., Jr Estrogen replacement therapy and fatal ovarian cancer. Am J Epidemiol. 1995;141:828–835. doi: 10.1093/oxfordjournals.aje.a117518. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez C, Patel AV, Calle EE, Jacob EJ, Thun MJ. Estrogen replacement therapy and ovarian cancer mortality in a large prospective study of US women. JAMA. 2001;285:1460–1465. doi: 10.1001/jama.285.11.1460. [DOI] [PubMed] [Google Scholar]
- 22.Mascarenhas C, Lambe M, Bellocco R, et al. Use of hormone replacement therapy before and after ovarian cancer diagnosis and ovarian cancer survival. Int J Cancer. 2006;119:2907–2915. doi: 10.1002/ijc.22218. [DOI] [PubMed] [Google Scholar]
- 23.Trentham-Dietz A, Newcomb PA, Egan KM, et al. Weight change and risk of postmenopausal breast cancer (United States) Cancer Causes Control. 2000;11:533–542. doi: 10.1023/a:1008961931534. [DOI] [PubMed] [Google Scholar]
- 24.Sprague BL, Trentham-Dietz A, Newcomb PA, Titus-Ernstoff L, Hampton JM, Egan KM. Lifetime recreational and occupational physical activity and risk of in situ and invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:236–243. doi: 10.1158/1055-9965.EPI-06-0713. [DOI] [PubMed] [Google Scholar]
- 25.Calle EE, Terrell DD. Utility of the National Death Index for ascertainment of mortality among cancer prevention study II participants. Am J Epidemiol. 1993;137:235–241. doi: 10.1093/oxfordjournals.aje.a116664. [DOI] [PubMed] [Google Scholar]
- 26.Breslow NE, Day NE. Volume I - The analysis of case-control studies. IARC Scientific Publications; (Lyon): 1980. Statistical methods in cancer research; pp. 5–338. [PubMed] [Google Scholar]
- 27.Breslow NE, Day NE. Volume II-The design and analysis of cohort studies. Lyon: IARC Scientific Publications; 1987 . Statistical methods in cancer research. [PubMed] [Google Scholar]
- 28.Coughlin SS, Giustozzi A, Smith SJ, Lee NC. A meta-analysis of estrogen replacement therapy and risk of epithelial ovarian cancer. J Clin Epidemiol. 2000;53:367–375. doi: 10.1016/s0895-4356(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 29.Riman T, Dickman PW, Nilsson S, et al. Hormone replacement therapy and the risk of invasive epithelial ovarian cancer in Swedish women. J Natl Cancer Inst. 2002;94:497–504. doi: 10.1093/jnci/94.7.497. [DOI] [PubMed] [Google Scholar]
- 30.Folsom AR, Anderson JP, Ross JA. Estrogen replacement therapy and ovarian cancer. Epidemiology. 2004;15:100–104. doi: 10.1097/01.ede.0000091606.31903.8e. [DOI] [PubMed] [Google Scholar]
- 31.Moorman PG, Schildkraut JM, Calingaert B, Halabi S, Berchuck A. Menopausal hormones and risk of ovarian cancer. Am J Obstet Gynecol. 2005;193:76–82. doi: 10.1016/j.ajog.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Pike MC, Pearce CL, Peters R, Cozen W, Wan P, Wu AH. Hormonal factors and the risk of invasive ovarian cancer: a population-based case-control study. Fertil Steril. 2004;82:186–195. doi: 10.1016/j.fertnstert.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Purdie DM, Bain CJ, Siskind V, et al. Hormone replacement therapy and risk of epithelial ovarian cancer. Br J Cancer. 1999;81:559–563. doi: 10.1038/sj.bjc.6690731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherman ME, Carreon JD, Lacey JV, Jr, Devesa SS. Impact of hysterectomy on endometrial carcinoma rates in the United States. J Natl Cancer Inst. 2005;97:1700–1702. doi: 10.1093/jnci/dji378. [DOI] [PubMed] [Google Scholar]
- 35.Luoto R, Raitanen J, Pukkala E, Anttila A. Effect of hysterectomy on incidence trends of endometrial and cervical cancer in Finland 1953–2010. Br J Cancer. 2004;90:1756–1759. doi: 10.1038/sj.bjc.6601763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redburn JC, Murphy MF. Hysterectomy prevalence and adjusted cervical and uterine cancer rates in England and Wales. Bjog. 2001;108:388–395. doi: 10.1111/j.1471-0528.2001.00098.x. [DOI] [PubMed] [Google Scholar]
- 37.Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst. 1998;90:1774–1786. doi: 10.1093/jnci/90.23.1774. [DOI] [PubMed] [Google Scholar]
- 38.Syed V, Ulinski G, Mok SC, Yiu GK, Ho SM. Expression of gonadotropin receptor and growth responses to key reproductive hormones in normal and malignant human ovarian surface epithelial cells. Cancer Res. 2001;61:6768–6776. [PubMed] [Google Scholar]
- 39.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2004. National Cancer Institute; Bethesda, MD : http://seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER web site, 2007. [Google Scholar]