Abstract
The adult mammalian brain continuously generates new neurons in the olfactory bulb and hippocampus throughout life. Adult neurogenesis, a highly dynamic process, has been shown to be exquisitely modulated by neuronal circuit activity at different stages, from proliferation of adult neural progenitors, to differentiation, maturation, integration and survival of newborn neurons in the adult brain. Strategic activity-dependent addition of new neurons into the existing neuronal circuitry represents a prominent form of structural plasticity and may contribute to specific brain functions, such as learning, memory and mood modulation. Here we review extrinsic mechanisms through which adult neurogenesis is regulated by environmental cues, physiological learning-related stimuli and neuronal activities.
Keywords: Plasticity, adult stem cells, neurotransmitter, neurogenesis, neural activity
Introduction
Since the pioneering studies by Altman et al. in the early 1960s, adult neurogenesis has now been unambiguously established in discrete brain regions in most mammals.1-7 New excitatory granule neurons and inhibitory granule and periglomerular interneurons are continuously added to existing circuits in the dentate gyrus and olfactory bulb (Figure 1A), respectively. Such continuous adult neurogenesis represents a surprising and intriguing type of plasticity conserved in adult mammalian brains.5-8 In contrast to the main form of neural plasticity through synaptic level of modification, adult neurogenesis confers plasticity through the addition of population of new neurons with functional synaptic inputs and outputs. Distinct features of these two types of plasticity may endow the brain with parallel yet complementary capacities for information processing. Common in all neural plasticity and similar to synaptic changes, adult neurogenesis is under the exquisite control of neural activity. Studies in the last few years have delineated the sequential steps of endogenous adult neurogenesis in these two brain regions, from proliferation and fate specification of adult neural progenitors, to differentiation, maturation, axon and dendritic targeting, formation of functional synaptic inputs and outputs, and selective survival of newborn neurons9, 10. Accumulative evidence has implicated adult neurogenesis in specific brain functions, such as olfactory learning, spatial memory formation and mediating behavioral effects of antidepressants.11-15 Accordingly, many environmental cues, physiological learning-related stimuli and neuronal activities dynamically influence different stages of adult neurogenesis.16-18 The underlying molecular and cellular mechanisms through which diverse types of activity selectively regulate different stages of adult neurogenesis are only beginning to be unraveled.
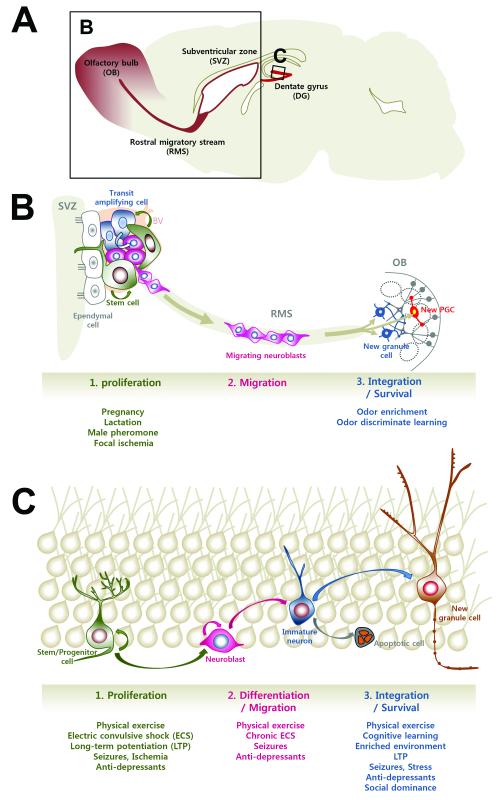
Figure 1. Regulation of adult neurogenesis by various types of neural activity.
(A) Schematic representation of a sagittal view of the adult mouse brain. New neurons are generated continuously throughout life in the subventricular zone (SVZ)-olfactory bulb (OB) system and the dentate gyrus (DG) of the hippocampus.
(B) Activity-dependent neurogenesis in the adult olfactory bulb. Transient amplifying cells give rise to neuroblasts, migrating toward the OB through the rostral migratory stream (RMS). Within the OB, these neuroblasts differentiate into two types of interneurons as the granule cell and periglomerular cell (PGC). Integration into the pre-existing circuits by newly entered neurons and their survival are predominantly influenced by odor experiences, such as enriched odor exposure or odor discriminate learning.
(C) Activity-dependent neurogenesis in the dentate gyrus. Neural progenitors in the SGZ proliferate and differentiate into neuroblasts that migrate a short distance into the inner granular cell layer, processes that are modulated by various types of neural activity. The survival and integration of new neurons is also regulated in an activity-dependent manner.
II. Activity-dependent regulation of adult olfactory bulb neurogenesis
New neurons generated in the adult olfactory bulb originate from progenitors in the subventricular zone (SVZ) of the lateral ventricle of the forebrain19. The physical distance between the place of their birth and place of final destination naturally creates two distinct compartments for potential extrinsic regulation. In the SVZ, adult progenitor cells are located in a developmental “vestige” where embryonic counterparts generate the majority of neurons, and not surprisingly their maintenance, proliferation and fate specification are subject to numerous developmental signaling controls in a largely activity-independent manner.19, 20 Highly specialized niche structure such as “glial tunnel”21 may also function to segregate the earlier stages of neurogenesis from influences of circuit neuronal activity. After neuroblasts migrate through the rostral migratory stream and reach the bulb where olfactory information processing takes place, the newly generated neurons switch to radial migration to their final positions and start to receive synaptic inputs and subject to activity-dependent survival and final integration into the pre-existing circuitry.
Olfactory bulbs process odorant information from the nose to the brain, thus the main type of activity results from sensory stimuli of odorant exposure. Using a technique of reversible olfactory deprivation, Cummings et al. showed that unilateral olfactory deprivation during the first postnatal month in rodents led to a dramatic reduction in the size of the olfactory bulb at the experimental side.22 Surprisingly, the bulb size had mostly recovered within forty days after normal stimulation was restored. Thymidine analog labeling revealed that the recovery was accompanied with the addition of a large population of new neurons. Other similar experiments showed that the increased number of neurons after deprivation was due to rescued survival from “default” apoptosis program.23-25 Thus, neuronal survival in the olfactory bulb can be profoundly influenced by afferent activities.
Several recent studies have directly examined whether the physiological exposure to an odor-enriched environment or learning tasks affects neurogenesis in the adult olfactory bulb.26 Using bromodeoxyuridine (BrdU) to birth date progenitors from SVZ and their progeny, Rochefort et al. found that long-term exposure to an odor-enriched environment dramatically increase the number of surviving new neurons at 3 weeks after BrdU injection. The number of BrdU-labeled progenitors was not altered 4 hours after a single BrdU injection, suggesting a lack of effects in cell proliferation. Interestingly, this effect appears to temporally transient since the number of newborn neurons returned to control levels 30 days after experiencing the odor-enriched environment.27 Furthermore, odor discrimination learning for an extended period, not just exposure to a single odor, is also able to significantly increase the survival of newborn neurons in the olfactory bulb.28 The effects are region-specific since enriched odor exposure did not influence hippocampal neurogenesis. Although it still remains under debate,29, 30 olfactory bulb neurogenesis might be associated with specific olfactory functions such as olfactory discrimination and olfactory memory. For example, olfactory enrichment induces not only an increase in the number of surviving newborn neurons but also enhancement of short-term olfactory memory. 6, 26, 27 Conversely, mutant mice deficient in a neural cell adhesion molecule (NCAM) with reduced olfactory neurogenesis exhibits reduced olfactory sensitivity and olfactory memory.11 Studies also reported a correlation between the age-dependent decline of OB neurogenesis and fine odorant discrimination ability.31 These functional correlations suggest that the activity from odorant-related environmental stimuli may not only increase the number of surviving neurons, but also enhance their integration into the circuitry to participate in information processing. Using immediate early gene activation to monitor the integration of new neurons, Magavi et al. showed that odor familiarization specifically increases the response of adult-born neurons but depresses the response of the overall population of granule neurons.32
In addition to odorants and olfactory learning, other physiological conditions have also been shown to modulate olfactory bulb neurogenesis in rodents. Compared to subordinate male mice, pheromones from dominant males stimulate neuronal production in the olfactory bulb of female mice by increasing cell proliferation in the SVZ (Figure 1B).33 In a similar vein, pregnancy of female mice promotes both SVZ proliferation and the production of new interneurons in the olfactory bulb through prolactin (Figure 1B).34 Although the SVZ progenitors express receptors for these sex-specific cues, it remains unclear to what extend neuronal activity is directly involved in mediating the action of these cues and the long-term outcome.
III. Activity-dependent regulation of adult neurogenesis in hippocampus
Neurogenesis in the adult hippocampus occurs in the granule cell layer of the dentate gyrus. New neurons are born in the subgranular zone (SGZ) and then migrate a short distance before maturing into excitatory dentate granule cells in the inner granule cell layer (Figure 1C). The entire neurogenesis process, including progenitor proliferation, fate specification, migration and neuronal differentiation, is spatially restricted compared to the extensive spreading of neurogenesis from the SVZ to the olfactory bulb. The dentate gyrus receives inputs from numerous regions of the brain.35 Particularly, the subgranular zone is known to be enriched in exuberant neuronal communications between mature granule neurons and local interneurons.36 The spatial proximity to high neuronal network circuit activity may place adult hippocampal neurogenesis under more elaborated, activity-dependent control than in the SVZ.
The hippocampus is a brain region crucial for acquisition of new episodic memory and the dentate gyrus plays a role in “pattern separation”,37 distinguishing similarly encoded contextual information. This functional requirement may engender the dentate gyrus to be superiorly sensitive to the surrounding environment and ongoing cortical activity. Consistent with this notion, the dentate hilus region is one of the major loci responsible for temporal lobe epilepsy.38 As part of the limbic system, another established role of dentate gyrus is its modulation of emotions, such as stress and depression.39 As reviewed below, major types of activity in the dentate gyrus, including those induced by enriched environment, voluntary exercises, cognitive and emotional processes, modulate different aspects of adult hippocampal neurogenesis.
Exposure to an enriched environment is known to produce structural and functional alterations in the brain; its specific effects on adult hippocampal neurogenesis were examined by Kempermman et al.40 Compared with littermates housed in standard cages, significantly more new neurons exist in the dentate gyrus of mice exposed to an enriched environment. Surprisingly, van Praag et al. further showed that voluntary exercise alone, without other components of enriched environment, is sufficient to enhance neurogenesis.41 This effect can be observed as early as day 1 after the start of exercise but the most profound effect is detected after 3 days of exercise, manifested by the enhanced proliferation of early neural progenitors. Continued exercise elicits a predominantly enhanced survival of newborn neurons and results in a net increased number of integrated neurons in the adult brain.
Cognitive activity, mainly from hippocampus-dependent learning, also modulates adult hippocampal neurogenesis. Training on associative learning tasks that require the hippocampus, such as trace eyeblink conditioning and Morris water maze learning, significantly increases the number of newly generated neurons in the adult dentate gyrus, whereas tasks that do not require the hippocampus does not change the number of new neurons.42 Analysis using BrdU-based birth dating further suggest that the learning behavior specifically promotes the survival of new neurons between 1-3 week after they are generated in the adult brain.42 Interestingly, the survival of earlier, 3-day-old newborn neurons is actually inhibited by the similar Morris water maze-learning paradigm, suggesting a stage-dependent effect and a finely sculpted developmental selection of newborn neurons for strategic addition.43 The learning process involves precisely timed and highly patterned neuronal activity; excessive or abnormal patterns of activity, such as those occurring during temporal lobe epileptic seizures, dramatically impact neurogenesis including progenitor proliferation and dendritic developemnt, synapse formation and survival of new neurons as documented in numerous clinical and experimental settings.44 On the other hand, negative emotional activity, including stress and depression, has been shown to be a potent inhibitor of adult hippocampal neurogenesis.39 The major effect of stress on neurogenesis appears to be decreased cell proliferation, while reduced cell survival of newborn neurons has also been reported. The discrepancy may be due to different stress paradigms used in different studies.39 Stressful events are known to induce depression; interestingly, a variety of antidepressant treatments elevate hippocampal neurogenesis. The most effective anti-depressant used in clinics, electroconvulsive treatment, promotes the entire adult neurogenic process including progenitor proliferation, survival and dendritic development of newborn neurons.44, 45
III. Cellular and molecular mechanisms
The activity-dependent regulation of adult neurogenesis in both olfactory bulb and dentate gyrus of hippocampus has now been widely observed and extensively studied. The specific molecular and cellular mechanisms through which activity regulates different stages of adult neurogenesis, however, are only beginning to be explored. Particularly, the extrinsic mechanisms of how diverse types of activity are translated into actions on sequential processes of adult neurogenesis remain unclear. Although various cell types in the brain, such as microglia, endothelial cells, and T cells, have been shown to play interesting roles,46-48 mature neurons are likely the major cellular players in extrinsic regulation of neurogenesis by mediating effects of neural activity. Here we focus our discussion on two major types of molecular mediators for activity-dependent adult neurogenesis: neurotransmitters and membrane-associated/diffusible factors from neurons.
GABA is the major neurotransmitter used by mature interneurons in the brain and has emerged as a key regulator for adult neurogenesis.18 GABA, activating both synaptic and extrasynaptic GABAA receptors, causes hyperpolarization of mature neurons while depolarizes neural progenitors and immature neurons in the adult brain10, 49. Electrophysiological analysis revealed that neural progenitors are initially activated by ambient GABA, and followed by sequential GABAergic and glutamatergic inputs.18 In the SVZ, GABA released from migrating neuroblasts regulates proliferation, which is probably not directly connected to circuit activity-dependent modification.50 In the SGZ, GABA is released from local interneurons and tonically activating progenitors and immature neurons. Importantly, both tonic and phasic GABA activation is essential for the maturation, dendritic development and synaptic integration of newborn granule cells.51, 52 Given extensive recurrent connections between granule cells and interneurons in the adult dentate gyrus,36 the ambient GABA levels might reflect general local circuit activity and translate neuronal stimuli into actions on neural progenitors, while phasic GABA activation serves as input specific modulators for immature new neurons. Interesting, recent studies have shown that both tonic and phasic GABA activation of neural progenitors and immature newborn neurons in the dentate gyrus is modulated by chemokine Stromal cell-derived factor-1 (SDF-1) co-released from local interneurons 53, 54. Current evidence suggests that regulation by GABA from interneurons creates a favorable condition to orchestrate the integration of new neurons into a mature neuronal environment in the SGZ. It would be interesting to examine whether activity-dependent maturation, integration and survival of new neurons also recruits GABA-mediated regulation in the olfactory bulb.
Glutamate is the major neurotransmitter used by excitatory principal neurons in the brain and has long been implicated in regulating adult hippocampus neurogenesis through NMDA receptors. Direct injection of NMDA negatively regulates cell proliferation in the adult rat dentate gyrus,55, 56 while induction of long-term potentiation at the glutamatergic perforant path to dentate granule cells promotes the proliferation of adult neural progenitors and survival of newborn neurons in an NMDAR-dependent fashion.57, 58 These results suggest that glutamate has both cell autonomous effects in immature neurons and non-cell autonomous effects through modulation of existing neuronal circuits. Although local mature neurons have predominantly heightened NMDA receptor signaling, ionotropic glutamate receptors are present in immature neurons and early progenitors. Interestingly, direct NMDAR signaling has been shown to regulate newborn neurons during two “critical” periods 10. In the first phase, NMDAR activation of new neurons promotes competitive survival of these new neurons during 1-3 weeks after their birth.59 In the second phase, NR2B-containing NMDAR activation is required for the enhanced synaptic plasticity of glutamatergic inputs to new neurons during 4-6 weeks after their birth, potentially serving as a substrate for learning from new experience.60, 61 In parallel to such direct impact of glutamate on newborn neurons, NMDAR activation in mature neurons is known to lead to gene expression program that produces diffusible factors profoundly influencing adult neurogenesis.
Cells membrane-associated or diffusible factors serve as major mediators for short-range cell-cell communication. Given that adult neural progenitors and their progeny are extensively regulated by a plethora of growth factors, neurotrophins and developmental cues,20, 62 nearby mature neurons are suited to contribute producing these diffusible niche factors or their antagonists in response to neuronal activity. BDNF is such a prime example. As a classic activity-dependent neurotrophin,62 its expression is bi-directionally sensitive to activity in dentate granule neurons and can exert pleiotropic yet strong influences on adult hippocampal neurogenesis.63, 64 In the olfactory bulb, extracellular matrix glycoprotein tenascin-R regulates the initiation of the detachment of neuroblasts from the migrating chain as well as their radial migration.65 Interestingly, Tenascin-R is expressed from adult olfactory bulb neurons in an activity-dependent manner, as it is markedly reduced by odor deprivation. Similarly, other extrinsic factors, including Wnts and their antagonists, fibroblast growth factors, vascular endothelial growth factors, neuropeptide VGF, may be regulated by various neuronal stimuli in the dentate gyrus and perhaps also in the olfactory bulb to modulate activity-dependent neurogenesis.62 Ample evidence suggests that the genetic program driving activity-dependent gene expression of diffusible factors in mature granule neurons requires NMDAR signaling, which is critically important for synaptic plasticity and information storage of neurons per se.66, 67 This may provide a mechanism by which encoded information from patterned neuronal stimuli is specifically and precisely translated into local neurogenic responses for strategic neuronal addition to the circuitry.
IV. Functional implication
The scope and modes of activity-dependent regulation of adult neurogenesis discussed above have interesting implications for functional contributions of newborn neurons. During adult neurogenesis, nearly half of the precociously generated neurons are destined to die,5, 28, 59 and this specific stage of life-or-death decision has predominantly been used to serve as the substrate of activity-dependent modification in both the olfactory bulb and hippocampus. Importantly, the survival of individual neurons is competitive so that only new neurons activated by information-relevant activity are selectively preserved and integrated to the circuitry. One intriguing idea is that the newly integrated neurons (4-6 week) with enhanced plasticity may encode information that is specific to what that particular neuron has encountered during its history of activity-dependent regulation such as competitive survival (1-3 week), thus representing a trace of memory.59, 60, 68 The same GABA, glutamate, or diffusible factor-based mechanisms may have been used during the earlier period (1-3 week) and later encoding of the memory trace (4-6 week) as for perpetuating the time-specific network activity or input-specific information.
V. Conclusions and Future directions
Activity-dependent addition of new neurons to the existing circuitry represents a prominent form of structural plasticity and may contribute to specific brain functions in the adult brain. Research over the past decade has established an intimate relationship between adult neurogenesis and neuronal activity resulted from various environmental cues, physiological learning-related stimuli and internal cognitive, emotional activities. The remarkable scope and modes of activity-dependent regulation of adult neurogenesis suggest that the adult brain has evolved mechanisms to exquisitely tailor neuronal addition into its information processing capacity, which in turn is contributed by the new population of neurons.
Neuronal derived GABA, glutamate signaling and diffusible factors have emerged as major molecular mediators for activity-dependent modification of adult neurogenesis. The molecular mechanisms underlying activity-dependent survival and integration remains to be further studied. Many other neurotransmitter systems are known to innervate SVZ, olfactory bulb and SGZ area, and their specific roles in regulating different phases of adult neurogenesis are being characterized. Finally, exploring the mutual relationship between activity and neurogenesis in context of neural circuit dynamics to infer the exact functional role of adult neurogenesis remains a great challenging, yet rewarding goal.
Acknowledgement
This work was supported by grants from the NIH, McKnight Scholar, NARSAD, Rett Syndrome Research Foundation, the Muscular Dystrophy Association and the Packard Center for ALS Research at Johns Hopkins (to H.S.) and from the NIH, Sloan, March of Dimes, and Adelson Medical Research Foundation (to G-l.M.).
References
- 1.Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–6. doi: 10.1038/207953a0. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–4. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 4.Temple S, Alvarez-Buylla A. Stem cells in the adult mammalian central nervous system. Curr Opin Neurobiol. 1999;9:135–41. doi: 10.1016/s0959-4388(99)80017-8. [DOI] [PubMed] [Google Scholar]
- 5.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 6.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–93. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 7.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–60. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–69. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 9.Duan X, et al. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18:108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge S, et al. Synaptic integration and plasticity of new neurons in the adult hippocampus. J Physiol. 2008;586:3759–65. doi: 10.1113/jphysiol.2008.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gheusi G, et al. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci U S A. 2000;97:1823–8. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang CL, et al. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–7. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 13.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 14.Kitabatake Y, et al. Adult neurogenesis and hippocampal memory function: new cells, more plasticity, new memories? Neurosurg Clin N Am. 2007;18:105–13. x. doi: 10.1016/j.nec.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–91. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Doetsch F, Hen R. Young and excitable: the function of new neurons in the adult mammalian brain. Curr Opin Neurobiol. 2005;15:121–8. doi: 10.1016/j.conb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 17.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–8. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 18.Ge S, et al. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–6. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 20.Ma DK, Ming GL, Song H. Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr Opin Neurobiol. 2005;15:514–20. doi: 10.1016/j.conb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–81. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 22.Cummings DM, Henning HE, Brunjes PC. Olfactory bulb recovery after early sensory deprivation. J Neurosci. 1997;17:7433–40. doi: 10.1523/JNEUROSCI.17-19-07433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Najbauer J, Leon M. Olfactory experience modulated apoptosis in the developing olfactory bulb. Brain Res. 1995;674:245–51. doi: 10.1016/0006-8993(94)01448-q. [DOI] [PubMed] [Google Scholar]
- 24.Corotto FS, Henegar JR, Maruniak JA. Odor deprivation leads to reduced neurogenesis and reduced neuronal survival in the olfactory bulb of the adult mouse. Neuroscience. 1994;61:739–44. doi: 10.1016/0306-4522(94)90397-2. [DOI] [PubMed] [Google Scholar]
- 25.Fiske BK, Brunjes PC. Cell death in the developing and sensory-deprived rat olfactory bulb. J Comp Neurol. 2001;431:311–9. [PubMed] [Google Scholar]
- 26.Rochefort C, et al. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–89. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rochefort C, Lledo PM. Short-term survival of newborn neurons in the adult olfactory bulb after exposure to a complex odor environment. Eur J Neurosci. 2005;22:2863–70. doi: 10.1111/j.1460-9568.2005.04486.x. [DOI] [PubMed] [Google Scholar]
- 28.Alonso M, et al. Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. J Neurosci. 2006;26:10508–13. doi: 10.1523/JNEUROSCI.2633-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim WR, et al. Impaired migration in the rostral migratory stream but spared olfactory function after the elimination of programmed cell death in Bax knock-out mice. J Neurosci. 2007;27:14392–403. doi: 10.1523/JNEUROSCI.3903-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Chevigny A, et al. Delayed onset of odor detection in neonatal mice lacking tenascin-C. Mol Cell Neurosci. 2006;32:174–86. doi: 10.1016/j.mcn.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Enwere E, et al. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–65. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magavi SS, et al. Adult-born and preexisting olfactory granule neurons undergo distinct experience-dependent modifications of their olfactory responses in vivo. J Neurosci. 2005;25:10729–39. doi: 10.1523/JNEUROSCI.2250-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mak GK, et al. Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat Neurosci. 2007;10:1003–11. doi: 10.1038/nn1928. [DOI] [PubMed] [Google Scholar]
- 34.Shingo T, et al. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–20. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- 35.Acsady L, Kali S. Models, structure, function: the transformation of cortical signals in the dentate gyrus. Prog Brain Res. 2007;163:577–99. doi: 10.1016/S0079-6123(07)63031-3. [DOI] [PubMed] [Google Scholar]
- 36.Houser CR. Interneurons of the dentate gyrus: an overview of cell types, terminal fields and neurochemical identity. Prog Brain Res. 2007;163:217–32. doi: 10.1016/S0079-6123(07)63013-1. [DOI] [PubMed] [Google Scholar]
- 37.McHugh TJ, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–9. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 38.Dudek FE, Sutula TP. Epileptogenesis in the dentate gyrus: a critical perspective. Prog Brain Res. 2007;163:755–73. doi: 10.1016/S0079-6123(07)63041-6. [DOI] [PubMed] [Google Scholar]
- 39.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–5. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 40.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–5. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 41.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 42.Gould E, et al. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–5. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 43.Dupret D, et al. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parent JM. Adult neurogenesis in the intact and epileptic dentate gyrus. Prog Brain Res. 2007;163:529–40. doi: 10.1016/S0079-6123(07)63028-3. [DOI] [PubMed] [Google Scholar]
- 45.Overstreet-Wadiche LS, et al. Seizures accelerate functional integration of adult-generated granule cells. J Neurosci. 2006;26:4095–103. doi: 10.1523/JNEUROSCI.5508-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi SH, et al. Non-cell-autonomous effects of presenilin 1 variants on enrichment-mediated hippocampal progenitor cell proliferation and differentiation. Neuron. 2008;59:568–80. doi: 10.1016/j.neuron.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziv Y, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–75. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 48.Warner-Schmidt JL, Madsen TM, Duman RS. Electroconvulsive seizure restores neurogenesis and hippocampus-dependent fear memory after disruption by irradiation. Eur J Neurosci. 2008;27:1485–93. doi: 10.1111/j.1460-9568.2008.06118.x. [DOI] [PubMed] [Google Scholar]
- 49.Platel JC, Dave KA, Bordey A. Control of neuroblast production and migration by converging GABA and glutamate signals in the postnatal forebrain. J Physiol. 2008;586:3739–43. doi: 10.1113/jphysiol.2008.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu X, et al. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–87. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tozuka Y, et al. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–15. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 52.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhattacharyya BJ, et al. The chemokine stromal cell-derived factor-1 regulates GABAergic inputs to neural progenitors in the postnatal dentate gyrus. J Neurosci. 2008;28:6720–30. doi: 10.1523/JNEUROSCI.1677-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kolodziej A, et al. Tonic activation of CXC chemokine receptor 4 in immature granule cells supports neurogenesis in the adult dentate gyrus. J Neurosci. 2008;28:4488–500. doi: 10.1523/JNEUROSCI.4721-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nacher J, et al. NMDA receptor antagonist treatment induces a long-lasting increase in the number of proliferating cells, PSA-NCAM-immunoreactive granule neurons and radial glia in the adult rat dentate gyrus. Eur J Neurosci. 2001;13:512–20. doi: 10.1046/j.0953-816x.2000.01424.x. [DOI] [PubMed] [Google Scholar]
- 56.Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–92. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chun SK, et al. Enhanced proliferation of progenitor cells following long-term potentiation induction in the rat dentate gyrus. Neurobiol Learn Mem. 2006;86:322–9. doi: 10.1016/j.nlm.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Bruel-Jungerman E, et al. Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J Neurosci. 2006;26:5888–93. doi: 10.1523/JNEUROSCI.0782-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tashiro A, et al. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–33. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 60.Ge S, et al. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–66. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kee N, et al. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–62. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 63.Li Y, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bergami M, et al. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:15570–5. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saghatelyan A, et al. Tenascin-R mediates activity-dependent recruitment of neuroblasts in the adult mouse forebrain. Nat Neurosci. 2004;7:347–56. doi: 10.1038/nn1211. [DOI] [PubMed] [Google Scholar]
- 66.Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev. 2006;86:1033–48. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- 67.Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–90. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–9. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]