Abstract
Rationale
We previously co-localized a quantitative trait locus (QTL) for sensitivity to the locomotor stimulant effect of methamphetamine (MA) with a QTL for expression of casein kinase 1 epsilon (Csnk1-ε) in the nucleus accumbens (NAc). Subsequently, we identified a single nucleotide polymorphism in CSNK1E (rs135745) that was associated with increased sensitivity to the subjective effects of d-amphetamine in healthy human subjects. Based on these results, we hypothesized that differential expression of Csnk1-ε causes differential sensitivity to MA-induced locomotor activity in mice.
Objective
In the present study, we used PF-670462 (PF), which is a selective inhibitor of Csnk1-ε, to directly evaluate the role of Csnk1-ε in the locomotor response to MA in male C57BL/6J mice.
Methods
We administered vehicle, PF, MA or MA+PF, either via intraperitoneal injections or bilateral intra-NAc microinjections. We also examined Darpp-32 phosphorylation in mice receiving intraperitoneal injections.
Results
Intraperitoneal PF (20-40 mg/kg) attenuated the locomotor response to MA (2 mg/kg) without affecting baseline activity. The high dose of PF also significantly inhibited MA-induced phosphorylation of Darpp-32, providing a potential mechanism by which Csnk1-ε contributes to MA-induced locomotor activity. Furthermore, microinjection of PF (5 μg/side) into the NAc completely blocked the locomotor response to MA (2.5 μg/side) without affecting baseline activity.
Conclusions
These results provide direct evidence that Csnk1-ε is crucial for the locomotor stimulant response to a moderate dose of MA and are consistent with the hypothesis that genetic polymorphisms in Csnk1-ε influence sensitivity to amphetamines in both mice and humans.
Keywords: nucleus accumbens, psychostimulant, activity, dopamine, Darpp-32, casein kinase 1 epsilon, genetic, qtl, amphetamine
Introduction
Multiple classes of commonly abused drugs, including psychostimulants such as amphetamine and methamphetamine (MA), increase locomotor activity in rodents (Wise and Bozarth 1987). This behavior is in part mediated by dopamine release in the nucleus accumbens (NAc), a brain region that is also critical for drug reward (Di Chiara and Imperato 1988). Thus, studying mechanisms underlying drug-induced locomotor activity may provide insights into the subjectively rewarding effects of drugs in humans.
Administration of MA causes release of dopamine (Volz et al. 2007), which activates dopamine D1 receptors on medium spiny neurons of the NAc and contributes to MA-induced locomotor activity (Koshikawa et al. 1989). D1 receptor-dependent activation of protein kinase A (PKA) phosphorylates dopamine- and cyclic AMP-regulated phosphoprotein 32 kD (Darpp-32) at Thr-34, transforming it into a potent inhibitor of protein phosphatase I (PP-1) (Hemmings et al. 1984; Nishi et al. 1997). Darpp-32 is highly expressed in dopamine-receiving neurons in the NAc (Ouimet et al. 1984; Walaas et al. 1983) and modulates psychostimulant-induced locomotor activity (Fienberg et al. 1998; Greengard 2001; Lindskog et al. 2002; Snyder et al. 2000; Zachariou et al. 2006) and reward (Zachariou et al. 2002). PP-1 inhibition leads to increased phosphorylation of a variety of targets, including glutamate receptors (Snyder et al. 2000; Snyder et al. 1998). The inhibition of PP-1 by Darpp-32 is positively regulated by casein kinases 1 and 2 which phosphorylate Ser 130 (mice) / 137 (rats) and Ser 97 (mice) / 102 (rats), respectively to increase PKA phosphorylation of Darpp-32 at Thr-34 (Desdouits et al. 1995a; Desdouits et al. 1995b; Girault et al. 1989).
Locomotor activation induced by amphetamines is highly heritable and multiple quantitative trait loci (QTL) influencing this trait have been identified (Phillips et al. 2008). Using mice selectively bred for high and low sensitivity to MA-induced locomotor activity (Kamens et al. 2005), we co-mapped a QTL for behavioral sensitivity to MA with a QTL for increased casein kinase 1 epsilon (Csnk1-ε) expression (Palmer et al. 2005). Gene expression profiling indicated an almost ten-fold increase in Csnk1-ε transcript abundance in the NAc in the mouse line selected for high MA sensitivity (Palmer et al. 2005). We also observed significantly higher transcript abundance of Darpp-32 in the mice selectively bred for high sensitivity. Based on these results, we examined polymorphisms in the human CSNK1E gene and found a significant association between a single nucleotide polymorphism (rs135745) and sensitivity to the euphorigenic effects of d-amphetamine (Veenstra-VanderWeele et al. 2006). Thus, multiple lines of genetic evidence suggest Csnk1-ε contributes to sensitivity to amphetamines.
In order to directly test this hypothesis, we used PF-670462 (PF), which is a selective inhibitor of Csnk1-ε (Badura et al. 2007) in conjunction with MA. We co -administered vehicle, PF, MA or MA+PF either peripherally or via intra- NAc microinjection in male C57BL/6J mice and recorded locomotor behavior immediately thereafter. The results clearly show that Csnk1-ε is critical for the acute locomotor response to MA.
Materials and Methods
Drugs
Methamphetamine (MA) hydrochloride (Sigma-Aldrich, St. Louis, MO) and PF-670462 (4-(3-cyclohexyl-5-(4-fluoro-phenyl)-3H-imidazol-4-yl)pyrimidin-2-ylamine; “PF”), provided as a gift by Pfizer (Groton, CT), were dissolved in physiological saline (Experiment 1) and PBS (Experiment 2). The MA dose (2 mg/kg) was chosen based on our previous study using this dose (Palmer et al. 2005). The PF doses (20-40 mg/kg, i.p. for the systemic study; 5 μg / side for the microinjection study) were chosen based on pilot studies in which we found lower doses to have little effect on MA-induced locomotor activity. We did not use higher doses of PF in order to avoid decreases in locomotor activity due to PF alone.
Mice
Male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME), aged 50-70 days were used. Mice were group housed, 4-5 mice per cage in the vivarium. Food and water were available ad libitum and the vivarium was maintained on a 12 h/12 h light/dark cycle. All testing was completed during the light phase (0600h-1800h). All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Chicago.
Locomotor Activity
Locomotor activity was measured using automated Versamax activity chambers (AccuScan, Columbus, OH). Each chamber was made of a clear acrylic arena (40 × 40 × 30 cm) placed inside a frame containing evenly spaced photocells and receptors making a grid of infrared photobeams from the front to the back and from the left to the right of the arena. Beam breaks were recorded on a computer and converted into distance traveled (cm). Each activity chamber was surrounded by a sound attenuating PVC / lexan environmental chamber (AccuScan). In each chamber overhead lighting provided dim illumination (∼80 lux) and a fan provided ventilation and masking of background noise.
Experiment 1: Effect of peripheral administration of the Csnk1-ε inhibitor PF on methamphetamine-induced locomotor activity
Behavioral testing occurred over 3 consecutive days at the same time each day. Mice were transported from the vivarium next door to a testing room and were allowed at least 30 min to habituate in their home cages. On the first and second days of testing, mice were removed from their home cages, weighed, and placed in individual holding cages filled with clean bedding. Mice then received an i.p. injection of physiological saline and were immediately placed in individual activity chambers and locomotor activity was monitored for 30 min. On the third day of testing, mice were randomly assigned to one of 6 experimental groups (n=12 per group) and received i.p. injections of the following: Saline (Saline); 20 mg/kg PF (PF20); 40 mg/kg PF (PF40); 2 mg/kg MA (MA); 20 mg/kg PF + 2 mg/kg MA (PF20+MA); 40 mg/kg PF + 2 mg/kg MA (PF40+MA). All systemic injections were administered in a volume of 10 ml/kg body weight. Following the appropriate treatment, mice were immediately placed in the activity chambers and locomotor activity was monitored for 30 min. PF doses were chosen based on pilot studies (data not shown) and a previous study (Badura et al. 2007).
Western blots
Immediately after testing (approx 1330-1600 hrs), bilateral nucleus accumbens samples were collected from a subset of these mice (Saline, PF20, PF40, PF40+MA=4 per group; PF20+MA=5; MA=6) and were immediately flash frozen with dry ice. Tissue processing for Western blotting began with sonication in RIPA buffer (50 mM Tris, pH 7.4, 1 mM EDTA, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate,0.1% SDS, 1 mM phenylmethylsulfonylfluoride, 1 mM Sodium orthovanadate, and protease and phosphatase inhibitor cocktail) for 5 s, incubation on ice for 30 min, and spinning at 13,000 rpm for 20 min at 4°C. Protein concentration in the supernatant was measured by DC protein assay (Bio-Rad, Hercules, CA) and 20 μg of protein per sample was separated by 12% sodium dodecyl sulfate-polyacrylamide gel elecrophoresis and transferred to an Immobilon-P polyvinylidene fluoride microporous membrane (Amersham Biosciences, Arlington Heights, IL). The membrane was blocked with Tris-buffered saline (TBS-T; 20 mM Tris-HCl, 500mM NaCl, pH 7.5, 0.1% Tween 20) containing 5% defatted milk powder at 4°C for 24 hr, followed by overnight incubation at 4°C with a polyclonal primary rabbit antibody raised against the Darpp-32 (cell signaling, Danvers, MA), or phospho-Thr-34-Darpp-32 (P-Darpp-32; Chemicon, Temecula, CA) or a mouse antibody against β-actin (Sigma, St. Louis, MO) diluted 1:1000, 1:500, and 1:2000, respectively, in TBS-T with 5% defatted milk powder. Blots were washed three times with TBS-T, incubated with peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody in TBS-T for 1 h at room temperature, and rinsed three times with TBS-T. All immunoblots were developed with ECL Plus (Amersham, Buckinghamshire, UK), and digital images were acquired on the Syngene Chemigenius2 Bioimaging system, with care to avoid signal saturation. Band intensity was quantified as peak area (net of background signal) in line scans of each lane, using SynGene geneTool software (Synoptics, Cambridge, England). Values for P-Darpp-32 and Darpp-32 were individually normalized to β-actin run on the same blots and then the ratio of normalized P-Darpp-32 to normalized total Darpp-32 was used as the dependent measure.
Experiment 2: Effects of intra-NAc administration of the Csnk1-ε inhibitor PF on methamphetamine-induced locomotor activity
Surgical Preparation
Mice were anesthetized with an i.p. injection of an anesthetic cocktail consisting of 10 mg/kg ketamine and 1 mg/kg xylazine in sterile double deionized water. The injection volume was 1.25-1.5 μl/g body weight. Supplemental injections at a volume of 0.1 mL were administered every 30-45 min to maintain anesthesia throughout the surgery (assessed via toe pinch reflex). Anesthetized mice were placed in a sterotaxic instrument (Stoelting, Wood Dale, IL) and vertically implanted with 26 G double guide cannulae (PlasticsOne, Roanoke, VA) using coordinates targeting the NAc (AP: + 1.4 mm relative to Bregma; ML: ± 0.95 mm; DV: -4.3 mm; (Paxinos and Franklin 2001) and held in place with a cyanoacrylic resin. Dummy cannulae were inserted into the double guide cannulae and secured with a dust cap to prevent blockage. Mice undergoing surgery on the same day were group housed (up to 4 mice per cage) post-operatively. Mice were allowed 1 week to recover and were checked daily for health and to confirm that the dust caps and dummy cannulae were still in place.
Behavioral testing
As in Experiment 1, testing occurred over 3 consecutive days. On each day, mice were transported from the vivarium next door to a testing room and were allowed at least 30 min to habituate in their home cages. On the first and second day of testing, mice were removed from their home cages, weighed, and placed into individual holding cages filled with clean bedding. A 33 G injector needle connected to an infusion pump (Harvard Apparatus, Holliston, MA) was inserted into the double guide cannulae and 0.5 μl of PBS was infused in each side for 1.5 min per side. Immediately thereafter, mice were placed into the activity chambers and locomotor activity was monitored for 30 min. On the third day of testing, mice were randomly assigned to one of four experimental groups and received bilateral intra-NAc infusions of the following treatments: PBS (Group PBS; n=6); 5 μg/side PF (PF; n=7); 2.5 μg/side MA (MA; n=5); or 5 μg/side PF + 2.5 μg/side MA (PF+MA; n=8). Drugs were infused at a volume of 0.5 μl/side at a rate of 1 μl/min and the injector needle was left in place for and additional 1 min to allow diffusion. After treatment, mice were placed in the activity chambers and locomotor behavior was monitored for 30 min in the same manner as described for Experiment 1.
Histology
Following behavioral testing on day 3, mice were euthanized with CO2. A small amount of India ink was infused through both cannulae and brains were extracted and placed in a sucrose / formalin solution until fixed. Brains were sectioned (60 μm) with a cryostat throughout the extent of NAc and then mounted on slides. Sections were stained with Cresyl violet and correct cannulae placement was decided by an author blind to the treatments and experimental results (Figure 2A). Five mice were excluded due to poor placement of cannulae (PBS, n=1; MA, n=3; PF, n=1).
Figure 2. Nucleus accumbens microinjections and effect of PF on MA-induced locomotor activity.
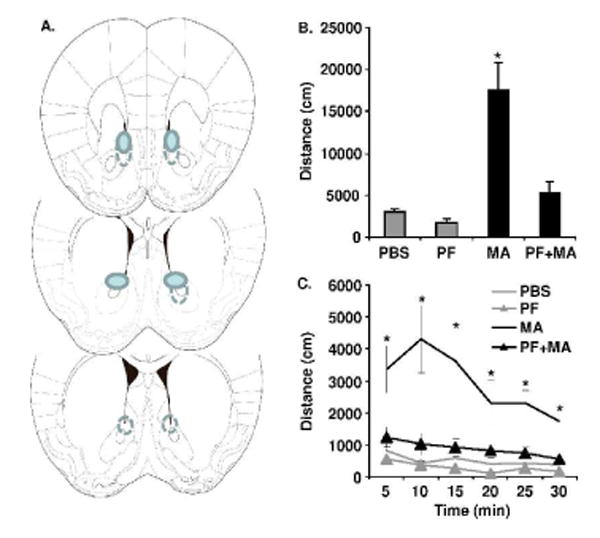
A). Schematic representations of coronal plates taken from the atlas of Paxinos and Franklin (2001) showing the region from +1.70 to + 0.74 mm anterior to Bregma, which contains the shell and core of the NAc. Filled symbols represent the position of the majority of cannula tips and dotted lines represent instances when placement was still within the NAc but deviated from the majority of placements. A total of 5 animals were excluded because the cannula tips were not within the desired area. B). Total distance traveled for the entire 30 min on Day 3. C). Total distance traveled in 5 min bins over 30 min. Intra-NAc administration of PF completely blocked intra-NAc MA-induced locomotor activity. * =significantly different from all other treatment groups (p < 0.05). N=6 for PBS; N=7 for PF; N=5 for MA; N=8 for PF+MA.
Statistical analysis
Behavioral data were analyzed using a repeated-measures analysis of variance (ANOVA) with a nested design to examine locomotor behavior across days and within a day. Western blots were analyzed with a two-way ANOVA. Repeated-measures ANOVA, one-way ANOVA, and Tukey-Kramer were used as post-hoc tests when significant main effects and interactions were obtained. An alpha level was set at 0.05 for all tests.
Results
As expected, there were no significant main effects or interactions on test days 1 and 2 (all subjects were treated with vehicle on these days) in either experiment. Thus, the data reported are the results following experimental treatments on Day 3.
Experiment 1: Effects of systemic administration of the Csnk1-ε inhibitor PF on methamphetamine-induced locomotor activity
The total distance traveled on Day 3 for the entire 30 min test period is shown in Figure 1A; the same data broken down into 5 min bins are shown in Figure 1B. For the full 30 min, there was a significant interaction between the PF dose and MA dose (F2,66=8.9; p<0.0005). The PF40+MA group showed significantly less locomotor activity than the other groups receiving MA and significantly more activity than the groups receiving saline (p < 0.05), reflecting attenuation of MA-induced locomotor activity.
Figure 1. Effect of intraperitoneal administration of PF on MA-induced locomotor activity and Darpp-32 phosphorylation.
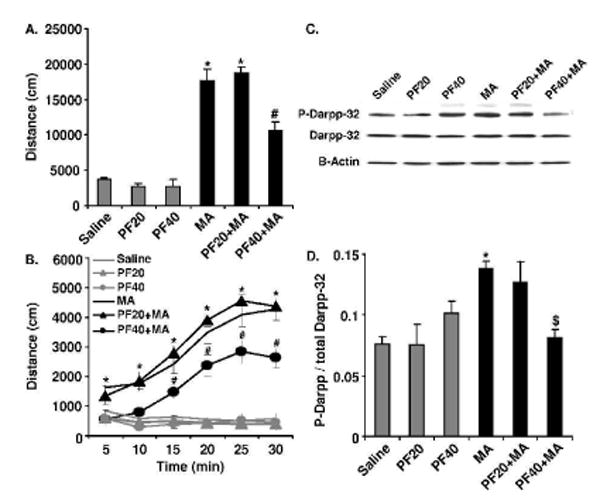
A). Total distance traveled for the entire 30 min test period on Day 3. B). Total distance traveled in 5 min bins over 30 min. 20 mg/kg of PF had no effect on MA-induced locomotor activity but 40 mg/kg of PF significantly attenuated the locomotor stimulant effect of MA. *=MA and PF20+MA are significantly different from all other treatment groups (p < 0.05). #=PF40+MA is significantly different from all other treatment groups (p < 0.05). N=12 per group. C). Representative Western blot of P-Darpp-32 in NAc from brains of mice tested in Experiment 2. D). Ratio of P-Darpp-32 relative to total Darpp-32. 20 mg/kg of PF had no effect on MA-induced Darpp-32 phosphorylation but 40 mg/kg of PF significantly attenuated it. *=MA group is significantly different from groups not receiving MA (p<0.05). $=MA+PF40 group is significantly different from the MA and MA+PF20 groups (p<0.05).
During the 0-5 and 5-10 min bins, the PF40+MA group was similar to the Saline, PF20 and PF40 groups, and was significantly lower than the MA and PF20+MA groups. In all subsequent time bins, the PF40+MA group showed significantly less locomotor activity that the MA and PF20+MA groups, but showed significantly more activity than the Saline, PF20 and PF40 groups. Thus PF completely blocked the response to MA for the first 10 minutes and thereafter significantly antagonized the response to MA. For all bins, the MA and PF20+MA groups showed significantly more activity than all other treatment groups but did not differ from each other, indicating no effect of the 20 mg/kg PF dose on MA-induced locomotor activity. The PF20 and PF40 groups never differed from the Saline group, indicating that there were no non-selective effects of PF on locomotor activity.
As shown in Figures 1C and 1D, treatment with MA and PF significantly affected P-Darpp-32 levels with MA increasing P-Darpp-32 and PF inhibiting this effect. Neither of the PF doses altered P-Darpp-32 levels in the absence of induction by MA. A two-way ANOVA for the factors MA dose (0 or 2) and PF dose (0, 20 and 40) identified a significant interaction between the two (F2,21=7.06; p<0.005). In order to determine the source of this interaction, we examined MA dose at each level of PF using a post-hoc ANOVA and found that MA increased P-Darpp-32 relative to the Saline group. We also explored the effect of each level of MA dose using a post-hoc ANOVA followed by Tukey posthoc tests. For the groups given 0 mg/kg MA (Saline, PF20, PF40) the ANOVA and Tukey results were not significant, indicating no effect of PF alone on the phosphorylation state of the Darpp-32. For the groups given 2 mg/kg MA (MA, PF20+MA, PF40+MA) there was a significant effect of PF treatment (F2,12=6.61;p<0.05). Tukey posthoc test indicated that the PF40+MA group had significantly less P-Darpp-32 than either the MA or the PF20+MA groups (Figure 1D).
Experiment 2: Effects of intra-NAc administration of the Csnk1-ε inhibitor PF on methamphetamine-induced locomotor activity
In the previous experiment, the higher dose of peripherally administered PF attenuated MA-induced locomotor activity. Because we previously identified differential expression of Csnk1-ε in the NAc of mice selectively bred for high or low MA-induced locomotor activity, we next sought to determine whether the NAc was a critical site of action for the effects of PF on Csnk1-ε by using intra-NAc microinjections of Saline, PF, MA or MA+PF. The total distance traveled for the entire 30 min test period is shown in Figure 2B and the same data broken into 5 min bins are shown in Figure 2C. Following intra-NAc infusion on Day 3, there was a significant interaction between PF and MA (F1,22=13.4; p < 0.005). Intra-NAc MA significantly increased locomotor activity relative to all other groups for the entire 30 min session (Figure 2B) and for every 5 min bin (Figure 2C; p < 0.05). In contrast, locomotor activity following intra-NAc infusions of PF+MA did not differ from either the PBS group or the PF group, indicating a complete blockade of MA-induced locomotor activity. There was no significant effect of PF alone on spontaneous locomotor activity, indicating its specificity for targeting the locomotor stimulant properties of MA.
Discussion
These results provide direct evidence that Csnk1-ε is essential for the locomotor stimulant response to MA and further suggest that phosphorylation of Darpp-32 is the likely mechanism for this effect. Intraperitoneal administration of PF, which is a selective inhibitor of Csnk1-ε (Badura et al. 2007), significantly attenuated MA-induced locomotor activity (Figure 1A, 1B) and also blocked phosphorylation of Darpp-32 at the highest dose (Figure 1C, 1D). Moreover, microinjection of PF into the NAc completely blocked the locomotor stimulant response to microinjected MA (Figure 2B, 2C). In both cases, administration of the same dose of PF alone had no effect on locomotor activity, demonstrating that inhibition of the effects of MA by PF were not secondary to a non-specific locomotor depressant effect of PF.
Members of the casein kinase 1 family phosphorylate Darpp-32 at Ser-130/137 which stabilizes the phosphorylated state of Thr-34 and thus, increases inhibition of PP1 (Desdouits et al. 1995a; Desdouits et al. 1995b). Phosphorylation of the Thr-34 site is crucial for Darpp-32's contribution to psychostimulant locomotor activity (Valjent et al. 2005; Zachariou et al. 2006). PP1 dephosphorylates many targets, including excitatory and inhibitory ionotropic receptors, voltage-gated ion channels, and kinases (Svenningsson et al. 2004). In the present studies we show that inhibition of Csnk1-ε by PF inhibits both MA-induced phosphorylation of Darpp-32 and also MA-induced locomotor stimulation.
Peripheral injections of PF blocked the response to MA at the early time bins and attenuated it at the later time bins (Figure 1B). It is possible that a higher dose of PF would have been required to completely block the response to MA, however the observation that the PF40+MA group had comparable levels of P-Darpp-32 to the Saline group suggests that PF had already completely blocked MA-induced phosphorylation of Darpp-32 in the NAc. It is known that sufficiently high doses of psychostimulants can still induce locomotor activity even in Darpp-32 null mutant mice (Fienberg et al. 1998; Zachariou et al. 2006). Therefore, either Darpp-32-independent signaling in the NAc, or NAc independent signaling could explain the failure of PF to completely block the MA-induced locomotor response.
Co-microinjection of PF+MA produced a complete blockade of the stimulant response to MA, demonstrating that the NAc is a key site for the action of Csnk1-ε. We observed greater MA-induced locomotor activity following central versus peripheral injections of MA alone. Thus, the greater antagonism of the behavioral response by PF following microinjection into the NAc cannot be attributed to a less effective dose of MA. Because we needed the brains for histology to confirm cannula placement, we did not examine P-Darpp-32 induction in the mice that received microinjections. However, the data from the systemic studies strongly suggest that MA stimulated phosphorylation of Darpp-32 and that this effect was blocked by PF.
PF is a selective inhibitor of Csnk1-ε and can penetrate the blood-brain barrier, which allowed us to use a peripheral route of administration. PF also inhibits the closely related Csnk1-δ with about 1.8-fold less potency and inhibits epidermal growth factor receptor (EGFR) and p38 mitogen-activated protein kinase (MAPK) with 19-fold and 25-fold less potency, respectively (Badura et al. 2007). A previous report demonstrated that intra-NAc injection of a selective inhibitor of p38 MAPK activation had no effect on amphetamine-induced locomotor activity (Gerdjikov et al. 2004), indicating that inhibition of p38 MAPK by PF is not responsible for its effect on MA-induced locomotor activity. As with any pharmacological study, it is difficult to eliminate the possibility that the observed effects are due to non-specific activity, just as gene knockout studies are limited by the potential for long-term adaptations that obscure the true function of the gene under normal physiological conditions. Our studies showing that PF also inhibits phosphorylation of Darpp-32 are most consistent with a selective effect of PF on Csnk1-ε.
In addition to phosphorylation of Darpp-32, Csnk1-ε is also involved in the circadian rhythm pathway where it phosphorylates and regulates the localization and stability of Per proteins (Gallego and Virshup 2007). Mutations in Csnk1-ε or its orthologs and pharmacological inhibition result in disruption of circadian rhythm (Badura et al. 2007; Kloss et al. 1998; Lowrey et al. 2000; Meng et al. 2008; Price et al. 1998). Interestingly, there are multiple lines of evidence that have implicated circadian rhythm genes in mediating the responses to drugs of abuse (Falcon and McClung 2008; McClung 2007; Yuferov et al. 2005). For example, mutations in Period, Clock, Cycle, and Doubletime (ortholog of Csnk1-ε) prevent sensitization to the behavioral effects of cocaine in Drosophila (Andretic et al. 1999), disruption of mPer1 or mPer2 blocks or enhances cocaine sensitization and reward in mice (Abarca et al. 2002), and mice lacking Clock show enhanced cocaine reward (McClung et al. 2005). Thus, it is possible that Csnk1-ε contributes to MA locomotor activity via phosphorylation of one or more Per proteins. In relation to our data, there is a circadian rhythm in both expression of mPer1 in the nucleus accumbens (Uz et al. 2003) and MA-induced locomotor activity (Kuribara and Tadokoro 1982). However, while MA-induced phosphorylation of Per proteins has not been demonstrated, acute methamphetamine administration increases expression of mPer1 (and not mPer2 or mPer3) in the dorsal striatum, but not the NAc at 60 min post-injection (Nikaido et al. 2001). This time course suggests that the blockade of MA locomotor activity by PF in the NAc is not due to an interaction of MA with mPer1. Furthermore, although the molecular functions of Per proteins are not fully understood, Csnk1-ε-induced phosphorylation of Per proteins results in either their nuclear entry where they act as transcriptional repressors of their own transcription or their own degradation (Gallego and Virshup 2007). This precludes a role of Per proteins as second messengers and makes it highly unlikely that they would affect the acute (30 min) locomotor response to MA.
Another signaling pathway that is influenced by Csnk1-ε is the Wnt signaling pathway. Traditionally, the Wnt pathway is known for its involvement in embryonic development and cell proliferation associated with cancerous tumors but it is now also associated with such conditions as diabetes and neuropsychiatric disorders (Coombs et al. 2008). In response to Wnt signaling via activation of frizzled (Fzl) receptors, Csnk1-ε phosphorylates Dishevelled (Dvl) and possibly multiple other substrates, resulting in inhibition of GS3K- β activity and stabilization of β-catenin. β-catenin then translocates to the nucleus and promotes the activation of Wnt target genes (Knippschild et al. 2005; Price 2006). Because this pathway also involves changes in gene transcription, it is unlikely that changes in Wnt signaling via Csnk1-ε inhibition would contribute to the rapid behavioral response to MA observed in the present. Thus, the Darpp-32 pathway is the strongest candidate for Csnk1-ε modulation of MA locomotor activity, especially in light of our data showing PF-mediated inhibition of Darpp-32 phosphorylation following MA administration.
In addition to their recreational and pathological use, stimulant drugs similar to MA are used to treat a variety of disorders, including attention deficit hyperactive disorder, Parkinson's disease, and sleep disorders such as narcolepsy. Moreover, drugs that antagonize the dopamine system are used as antipsychotics for treating such conditions as schizophrenia and mania. Therefore, manipulation of Csnk1-ε may prove to be a useful pharmaceutical strategy for treating a variety of conditions that respond to dopaminergic modulation. It is possible that modulation of second messenger pathways, including the Csnk1-ε /Darpp-32 pathway, would provide more efficacious targets for intervention. Finally, a functional variant of CSNK1E was identified in humans which predicts susceptibility to delayed sleep phase syndrome (DSPS) and non-24-h sleep-wake syndrome (N-24) (Takano et al. 2004) and thus, pharmacological manipulation of CSNK1E may be useful for treating these conditions.
These data identify a potent effect of inhibition of Csnk1-ε on the locomotor response to MA administration as well as a likely signaling mechanism. Because dopaminergic signaling is of great importance for many different psychiatric disorders, future clinical applications may be developed for PF-670462 or a similar compound. Additional research may also focus on the genetic polymorphisms that we have previously identified in human sensitivity to amphetamine. In this regard, the current results are significant because they further bolster evidence for the hypothesis that genetic variability in Csnk1-ε influences sensitivity to psychostimulants in mice and humans.
Acknowledgments
The authors would like to thank Carolyn Cain, Ryan Walters, and Pei-Chun Chen for technical assistance on the present study and in previous pilot studies. This work was supported by DA021336-02 (A.A.P.), DA09397 (P.V.), T32DA007255 (C.D.B.), the Biological Sciences Collegiate Division Research endowments at the University of Chicago (M.E.G.), and the National Institute of General Medical Sciences Medical Scientist National Research Service Award T32GM07281 (M.G.D.).
Funding: DA021336-02 (A.A.P.), DA09397 (P.V.), 2T32DA007255 (C.D.B.), 5T32GM07281 (M.G.D.).
Abbreviations
- MA
methamphetamine
- Csnk1-ε
casein kinase 1-epsilon
- Darpp-32
Dopamine and cyclic AMP-regulated phosphoprotein-32
- PF
PF-670462
- QTL
quantitative trait loci
- NAc
nucleus accumbens
Footnotes
The experiments comply with the current laws in the United States. The authors declare they have no conflict of interest.
References
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002;99:9026–30. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretic R, Chaney S, Hirsh J. Requirement of circadian genes for cocaine sensitization in Drosophila. Science. 1999;285:1066–8. doi: 10.1126/science.285.5430.1066. [DOI] [PubMed] [Google Scholar]
- Badura L, Swanson T, Adamowicz W, Adams J, Cianfrogna J, Fisher K, Holland J, Kleiman R, Nelson F, Reynolds L, St Germain K, Schaeffer E, Tate B, Sprouse J. An inhibitor of casein kinase I epsilon induces phase delays in circadian rhythms under free-running and entrained conditions. J Pharmacol Exp Ther. 2007;322:730–8. doi: 10.1124/jpet.107.122846. [DOI] [PubMed] [Google Scholar]
- Coombs GS, Covey TM, Virshup DM. Wnt signaling in development, disease and translational medicine. Curr Drug Targets. 2008;9:513–31. doi: 10.2174/138945008784911796. [DOI] [PubMed] [Google Scholar]
- Desdouits F, Cohen D, Nairn AC, Greengard P, Girault JA. Phosphorylation of DARPP-32, a dopamine- and cAMP-regulated phosphoprotein, by casein kinase I in vitro and in vivo. J Biol Chem. 1995a;270:8772–8. doi: 10.1074/jbc.270.15.8772. [DOI] [PubMed] [Google Scholar]
- Desdouits F, Siciliano JC, Greengard P, Girault JA. Dopamine- and cAMP-regulated phosphoprotein DARPP-32: phosphorylation of Ser-137 by casein kinase I inhibits dephosphorylation of Thr-34 by calcineurin. Proc Natl Acad Sci U S A. 1995b;92:2682–5. doi: 10.1073/pnas.92.7.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon E, McClung CA. A role for the circadian genes in drug addiction. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fienberg AA, Hiroi N, Mermelstein PG, Song W, Snyder GL, Nishi A, Cheramy A, O'Callaghan JP, Miller DB, Cole DG, Corbett R, Haile CN, Cooper DC, Onn SP, Grace AA, Ouimet CC, White FJ, Hyman SE, Surmeier DJ, Girault J, Nestler EJ, Greengard P. DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–42. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8:139–48. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- Gerdjikov TV, Ross GM, Beninger RJ. Place preference induced by nucleus accumbens amphetamine is impaired by antagonists of ERK or p38 MAP kinases in rats. Behav Neurosci. 2004;118:740–50. doi: 10.1037/0735-7044.118.4.740. [DOI] [PubMed] [Google Scholar]
- Girault JA, Hemmings HC, Jr, Williams KR, Nairn AC, Greengard P. Phosphorylation of DARPP-32, a dopamine- and cAMP-regulated phosphoprotein, by casein kinase II. J Biol Chem. 1989;264:21748–59. [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–30. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Greengard P, Tung HY, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310:503–5. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Sensitivity to psychostimulants in mice bred for high and low stimulation to methamphetamine. Genes Brain Behav. 2005;4:110–25. doi: 10.1111/j.1601-183X.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- Kloss B, Price JL, Saez L, Blau J, Rothenfluh A, Wesley CS, Young MW. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell. 1998;94:97–107. doi: 10.1016/s0092-8674(00)81225-8. [DOI] [PubMed] [Google Scholar]
- Knippschild U, Gocht A, Wolff S, Huber N, Lohler J, Stoter M. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17:675–89. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Koshikawa N, Mori E, Oka K, Nomura H, Yatsushige N, Maruyama Y. Effects of SCH23390 injection into the dorsal striatum and nucleus accumbens on methamphetamine-induced gnawing and hyperlocomotion in rats. J Nihon Univ Sch Dent. 1989;31:451–7. doi: 10.2334/josnusd1959.31.451. [DOI] [PubMed] [Google Scholar]
- Kuribara H, Tadokoro S. Circadian variation in methamphetamine- and apomorphine-induced increase in ambulatory activity in mice. Pharmacol Biochem Behav. 1982;17:1251–6. doi: 10.1016/0091-3057(82)90129-0. [DOI] [PubMed] [Google Scholar]
- Lindskog M, Svenningsson P, Pozzi L, Kim Y, Fienberg AA, Bibb JA, Fredholm BB, Nairn AC, Greengard P, Fisone G. Involvement of DARPP-32 phosphorylation in the stimulant action of caffeine. Nature. 2002;418:774–8. doi: 10.1038/nature00817. [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–92. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA. Circadian rhythms, the mesolimbic dopaminergic circuit, and drug addiction. ScientificWorldJournal. 2007;7:194–202. doi: 10.1100/tsw.2007.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A. 2005;102:9377–81. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng QJ, Logunova L, Maywood ES, Gallego M, Lebiecki J, Brown TM, Sladek M, Semikhodskii AS, Glossop NR, Piggins HD, Chesham JE, Bechtold DA, Yoo SH, Takahashi JS, Virshup DM, Boot-Handford RP, Hastings MH, Loudon AS. Setting clock speed in mammals: the CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron. 2008;58:78–88. doi: 10.1016/j.neuron.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido T, Akiyama M, Moriya T, Shibata S. Sensitized increase of period gene expression in the mouse caudate/putamen caused by repeated injection of methamphetamine. Mol Pharmacol. 2001;59:894–900. doi: 10.1124/mol.59.4.894. [DOI] [PubMed] [Google Scholar]
- Nishi A, Snyder GL, Greengard P. Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J Neurosci. 1997;17:8147–55. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet CC, Miller PE, Hemmings HC, Jr, Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J Neurosci. 1984;4:111–24. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AA, Verbitsky M, Suresh R, Kamens HM, Reed CL, Li N, Burkhart-Kasch S, McKinnon CS, Belknap JK, Gilliam TC, Phillips TJ. Gene expression differences in mice divergently selected for methamphetamine sensitivity. Mamm Genome. 2005;16:291–305. doi: 10.1007/s00335-004-2451-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- Phillips TJ, Kamens HM, Wheeler JM. Behavioral genetic contributions to the study of addiction-related amphetamine effects. Neurosci Biobehav Rev. 2008;32:707–759. doi: 10.1016/j.neubiorev.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Blau J, Rothenfluh A, Abodeely M, Kloss B, Young MW. double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell. 1998;94:83–95. doi: 10.1016/s0092-8674(00)81224-6. [DOI] [PubMed] [Google Scholar]
- Price MA. CKI, there's more than one: casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev. 2006;20:399–410. doi: 10.1101/gad.1394306. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci. 2000;20:4480–8. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder GL, Fienberg AA, Huganir RL, Greengard P. A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J Neurosci. 1998;18:10297–303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–96. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Takano A, Uchiyama M, Kajimura N, Mishima K, Inoue Y, Kamei Y, Kitajima T, Shibui K, Katoh M, Watanabe T, Hashimotodani Y, Nakajima T, Ozeki Y, Hori T, Yamada N, Toyoshima R, Ozaki N, Okawa M, Nagai K, Takahashi K, Isojima Y, Yamauchi T, Ebisawa T. A missense variation in human casein kinase I epsilon gene that induces functional alteration and shows an inverse association with circadian rhythm sleep disorders. Neuropsychopharmacology. 2004;29:1901–9. doi: 10.1038/sj.npp.1300503. [DOI] [PubMed] [Google Scholar]
- Uz T, Akhisaroglu M, Ahmed R, Manev H. The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology. 2003;28:2117–23. doi: 10.1038/sj.npp.1300254. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–6. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Qaadir A, Palmer AA, Cook EH, Jr, de Wit H. Association between the casein kinase 1 epsilon gene region and subjective response to D-amphetamine. Neuropsychopharmacology. 2006;31:1056–63. doi: 10.1038/sj.npp.1300936. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Hanson GR, Fleckenstein AE. The role of the plasmalemmal dopamine and vesicular monoamine transporters in methamphetamine-induced dopaminergic deficits. J Neurochem. 2007;101:883–8. doi: 10.1111/j.1471-4159.2006.04419.x. [DOI] [PubMed] [Google Scholar]
- Walaas SI, Aswad DW, Greengard P. A dopamine- and cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature. 1983;301:69–71. doi: 10.1038/301069a0. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–92. [PubMed] [Google Scholar]
- Yuferov V, Butelman ER, Kreek MJ. Biological clock: biological clocks may modulate drug addiction. Eur J Hum Genet. 2005;13:1101–3. doi: 10.1038/sj.ejhg.5201483. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Benoit-Marand M, Allen PB, Ingrassia P, Fienberg AA, Gonon F, Greengard P, Picciotto MR. Reduction of cocaine place preference in mice lacking the protein phosphatase 1 inhibitors DARPP 32 or Inhibitor 1. Biol Psychiatry. 2002;51:612–20. doi: 10.1016/s0006-3223(01)01318-x. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Sgambato-Faure V, Sasaki T, Svenningsson P, Berton O, Fienberg AA, Nairn AC, Greengard P, Nestler EJ. Phosphorylation of DARPP-32 at Threonine-34 is required for cocaine action. Neuropsychopharmacology. 2006;31:555–62. doi: 10.1038/sj.npp.1300832. [DOI] [PubMed] [Google Scholar]


