Abstract
Deferoxamine (DFO) is a high-affinity iron chelator approved by the Food and Drug Administration for treating iron overload. Preclinical research suggests that systemically administered DFO prevents and treats ischemic stroke damage and intracerebral hemorrhage. However, translation into human trials has been limited, probably because of difficulties with DFO administration. A noninvasive method of intranasal administration has emerged recently as a rapid way to bypass the blood-brain barrier and target therapeutic agents to the central nervous system. We report here that intranasal administration targets DFO to the brain and reduces systemic exposure, and that intranasal DFO prevents and treats stroke damage after middle cerebral artery occlusion (MCAO) in rats. A 6-mg dose of DFO resulted in significantly higher DFO concentrations in the brain (0.9–18.5 μM) at 30 min after intranasal administration than after intravenous administration (0.1–0.5 μM, p < 0.05). Relative to blood concentration, intranasal delivery increased targeting of DFO to the cortex approximately 200-fold compared with intravenous delivery. Intranasal administration of three 6-mg doses of DFO did not result in clinically significant changes in blood pressure or heart rate. Pretreatment with intranasal DFO (three 6-mg doses) 48 h before MCAO significantly decreased infarct volume by 55% versus control (p < 0.05). In addition, post-treatment with intranasal administration of DFO (six 6-mg doses) immediately after reperfusion significantly decreased infarct volume by 55% (p < 0.05). These experiments suggest that intranasally administered DFO may be a useful treatment for stroke, and a prophylactic for patients at high risk for stroke.
Deferoxamine (DFO) is a high-affinity iron chelator that is approved by the Food and Drug Administration for the treatment of chronic iron overload. In rodent models of ischemic stroke, systemically administered DFO has been shown to reduce stroke damage when administered both before and after middle cerebral artery occlusion (Palmer et al., 1994; Prass et al., 2002; Mu et al., 2005; Freret et al., 2006). In addition, DFO has also been shown to be neuroprotective in rat models of intracerebral and subarachnoid hemorrhage (Wan et al., 2006; Hishikawa et al., 2008), traumatic brain injury (Long et al., 1996), Parkinson's disease (Ben-Shachar et al., 1991; Jiang et al., 2006), and age-induced loss of memory (de Lima et al., 2008). A single clinical trial of DFO for treatment of Alzheimer's disease showed that twice daily intramuscular injections slowed cognitive decline by 50% over 2 years (Crapper McLachlan et al., 1991). The use of DFO for stroke treatment has not been clinically tested, probably because of problems administering DFO systemically including: 1) injection as an intravenous bolus causes acute hypotension, and 2) DFO is rapidly filtered by the kidney and eliminated in the urine (Dragsten et al., 2000).
We have been investigating a novel intranasal drug delivery approach to noninvasively target DFO to the central nervous system (CNS). Intranasal delivery allows drugs that do not readily cross the blood-brain barrier (BBB), to bypass the BBB and be delivered extracellularly to the CNS along the olfactory and trigeminal nerves within minutes (Ross et al., 2004; Thorne et al., 2004; Dhanda et al., 2005; Hanson and Frey, 2008; Thorne et al., 2008). A wide variety of therapeutic agents, including both small and large molecules, are rapidly delivered to the CNS by intranasal delivery and have shown therapeutic effects in both animals and humans. In a rat model of middle cerebral artery occlusion (MCAO), intranasal administration of insulin-like growth factor-1 reduced infarct volume and neurologic deficit (Liu et al., 2004). In a mouse model of Alzheimer's disease, intranasal administration of nerve growth factor largely reversed neurodegeneration and memory loss (Capsoni et al., 2000; DeRosa et al., 2005). In both healthy human adults (Benedict et al., 2004, 2007) and in patients with amnestic mild cognitive impairment or in the early stages of Alzheimer's disease (Reger et al., 2006, 2008), intranasally administered insulin improved memory. Advantages of intranasal administration of DFO include targeting of the CNS, reducing systemic exposure with possible side effects, and the noninvasive method of administration.
The aim of this study was to test the effects of intranasally administered DFO on ischemic brain damage in the rat to determine whether it is a viable noninvasive alternative to systemic injections for the treatment and prevention of stroke. We show that intranasally administered DFO targeted the CNS and was neuroprotective when administered both before and after induction of ischemia.
Materials and Methods
Experimental Design. This study was conducted as four separate experiments. First, to determine the concentration and distribution of intranasally administered DFO reaching the CNS, anesthetized rats received one 6-mg i.n. or i.v. dose of a solution of DFO with a small portion bound to 59Fe and were euthanized at 25 min after the onset of administration. After perfusion, tissues were dissected and DFO concentrations calculated by use of tissue weight, scintillation counting, and the specific activity of standards. Second, the effects of intranasally administered DFO on physiologic parameters were examined after three treatments with either 10% intranasally administered DFO (6 mg per dose) or intranasally administered water (vehicle control) at 3-h intervals. Mean blood pressure and heart rate were monitored under anesthesia by a catheter in the right femoral artery. Third, to assess the effect of intranasal DFO pretreatment on infarct size, rats were pretreated with three doses of either 10% intranasally administered DFO (6 mg per dose) or water (vehicle control) under anesthesia at three h intervals 48 h before MCAO. Rats were euthanized 5 days after the surgery, brain slices were stained with 2,3,5-triphenyltetrazolium chloride (TTC) as described below, and infarct volume was calculated correcting for edema. Two additional pretreatment regimens were tested including a single dose of 10% DFO (6 mg) and three doses of 3% DFO (1.8 mg per dose). Last, to determine the effect of intranasal DFO posttreatment effect on infarct size, rats were dosed with either 10% intranasally administered DFO (6 mg per dose) or intranasally administered water (vehicle control) under anesthesia immediately after reperfusion and at 2 and 4 h after reperfusion. At 24 h after MCAO these same rats received three additional doses of intranasally administered DFO at 3-h intervals and were euthanized 48 h after MCAO. Infarct volumes were determined as described for the pretreatment study.
Animals. Experiments were conducted in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals using male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA). Pentobarbital anesthesia (sodium pentobarbital, 50 mg/kg) was used for the terminal biodistribution study. For measurement of physiologic parameters and effects on infarct volume with DFO treatment, surgery and intranasal drug delivery were performed under isoflurane anesthesia (4% for induction, 1.5–2.0% for maintenance, in 70% nitrous oxide and 30% oxygen; Baxter, McGaw Park, IL). For all studies, body temperature was controlled (0.5°C by a feedback-regulating heating pad (Harvard Apparatus, Holliston, MA) connected to a rectal probe and set at 37°C.
Assessment of DFO Concentrations in the CNS. Anesthetized rats were placed on their back and the descending aortas were cannulated for collection of blood samples during dosing and to facilitate perfusion. For intranasal administration (n = 10), a solution containing an average of 6 mg of DFO (9.3 μmol) with 36 μCi of 59Fe (0.02 μmol 59FeCl3, PerkinElmer NEZ037; PerkinElmer Life and Analytical Sciences, Waltham, MA) was administered as ten 6-μl drops for a total volume of 60 μl, alternating nares with 2 min between drops (i.e., five drops per nare). For intravenous administration (n = 8), the femoral vein was cannulated, and a solution containing an average of 6 mg of DFO (9.1 μmol) with 40 μCi of 59Fe (0.022 μmol) in a total of 0.5 ml was infused over 14 min (the same duration as intranasal administration). In both formulations, DFO was dissolved in 25 mM sodium acetate buffer and equal volumes of 59FeCl3 and 0.5 M NaOH were added. Because DFO was present at a 465-fold excess above 59Fe and the binding affinity (Kd) of DFO for iron is 1031, it is likely that all of the iron remained bound. At 25 min after the onset of intranasal or intravenous administration, rats were perfused with 60 ml of saline followed by 360 ml of 4% paraformaldehyde at a rate of 15 ml/min with use of a syringe pump.
Dura mater was collected as bone was chipped away and the intact brain was removed from the skull. After olfactory bulbs were detached, six 2-mm coronal slices were made with use of a brain matrix. Anatomical regions, including the anterior olfactory nucleus, frontal cortex, parietal cortex, striatum, septal nucleus, hippocampus, and thalamus hypothalamus, were dissected from the slices. The remaining unsliced tissue was divided into midbrain, pons, medulla, and cerebellum. The spinal cord was dissected away from the body and divided into cervical, thoracic, and lumbar sections after the dura was removed. Three sets of lymph nodes were collected including superficial and deep cervical lymph nodes from the neck and the axillary nodes from the armpits. Samples of peripheral tissue collected included liver, kidney, muscle, heart, and lung. All tissues were weighed and placed in vials with scintillation fluid; 59Fe-DFO was quantified with a scintillation counter (Beckman Coulter, Fullerton, CA). Concentrations of DFO were calculated based on tissue weights and levels of 59Fe-DFO reaching the brain by use of the specific activity of known standards.
The mean and standard deviation of the concentration of each tissue were calculated. Outliers were defined as values outside two standard deviations of the mean and removed from analysis. The data from one entire rat in the intravenous group was removed from analysis because the blood concentration indicated that the femoral vein infusion failed. The larger number of animals in the intranasal group was the result of attempting autoradiographic image analysis of brain tissue in two rats, which did not yield usable images. Differences in mean DFO concentrations between the intravenous and intranasal groups for each tissue type were determined by t tests, performed with PROC MULTTEST in SAS 9.1 (SAS Institute, Cary, NC), using the bootstrap method of p value correction for multiple comparisons. Targeting ratios, an indicator of direct delivery from the nose to the brain versus delivery from the nose to the blood to the brain, were calculated by dividing the ratio of DFO concentrations in each brain tissue to the DFO concentration in blood after intranasal administration by the same ratio after intravenous administration.
Measurement of Physiologic Parameters after DFO Treatment. Catheters were inserted into the right femoral artery of anesthetized rats (n = 6). Blood pressure was monitored through a Harvard Apparatus blood pressure transducer. Under anesthesia, three doses of either 60 μl of 10% DFO (6 mg per dose) or intranasally administered water (vehicle control) were administered at 3-h intervals. Each rat received both treatments in a randomized crossover design on 2 consecutive days. Mean blood pressure was recorded via hand entry every 15 s. Body temperature was controlled ±0.5°C by a feedback-regulating heating pad (Harvard Apparatus) connected to a rectal probe and set at 37°C. In a separate group of two rats, a DSI telemetric pressure-sensing transmitter (TL11M2-C50-PXT; Data Sciences International, St. Paul, MN) was implanted 5 days before dosing in the abdominal aorta for monitoring of blood pressure and heart rate during three doses of intranasally administered DFO. Mean blood pressure and heart rate were captured electronically via DSI computer software. Descriptive statistics (mean, standard error) were calculated and overall predose and postdose differences (averaged over all rats, treatments, and treatment sequences) were analyzed by a t test with pooled variance by use of SAS v 9.1. Deferoxamine (a gift from Biomedical Frontiers, Minneapolis, MN) was dissolved as a 10% solution (w/v) in distilled water with a final pH of 4.65. Each 60-μl dose was administered as 6-μl drops with a pipette while occluding the opposite naris, waiting 2 min between drops and alternating nares. Animals were returned to their cages between doses.
MCAO Model. Rats (259–300 g) were anesthetized with isoflurane and MCAO was performed as described previously (Longa et al., 1989). To occlude the middle cerebral artery, an incision on the neck was made, and the left common carotid artery was exposed. The occipital and superior thyroidal branches of the external carotid artery (ECA) were electrocauterized, and the pterygopalatine branch of the internal carotid artery was ligated by using a 6-O silk suture. A 3-O poly(l-lysine)-coated nylon suture with a round tip [the tip of the suture was rounded by heating before coating with poly(lysine)] was then inserted from the proximal ECA, pushed to the bifurcation of the external and internal carotids, and inserted into the internal carotid artery (Belayev et al., 1996). The suture was then further advanced into the middle cerebral artery until mild resistance was encountered. The total distance the suture was advanced from the carotid bifurcation into the middle cerebral artery was 20 to 21 mm. The skin incision was closed with a silk suture. At the end of the surgical procedure, animals were allowed to recover and returned to their cages. Sutures remained in place for 120 min. After 120 min, animals were reanesthetized to pull and remove sutures, and to ligate ECAs. After the wounds were closed, animals were returned to their cages for recovery.
Intranasal Treatment Regimens in the MCAO Model. One group of rats was pretreated with three doses of either 10% intranasally administered DFO (6 mg per dose, n = 9) or intranasally administered water (vehicle control, n = 9) under anesthesia at 3-h intervals 48 h before MCAO. Two additional pretreatment regimens were tested in separate groups of rats including a single dose of 10% DFO (6 mg, n = 14) and three doses of 3% DFO (1.8 mg per dose, n = 14), each with an equivalent intranasally administered water control (n = 14). One post-treatment regiment was tested in which a group of rats received six doses of either 10% intranasally administered DFO (6 mg per dose, n = 9) or intranasally administered water (vehicle control, n = 9) under anesthesia. The doses were administered at the following times: immediately after reperfusion and at 2, 4, 22, 25, and 28 h after reperfusion. Larger sample sizes were chosen for the two lower-dose pretreatment groups in anticipation of a reduced effect size. Each intranasal dose was given under anesthesia and consisted of 60 μl administered as 6-μl drops with a pipette while occluding the opposite naris, waiting 2 min between drops, and alternating nares.
Neurologic Assessment. The neurologic function of animals was assessed at 60 and 120 min after MCAO to confirm a behavioral deficit. The severity of neurologic deficit was evaluated by use of a four-point scoring system, as described previously (Bederson et al., 1986), while the animal was suspended by its tail. The measurement was repeated at 120 h after MCAO before euthanasia. An additional assessment at 24 h was made only in the post-treatment group. Comparisons between intranasal DFO and control groups were made at each time point by use of a two-tailed unpaired Student's t test assuming equal variances.
Analysis of Infarct Volume. Five days or 48 h after MCAO surgery, rats were euthanized, the whole brain removed, and a brain matrix used to make 2-mm sections. Brain slices were stained by use of a 2% TTC (Sigma-Aldrich, St. Louis, MO) solution and were then transferred to a 4% paraformaldehyde solution. Slices were scanned into a computer, and infarct volume was determined by use of the National Institutes of Health imaging program. To minimize the effects of brain edema, the volume of the infarcted hemisphere was normalized to the volume of the contralateral hemisphere. Brain infarct volume was calculated, correcting for edema, as described previously (Selim and Ratan, 2004). In brief, infarct area was multiplied by one minus the difference of ipsilateral and contralateral hemispheres divided by the area of the ipsilateral hemisphere, then the product was multiplied by tissue thickness, resulting in volume. Edema was not individually measured in the cortex and striatum. Total infarct volume, and cortex and striatum infarcts were all corrected according to edema in the entire hemisphere. Animals in which MCAO surgery was not successful, those that displayed subdural hemorrhage on examination of the brain or did not have either an infarct or neurologic deficit, were not included in subsequent analysis. Statistical significance was determined by a two-tailed unpaired Student's t test assuming equal variances. A p value < 0.05 was considered significant.
Results
Intranasal Administration Targets DFO to the CNS. After intranasal administration, high concentrations of 59Fe-DFO were observed in the olfactory bulb and trigeminal nerves demonstrating that intranasally administered DFO travels to the CNS along these neural pathways. Based on the levels of 59Fe-DFO detected, one 6-mg dose of intranasally administered DFO (60 μl of 10% solution) resulted in cortical DFO concentrations ranging from 1.5 to 3.4 μM and striatal concentrations of 1.7 μM (Table 1); concentrations in other brain regions ranged from 0.9 to 4.9 μM. A rostral to caudal concentration gradient of DFO was observed in the spinal cord, and systemic exposure was minimal after intranasal administration. In comparison, intravenous administration of a similar dose resulted in DFO concentrations in the brain ranging from only 0.1 to 0.5 μM with high exposure to the blood (44 μM) and organs, such as kidney (156 μM). Intranasal administration of DFO resulted in significantly higher concentrations in tissues of the CNS, and significantly lower concentrations in blood and peripheral tissues (p < 0.05). Intranasal delivery increased targeting of DFO to all CNS tissues with the targeting ratios, concentrations in brain tissue relative to concentrations in blood, ranging from 86 to 949.
TABLE 1.
DFO concentrations (mean μM ± S.E.) 25 min after intranasal (n = 7–10) or intravenous (n = 6–7) administration of DFO (6 mg, 40 μCi) to anesthetized rats
| Tissue | Intranasala | Intravenousa | Targeting Ratiob |
|---|---|---|---|
| Brain tissue | |||
| Trigeminal nerve | 20.5 ± 2.8 | 5.5 ± 0.9* | 104 |
| Olfactory bulb | 18.5 ± 2.6 | 0.54 ± 0.07** | 949 |
| Anterior olfactory nucleus | 4.9 ± 0.6 | 0.25 ± 0.05** | 536 |
| Frontal cortex | 3.4 ± 0.7 | 0.35 ± 0.11# | 271 |
| Parietal cortex | 1.5 ± 0.3 | 0.25 ± 0.06** | 173 |
| Striatum | 1.7 ± 0.2 | 0.14 ± 0.07** | 332 |
| Septal nucleus | 2.2 ± 0.2 | 0.39 ± 0.10** | 156 |
| Hippocampus | 1.0 ± 0.1 | 0.19 ± 0.05** | 141 |
| Thalamus | 1.1 ± 0.1 | 0.18 ± 0.04** | 174 |
| Hypothalamus | 4.7 ± 0.6 | 0.55 ± 0.09** | 241 |
| Midbrain | 1.3 ± 0.1 | 0.28 ± 0.06** | 125 |
| Pons | 1.8 ± 0.2 | 0.34 ± 0.06** | 145 |
| Medulla | 1.8 ± 0.2 | 0.35 ± 0.05** | 141 |
| Cerebellum | 0.91 ± 0.10 | 0.29 ± 0.06** | 86 |
| Spinal cord | |||
| Cervical | 1.2 ± 0.4 | 0.54 ± 0.10 | 62 |
| Thoracic | 0.22 ± 0.06 | 0.54 ± 0.13 | 11 |
| Lumbar | 0.09 ± 0.01 | 0.68 ± 0.14** | 3.8 |
| Dura mater | |||
| Spinal dura | 1.9 ± 0.4 | 10.0 ± 2.8 | 5.2 |
| Dorsal dura | 16.3 ± 3.6 | 5.8 ± 1.2 | 77 |
| Ventral dura | 42.3 ± 6.9 | 5.1 ± 0.7** | 232 |
| Lymph nodes | |||
| Superficial | 1.1 ± 0.2 | 22.2 ± 4.4* | 1.3 |
| Cervical | 30.6 ± 7.0 | 14.1 ± 2.3 | 60 |
| Axillary | 0.46 ± 0.07 | 13.7 ± 3.4# | 0.9 |
| Peripheral tissues | |||
| Blood at 25 min | 1.6 ± 0.1 | 43.7 ± 8.4** | 1.0 |
| Liver | 0.37 ± 0.02 | 2.1 ± 0.4* | 5.0 |
| Kidney | 0.63 ± 0.10 | 156.2 ± 46.3# | 0.1 |
| Muscle | 0.43 ± 0.05 | 4.6 ± 1.0* | 2.6 |
| Heart | 0.52 ± 0.14 | 2.2 ± 0.3* | 6.7 |
| Lung | 1.0 ± 0.2 | 3.5 ± 0.6* | 8.2 |
Differences in mean concentrations between intravenous and intranasal administration for each tissue were determined by t tests, performed with PROC MULTTEST in SAS 9.1, using the bootstrap method of p value correction for multiple comparisons
, p < 0.10
, p < 0.05
, p < 0.01
Targeting ratio is calculated as the ratio of tissue concentration to blood concentration after intranasal administration divided by the same ratio after intravenous administration
Measurement of Physiologic Parameters with Intranasal DFO Treatment. Mean arterial blood pressure increased 0.89 mm Hg after intranasally administered DFO (predose, 99.98 ± 0.38 mm Hg; postdose, 100.88 ± 0.24 mm Hg; n = 6, p = 0.02) and by 0.39 mm Hg after intranasally administered water (predose, 101.97 ± 0.22 mm Hg; postdose, 102.36 ± 0.14 mm Hg; n = 6, p = 0.13). Heart rate increased by 1.2% after intranasally administered DFO (predose, 424.7 ± 1.7 beats/min; postdose, 429.7 ± 0.8 beats/min; n = 2, p = 0.045) and decreased by 0.6% after intranasally administered water (predose, 427.0 ± 2.9 beats/min; postdose, 425.3 ± 1.1 beats/min; n = 2, p = 0.74).
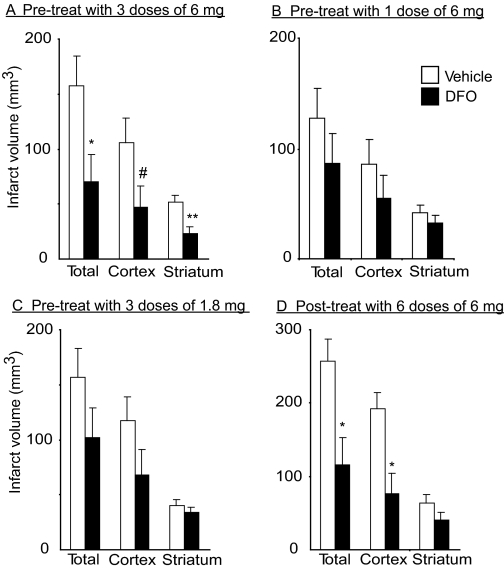
Neuroprotective Effect of Intranasal DFO Pretreatment. In the absence of pretreatment with intranasally administered DFO (in rats treated with vehicle control: intranasal water, n = 9), transient MCAO for 120 min caused extensive infarction of the ipsilateral cortex and striatum (Fig. 1A) as assessed 5 days after reperfusion. In rats pretreated with three doses of intranasally administered DFO 48 h before surgery, there was a notably smaller infarction (n = 9; Fig. 1B). The total, cortical, and striatal infarct volumes (mean ± S.E.) in intranasally water-treated rats were 157 ± 27, 106 ± 22, and 52 ± 6 mm3, respectively, versus 71 ± 25, 47 ± 19, and 23 ± 6 mm3, respectively, in intranasally DFO-pretreated rats (mean ± S.E.). This represents a 55% decrease in total infarct volume (p = 0.03), a 55% decrease in cortical infarct volume (p = 0.06), and a 55% decrease in striatal infarct volume (p = 0.005) (Fig. 2A). Two additional pretreatment dosing regimens were examined: a single 6-mg dose of 10% DFO administered intranasally 48 h before surgery (n = 14; control, n = 12), and three 1.8-mg doses of 3% DFO administered intranasally at 3-h intervals starting 48 h before surgery (n = 14; control, n = 14). Although there was a trend for these additional intranasal DFO treatment regimens to reduce infarct volume (Fig. 2, B and C), the effects of these regimens were not found to be statistically significant. Two animals, one from each treatment group, were excluded from analysis because they had no infarct and no neurologic deficit. There were no significant effects of edema with any of the intranasal DFO pretreatment dosing regimens.
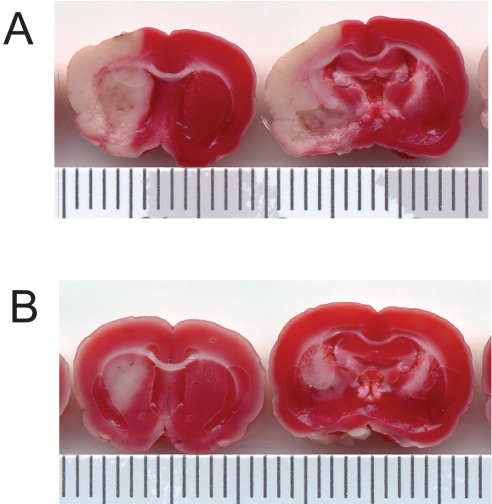
Fig. 1.
Pretreatment with three 6-mg intranasal doses of 10% DFO under anesthesia 48 h before middle cerebral artery occlusion significantly reduces infarct volume at 5 days after reperfusion. Tissue slices were stained with TTC and areas of infarction appear white. A, pretreatment with intranasally administered water control (n = 9). B, pretreated with intranasally administered DFO (n = 9).
Fig. 2.
Intranasal DFO treatment reduces total, cortical, and striatal infarct volume at 5 days or 48 h after middle cerebral artery occlusion (mean ± S.E.). Intranasal doses were administered under anesthesia. A, pretreatment with three 6-mg intranasal doses of 10% DFO (n = 9) compared with intranasally administered water control (n = 9). B, pretreatment with one 6-mg intranasal dose of 10% DFO (n = 14) compared with intranasally administered water control (n = 12). C, pretreatment with three 1.8-mg intranasal doses of 3% DFO (n = 14) compared with intranasally administered water control (n = 14). D, post-treatment with six 6-mg intranasal doses of 10% DFO (n = 9) compared with intranasally administered water control (n = 6). p values from Student's unpaired t test with #, p < 0.10, *, p < 0.05, and **, p < 0.01.
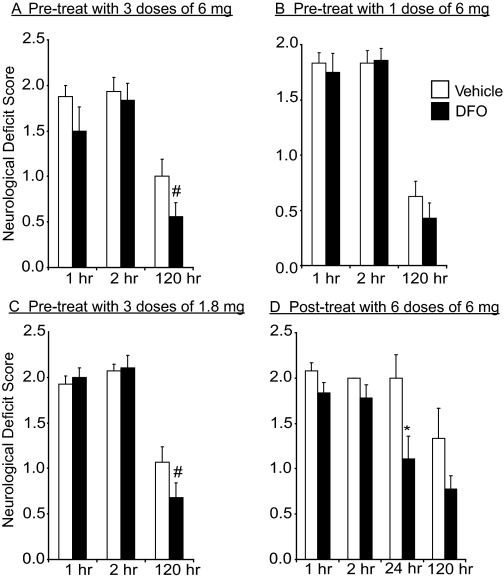
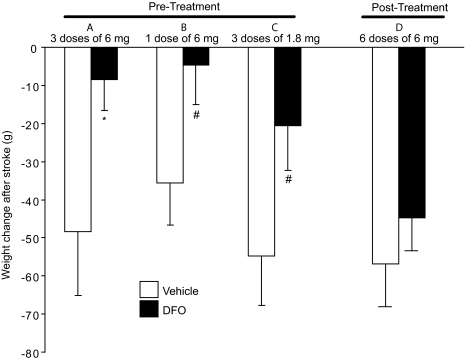
In addition to a reduction in infarct volume, a trend of improved neurologic deficit at 120 h after MCAO was observed with intranasal DFO pretreatment (Fig. 3, A–C). Animals treated with both three-dose pretreatment regimens (6 and 1.8 mg) showed improved function (p = 0.09 and p = 0.10, respectively). A significant effect on postischemic weight loss in rats pretreated with intranasal DFO was also observed compared with controls (Fig. 4A). Vehicle-treated rats lost an average of 48 ± 17 g in the 5 days after surgery as opposed to intranasal-DFO-treated rats (three 6-mg doses) that only had an average weight loss of 8 ± 9g (p = 0.048). This represents an 83% difference in weight loss following stroke. There was also a trend of reduced weight loss after MCAO for the two additional pretreatment dosing regimens (Fig. 4, B and C; p = 0.06 for both).
Fig. 3.
Intranasal DFO treatment reduces neurologic deficit score at 5 days or 48 h after middle cerebral artery occlusion (mean ± S.E.). Intranasal doses were administered under anesthesia. A, pretreatment with three 6-mg intranasal doses of 10% DFO (n = 9) compared with intranasally administered water control (n = 9). B, pretreatment with one 6-mg intranasal dose of 10% DFO (n = 14) compared with intranasally administered water control (n = 12). C, pretreatment with three 1.8-mg intranasal doses of 3% DFO (n = 14) compared with intranasally administered water control (n = 14). D, post-treatment with six 6-mg intranasal doses of 10% DFO (n = 9) compared with intranasally administered water control (n = 6). p values from Student's unpaired t test with #, p < 0.10 and *, p < 0.05.
Fig. 4.
Intranasal DFO treatment reduces postischemic weight loss at 5 days or 48 h after middle cerebral artery occlusion (mean ± S.E.). Intranasal doses were administered under anesthesia. A, pretreatment with three 6-mg intranasal doses of 10% DFO (n = 9) compared with intranasally administered water control (n = 9). B, pretreatment with one 6-mg intranasal dose of 10% DFO (n = 14) compared with intranasally administered water control (n = 12). C, pretreatment with three 1.8-mg intranasal doses of 3% DFO (n = 14) compared with intranasally administered water control (n = 14). D, post-treatment with six 6-mg intranasal doses of 10% DFO (n = 9) compared with intranasally administered water control (n = 6). p values from Student's unpaired t test with #, p < 0.10 and *, p < 0.05.
Neuroprotective Effect of Intranasal DFO Posttreatment. Post-treatment with intranasally administered 10% DFO, beginning at the time of reperfusion, significantly reduced infarct volume, with a predominantly cortical effect (Fig. 2D). The total, cortical, and striatal infarct volumes in intranasally water-treated rats (n = 6) were 257 ± 31, 193 ± 21, and 64 ± 11 mm3, respectively, versus 116 ± 36, 76 ± 28, and 40 ± 10 mm3, respectively, in intranasally DFO posttreated rats (n = 9). This represents a 55% decrease in total infarct volume (p = 0.017) and a 60% decrease in cortical infarct volume (p = 0.01). There was a 37% decrease in striatal infarct volume, but this was not found to be statistically significant (p = 0.15). Three rats in the intranasal water group were not included in the analysis: two animals died immediately after reperfusion and one animal died at 24 h after MCAO. No significant effect of post-treatment with DFO was found on edema, neurologic deficit, or weight (Figs. 3D and 4D).
Discussion
We have demonstrated that intranasally administered DFO, a high-affinity iron chelator, targets the CNS and reduces infarct volume after MCAO in rats. Intranasal administration of DFO resulted in significantly higher CNS concentrations and significantly reduced systemic exposure compared with intravenous administration (p < 0.05). Both pretreatment with intranasally administered DFO, 48 h before MCAO, and post-treatment with intranasally administered DFO immediately after reperfusion after MCAO provided significant protection from ischemic damage. In the highest pretreatment group (18 mg total), not only was total infarct volume significantly reduced (55%), but infarct volumes in both the cortex (55%) and striatum (55%) were significantly reduced. The level of efficacy reported here with intranasally administered DFO (18 mg) is greater than previous reports demonstrating 22% and 35% reduction of infarct volume with 100 mg i.p. of DFO pretreatment administered 48 h and 72 h before MCAO, respectively (Prass et al., 2002). This increased efficacy is consistent with improved targeting of DFO to the brain with intranasal administration. Although statistically significant changes in mean arterial blood pressure (+0.89 mm Hg) and heart rate (+1.2%) were noted with intranasally administered DFO, the small changes are not clinically meaningful. Intranasal treatment with DFO beginning immediately after reperfusion (2 h after MCAO) also resulted in a significant reduction in total (55%) and cortical (60%) infarct volume, although the striatum was not significantly protected and it will be important to further define the window of opportunity for intranasal DFO treatment in future studies. In addition to reducing infarct volume, both intranasal DFO pretreatment and post-treatment significantly improved neurologic deficit and pretreatment prevented weight loss after stroke. These results suggest that intranasal administration of DFO is a promising noninvasive alternative to systemic administration for the treatment of ischemic stroke.
Disturbances in iron homeostasis have been linked to neuronal damage after ischemic brain injury. An abundance of preclinical data supports a pathogenic relationship between increased iron and ischemic neuronal injury (Selim and Ratan, 2004). Despite a large body of evidence indicating that dysregulation of brain iron metabolism and transport plays a role in mediating neuronal damage and cell death in ischemic stroke, only a handful of studies have addressed the clinical relevance of iron and its toxicity in patients with ischemic stroke. With its high affinity for iron (Kd = 1031), over 40 years of clinical use, ability to be used for long periods of time with manageable side effects (Kontoghiorghes, 1995), and evidence of neuroprotection in preclinical studies of ischemic stroke, DFO is a strong candidate for human trials.
Several mechanisms underlying the neuroprotective functions of DFO have been proposed. These include chelation of the small fraction of unbound iron (the labile iron pool) responsible for catalyzing the production of reactive oxygen species and the subsequent modulation of gene expression, including the hypoxia-inducible factor (HIF-1α) pathway (Mu et al., 2005; Baranova et al., 2007). Systemically administered DFO elevated HIF-1α protein levels and vascular endothelial growth factor mRNA levels, and attenuated vasospasm and reduction of blood flow after subarachnoid hemorrhage in rats (Hishikawa et al., 2008). In a rat model of cerebral ischemia, the neuroprotective effect of pretreatment with systemically administered DFO was accompanied by an increase in synthesis of HIF-1α and erythropoietin (Li et al., 2008). Future mechanistic studies of intranasally administered DFO should also include investigation of lipid and protein oxidation levels and reductions in tissue peroxynitrite generated because of endothelial and neuronal generation of nitric oxide in ischemic stroke. We are currently examining the effects of intranasally administered DFO on gene and protein expression, which will be reported separately because these studies are extensive.
Although stroke is one of the leading causes of death and disability in the United States, the options for therapeutic intervention are limited. Only approximately 5% of stroke patients meet the criteria for treatment with tissue plasminogen activator (Grotta et al., 2001), the only approved drug treatment for ischemic stroke, which must be administered intravenously within 3 h. Recently, percutaneous clot removal by device has emerged as a treatment option for patients in which the interval for thrombolytic therapy has elapsed (Stead et al., 2008). For hemorrhagic stroke, treatment by surgical evacuation is considered, depending on the size of the hemorrhage (Manno et al., 2005). Deferoxamine is a good candidate for the treatment of stroke for several reasons. Unlike with the use of pharmacologic thrombolysis, the underlying cause of stroke may not need to be determined before treatment with DFO. DFO does not increase the risk of bleeding, and preclinical studies using systemic delivery of DFO showed benefit for treatment of both hemorrhagic and ischemic stroke. Although the one intranasal DFO post-treatment dosing regimen equivalent to 110 mg/kg reported in this study to reduce total infarct volume by 55% when administered at the time of reperfusion (2 h after MCAO) does not adequately define the potential therapeutic window for intranasal DFO treatment of stroke in humans, previous reports of systemically administered DFO indicate a large therapeutic window for treatment of stroke. Injection of 300 mg/kg s.c. DFO was reported to reduce infarct volume by 20% when administered 24 h after MCAO (Freret et al., 2006) and injection of 100 mg/kg i.p. DFO 3 days after MCAO decreased infarct volume by 32% (Li et al., 2008). Limitations of this study include the rigor of measurement of intranasal DFO effects on neurologic function and physiologic parameters; additional investigations are warranted. In addition, the model of ischemia used here, temporary MCAO, mimics thrombolysis with reperfusion, and proven efficacy of intranasally administered DFO in a permanent MCAO model (i.e., without reperfusion) would further justify use in stroke in humans.
The neuroprotective effects of pretreatment with DFO observed in this study and others suggest it may be a noninvasive prophylactic treatment for patients at high risk for stroke, including those that have previously had a stroke, experienced a transient ischemic event, had cardiac surgery, or had atrial fibrillation. For example, coronary artery bypass graft (CABG) surgery is performed on more that 800,000 patients worldwide each year, and many of these procedures are associated with neurologic complications that range from stroke (1–5% of patients) to general cognitive impairment (more than 30% of patients) (Mark and Newman, 2002). Although rodents subjected to global forebrain ischemia (four-vessel occlusion), would be a better model of human CABG to examine the effects of intranasally administered DFO, pretreatment with systemically administered DFO protects against focal ischemia with global hypoxia in neonatal rodents (Palmer et al., 1994; Mu et al., 2005). DFO may also offer potential cardiac protection from ischemic insult because DFO (100 mg/kg) administered intraperitoneally has been shown to reduce ischemia-reperfusion-induced myocardial infarction in rat heart (Tang et al., 2008), although intranasal administration resulted in four times lower concentrations in the heart compared with intravenous administration.
Intranasal administration of DFO provides many advantages over systemic administration. In general, DFO is not injected intravenously for two reasons. First, it is a small molecule and is eliminated rapidly through the kidney, with a plasma half-life that is less than 10 min in humans (Mahoney et al., 1989; Dragsten et al., 2000). Second, the injection of an intravenous bolus can cause acute hypotension that is rapid and can be lethal (Mahoney et al., 1989). Subcutaneous and intramuscular injections of DFO are often associated with local injection site reactions. These negative attributes of DFO have limited its utility as a neuroprotective agent. However, intranasal administration is a viable alternative for the delivery and targeting of DFO to the brain. Intranasal delivery allows drugs that do not readily cross the BBB, to bypass the BBB and be rapidly delivered to the CNS along the olfactory and trigeminal nerves (Frey et al., 1997; Dhanda et al., 2005). In addition, it directly targets drugs that do cross the BBB to the CNS, eliminating the need for systemic delivery and thereby reducing unwanted systemic side effects, such as those manifested by DFO. Although there is some controversy in the field of the bioavailability of intranasally administered drugs (Merkus et al., 2003), this is mainly a result of key evidence being omitted from consideration because of experimental variables such as dosing volume; when all data reports are included, the majority indicate direct delivery of a wide range of molecules from the nose to the brain. In this study, we have demonstrated that, compared with intravenous administration, intranasal administration of DFO increased targeting to the frontal cortex by 271-fold.
The notable neuroprotection attained by intranasally administered DFO in rodents has great relevance to patients with neurologic disorders and patients at risk for stroke; for example, patients undergoing any type of vascular surgery. Further studies will be needed to determine the mechanisms of action through which DFO conveys neuroprotection and the optimal dose and timing of administration. Given the large number of patients with stroke that do not qualify for thrombolytic therapy and those at risk for stroke, such as patients undergoing CABG surgery or other types of vascular surgery, the findings in this study are particularly relevant.
Acknowledgments
We thank Jared Fine for assistance with figures and critical review of this manuscript before submission for publication, and Christopher Anderson for assistance with statistical analysis.
This work was supported by the National Institutes of Health [Grant R21-NS04761401A1] and by the Alzheimer's Research Center.
L.R.H., A.R., W.H.F., and S.S.P. have filed a patent related to the findings discussed in this article.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.149807.
ABBREVIATIONS: DFO, deferoxamine; MCAO, middle cerebral artery occlusion; CNS, central nervous system; BBB, blood-brain barrier; HIF-1α, hypoxia-inducible factor; TTC, 2,3,5-triphenyltetrazolium chloride; ECA, external carotid artery; CABG, coronary artery bypass graft.
References
- Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, and Chavez JC (2007) Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci 27 6320-6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, and Bartkowski H (1986) Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17 472-476. [DOI] [PubMed] [Google Scholar]
- Belayev L, Alonso OF, Busto R, Zhao W, and Ginsberg MD (1996) Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke 27 1616-1623. [DOI] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, and Kern W (2004) Intranasal insulin improves memory in humans. Psychoneuroendocrinology 29 1326-1334. [DOI] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Schmitz K, Schultes B, Ratter F, Fehm HL, Born J, and Kern W (2007) Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology 32 239-243. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar D, Eshel G, Finberg JP, and Youdim MB (1991) The iron chelator desferrioxamine (Desferal) retards 6-hydroxydopamine-induced degeneration of nigrostriatal dopamine neurons. J Neurochem 56 1441-1444. [DOI] [PubMed] [Google Scholar]
- Capsoni S, Ugolini G, Comparini A, Ruberti F, Berardi N, and Cattaneo A (2000) Alzheimer-like neurodegeneration in aged antinerve growth factor transgenic mice. Proc Natl Acad Sci U S A 97 6826-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapper McLachlan DR, Dalton AJ, Kruck TP, Bell MY, Smith WL, Kalow W, and Andrews DF (1991) Intramuscular desferrioxamine in patients with Alzheimer's disease. Lancet 337 1304-1308. [DOI] [PubMed] [Google Scholar]
- de Lima MN, Dias CP, Torres JP, Dornelles A, Garcia VA, Scalco FS, Guimarães MR, Petry RC, Bromberg E, Constantino L, et al. (2008) Reversion of age-related recognition memory impairment by iron chelation in rats. Neurobiol Aging 29 1052-1059. [DOI] [PubMed] [Google Scholar]
- De Rosa R, Garcia AA, Braschi C, Capsoni S, Maffei L, Berardi N, and Cattaneo A (2005) Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD11 anti-NGF transgenic mice. Proc Natl Acad Sci U S A 102 3811-3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanda DS, Frey WH II, Leopold D, Kompella UB (2005) Nose-to-brain delivery: Approaches for drug deposition in the human olfactory epithelium. Drug Del Tech 5 64-72. [Google Scholar]
- Dragsten PR, Hallaway PE, Hanson GJ, Berger AE, Bernard B, and Hedlund BE (2000) First human studies with a high-molecular-weight iron chelator. J Lab Clin Med 135 57-65. [DOI] [PubMed] [Google Scholar]
- Freret T, Valable S, Chazalviel L, Saulnier R, Mackenzie ET, Petit E, Bernaudin M, Boulouard M, and Schumann-Bard P (2006) Delayed administration of deferoxamine reduces brain damage and promotes functional recovery after transient focal cerebral ischemia in the rat. Eur J Neurosci 23 1757-1765. [DOI] [PubMed] [Google Scholar]
- Frey WH II, Liu J, Chen X, Thorne RG, Fawcett JR, Ala TA, and Rahman Y-E (1997) Delivery of 125I-NGF to the brain via the olfactory route. Drug Delivery 4 87-92. [Google Scholar]
- Grotta JC, Burgin WS, El-Mitwalli A, Long M, Campbell M, Morgenstern LB, Malkoff M, and Alexandrov AV (2001) Intravenous tissue-type plasminogen activator therapy for ischemic stroke: Houston experience 1996 to 2000. Arch Neurol 58 2009-2013. [DOI] [PubMed] [Google Scholar]
- Hanson LR and Frey WH II (2008) Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci 9 (Suppl 3): S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishikawa T, Ono S, Ogawa T, Tokunaga K, Sugiu K, and Date I (2008) Effects of deferoxamine-activated hypoxia-inducible factor-1 on the brainstem after subarachnoid hemorrhage in rats. Neurosurgery 62 232-241. [DOI] [PubMed] [Google Scholar]
- Jiang H, Luan Z, Wang J, and Xie J (2006) Neuroprotective effects of iron chelator Desferal on dopaminergic neurons in the substantia nigra of rats with iron-overload. Neurochem Int 49 605-609. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes GJ (1995) Comparative efficacy and toxicity of desferrioxamine, deferiprone and other iron and aluminium chelating drugs. Toxicol Lett 80 1-18. [DOI] [PubMed] [Google Scholar]
- Li YX, Ding SJ, Xiao L, Guo W, and Zhan Q (2008) Desferoxamine preconditioning protects against cerebral ischemia in rats by inducing expressions of hypoxia inducible factor 1 alpha and erythropoietin. Neurosci Bull 24 89-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XF, Fawcett JR, Hanson LR, and Frey WH II (2004) The window of opportunity for treatment of focal cerebral ischemic damage with noninvasive intranasal insulin-like growth factor-I in rats. J Stroke Cerebrovasc Dis 13 16-23. [DOI] [PubMed] [Google Scholar]
- Long DA, Ghosh K, Moore AN, Dixon CE, and Dash PK (1996) Deferoxamine improves spatial memory performance following experimental brain injury in rats. Brain Res 717 109-117. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, and Cummins R (1989) Riversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20 84-91. [DOI] [PubMed] [Google Scholar]
- Mahoney JR Jr, Hallaway PE, Hedlund BE, and Eaton JW (1989) Acute iron poisoning. Rescue with macromolecular chelators. J Clin Invest 84 1362-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno EM, Atkinson JL, Fulgham JR, and Wijdicks EF (2005) Emerging medical and surgical management strategies in the evaluation and treatment of intracerebral hemorrhage. Mayo Clin Proc 80 420-433. [DOI] [PubMed] [Google Scholar]
- Mark DB and Newman MF (2002) Protecting the brain in coronary artery bypass graft surgery. JAMA 287 1448-1450. [DOI] [PubMed] [Google Scholar]
- Merkus P, Guchelaar HJ, Bosch DA, and Merkus FW (2003) Direct access of drugs to the human brain after intranasal drug administration?. Neurology 60 1669-1671. [DOI] [PubMed] [Google Scholar]
- Mu D, Chang YS, Vexler ZS, and Ferriero DM (2005) Hypoxia-inducible factor 1 alpha and erythropoietin up-regulation with deferoxamine salvage after neonatal stroke. Exp Neurol 195 407-415. [DOI] [PubMed] [Google Scholar]
- Palmer C, Roberts RL, and Bero C (1994) Deferoxamine posttreatment reduces ischemic brain injury in neonatal rats. Stroke 25 1039-1045. [DOI] [PubMed] [Google Scholar]
- Prass K, Ruscher K, Karsch M, Isaev N, Megow D, Priller J, Scharff A, Dirnagl U, and Meisel A (2002) Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro. J Cereb Blood Flow Metab 22 520-525. [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Frey WH II, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD, et al. (2006) Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging 27 451-458. [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA, Plymate SR, Cherrier MM, Schellenberg GD, Frey WH II, et al. (2008) Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis 13 323-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross TM, Martinez PM, Renner JC, Thorne RG, Hanson LR, and Frey WH II (2004) Intranasal administration of interferon beta bypasses the blood-brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. J Neuroimmunol 151 66-77. [DOI] [PubMed] [Google Scholar]
- Selim MH and Ratan RR (2004) The role of iron neurotoxicity in ischemic stroke. Ageing Res Rev 3 345-353. [DOI] [PubMed] [Google Scholar]
- Stead LG, Gilmore RM, Bellolio MF, Rabinstein AA, and Decker WW (2008) Percutaneous clot removal devices in acute ischemic stroke: a systematic review and meta-analysis. Arch Neurol 65 1024-1030. [DOI] [PubMed] [Google Scholar]
- Tang WH, Wu S, Wong TM, Chung SK, and Chung SS (2008) Polyol pathway mediates iron-induced oxidative injury in ischemic-reperfused rat heart. Free Radic Biol Med 45 602-610. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Hanson LR, Ross TM, Tung D, and Frey WH II (2008) Delivery of interferon-beta to the monkey nervous system following intranasal administration. Neuroscience 152 785-797. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Pronk GJ, Padmanabhan V, and Frey WH II (2004) Delivery of insulin-like growth factor-1 to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 127 481-496. [DOI] [PubMed] [Google Scholar]
- Wan S, Hua Y, Keep RF, Hoff JT, and Xi G (2006) Deferoxamine reduces CSF free iron levels following intracerebral hemorrhage. Acta Neurochir Suppl 96 199-202. [DOI] [PubMed] [Google Scholar]