Abstract
Voiding dysfunctions, including increased voiding frequency, urgency, or incontinence, are prevalent in the postmenopausal population. β3-Adrenergic receptor (β3AR) agonists, which relax bladder smooth muscle, are being developed to treat these conditions. We utilized the rat ovariectomy (OVX) model to investigate the effect of ovarian hormone depletion on bladder function and the potential for β3AR agonists to treat bladder hyperactivity in this setting. OVX increased voiding frequency and decreased bladder capacity by ∼25% in awake rats and induced irregular cystometrograms in urethane-anesthetized rats. Reverse transcription-polymerase chain reaction revealed three βARs subtypes (β1,2,3) in bladder tissue, and immunostaining indicated β3AR localization in urothelium and detrusor. Receptor expression was not different in OVX and SHAM rats. The β3AR agonist selectivity of BRL37344 [(±)-(R*,R*)-[4-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]phenoxy]acetic acid sodium hydrate], TAK-677 [(3-((2R)-(((2R)-(3-chlorophenyl)-2-hydroxyethyl)amino)propyl)-1H-indol-7-yloxy)acetic acid], and FK175 [acetic acid, 2-[[(8S)-8-[[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]-6,7,8,9-tetrahydro-5H-benzocyclohepten-2-yl]oxy], ethyl ester, hydrochloride] was confirmed by examining the relative potency for elevation of cAMP in CHOK1 cells overexpressing the various rat βARs. Intravenous injection of each of the β3AR agonists (0.1–500 μg/kg) in anesthetized rats decreased voiding frequency, bladder pressure, and amplitude of bladder contractions. In bladder strips, β3AR agonists (10-12-10-4 M) decreased baseline tone and reduced spontaneous contractions. BRL37344 (5 mg/kg) and TAK-677 (5 mg/kg) injected intraperitoneally in awake rats decreased voiding frequency by 40 to 70%. These effects were not altered by OVX. The results indicate that OVX-induced bladder dysfunction, including decreased bladder capacity and increased voiding frequency, is not associated with changes in β3AR expression or the bladder inhibitory effects of β3AR agonists. This suggests that β3AR agonists should prove effective for the treatment of overactive bladder symptoms in the postmenopausal population.
Lower urinary tract (LUT) dysfunctions, including increased voiding frequency, urgency, incontinence and nocturia, increase in the elderly population and following menopause (Stewart et al., 2003). These dysfunctions could result from hormonally induced changes in bladder contractile and/or relaxing mechanisms. Bladder contractions are triggered by parasympathetic nerves, which release ACh that in turn activates postjunctional muscarinic receptors (mAChRs) in the detrusor. Bladder relaxation is induced by release of norepinephrine from sympathetic nerves, which activates β-adrenergic receptors (βAR) (Fowler et al., 2008). Although drugs that block mAChRs are presently mainstream therapy for the treatment of overactive bladder (OAB) symptoms, considerable attention is now being focused on βAR agonists as an alternative treatment.
Three subtypes of βARs, β1AR, β2AR, and β3AR, are expressed in bladder of several species, including human and rat (Yamaguchi and Chapple, 2007). β3ARs, which are considered the predominant subtype in human bladder, are coupled to excitatory Gs and inhibitory Gi proteins (Vrydag and Michel, 2007). Muscle relaxation is achieved via stimulation of Gs, which increases cAMP. Activation of large conductance Ca2+-activated K+ channels has also been implicated in the mechanism of action (Frazier et al., 2008). Stimulation of β3ARs using selective agonists, including BRL37344, CL316243, FK175, or YM178 improved bladder function (i.e., decreased voiding frequency) in normal rats and in rats with detrusor overactivity (Fujimura et al., 1999; Woods et al., 2001; Kaidoh et al., 2002; Takasu et al., 2007; Leon et al., 2008). β3AR agonists also relaxed rat bladder smooth muscle strips (Fujimura et al., 1999; Longhurst and Levendusky, 1999; Woods et al., 2001; Takeda et al., 2003). Relaxation of rat detrusor can be mediated by all βARs (Yamaguchi and Chapple, 2007). In tissues where β2ARs and β3ARs are coexpressed, the β2/β3 receptor heterodimer can mediate unique signals (Breit et al., 2004). Furthermore, substantial species selectivity exists for β3AR-mediated ligand binding and signaling between rodent and human (Rozec and Gauthier, 2006). Hence, for pharmacological studies, the selectivity of the ligand for rat β3AR should be verified, particularly when using ligands optimized for human β3AR.
Hormonal status and aging affect bladder structure and function, including axon degeneration (Zhu et al., 2001; Fleischmann et al., 2002) and changes in the cholinergic (Diep and Constantinou, 1999; Yoshida et al., 2007) and adrenergic innervation (Matsubara et al., 2002; Dmitrieva, 2007). Hormonal depletion after ovariectomy (OVX) is associated with increased voiding frequency in awake and anesthetized rats (Diep and Constantinou, 1999; Liang et al., 2002; Dmitrieva, 2007; Yoshida et al., 2007). Bladder strips from OVX and old rats exhibit decreased responsiveness to cholinergic stimulation (Diep and Constantinou, 1999), decreased tetrodotoxin-sensitive ACh release from nerve fibers, and increased tetrodotoxin-insensitive basal and stretch-evoked ACh release from the urothelium (Yoshida et al., 2007). Other studies demonstrated hormonal- and/or age-related alterations in G proteins, βAR density, and/or βAR responsiveness (Nishimoto et al., 1995; Derweesh et al., 2000; Frazier et al., 2006). Increased levels of Gi protein, which inhibits cAMP production, were suggested as an underlying mechanism for the decreased βAR-induced relaxation of the bladder in old (24 months) male Fisher 344 rats (Derweesh et al., 2000). Bladder strips from old (>22 months) Fisher 344 (Nishimoto et al., 1995; Derweesh et al., 2000) or Wistar (Frazier et al., 2006) male rats were less relaxed by the nonselective βAR agonists isoproterenol or norepinephrine and by the selective β3AR agonists (BRL37344 and CGP12177) than bladder strips from young rats. However, other studies found no change in isoproterenol or norepinephrine-induced relaxation of bladder strips from 10- and 30-month-old female Wistar/Rij rats (Lluel et al., 2000). In addition, bladder strips from young (2–3 months) OVX rats were slightly more relaxed by BRL37344 than strips from SHAM animals (Matsubara et al., 2002).
These studies suggest that the influence of age and hormonal changes on βAR pathways in the bladder is poorly defined. Several β3AR agonists are currently in clinical trials for the treatment of OAB (Yamaguchi and Chapple, 2007). Although these trials enroll patients of both genders, the bladders of postmenopausal women may be uniquely influenced by hormonal status. This study investigated the effects of OVX on: 1) β3AR expression in the bladder, 2) voiding pattern in awake and anesthetized rats, 3) detrusor contractility, and 4) the effects of selective β3AR agonists on reflex voiding and detrusor contractility. The results indicate that the OVX-induced increase in voiding frequency and decrease in bladder capacity are not associated with either an alteration in β3AR expression or β3AR agonist-induced suppression of bladder smooth muscle activity in vivo or in vitro, implying a preservation of β3AR function in the postmenopausal state.
Materials and Methods
Experimental Animals. Female Sprague-Dawley rats (retired breeders, RB, 8–9 months old when received, 300–450 g; and virgins (V) 2–3 months old, 200–250g; Harlan, Indianapolis, IN) were used in this study. Care and handling of the animals were in accordance with the University of Pittsburgh Institutional Animal Care and Use Committee.
OVX. OVX and sham (SHAM) surgeries were performed in 9- to 10-month-old RB rats anesthetized with isoflurane (2–4% in O2). The ovaries were removed bilaterally via dorsal incisions of the skin and muscle ∼1 cm lateral to the vertebral column. For sham surgeries, the ovaries were inspected and left in place. The muscle and skin were sutured using silk thread, and rats were given a single injection of antibiotic (ampicillin trihydrate, Polyflex, 100 mg/kg) and returned to their cages. The effects of OVX on the reproductive organs (uterus, fallopian tubes) were assessed during the terminal experiment in each rat. Visual inspection in the OVX animals indicated that the ovaries were missing and that the uterus and the fallopian tubes appeared atrophied, with yellow orange deposits. There was a significant difference between uterine weight of OVX rats compared with SHAM or RB rats (90.8 ± 7.5 mg, n = 11 OVX rats; 217.9 ± 14.2 mg, n = 9 SHAM rats; 195.7 ± 11.8 mg, n = 7 RB rats; p < 0.05 for OVX versus SHAM and p < 0.05 for OVX versus RB; unpaired t tests). The bladder appearance and bladder weight were not different in OVX or SHAM rats (98.7 ± 2.2 mg, n = 10 OVX; 100.3 ± 2.5 mg, n = 10 SHAM; p > 0.05 unpaired t test). However, when normalized to the body weight, the bladder weights of OVX rats were significantly smaller than those of SHAM rats due to larger body weight in OVX rats (0.28 ± 0.005 mg/g, n = 10 OVX rats; 0.32 ± 0.01 mg/g, n = 10 SHAM rats; p < 0.05 unpaired t test; body weights: 347.6 ± 4.6 g, n = 10 OVX rats; 309.2 ± 7.5 g, n = 10 SHAM rats, p < 0.05 unpaired t test).
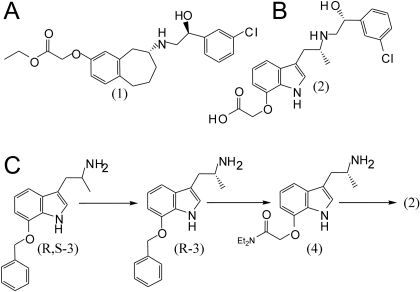
Drugs, Chemistry, and Synthesis of Specific β3AR Agonists. The β3AR agonists FK175 and TAK-677 were synthesized as described below. Other drugs used in this study include the β3AR agonist BRL37344 (Sigma-Aldrich, St. Louis, MO), the β3AR antagonist, SR59230A oxalate salt (Sigma-Aldrich), the β1AR antagonist, atenolol [4-[2′-hydroxy-3′-(isopropylamino)propoxy]phenylacetamide, (±)-4-[2-hydroxy-3-[(1-methylethyl)amino]propoxy]benzeneacetamide; Sigma-Aldrich], the β2AR antagonist ICI 118551 (Sigma-Aldrich) and the nonselective βAR agonist isoproterenol hydrochloride (Sigma-Aldrich). FK175 (1) (Fig. 1A) was synthesized at Girindus America Inc. (Cincinnati, OH), according to previously described methods (Hashimoto et al., 2003), and was stored and handled as a white solid at room temperature. TAK-677 (2) (Fig. 1, B and C) was synthesized using a combination of several published methods. The racemic tryptamine moiety (R,S-3) was synthesized and resolved into the desired individual enantiomer (R-3), according to a previous method (Fujii et al., 2001), and was then successfully converted to the free base (4) using a method described previously (Harada et al., 2004). Ring-opening of (R)-chlorostyrene oxide with the chiral amine, followed by treatment with aqueous NaOH, provided the compound TAK-677 as described previously by the Dainippon Pharmaceuticals Co. (Osaka, Japan) (Harada et al., 2005).
Fig. 1.
Chemical structures of FK175 and TAK-677 and synthesis of TAK-677. A, chemical structure of FK175. B, chemical structure of TAK-677. C, synthesis of TAK-677.
Metabolic Cages Studies. Rats were placed weekly in metabolic cages for 2 to 4 times before ovariectomy and followed weekly or biweekly for 5 to 10 weeks after OVX (rats were 8–9 months old before surgery, 9–10 months old at the time of surgery, and ∼10.5–13.5 months old when sacrificed, with two animals at 15 months of age). The light cycle was from 7:00 AM to 7:00 PM; food and water were provided ad libitum. Drugs or vehicle were administered intraperitoneally just before 7:00 PM under isoflurane anesthesia. Voided urine was collected in cups attached to force displacement transducers (Grass Technologies, Warwick, RI) connected to a computer. Data were recorded for offline analysis using Windaq data acquisition software (DATAQ Instruments Inc., Akron, OH), and analysis was performed using Excel (Microsoft, Redmond, WA). Data were averaged for 24 h and also analyzed for 12-h periods during the day (7:00 AM—7:00 PM) and night (7:00 PM—7:00 AM). Voiding frequency, total voided volume, and volume per void were analyzed. Voiding frequency was calculated as the number of voiding events per hour during 24 h and during the 12-h day and 12-h night periods. Volume per void, which defines bladder capacity, was calculated as an average of the voids occurring during these periods. For each rat, data from two to four measurements in metabolic cages were averaged and taken as one data point. For drug treatment, in preliminary studies, we tested BRL37344 at doses of 0.1, 0.5, 1, 2, and 5 mg/kg i.p. and established that 5 mg/kg had a consistent and significant effect on voiding frequency. Thus, we used this dose for the experiments included in this article. The effect of BRL37344 lasted for 4 to 6 h; therefore, the data were summarized for 4 h after drug or vehicle treatment. TAK-677 was administered at 5 mg/kg i.p. The vehicles used in this study were saline (0.9% NaCl) for BRL37344, 20% DMSO plus 10% β-cyclodextrin in water, or 33% DMSO in saline for TAK-677. For each rat, the drug-induced percentage decrease in voiding frequency in the 4-h interval after drug treatment was calculated relative to the voiding frequency in the 4-h interval after vehicle treatment. Some rats were treated with both drugs, BRL37344 and TAK-677, in different weeks.
Continuous infusion cystometry (CMG) was performed as described previously (Kullmann et al., 2008). Rats were anesthetized with urethane (1–1.2 g/kg s.c.; Sigma-Aldrich). The jugular vein was catheterized with a polyethylene-10 catheter for intravenous drug delivery. The urinary bladder was catheterized through the dome using a polyethylene-50 catheter. The catheter was connected to a pump for saline infusion and to a pressure transducer for bladder pressure recording. Voiding responses were elicited by continuously infusing saline (0.9% NaCl) at a rate of 0.04 ml/min at room temperature (∼22°C). Control CMGs were performed for a period of 1.5 to 2 h before drug application. Drugs dissolved in saline or specific vehicles were administered intravenously in small volumes (100–200 μl) followed by 100 μl of saline to flush the catheter. BRL37344 was dissolved in saline. FK175 and TAK-677 were dissolved in 100% DMSO (Sigma-Aldrich) and subsequently diluted in saline. The final percentage of DMSO was 0.05% for 10 μg/kg drug, 0.5% for 100 μg/kg drug, 2.5% for 250 μg/kg drug, and 5% for 500 μg/kg drug. Saline (100–300 μl; n = 5 rats) and DMSO (0.05–5% for 300 μl; n = 4–8 rats) intravenously did not significantly alter CMG parameters (Fig. 8). Drug-induced dose-response curves were constructed using increasing doses of agonists delivered after three to four voidings occurred during instillation of each dose (total time, ∼50–120 min for each dose). Data were recorded for offline analysis using Windaq, and analysis was performed using Excel and Origin (version 7; OriginLab Corp., Northampton, MA) and Prism 4 (GraphPad Software, Inc., San Diego, CA). The CMG parameters analyzed were: intercontraction interval (ICI; defined as the interval between two voiding episodes), amplitude of contractions (A; defined as the difference between bladder pressure at the peak of the contraction minus baseline bladder pressure), pressure threshold (PTh; defined as the bladder pressure necessary to evoke a voiding contraction), baseline bladder pressure (BP; defined as the lowest bladder pressure just after voiding), and nonvoiding contractions (NVCs; defined as small amplitude, >0.5 cm H2O, bladder contractions before micturition). NVCs were quantified in the last 200 s before micturition contraction using amplitude (threshold set to 0.5 cm H2O) and area under the curve. For each parameter, at least three measurements during the control period and after drug administration were averaged. Data are reported as percentage change relative to control, which was set to 100%. The coefficient of variation of ICIs was calculated as the standard deviation divided by the mean using data from the control period.
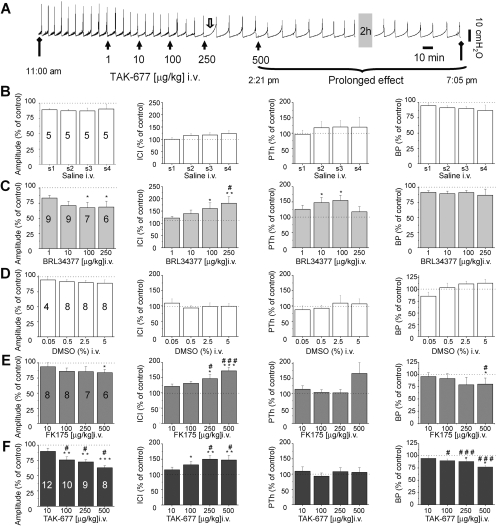
Fig. 8.
β3AR agonists alter the voiding pattern of urethane-anesthetized RB rats. A, examples of the effect of TAK-677 in a RB rat. Small black arrows indicate the time when a dose (actual dose, not cumulative dose) of drug was delivered intravenously. Open arrow indicates increases in PTh, which occurred randomly in most rats. Diamond shape arrows indicate the actual time of the recordings to illustrate the long-lasting effect of the drug. Gray shadow indicates a break of 2 h in the recordings. B, summary of the effects of saline injected intravenously, the vehicle for BRL37344 on CMG parameters. C, summary of the effects of BRL37344 on CMG parameters. D, summary of the effects of DMSO injected intravenously, the vehicle for FK175 and TAK-677 on CMG parameters. E, summary of the effects of FK175 on CMG parameters. F, summary of the effects of TAK-677 on CMG parameters. Numbers on the bar graphs indicate the number of rats tested with a specific dose of the agonist. Percentage of control refers to percentage change of a parameter after drug application relative to the period before drug application. Each rat served as its own internal control. Asterisks indicate statistical significance comparing the effect of a certain concentration of the drug with control using ANOVA followed by Tukey post hoc test. # indicates statistically significant differences between drug and vehicle, tested using unpaired t test.
Bladder strips were prepared as described previously (Birder et al., 2007). The bladder was removed from isoflurane-anesthetized rats, placed in warm aerated Krebs' solution (118 mM NaCl, 4.7 mM KCl, 1.9 mM CaCl2, 1.2 mM MgSO4, 24.9 mM NaHCO3, 1.2 mM KH2PO4, 11.7 mM dextrose, pH 7.4, when aerated with 95% O2, 5% CO2), and cut into three or four longitudinal strips (∼1.5 × 8–10 mm), including urothelium. Strips were tied at each end and mounted in a vertical double-jacketed organ bath (15-ml volume) in aerated Krebs' solution at 37°C. After mounting, the strips were washed several times every 5 to 10 min and allowed to equilibrate for more than 2 h before drug testing. An initial force of 10 mN (1 g) was set as baseline tension, and contractions were measured with a force displacement transducer (Grass, Astromed, RI). Drugs from concentrated stock solutions were directly added to the organ bath every 12 to 15 min. BRL37344 was dissolved in distilled water at 10-2 M and diluted in Krebs' solution to 10-12 to 10-4 M; FK175 and TAK-677 were dissolved in DMSO at 10-2 and 5 × 10-2 M, respectively, and diluted in Krebs' solution to 10-12 to 10-4 M. Vehicles, including saline and DMSO (final concentrations of DMSO were from 1 × 10-9 to 1%), had no significant effects on bladder strip activity (Fig. 10). Data were recorded and analyzed using Windaq and Excel. The parameters analyzed were baseline tone and amplitude of the spontaneous contractions. For determining the effect of a drug on these parameters, a 3-min window was selected at the time when the effect of a drug was maximal or at ∼3 to 4 min after drug application. In this window, four to eight measurements of baseline pressure and amplitude of contractions were averaged and taken as one data point. The threshold for the amplitude of spontaneous activity was set to 0.05 g. Results are reported relative to the values before drug application, which were set to 100%.
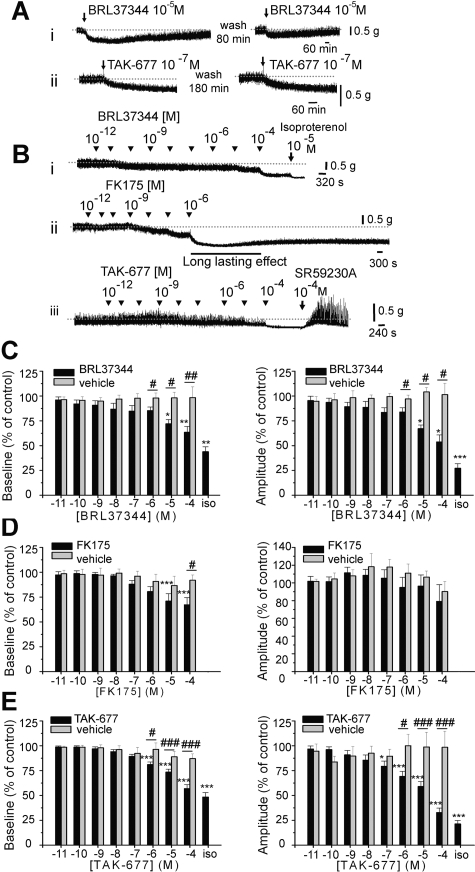
Fig. 10.
β3AR agonists decrease baseline tone and spontaneous activity in bladder strips from RB rats. A, the effects of β3AR agonists BRL37344 (i) and TAK-677 (ii) are long-lasting and repeatable. B, β3AR agonists BRL37344 (i), FK175 (ii), and TAK-677 (iii) decrease baseline tone and amplitude of spontaneous contractions in a concentration-dependent manner. Examples are from control RB rats. Isoproterenol, a nonspecific βAR agonist further decreased the baseline tone and the amplitude of the contractions (i). The effects are reversed by the β3AR antagonist SR59230A (iii). C to E, summary of the effects of β3AR agonists BRL37344 (C; n = 22 strips), FK175 (D; n = 8 strips), and TAK-677 (E; n = 27 strips) on the baseline tone (left panels) and on the amplitude of spontaneous contractions (right panels) in strips from RB rats. Vehicle (saline for BRL37344, n = 9 strips and DMSO, 1 × 10-9 to 1% for FK175, n = 3 strips, and for TAK-677, n = 6 strips) had no effect. Isoproterenol further decreased the baseline tone and the amplitude of the contractions. In C to E, # indicates statistically significant changes comparing the effect of the drug with the effect of the vehicle, using unpaired t test. * indicates statistically significant changes from control tested using ANOVA followed by Tukey-Kramer post hoc test.
qPCR. Whole bladders were collected from 10 OVX rats and 10 SHAM rats, sacrificed 6 weeks after surgery. RNA from each tissue was generated using TRIzol reagent followed by mRNA Catcher PLUS Kit (Invitrogen, Carlsbad, CA). cDNA from each corresponding sample was then generated using 0.5 μg of mRNA and SuperScript III RT (Invitrogen). Approximately 100-ng input cDNA was used for qPCR using custom-made primer sets for β1AR, β2AR, and β3AR (Invitrogen) and the Certified LUX primer set for 18S rRNA FAM (Invitrogen) using Platinum Quantitative PCR SuperMix-UDG (Invitrogen). The sequences of the primers used in this study were: β1AR accession number NM_012701: forward primer, NM_012701.1_198FL, cgccGTATGGGCCTACTCCTGG[FAM]G, and reverse primer, NM_012701.1_198FL/219RU, ATCACCAACACGTTGCCCACT; β2AR accession number NM_012492: forward primer, NM_012492.2_398FL, cggttAAGTTCGAGCGACTACAAAC[FAM]G, and reverse primer, NM_012492.2_398FL/418RU, AGATCAGCACACGCCAAGGAG; β3AR accession number NM_013108: forward primer, NM_013108.1_646FL, cgtaacCACCAACCCTCTGCGTTA-[FAM]G, and reverse primer, NM_013108.1_646FL/679Rua, ACGATCCACACCAGGACTACTGC.
For data analysis, average threshold cycle (Ct) values for all sample sets were calculated, and standard deviation was determined. All primer sets performed at 90% efficiency or greater had an R2 value of 0.99 or greater and showed a single peak in the dissociation curve. Relative expression levels of all three genes in each tissue were compared to that of the housekeeping gene 18S using the ΔCt calculation according to eqs. 1, 2, 3:
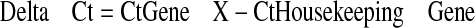 |
(1) |
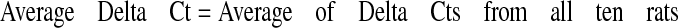 |
(2) |
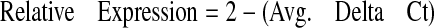 |
(3) |
Relative expression to 18S was averaged for each cohort, and statistical analysis was performed as follows. The RNA expression was first (natural) log-transformed in order to conform to normality assumptions. The estimated geometric means ± S.E. of log-transformed means were calculated, and pairwise comparisons were made. P ≤ 0.05 was considered statistically significant. No multiple comparison adjustment was made. Because standard curves for all βAR primer sets were not identical (Y-intercept values for β1, β2, and β3 were 36.0, 37.4, and 33.5, respectively; data not shown), conclusions about relative receptor levels could not be made.
Binding Studies. To determine Ki values for unlabeled ligands shown in Table 1, Chinese hamster ovary cells (CHOK1) cells were transfected with the rat (r) or human (h) βAR cDNAs using the Lipofectamine 2000 method (5 μg of cDNA/100-mm dish; Invitrogen). Stable clones were isolated for rβ3AR and hβ3AR, and transient rβ1AR and rβ2AR transfectants were generated for subsequent binding studies. On the day after transient transfection of CHOK1 rβ1AR and rβ2AR, cells were plated [15,000 cells/well (c/w) for transients and 40,000 c/w for rβ3AR, hβ3AR stable cells] into 48-well plates and cultured at 37°C, 5% CO2. Whole-cell binding was performed the next day at 4°C for 5 h with 30 to 80 pM [125I]CYP for the β1AR and β2AR or 610 to 940 pM [125I]CYP for the β3AR (GE Healthcare, Piscataway, NJ) in the presence of 1 mM ascorbic acid, 0.5% BSA, 50 mM HEPES, 1 mM CaCl2, and 5 mM MgCl2. Cells were washed three times with ice-cold phosphate-buffered saline. Cell lysates were collected with ice-cold 2% Nonidet P-40/phosphate-buffered saline, and counts were measured using a Wizard gamma counter. Specific binding for rat βARs in CHOK1 cells utilized for immunohistochemical analysis was determined by whole-cell binding using rβ3AR stable cells or transient rβ1AR or rβ2AR transfectants plated at 30,000 c/w in the presence of 36.1 pM [125I]CYP with or without 20 mM isoproterenol. Specific binding for human βARs in CHOK1 cells utilized for immunohistochemical analysis was determined by whole-cell binding using transient hβ1AR, hβ2AR or hβ3AR transfectants plated at 30,000 c/w in the presence of 30 to 80 pM [125I]CYP with or without 20 mM isoproterenol.
TABLE 1.
Binding data for β3AR agonists in CHOK1 cells transfected with rat and human β3AR
|
Ligand
|
Rat β3AR
|
Human β3AR
|
||||
|---|---|---|---|---|---|---|
| Log IC50 ± S.E.M. | Ki | n | Log IC50 ± S.E.M. | Ki | n | |
| M | M | |||||
| Isoproterenol | –4.15 ± 0.506 | 7.09 × 10–5 | 9 | –5.34 ± 0.302 | 4.58 × 10–6 | 7 |
| BRL37344 | –5.60 ± 0.281 | 2.51 × 10–6 | 7 | –6.16 ± 0.211 | 6.95 × 10–7 | 9 |
| TAK-677 | –6.09 ± 0.273 | 8.17 × 10–7 | 8 | –6.89 ± 0.449 | 1.30 × 10–7 | 8 |
| FK175 | –4.97 ± 0.350 | 1.07 × 10–5 | 6 | –6.35 ± 0.215 | 4.46 × 10–7 | 8 |
n, number of experiments
cAMP Assay. Transiently transfected CHOK1 cells with rβ1AR and hβ1AR and stably expressing rβ2AR, hβ2AR, rβ3AR, and hβ3AR CHOK1 cells were used for cAMP studies. The next day after transient transfection (120,000 c/w rβ1AR, and hβ1AR), CHOK1 stable cells (100,000 c/w rβ3AR, hβ3AR, rβ2AR, and rβ2AR) were plated into 24-well plates and cultured at 37°C, 5% CO2. Cells were starved for 30 to 60 min at 37°C in serum-free Dulbecco's modified Eagle's medium/F-12, 0.2% BSA, pretreated at 37°C for 30 min in 500 μM 3-isobutyl-1-methylxanthine in Dulbecco's modified Eagle's medium/F-12 (no phenol red), 0.2% BSA, and 25 mM HEPES, and then stimulated with ligands in the same buffer for 30 min at 37°C. Reactions were stopped, and cAMP was measured using the GE Healthcare Biotrack kit (GE Healthcare Bio-Sciences Corp.).
Immunohistochemistry. Whole bladders were collected from three OVX rats and three SHAM rats (sacrificed 6 weeks after surgery), immediately fixed in formalin (10% neutral buffered formalin; VWR, West Chester, PA), and sent for staining (LifeSpan BioSciences, Seattle, WA). Tissue was embedded in paraffin and sectioned at 4 μm. Because of recently demonstrated lack of specificity of commercially available antisera against the β3AR (Pradidarcheep et al., 2009), we chose to confirm that our antibodies recognized the antigen of interest using a heterologous expression system and to verify that a similar staining pattern was obtained in tissue using multiple antibodies generated against distinct epitopes on the β3AR (Michel et al., 2009). The antibodies used were CH-AB15688 (chicken antibody AB15688; Millipore, Billerica, MA) and LS-A4198 (rabbit polyclonal antibody LS-A4198; MBL International, Woburn, MA). The CH-AB15688 antibody was generated against a synthetic peptide in the carboxyl terminus of the mouse β3AR, whereas the LS-A4198 antibody maps to the NH2 terminus (amino acids 1–20) of the human β3AR. Both antibodies were tested for reactivity and specificity against each of the βARs when overexpressed in CHOK1 cells (Fig. 4; Supplemental Fig. 1; discussed under Results). Titration experiments were conducted to establish concentrations that would result in minimal background and maximal detection of signal. From the serial dilutions of 20, 10, 5, and 2.5 μg/ml, the concentration of 5 μg/ml was selected for the study. After deparaffinization with three changes of xylene (3 min each) and rehydration in a descending ethanol series (100% × 3, 95% × 3, 80% × 3, and distilled H2O for 5 min each), the samples were subjected to antigen retrieval via exposure to sodium citrate (0.01 M, pH 6.0) at boiling point for 20 min. Samples were allowed to stand at room temperature for 20 min before washing in Tris-buffered saline with Tween 20 for 1 min and protein blocking for 20 min in Tris-buffered saline with Tween 20 (Dako North America, Inc., Carpinteria, CA). The principal detection system consisted of a Vector anti-chicken secondary antibody (BA-9010; Vector Laboratories, Burlingame, CA) when the CH-AB15688 antibody was used or a Vector anti-rabbit secondary antibody (BA-1000; Vector Laboratories) when the LS-A4198 antibody was used. This was followed by a Vector ABC-AP kit (AK-5000; Vector Laboratories) with a Vector Red substrate kit (SK-5100; Vector Laboratories), which produced a fuchsia-colored deposit. Tissues were also stained with positive control antibodies (for the adhesion molecule CD31 and for the intermediate filament protein vimentin) to ensure that tissue antigens were preserved and accessible for immunohistochemical analysis. Only tissues that were positive for CD31 and vimentin staining were selected for the remainder of the study. The negative control consisted of performing the entire immunohistochemistry procedure on adjacent sections in the absence of primary antibody. Slides were imaged by LifeSpan BioSciences with a DVC 1310C digital camera coupled to a Nikon microscope and stored as tiff files using Photoshop (Adobe Systems Inc., San Jose, CA).
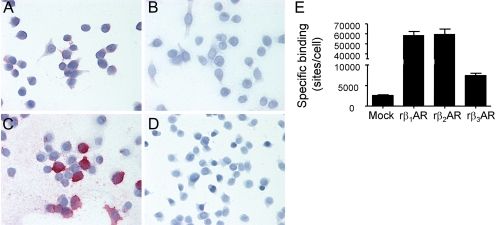
Fig. 4.
Validation of specific staining for the rat β3AR with the CH-AB15688 antibody. A to D, the antibody CH-AB15688 was generated against a synthetic peptide in the mouse COOH terminus. The antibody was used at a concentration of 0.625 μg/ml. Specific staining (fuchsia) was only observed in CHOK1 cells transiently overexpressing the rat β3AR (C), with no staining observed in cells transiently overexpressing rat β1AR (A), or β2AR (B), or in cells transfected with empty vector (D). Magnification: 40×. E, whole-cell binding data using 36.1 pM [125I]CYP in a sample of CHOK1 cells used for IHC-antibody validation studies, transiently transfected with empty vector (Mock), or cDNA for the rat β1AR, β2AR, or β3AR. The data illustrate overexpression of each of the various receptors in these cells, despite the absence of staining by the β3AR antibody. The error bars represent the mean ± S.E.M. of three determinations.
Statistics. Statistical significance was analyzed using paired, unpaired t test or ANOVA followed by Tukey-Kramer Multiple Comparisons Test when appropriate. Values of p < 0.05 were considered significant. Throughout the text, values are expressed as mean ± S.E.M. and *, p < 0.05, **, p < 0.01, and ***, p < 0.001.
Results
Receptor Binding Studies. The affinity of the β3AR agonists BRL37344, FK175, and TAK-677 was tested in binding studies performed in CHOK1 cells transfected with rat or human β3ARs (Table 1). At the human receptor, all three of the β3AR-selective ligands exhibited comparable affinity. In contrast, at the rat receptor, TAK-677 exhibited the highest affinity while FK175 exhibited a substantially lower relative affinity than the other two ligands. Furthermore, all three compounds were less potent at the rat versus the human receptor (Table 1).
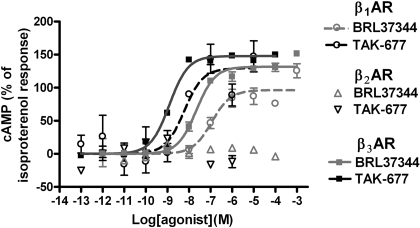
cAMP Studies. The potency and selectivity of the β3AR agonists BRL37344, FK175, and TAK-677 for different βAR subtypes were tested using the cAMP assay in CHOK1 cells transfected with the rat and human β1ARs, β2ARs, and β3ARs, respectively (Table 2). These ligands stimulated the rat and human β3ARs, with a similar potency series, i.e., TAK-677 > BRL37344 > FK175. In the rat, BRL37344 had efficacy at the β3AR that was comparable to that of isoproterenol, whereas TAK-677 was a superagonist at the β3AR relative to isoproterenol, producing >100% efficacy in eight of nine experiments (Table 2). At the human β3AR, BRL37344 was only a partial agonist relative to isoproterenol, whereas TAK-677 maintained full agonist efficacy (Table 2). Both agonists were inactive at the rat β2AR and exhibited ∼10-fold greater selectivity for the β3AR versus β1AR where they exhibited comparable efficacy to isoproterenol (Table 2; Fig. 2). Although all three ligands also exhibit selectivity for the human β3AR versus the other βAR subtypes, there seems to be some residual ability to stimulate the human β2AR, in contrast to what is observed in the rat (Table 2). Although TAK-677 is a partial agonist at the human β1AR (relative to isoproterenol), it seems to be a full agonist at the rat β1AR, albeit with similar relative selectivity for the β3/β1 receptor in both species (Table 2). Despite the differences between the activities at the human and rat βAR subtypes, the data indicate that these ligands have at least 10 times greater selectivity for the β3AR versus the β1AR at the rat receptor in these assays in CHOK1 cells overexpressing βARs.
TABLE 2.
cAMP measurements illustrating the potency and efficacy of β3AR agonists at different rat and human βARs Efficacy of each ligand is expressed relative to that of the nonselective full agonist isoproterenol.
|
Ligand
|
Receptor
|
Rat βAR
|
Human βAR
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Log EC50 ± S.E.M. | EC50 | n | Efficacy (% of Isoproterenol Response ± S.E.M.) | Log EC50 ± S.E.M. | EC50 | n | Efficacy (% of Isoproterenol Response ± S.E.M.) | ||
| BRL37344 | β3AR | –7.39 ± 0.12 | 4.1 ± 10–8 | 11 | 104.9 ± 11.87 | –7.35 ± 0.25 | 4.52 × 10–8 | 6 | 58.45 ± 9.92 |
| β2AR | NR1 | 3 | 31.1 ± 31.1 | –4.95 ± 0.64 | 1.12 × 10–5 | 5 | 80.19 ± 24.26 | ||
| β1AR | –6.17 ± 0.43 | 6.72 × 10–7 | 3 | 110.5 ± 35.65 | –5.29 ± 0.82 NR4 | 5.11 × 10–6 | 4 | 111.54 ± 63.38 | |
| TAK-677 | β3AR | –8.67 ± 0.26 | 2.12 × 10–9 | 9 | 135.57 ± 15.41 | –8.38 ± 0.41 | 4.16 × 10–9 | 6 | 105.58 ± 17.9 |
| β2AR | NR2 | 3 | 0 | –6.67 ± 0.33 | 2.12 × 10–7 | 4 | 71.48 ± 20.21 | ||
| β1AR | –7.83 ± 0.34 | 1.47 × 10–8 | 3 | 134.12 ± 35.20 | –7.05 ± 1.18 NR5 | 8.9 × 10–8 | 3 | 31.99 ± 16.31 | |
| FK175 | β3AR | –6.33 ± 0.29 | 4.71 × 10–7 | 3 | 56.78 ± 24.5 | –6.8 ± 0.66 | 1.57 × 10–7 | 3 | 72.65 ± 20.01 |
| β2AR | NR3 | 2 | 0 | –6.22 | 6.04 × 10–7 | 1 | 137.03 N6 | ||
| β1AR | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | |||
n = number of experiments; NR1, no response in two of three experiments; NR2, no response in three of three experiments; NR3, no response in two of two experiments; NR4, no response in one of four experiments; NR5, no response in one of three experiments; N6, one experiment; N.A., not assessed
Fig. 2.
β3AR agonists increase cAMP in CHOK1 cells. TAK-677 and BRL37344 increase cAMP in cells transfected with rat β3ARs (solid black and gray lines, respectively) in a concentration-dependent manner; they are less potent in cells transfected with rat β1AR (dashed black and gray lines, respectively) and completely ineffective in cells transfected with rat β2AR (empty black and gray triangles, respectively).
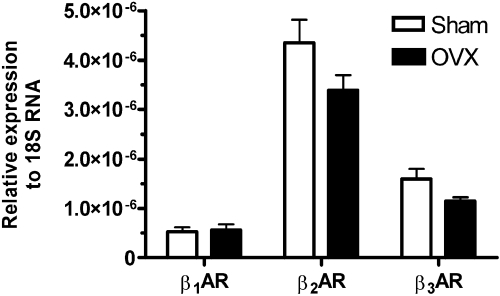
Expression of βARs in Whole Bladder Tissue in OVX and SHAM Rats. To determine which βARs are expressed in the rat bladder tissue and whether OVX alters their expression, we first investigated the mRNA levels of βARs. qPCR indicated the expression of all βARs in whole-bladder tissue from both SHAM and OVX rats (Fig. 3). Because the primer pairs do not lie at exactly the same position on the standard curve, no conclusions can be drawn about the relative abundance of the β1AR versus the β2AR versus the β3AR mRNA expression. The average value of mRNA levels for β2ARs and β3ARs had a decreased trend in OVX rats (p > 0.05).
Fig. 3.
mRNA of β1ARs, β2ARs and β3ARs is expressed in the rat bladder, and the expression is not altered by ovariectomy. mRNA expression of β1ARs, β2ARs, and β3ARs in bladder tissue from SHAM (n = 10 rats; white bars) and OVX (n = 10 rats; black bars) rats. Expression levels, which are relative to 18S, were not significantly different in SHAM and OVX tissue (for β1ARs p > 0.05, for β2ARs p = 0.058 and for β3ARs p = 0.075; unpaired t test).
The protein expression of β3AR was studied using immunohistochemistry. Recent publications have shown that G protein-coupled receptor antibodies lack specificity (Michel et al., 2009; Pradidarcheep et al., 2009). To ensure that the staining we observed is specific for the β3ARs, we used two different antibodies generated against distinct epitopes in the β3AR: CH-AB15688 and LS-A4198 (see Materials and Methods). The specificity of the CH-AB15688 antibody for the rat β3AR was tested in CHOK1 cells overexpressing the individual rat βARs (Fig. 4). The specificity of the LS-A4198 antibody for the human β3AR was similarly validated in the heterologous cell system (Supplemental Fig. 1). The validation data indicated that the anti-β3AR antibodies used in this study did not cross-react with either the β1AR or the β2AR and specifically recognized the rat or human β3AR protein, respectively. The LS-A4198 antibody did not recognize the rat β3AR when overexpressed in CHOK1 cells, despite exhibiting a staining pattern in bladder (Fig. 5; Supplemental Fig. 2) and other tissues (e.g., adipose tissue; data not shown) similar to that obtained with the anti-rat antibody CH-AB15688. The inability of this antibody to recognize the rat receptor in the heterologous system may be an artifact of receptor overexpression, which perhaps obscures the amino-terminal epitope, especially because the β3AR has been demonstrated to form homodimers in a heterologous system (Breit et al., 2004). In addition, it is thought that the β3AR is expressed endogenously at much lower levels in native tissues than in engineered cell lines. This evidence is based on radioligand binding assays, which use a high concentration of nonselective radiotracer, thus making it much more difficult to detect the β3AR levels in native tissues versus the heterologous cell systems (Vrydag and Michel, 2007). Comparison of the staining patterns of the two antibodies in Fig. 5 and Supplemental Fig. 2 illustrates a similar staining pattern throughout the bladder regions, further indicating that the observed staining is specific for the β3AR.
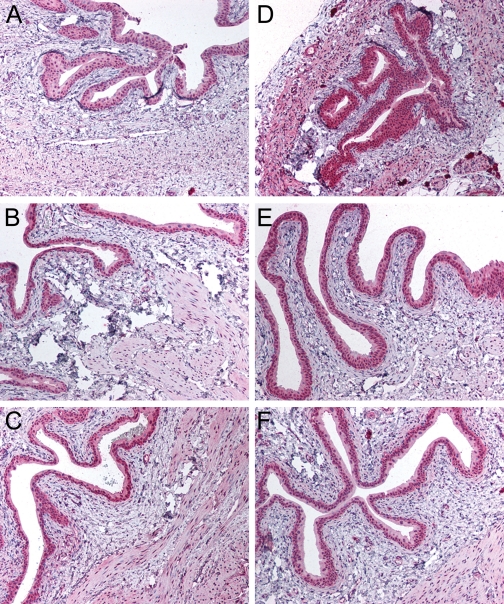
Fig. 5.
Protein expression of the β3ARs in the rat bladder is not changed after OVX (CH-AB15688 antibody). β3AR staining in the bladder trigone (A and D), lateral wall (B and E), or dome (C and F) of a SHAM (A–C) or OVX (D–F) rat is shown. Antibody CH-AB15688 showed strong staining (fuchsia) in the urothelium in all regions of the bladder, whereas faint staining was observed in different areas of the detrusor, with occasional higher intensity staining areas observed in the detrusor in the region near the trigone (D) compared to the lateral wall (E) and dome (F). This occasional regional difference in the detrusor was independent of whether the animal came from a SHAM or OVX group. Staining was not different in the OVX versus SHAM rats. Similar results were observed in two additional rats from each group. All pictures were taken at 10× magnification.
Figure 5 demonstrates that the receptors are present in the urothelium and smooth muscle in all regions of the rat bladder. Qualitatively, the expression appeared uniform in the urothelium in all bladder regions. Within the detrusor, expression occasionally appeared somewhat higher in the trigone versus the other bladder regions (Fig. 5, compare D with F; Supplemental Fig. 2, compare A with C and D with F), but this did not appear to be dependent on hormonal status. It is important to note that no qualitative differences were seen between the OVX and SHAM rats, suggesting that β3AR levels and their tissue distribution in the bladder are preserved upon ovarian hormone depletion.
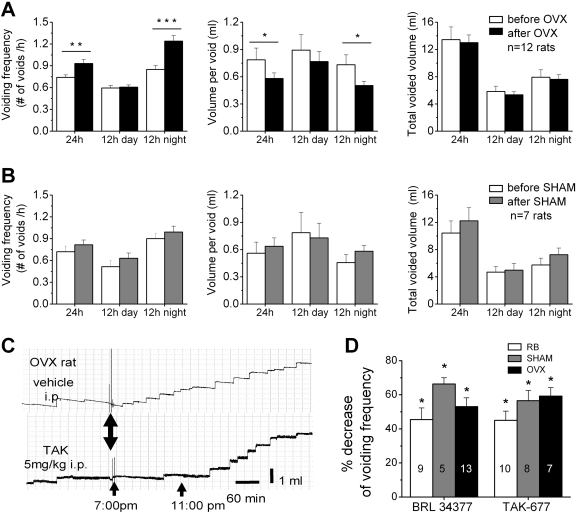
Effects of OVX and β3AR Agonists on Voiding Function in Vivo in Conscious Rats in Metabolic Cages. To determine whether OVX alters the voiding pattern, voiding was monitored in metabolic cages. During 24-h measurements, OVX increased voiding frequency and reduced voided volume by ∼25% without changing the total voided volume (n = 12 rats tested before and after OVX surgery; Fig. 6A). The effects were significant during the 12-h night period when the rats are more active (paired t test p < 0.05) but not significant during 12-h day period. These changes were observed starting at 5 to 6 weeks after OVX. No significant changes were observed in SHAM rats (n = 7 rats tested before and after SHAM surgery; paired t test p > 0.05; Fig. 6B). In preliminary studies, we tested several BRL37344 concentrations (see Materials and Methods) and determined that 5 mg/kg BRL37344 had a significant effect on voiding frequency, clearly distinguished from that of the vehicle (data analyzed by an observer blind to the treatment). Treatment with the β3AR agonists, BRL37344 (5 mg/kg i.p.) and TAK-677 (5 mg/kg i.p.) significantly decreased voiding frequency (by ∼40–70% compared to vehicle treatment; Fig. 6, C and D) in RB, SHAM, and OVX rats in the first 4 h after treatment. In general, the effect was detectable soon after drug injections and lasted for ∼4 to 6 h, after which the voiding frequency returned to control (Fig. 6C). Rats from the three groups (RB, SHAM, and OVX) were equally responsive to both BRL37344 and TAK-677 (ANOVA; p > 0.05).
Fig. 6.
Ovariectomy and β3AR agonists alter the voiding pattern of awake rats in metabolic cages. A, summary of voiding frequency, volume per void, and total voided volume during 24 h, the 12-h day and 12-h night periods, for n = 12 rats tested before OVX (white bars) and after OVX (black bars). Asterisks indicate statistically significant changes (paired t test). B, similar data as in A from n = 7 SHAM rats. C, example of the effects of vehicle (33% DMSO in saline; top trace) and TAK-677 (5 mg/kg; bottom trace) on the voiding pattern of an OVX rat. Double-sided arrow indicates the time at which the treatment was performed (5–15 min before 7:00 PM; noise transients are due to transducer movement when the rat was removed and returned to the cage for treatment). Arrows at 7:00 PM and 11:00 PM indicate the time interval after treatment used for data analysis. D, summary of the effect of BRL37344 (5 mg/kg) and TAK-677 (5 mg/kg) on voiding frequency in RB, SHAM, and OVX rats. Data are summarized for the 4-h interval after 7:00 PM. Numbers inside columns indicate the number of rats in each group. Asterisks indicate statistical significance comparing the effect of the drug with the effect of vehicle using unpaired t test.
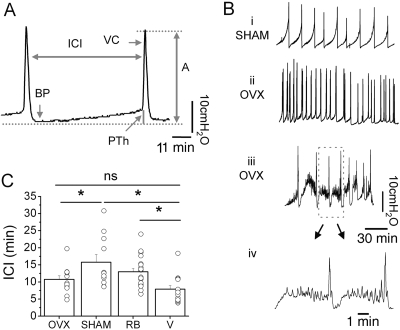
Effects of OVX and β3AR Agonists on Voiding Function in Vivo in Anesthetized Rats during Cystometric Studies. CMG in urethane-anesthetized rats revealed that OVX affected filling and voiding parameters (Fig. 7). OVX rats had significantly greater variability of ICIs than SHAM rats (coefficient of variation of ICIs in OVX versus ICIs in SHAMs were 0.44 ± 0.06 versus 0.26 ± 0.04, unpaired t test, p < 0.05). The ICIs of OVX rats were also significantly shorter than the ICIs of SHAM rats but not significantly different from the ICIs of young (2 months) virgin rats (Fig. 7C; Table 3). The ICIs of SHAM and RB rats were also significantly different from the ICIs of virgin rats (Fig. 7C; Table 3). Other CMG parameters, A, BP, and PTh, were not different between SHAM, OVX, RB, or virgin rats (Table 3). NVCs (amplitude range 0.5–9 cm H2O) that appeared during bladder filling before the micturition contraction were observed in most OVX rats (11 of 13) and in proportion of the SHAM rats (4 of 11) (Fig. 7B and quantification in Fig. 9, F and G).
Fig. 7.
Ovariectomy alters the voiding pattern of urethane-anesthetized rats. A, parameters used for quantifying the effects of drugs on voiding in a typical CMG recording: A, ICI, PTh, and BP. B, examples of voiding patterns recorded in the control period before adding drugs in a SHAM (i) and in two OVX rats (ii, iii, and iv). Note that OVX rats are more irregular (ii) have nonvoiding contractions between voidings (iii, iv), and the ICI is shorter. iv shows an expansion of the dashed area in iii. C, summary of ICIs of OVX (n = 13), SHAM (n = 11), RB (n = 20), and virgin (V; n = 14) rats before drug treatment. Bar graphs show the mean values for each group, and individual symbols show data from individual rats. Asterisks indicate statistically significant difference between the groups indicated in figure (unpaired t test p < 0.05). ns stands for statistically not significant. Comparisons between groups other that those indicated in the figure were not statistically significant.
TABLE 3.
Urodynamic parameters of OVX, SHAM, RB, and virgin (V) rats
| OVX (n = 13) (10–15 mo) | SHAM (n = 11) (10–15 mo) | RB (n = 20) (9–13 mo) | V (n = 14) (2–3 mo) | |
|---|---|---|---|---|
| ICI (s) | 646.07 ± 62.60*,a | 946.53 ± 135.84b | 778.92 ± 59.13b | 473.91 ± 62.69 |
| A (cm H2O) | 25.31 ± 1.19 | 24.10 ± 1.00 | 28.82 ± 2.10 | 26.90 ± 0.84 |
| PTh (cm H2O) | 5.85 ± 0.55 | 6.89 ± 0.81 | 5.02 ± 0.27 | 4.87 ± 0.50 |
| BP (cm H2O) | 4.39 ± 0.51 | 3.02 ± 0.29 | 3.91 ± 0.22 | 3.63 ± 0.29 |
p 0.0458; unpaired t test comparing ICI of OVX with ICI of SHAM rats
p 0.06 when comparing ICIs of OVX with ICIs of virgins rats
< 0.05 unpaired t test when comparing ICI measurements in SHAM rats with virgins rats (p = 0.0025) or RB rats with virgins rats (p = 0.0015)
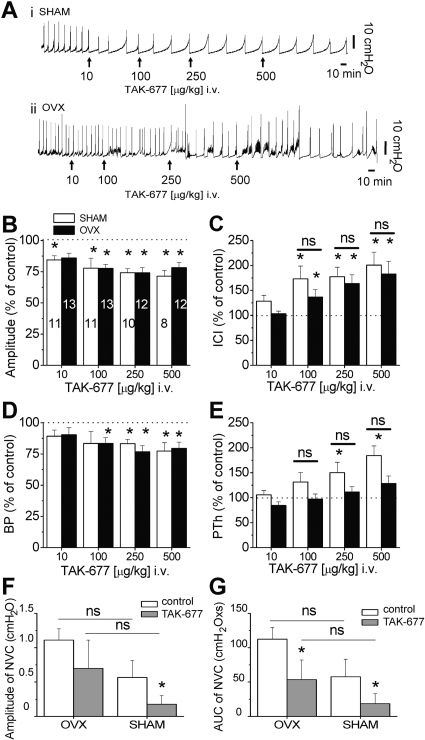
Fig. 9.
Ovariectomy does not change the effects of the β3AR agonist, TAK-677, on voiding in urethane-anesthetized rats. A, examples of the effect of TAK-677 on CMG in SHAM (i) and OVX (ii) rats. Small black arrows indicate the time when the drug was delivered intravenously. The dose indicated is the actual dose delivered (not cumulative). B to D, summary of the effect of TAK-677 on the amplitude of contractions (B), ICI (C), BP (D), and PTh (E) in SHAM (white bars) and OVX (black bars) rats. Numbers on graph bars in B indicate the number of rats tested at each specific dose and are the same for C, D, and E. Asterisks indicate statistical significance, p < 0.05, comparing each parameter before and after drug application, using paired t test. ns indicates no statistically significant differences between data indicated in the figure, using unpaired t test (p > 0.05). F and G, amplitude (F) and area under the curve (G) of nonvoiding contractions in OVX (n = 11) and SHAM (n = 4) rats are reduced by TAK-677 (500 μg/kg). Asterisk indicates statistically significant differences between control and drug using paired t test. ns indicates no statistically significant differences between SHAM and OVX rats using unpaired t test (p > 0.05).
Dose-response curves of β3AR agonists, BRL37344, FK175, and TAK-677, or vehicles (saline and DMSO) injected intravenously were constructed in RB rats (Fig. 8). The vehicles did not significantly alter CMG parameters (Fig. 8, B and D). β3AR agonists increased ICI and decreased A and BP in a dose-dependent manner (Fig. 8, A, C, E, and F). The effect on PTh was variable. In some animals, the drugs increased PTh prominently (Fig. 8A, open arrows); however, because of considerable variability, the effects did not reach statistical significance, except in the cases indicated in Fig. 8. The agonist-induced effects on CMG parameters occurred rapidly (i.e., in the first voiding immediately after drug delivery) and were long-lasting (more than 4 h; Fig. 8A). At 100 μg/kg, no significant differences on A, ICI, and BP were detected among the three agonists; whereas only BRL37344 increased PTh significantly (ANOVA followed by Tukey-Kramer Multiple Comparisons Test, p < 0.05). For TAK-677, the effect on ICI at 250 μg/kg was not different from the effect of 500 μg/kg (Fig. 8F), suggesting that these doses produced a maximal effect and that TAK-677 was more potent than the other two β3AR agonists.
The effects of a range of doses of TAK-677 were also examined in SHAM and OVX rats. In both groups, TAK-677 increased ICI and decreased A and BP in a dose-dependent manner (Fig. 9). TAK-677 increased PTh in a dose-dependent manner in SHAM rats, paralleling the increase in ICI, whereas in OVX rats the effect of the drug was less prominent but still dose-dependent (Fig. 9, C and E). There were no significant differences in the effects of TAK-677 between SHAM and OVX rats for any of the parameters analyzed. β3AR agonists also reduced the NVCs (Fig. 9, F and G). TAK-677 (500 μg/kg) reduced NVCs by ∼50% in OVX rats (amplitude reduced by 45.5 ± 26.9% and area reduced by 56.7 ± 18.6%) and by ∼75% in SHAM rats (amplitude reduced by 70.4 ± 18.8% and area reduced by 70.9 ± 21.5%) (Fig. 9, F and G).
In some control rats (9 of 29 RB; 3 of 11 SHAM) and OVX rats (1 of 13), high doses of β3AR agonists (>100 μg/kg for BRL37344 and for TAK-677 and >250 μg/kg for FK175) increased baseline pressure and decreased ICI. When the bladder was expressed manually, residual urine was observed, indicating impaired voiding. For these rats, data were included in the summary only for the concentrations before voiding impairment occurred. The decreasing numbers of animals with increasing doses of agonists in bar graphs in Figs. 8 and 9 reflect this adjustment. The effect of high doses of β3AR agonists to impair micturition was not explored in further detail.
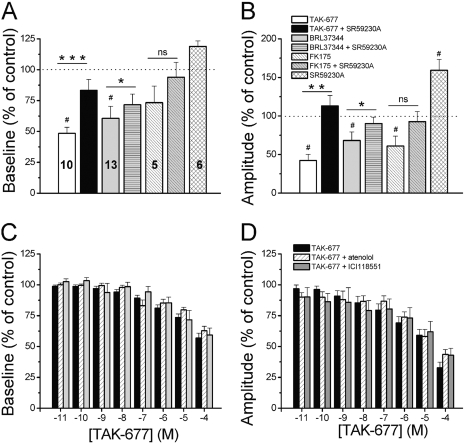
Effects of OVX and β3AR Activation in Bladder Strips in Vitro. To determine whether the observed effects of β3AR agonists are due to the activation of β3ARs in the smooth muscle and/or central nervous system, we performed experiments in bladder strips. β3AR agonists BRL37344, FK175, and TAK-677 decreased baseline tone and amplitude of spontaneous contractions in bladder strips from RB rats, with potency series TAK-677 ≥ BRL37344 > FK175 (Fig. 10). The effects were long-lasting (more than 30 min), repeatable, and concentration-dependent (Fig. 10, A–E). The threshold concentration was 10-6 M for BRL37344 and TAK-677 and 10-5 M for FK175. The effects of TAK-677 were not affected by the presence of the β1AR antagonist, atenolol (100 μM; n = 14 strips), or by the presence of the β2AR antagonist ICI 118551 (1 μM; n = 11 strips) (Fig. 11, C and D). The β3AR antagonist SR59230A (10–100 μM) partially reversed the effects of the agonists on baseline tone and completely reversed the effects of the agonists on the amplitude of the spontaneous contractions. However, it also increased spontaneous activity when administered in the absence of an agonist (Figs. 10Biii and 11, A and B). Application of isoproterenol (100 μM), a nonspecific βAR agonist, in the presence of a maximal concentration of β3AR agonists (100 μM BRL37344 or 100 μM TAK-677) further decreased baseline tone and amplitude of the contractions (Fig. 10, C–E), indicating that, in addition to β3AR, there are other βARs that contribute to smooth muscle relaxation.
Fig. 11.
β3AR but not β1AR and β2AR antagonists affect the β3AR agonist-induced responses in bladder strips. A and B, the β3AR antagonist SR59230A (100 μM) partially reverses the effects of TAK-677, BRL37344, and FK175 (at 100 μM each) on the baseline tone (A) and completely reverses the effects of these β3AR agonists on the amplitude of spontaneous contractions (B). The drug alone also increases the amplitude of contractions. * indicates statistically significant differences between the agonist and the antagonist. # indicates statistically significant differences between the control and agonist. Numbers on bars indicate the number of strips tested for each β3AR agonist. C and D, TAK-677 effects on baseline tone (C) and on the amplitude of contractions (D) were not significantly different (ANOVA; p > 0.05 at all TAK-677 concentrations) in the absence (black bar, same data as in Fig. 10E; n = 27 strips) and in the presence of the β1AR antagonist atenolol (100 μM, white hatched bars; n = 14 strips) or the β2AR antagonist ICI 118551 (1 μM, gray bars; n = 11 strips). The effects of 10-7 to 10-4 M TAK-677 were significantly different from control in all conditions (ANOVA followed by Tukey-Kramer post hoc test).
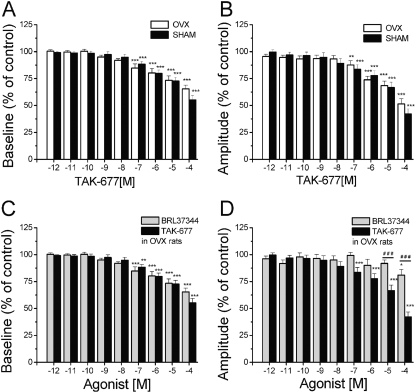
Ovariectomy had no effect on the amplitude of spontaneous muscle contractions (amplitude: 0.41 ± 0.08 g in OVX strips and 0.42 ± 0.06 g in SHAM strips; unpaired t test p > 0.05; n = 22 strips each). Similar to the results in strips from RB rats, TAK-677 and BRL37344 decreased baseline tone and amplitude of spontaneous contractions in OVX and SHAM rats. The drug effects were similar in the two groups (Fig. 12). In general, in strips from all groups, TAK-677 was more potent than BRL37344, especially in suppressing the amplitude of spontaneous contractions (Figs. 10, C and E, and 12).
Fig. 12.
Ovariectomy does not change the effects of β3AR agonists TAK-677 and BRL37344 in bladder strips. A, summary of the effects of TAK-677 on baseline tone in OVX (white bars; n = 25 strips) and in SHAM (black bars; n = 25 strips) rats. B, summary of the effects of TAK-677 on amplitude of spontaneous contractions in OVX (white bars; n = 25 strips) and in SHAM (black bars; n = 25 strips) rats. No statistically significant differences were seen between SHAM and OVX for any of the concentrations tested. C and D, comparisons between the effects of TAK-677 (black bars; n = 25 strips) and the effects of BRL37344 (gray bars; n = 10 strips) on baseline tone (C) and on amplitude of spontaneous contractions (D) in OVX rats. For all panels, * indicate significant changes from control tested using ANOVA followed by Tukey-Kramer post hoc test. # indicates statistically significant changes between the effect of TAK-677 and the effect of BRL37344 tested with unpaired t test.
Discussion
Using the rat ovariectomy model, we demonstrated that hormonal changes increase voiding frequency and decrease bladder capacity in conscious and anesthetized rats without altering the inhibitory effects of β3AR agonists on voiding or on the activity of bladder smooth muscle strips. We further showed that the expression and cellular localization of β3AR protein is maintained throughout the bladder after ovariectomy. These results indicate that, although some functions of the LUT are sensitive to hormonal changes induced by ovariectomy, β3AR-mediated inhibition of the bladder is resistant to these changes.
Estrogen receptors are expressed throughout the LUT, as well as in bladder sensory neurons and in many central nervous system pathways involved in bladder function (Pelletier, 2000; Bennett et al., 2003). Thus, multiple targets and mechanisms may be affected by hormonal changes. In awake middle-aged rats (10–13 months old) in metabolic cages, we detected an ∼25% increase in voiding frequency and a decrease in bladder capacity following OVX (Fig. 6), similar to previous reports (Liang et al., 2002; Yoshida et al., 2007). The effects were significant only during night time when the rats are active, suggesting that if similar changes occur in humans, hormonal depletion would not cause nocturia.
Under urethane anesthesia during CMGs, OVX rats exhibited NVCs during bladder filling (Figs. 7 and 9), resembling detrusor overactivity in spinal cord-injured rats (Cheng and de Groat, 2004), and had shorter ICIs, suggestive of reduced bladder capacity. NVCs are usually encountered in only a small percentage of control rats but can be unmasked or enhanced by intravesical administration of a mAChR agonist, which acts on targets close to the luminal surface of the bladder (Kullmann et al., 2008). This raises the possibility that OVX might facilitate bladder activity by enhancing cholinergic excitatory sensory mechanisms in urothelium or in suburothelial afferent nerves. This idea receives some indirect support from the finding that tetrodotoxin-insensitive basal- and stretch-evoked ACh release from the urothelium is increased in bladder strips from OVX rats (Yoshida et al., 2007).
It is less likely that changes in efferent nerves are responsible for the facilitatory effects of OVX, because other studies demonstrated that bladder strips from OVX rats exhibit decreased responsiveness to muscarinic receptor stimulation (Diep and Constantinou, 1999) or to electrical field stimulation of intramural nerves. The latter effect has been attributed to a decrease in ACh release from nerve fibers (Yoshida et al., 2007). Axonal degeneration in the detrusor, including disrupted axolemma, depleted synaptic vesicles, and disrupted neuronal mitochondria, as well as an ∼25% reduction in the smooth muscle mass at 4 months after OVX in aged (13–14 months) female Fisher rats may account for the reduction in efferent nerve-evoked responses (Zhu et al., 2001; Fleischmann et al., 2002). Taken together, the results suggest that the facilitatory effect of OVX is less likely due to enhancement of the excitatory efferent neurotransmission or increase in bladder smooth muscle excitability but may be related to changes in either peripheral sensory mechanisms (urothelium or afferent nerves) or central neural pathways controlling bladder reflexes.
OVX could also alter β-adrenergic inhibitory mechanisms in the bladder (Matsubara et al., 2002). mRNA of all three types of βARs is expressed in the rat bladder (Fig. 3), as reported in previous studies in several species, including rats and humans (Yamaguchi and Chapple, 2007). However, OVX did not change mRNA levels of any of the βARs in bladder tissue (Fig. 3), nor did it change β3AR protein expression (Fig. 5).
To investigate whether OVX induces changes in β3AR function, we evaluated the effects of three β3AR agonists, BRL37344, FK175, and TAK-677, which were well characterized for receptor selectivity, affinity, and efficacy in binding and cAMP generation in CHOK1 cells overexpressing rat and human βARs (Fig. 2; Tables 1 and 2). Although the affinity of these ligands was higher at the human than at the rat β3AR (Table 1), these ligands stimulated the rat and human β3ARs with a similar potency series, i.e., TAK-677 > BRL37344 > FK175 (Table 2). Comparison of the cAMP data (Table 2) with binding data (Table 1) at rat and human β3ARs indicates that all three ligands stimulated the receptor at considerably lower concentrations than would be predicted by the binding affinity, suggesting that β3AR is activated at low fractional receptor occupancy. On the other hand, the concentrations of β3AR agonists necessary to elicit a significant relaxing effect in bladder strips was at least an order of magnitude higher (10-6 M; Figs. 10, 11, 12). This discrepancy could be related to different methods and/or tissues used for testing the agonists (i.e., CHOK1 cells solely overexpressing β3ARs versus bladder strips containing multiple βARs). Alternatively, cAMP production may not be the only mechanism underlying β3AR agonist-induced inhibition of the bladder (Breit et al., 2004; Frazier et al., 2008). However, in bladder strips, the effects of TAK-677 were not significantly different in the presence of either β1AR or β2AR antagonists (Fig. 11, C and D), suggesting that the ligands are behaving as selective β3AR agonists in rat bladder.
Treatment of awake control and OVX rats with BRL37344 (5 mg/kg) or TAK-677 (5 mg/kg) produced significant and similar reductions in voiding frequency (by ∼40–70%) (Fig. 6). In CMG studies, in anesthetized rats, all β3AR agonists (1–500 μg/kg) significantly reduced NVCs, baseline pressure, voiding frequency, and amplitude of voiding contractions, with no major differences between OVX and SHAM rats (Figs. 8 and 9). The lack of effect of OVX on β3AR inhibition in vivo was confirmed in in vitro experiments in bladder strips where β3AR agonists had similar effects in tissues from OVX and SHAM rats (Fig. 12).
The effect of β3AR agonists on voiding could be attributable in part to a direct action on the smooth muscle, as indicated by the decrease in baseline bladder pressure during CMGs (Figs. 8 and 9) and by the decrease in baseline tone and spontaneous contractions in bladder strips (Figs. 10, 11, 12). In addition, effects on other components of the bladder (i.e., interstitial cells of Cajal or urothelial cells known to modulate the smooth muscle spontaneous contractions) (Hawthorn et al., 2000) are also possible. In bladder strips, isoproterenol had an additional relaxing effect in the presence of high concentrations of TAK-677 (Fig. 10), and reverse transcription-PCR data show the presence of other βAR subtypes (Fig. 3); thus, we cannot rule out the possibility that other βARs may contribute to βAR-mediated bladder relaxation. The inhibitory effects of β3AR agonists on bladder strips were reversed by a β3AR antagonist, SR59230A, indicating that the effects of these agonists were mediated by activation of β3ARs. It is unexpected that SR59230A alone increased the amplitude of contractions (Fig. 11, A and B), raising the possibility that β3ARs are tonically active, possibly due to the spontaneous release of norepinephrine from adrenergic nerves. β3AR agonists could also indirectly affect bladder muscle and influence reflex voiding by acting on urothelium or afferent nerves. β3ARs are highly expressed in the urothelium (Fig. 5), and activation of urothelial βARs releases NO (Birder et al., 2002), which in turn can alter afferent nerve excitability (Yoshimura et al., 2001). Furthermore, urothelium can interact with bladder muscle via the release of inhibitory transmitters (Hawthorn et al., 2000). Further studies are required to determine the precise sites and mechanisms of action of β3AR agonists in the bladder.
β3AR agonists have been effective in reducing voiding not only in normal rats (Woods et al., 2001; Takasu et al., 2007; Leon et al., 2008) but also in various rat models of detrusor overactivity. CL316243 (0.1–100 μg/kg), FK175 (1–10 mg/kg), and YM178 (0.03–3 mg/kg) increased ICI, decreased bladder pressure, and/or decreased detrusor overactivity in CMG studies in spontaneously hypertensive rats (Leon et al., 2008), in rats with bladder outlet obstruction (Woods et al., 2001), and in rats with neurogenic detrusor overactivity following ibotenic acid brain lesions (Fujimura et al., 1999) and cerebral infarction (Kaidoh et al., 2002). Our study adds OVX to this list by demonstrating that OVX-induced changes in voiding pattern can be ameliorated by β3AR agonists. The decrease in the amplitude of bladder contractions after moderate doses of β3AR agonist and the apparent urinary retention after high doses is a potential complication in the use of β3AR agonists, suggesting that the dose of these compounds should be cautiously chosen to avoid effects leading to voiding impairment. Currently, β3AR agonists are under development for the treatment of symptoms of OAB. The recent successful clinical trials with YM178 (Phase II) show that β3AR agonists improve bladder function in patients with OAB without the major side effects usually reported when using antimuscarinics (Chapple et al., 2008).
In summary, our results indicate that OVX facilitates voluntary and reflex voiding. These effects are not accompanied by a change in β3AR receptor expression in the bladder or a decrease in β3AR-mediated inhibition of bladder activity. From a clinical perspective, these results suggest that the hormonal changes that occur in the postmenopausal female population should neither decrease the efficacy nor alter the potency of β3AR agonists in the treatment of OAB symptoms.
Supplementary Material
Acknowledgments
We thank members of W. C. de Groat laboratory and Dr. Karl-Erik Andersson for valuable discussions. We also thank the staff of Life-Span BioSciences for excellent technical assistance with the IHC samples.
This work was supported in part by Procter & Gamble Pharmaceuticals, Incorporated; the American Urology Association [Research Scholar Award] (to F.A.K.); and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK49430].
Preliminary findings have been published in abstract form: Kullmann FA, Limberg BJ, Shah M, Contract D, Downs TR, Wos JA, Rosenbaum JS, and de Groat WC (2008) Effects of beta3 adrenergic receptor activation in rat urinary bladder after ovariectomy; 2008 Society for Neuroscience Meeting; 2008 Nov 15–19; Washington, DC. Society for Neuroscience, Washington, DC.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.155010.
ABBREVIATIONS: LUT, lower urinary tract; βAR, β-adrenergic receptor; OAB, overactive bladder; OVX, ovariectomy; SHAM, sham surgery; RB, retired breeders; SD, Sprague-Dawley; CMG, continuous infusion cystometry; ICI, intercontraction interval; IHC, immunohistochemistry; A, amplitude of contractions; BP, baseline bladder pressure; PTh, pressure threshold; CHOK1, Chinese hamster ovary cells; NVC, nonvoiding contraction; ACh, acetylcholine; mAChR, muscarinic acetylcholine receptor; BRL37344, (±)-(R*,R*)-[4-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]-amino]propyl]phenoxy]acetic acid sodium hydrate; TAK-677, (3-((2R)-(((2R)-(3-chlorophenyl)-2-hydroxyethyl)amino)propyl)-1H-indol-7-yloxy)acetic acid; SR59230A, oxalate salt, 3-(2-ethylphenoxy)-1[(1S)-1,2,3,4-tetrahydronaphth-1-ylamino]-(2S)-2-propanol oxalate; ICI 118551, (±)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol hydrochloride; CL316243, disodium 5-[(2R)-2-[[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]-1,3-benzo dioxole-2,2-dicarboxylate; YM178, (R)-2-(2-aminothiazol-4-yl)-4′-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl} acetanilide; CGP12177, 4-[3-[(1,1-dimethylethyl)-amino]-2-hydroxypropoxy]-1,3-dihydro-2H-benzimidazol-2-one hydrochloride; DMSO, dimethyl sulfoxide; BSA, bovine serum albumin; PCR, polymerase chain reaction; r, rat; h, human; ANOVA, analysis of variance; c/w, cells/well; V, virgin; FK-175, acetic acid, 2-[[(8S)-8-[[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]-6,7,8,9-tetrahydro-5H-benzocyclohepten-2-yl]oxy], ethyl ester, hydrochloride; FAM, fluorescein amidite; [125I]CYP, iodocyanopindolol; qPCR, quantitative polymerase chain reaction.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
References
- Bennett HL, Gustafsson JA, and Keast JR (2003) Estrogen receptor expression in lumbosacral dorsal root ganglion cells innervating the female rat urinary bladder. Auton Neurosci 105 90-100. [DOI] [PubMed] [Google Scholar]
- Birder L, Kullmann FA, Lee H, Barrick S, de Groat W, Kanai A, and Caterina M (2007) Activation of urothelial transient receptor potential vanilloid 4 by 4α-phorbol 12,13-didecanoate contributes to altered bladder reflexes in the rat. J Pharmacol Exp Ther 323 227-235. [DOI] [PubMed] [Google Scholar]
- Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, Apodaca G, and Kanai AJ (2002) Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci 22 8063-8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit A, Lagacé M, and Bouvier M (2004) Hetero-oligomerization between β2- and β3-adrenergic receptors generates a β-adrenergic signaling unit with distinct functional properties. J Biol Chem 279 28756-28765. [DOI] [PubMed] [Google Scholar]
- Chapple CR, Yamaguchi O, Ridder A, Liehne J, Carl S, Mattiasson A, Aramburu MAL, Lucas M, and Everaert K (2008) Clinical proof of concept study (BLOSSOM) shows novel β3 adrenoceptor agonist YM178 is effective and well tolerated in the treatment of symptoms of overactive bladder, in The 2008 European Association of Urology Conference; 2008 March 26–29; Milan, Italy. Poster no. 674, European Association of Urology, Arnhem, The Netherlands.
- Cheng CL and de Groat WC (2004) The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp Neurol 187 445-454. [DOI] [PubMed] [Google Scholar]
- Derweesh IH, Wheeler MA, and Weiss RM (2000) Alterations in G-proteins and β-adrenergic responsive adenylyl cyclase in rat urinary bladder during aging. J Pharmacol Exp Ther 294 969-974. [PubMed] [Google Scholar]
- Diep N and Constantinou CE (1999) Age dependent response to exogenous estrogen on micturition, contractility and cholinergic receptors of the rat bladder. Life Sci 64 PL279-PL289. [DOI] [PubMed] [Google Scholar]
- Dmitrieva N (2007) Increased alpha1-adrenergic activity in the rat bladder by depletion of ovarian hormones. J Urol 178 2677-2682. [DOI] [PubMed] [Google Scholar]
- Fleischmann N, Christ G, Sclafani T, and Melman A (2002) The effect of ovariectomy and long-term estrogen replacement on bladder structure and function in the rat. J Urol 168 1265-1268. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, and de Groat WC (2008) The neural control of micturition. Nat Rev Neurosci 9 453-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier EP, Peters SL, Braverman AS, Ruggieri MR Sr, and Michel MC (2008) Signal transduction underlying the control of urinary bladder smooth muscle tone by muscarinic receptors and beta-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol 377 449-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier EP, Schneider T, and Michel MC (2006) Effects of gender, age and hypertension on beta-adrenergic receptor function in rat urinary bladder. Naunyn Schmiedebergs Arch Pharmacol 373 300-309. [DOI] [PubMed] [Google Scholar]
- Fujii A, Fujima Y, Harada H, Ikunaka M, Inoue T, Kato S, and Matsuyama K (2001) A scaleable synthesis of (R)-3-(2-aminopropyl)-7-benzyloxyindole via resolution. Tetrahedron: Asymmetry 12 3235-3240. [Google Scholar]
- Fujimura T, Tamura K, Tsutsumi T, Yamamoto T, Nakamura K, Koibuchi Y, Kobayashi M, and Yamaguchi O (1999) Expression and possible functional role of the beta 3-adrenoreceptor in human and rat detrusor muscle. J Urol 161 680-685. [PubMed] [Google Scholar]
- Harada H, Fujii A, Odai O, and Kato S (2004) Process development of a scaleable route to (2R)-[3-(2-aminopropyl)-1H-indol-7-yloxy]-N,N-diethylacetamide: a key intermediate for AJ-9677, a potent and selective human and rat β3-adrenergic receptor agonist. Organic Process Res Dev 8 238-245. [Google Scholar]
- Harada H, Hirokawa Y, Suzuki K, Hiyama Y, Oue M, Kawashima H, Kato H, Yoshida N, Furutani Y, and Kato S (2005) Discovery of a novel and potent human and rat beta3-adrenergic receptor agonist, [3-[(2R)-[[(2R)-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]-1H-indol-7-yloxy]acetic acid. Chem Pharm Bull (Tokyo) 53 184-198. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Itoh N, Okamoto T, Ieda S, Kanda A, Baba Y, Ishibashi N, and Okawa K (2003), inventors; Fujisawa Pharmaceutical Company, LTD., assignee. Process for producing benzocycloheptene derivative. World Patent WO03035600A1. 2003.
- Hawthorn MH, Chapple CR, Cock M, and Chess-Williams R (2000) Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol 129 416-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidoh K, Igawa Y, Takeda H, Yamazaki Y, Akahane S, Miyata H, Ajisawa Y, Nishizawa O, and Andersson KE (2002) Effects of selective β2 and β3-adrenoceptor agonists on detrusor hyperreflexia in conscious cerebral infarcted rats. J Urol 168 1247-1252. [DOI] [PubMed] [Google Scholar]
- Kullmann FA, Artim DE, Birder LA, and de Groat WC (2008) Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J Neurosci 28 1977-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon LA, Hoffman BE, Gardner SD, Laping NJ, Evans C, Lashinger ES, and Su X (2008) Effects of the β3-adrenergic receptor agonist disodium 5-[(2R)-2-[[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]-1,3-benzo dioxole-2,2-dicarboxylate (CL-316243) on bladder micturition reflex in spontaneously hypertensive rats. J Pharmacol Exp Ther 326 178-185. [DOI] [PubMed] [Google Scholar]
- Liang W, Afshar K, Stothers L, and Laher I (2002) The influence of ovariectomy and estrogen replacement on voiding patterns and detrusor muscarinic receptor affinity in the rat. Life Sci 71 351-362. [DOI] [PubMed] [Google Scholar]
- Lluel P, Palea S, Barras M, Grandadam F, Heudes D, Bruneval P, Corman B, and Martin DJ (2000) Functional and morphological modifications of the urinary bladder in aging female rats. Am J Physiol Regul Integr Comp Physiol 278 R964-R972. [DOI] [PubMed] [Google Scholar]
- Longhurst PA and Levendusky M (1999) Pharmacological characterization of beta-adrenoceptors mediating relaxation of the rat urinary bladder in vitro. Br J Pharmacol 127 1744-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara S, Okada H, Shirakawa T, Gotoh A, Kuno T, and Kamidono S (2002) Estrogen levels influence beta-3-adrenoceptor-mediated relaxation of the female rat detrusor muscle. Urology 59 621-625. [DOI] [PubMed] [Google Scholar]
- Michel MC, Wieland T, and Tsujimoto G (2009) How reliable are G-protein-coupled receptor antibodies? Naunyn Schmiedebergs Arch Pharmacol 379 385-388. [DOI] [PubMed] [Google Scholar]
- Nishimoto T, Latifpour J, Wheeler MA, Yoshida M, and Weiss RM (1995) Age-dependent alterations in beta-adrenergic responsiveness of rat detrusor smooth muscle. J Urol 153 1701-1705. [PubMed] [Google Scholar]
- Pelletier G (2000) Localization of androgen and estrogen receptors in rat and primate tissues. Histol Histopathol 15 1261-1270. [DOI] [PubMed] [Google Scholar]
- Pradidarcheep W, Stallen J, Labruyère WT, Dabhoiwala NF, Michel MC, and Lamers WH (2009) Lack of specificity of commercially available antisera against muscarinergic and adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol 379 397-402. [DOI] [PubMed] [Google Scholar]
- Rozec B and Gauthier C (2006) beta3-Adrenoceptors in the cardiovascular system: putative roles in human pathologies. Pharmacol Ther 111 652-673. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, Hunt TL, and Wein AJ (2003) Prevalence and burden of overactive bladder in the United States. World J Urol 20 327-336. [DOI] [PubMed] [Google Scholar]
- Takasu T, Ukai M, Sato S, Matsui T, Nagase I, Maruyama T, Sasamata M, Miyata K, Uchida H, and Yamaguchi O (2007) Effect of (R)-2-(2-aminothiazol-4-yl)-4′-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl} acetanilide (YM178), a novel selective β3-adrenoceptor agonist, on bladder function. J Pharmacol Exp Ther 321 642-647. [DOI] [PubMed] [Google Scholar]
- Takeda H, Matsuzawa A, Igawa Y, Yamazaki Y, Kaidoh K, Akahane S, Kojima M, Miyata H, Akahane M, and Nishizawa O (2003) Functional characterization of beta-adrenoceptor subtypes in the canine and rat lower urinary tract. J Urol 170 654-658. [DOI] [PubMed] [Google Scholar]
- Vrydag W and Michel MC (2007) Tools to study beta3-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol 374 385-398. [DOI] [PubMed] [Google Scholar]
- Woods M, Carson N, Norton NW, Sheldon JH, and Argentieri TM (2001) Efficacy of the beta3-adrenergic receptor agonist CL-316243 on experimental bladder hyperreflexia and detrusor instability in the rat. J Urol 166 1142-1147. [PubMed] [Google Scholar]
- Yamaguchi O and Chapple CR (2007) Beta3-adrenoceptors in urinary bladder. Neurourol Urodyn 26 752-756. [DOI] [PubMed] [Google Scholar]
- Yoshida J, Aikawa K, Yoshimura Y, Shishido K, Yanagida T, and Yamaguchi O (2007) The effects of ovariectomy and estrogen replacement on acetylcholine release from nerve fibres and passive stretch-induced acetylcholine release in female rat bladder. Neurourol Urodyn 26 1050-1055. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Seki S, and de Groat WC (2001) Nitric oxide modulates Ca(2+) channels in dorsal root ganglion neurons innervating rat urinary bladder. J Neurophysiol 86 304-311. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Ritchie J, Marouf N, Dion SB, Resnick NM, Elbadawi A, and Kuchel GA (2001) Role of ovarian hormones in the pathogenesis of impaired detrusor contractility: evidence in ovariectomized rodents. J Urol 166 1136-1141. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.