Abstract
Drugs that inhibit dopamine (DA) reuptake through actions at the dopamine transporter (DAT) have been proposed as candidates for development as pharmacotherapies for cocaine abuse. Accordingly, it is important to understand the potential pharmacological interactions of cocaine with other drugs acting at the DAT. Effects of combinations of cocaine with a cocaine analog, 2β-carbomethoxy-3β-(4-fluorophenyl)tropane (WIN 35,428), were compared quantitatively with the combinations of cocaine with the N-butyl,4′,4″-diF benztropine analog, 3-(bis(4-fluorophenyl)methoxy)-8-butyl-8-azabicyclo[3.2.1]octane (JHW 007), to determine whether their effects on DA levels in the shell of the nucleus accumbens (NAC) in mice differed. Each of the drugs alone produced dose-related elevations in NAC DA levels. In contrast to the other drugs, JHW 007 was less effective, producing maximal effects that approached 400% of control versus ∼700% with the other drugs. In addition, the JHW 007 dose-effect curve was not as steep as those for cocaine and WIN 35,428. Combinations of cocaine with its analog, WIN 35,428, were most often greater than those predicted based on dose additivity. In contrast, combinations of cocaine with JHW 007 were most often subadditive. This outcome is consistent with recent studies suggesting that structurally divergent DA uptake inhibitors bind to different domains of the DAT, which can result in different DAT conformations. The conformational changes occurring with JHW 007 binding may result in functional outcomes that alter its abuse liability and its effects in combination with cocaine.
Preclinical studies of stimulant drugs have demonstrated a close relation between actions at the dopamine (DA) transporter (DAT) and their behavioral effects related to abuse liability (e.g., Ritz et al., 1987; Bergman et al., 1989; Wilcox et al., 2002). In addition, neuroimaging studies in human cocaine users have reported a relation between subjective responses related to abuse liability and DAT actions (e.g., Volkow et al., 1997). These observations, together with several others, suggest that inhibition of DA transport is fundamental to the abuse of cocaine-like stimulant drugs.
Given the role of the DAT in stimulant drug abuse, it has often been the target of drug discovery for medications to treat cocaine abuse. Several DA indirect agonists have been proposed as potential agonist therapies— drugs that produce similar effects and consequently would be expected to decrease cocaine abuse, as methadone does for opioid abuse (e.g., Carroll et al., 1999; Howell and Wilcox, 2001). Certain DAT inhibitors have been suggested as treatments that alter cocaine effects in a manner similar to that of a partial agonist. For example, Rothman et al. (1991) and Baumann et al. (1994) examined combinations of cocaine with the DA uptake inhibitor, GBR 12909. In those studies GBR 12909 was reported to blunt the cocaine-induced elevations of extracellular DA in vivo.
The potential use of drugs acting at the DAT emphasizes a need for a better understanding of the pharmacology of combinations of drugs acting at the DAT. The published literature on combinations of these drugs has primarily examined DAT binding and in vivo effects such as elevations in DA levels or various behavioral effects related to abuse. Binding studies have indicated that DAT inhibitors in general are mutually displacing agents (e.g., Reith et al., 1986; Ritz et al., 1987), although some more complex interactions have been identified (e.g., Berger et al., 1990).
In vivo studies of combinations of DA uptake inhibitors have shown a variety of effects, in addition to the blocking by GBR 12909 of the effects of cocaine on extracellular levels of DA (Rothman et al., 1991). Others have indicated that the attenuation of the effect of cocaine is not reliably obtained in vitro (Gifford et al., 1993). With behavioral measures, several studies have reported additive effects and others have reported less than additive effects of combinations of DA uptake inhibitors (e.g., Tolliver et al., 1999; Ginsburg et al., 2005), although most studies have not approached the question in a quantitative manner. In one of the few isobolographic studies, Holtzman (2001) reported effects of cocaine and GBR 12909 in combination that were more than dose-additive in rats trained to discriminate cocaine from saline injections. However, the synergism with these two drugs was not obtained in another study that examined a number of indirect DA agonists by use of a cocaine-discrimination procedure (Li et al., 2006). In that study combinations of cocaine with a variety of other DA uptake inhibitors were not different from dose-additive.
In contrast to those findings, studies of some analogs of benztropine (BZT) have suggested less than additive effects when administered with cocaine. For example, Katz et al. (2004) found that several N-substituted BZT analogs, in contrast to the cocaine analog, WIN 35,428, did not shift the cocaine dose-effect curve leftward in rats discriminating cocaine from saline injections. Desai et al. (2005) found that the N-butyl analog of BZT, JHW 007, actually blocked the locomotor-stimulant effects of cocaine, and a recent study reported decreases in the self-administration of cocaine with administration of the drug (Hiranita et al., 2009).
The present study quantitatively compared the effects of combinations of JHW 007 or the cocaine analog, WIN 35,428, with cocaine on the stimulation of DA levels in the nucleus accumbens (NAC) shell. This structure is involved in the acute behavioral and reinforcing effects of cocaine related to its abuse (Pontieri et al., 1995). Because many of the behavioral and reinforcing effects of cocaine are thought to result from its indirect DA agonist actions, the effects of the drugs on DA levels in the NAC were assessed quantitatively by brain microdialysis to gain a better appreciation of the range of effects of combinations of drugs acting at this site.
Materials and Methods
Subjects. Male Swiss-Webster mice (Charles River, MA), experimentally naive at the start of the study and weighing 30 to 40 g, were housed in groups of four and had free access to food and water (except during sample collection). The housing rooms were temperature- and humidity-controlled, and maintained on a 12-h light/dark cycle (lights were on from 7:00 AM to 7:00 PM). Experiments were conducted during the light phase. The housing facilities were fully accredited by AAALAC International, and all experimentation was conducted in accordance with the Guidelines of the Animal Care and Use Committee of the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, and the Guide for Care and Use of Laboratory Animals (National Research Council, 1996). Mice were used only once and were treated with only one drug dose or drug combination.
Surgery. Mice were implanted with microdialysis probes during surgery sessions that started at approximately 9:00 AM and lasted approximately 45 min each. Under a mixture of ketamine and xylazine (60.0 and 12.0 mg/kg i.p., respectively) anesthesia, mice were placed in a stereotaxic apparatus where the skull was exposed and a small hole was drilled to expose the dura. Mice were then randomly implanted in the right or left side of the brain with a concentric dialysis probe, under continuous perfusion (which lasted for another 20 to 30 min after end of the implant). The probe was aimed at the NAC shell, according to Paxinos and Franklin (2001) (anterior = +1.5, lateral =±0.6, vertical =-5.2) (uncorrected coordinates, in millimeters from bregma). After surgery, mice were allowed to recover overnight in square cages equipped with overhead quartz-lined fluid swivels (Instech Laboratories Inc., Plymouth Meeting, PA) for connections to the dialysis probes. All subsequent studies were conducted in these cages.
In Vivo Microdialysis. Concentric dialysis probes were prepared with AN69 dialyzing membranes (Hospal Dasco, Bologna, Italy) as described for rats (Tanda et al., 2005, 2007) and modified in size for mice. The exposed dialyzing surface of the membrane, i.e., the surface not covered by glue, was limited to the lowest 1.0-mm portion of the probes, and the probes were less than 18 mm in total length. The part of the probe that was implanted within the brain consisted only of a soft microdialysis membrane (approximately 200–250 μm o.d.), surrounding silica fused tubing, with closure at the bottom by a cone-shaped glue tip. These probes were sufficiently rigid for reliable localized placement into the brain and did not necessitate the use of guide cannulas or hard materials such as stainless steel.
Experiments were performed on freely moving mice in the same cages in which they recovered from surgery. Microdialysis test sessions started at 9:00 AM, approximately 20 to 24 h after the surgical implant on the previous day. Probes were connected to fluid swivels (375/D/22QM; Instech Laboratories), and Ringer's solution (147.0 mM NaCl, 2.2 mM CaCl2, and 4.0 mM KCl) was delivered by a 1.0-ml syringe, operated by a BAS Bee Syringe Pump Controller (BAS Bioanalytical Systems, West Lafayette, IN), through the dialysis probes at a constant flow rate of 1 μl/min. Dialysis samples were not collected for at least 30 min after the start of Ringer's solution delivery. At that time, collection of dialysate samples started, and 10-μl samples were collected every 10 min thereafter and immediately analyzed as detailed below. When three of these consecutive samples indicated stable (less than 10% variability) DA values (typically after approximately 1–2 h), mice were treated with drug or saline. Sample collection continued at 10-min intervals typically during the first 3 h after treatment, and every 20 min thereafter (only 10 of the 20 μl collected were analyzed).
Cocaine and WIN 35,428 had a rapid onset with maximal stimulation of DA levels during the first 30 min after drug administration. We therefore studied the effects of these drugs in combination immediately after their successive injections. The effects of combinations of JHW 007 and cocaine were studied with the JHW 007 given 4 h before cocaine, because the slower rate of DAT occupancy of this drug (Desai et al., 2005), with the samples collected and analyzed during the 30 min that immediately followed cocaine injection. Because some adaptation to the effects of JHW 007 may have occurred during the 4-h period after its administration and before cocaine injection, we subsequently studied the effects of JHW 007 in combination with cocaine when administered 10 min apart, and with DA samples collected and analyzed during the 30 min that immediately followed cocaine injection. The rationale for this latter experiment comes also from in vivo binding studies (Desai et al., 2005) that, although they indicate a slower rate of DAT occupancy with JHW 007, also show that there was some displacement of label within minutes after JHW 007 injection, and in vitro binding studies of [3H]JHW 007 have confirmed two phases to its association (Kopajtic et al., 2006).
Analytical Procedure. Dialysate samples (10 μl) were injected without purification into a high-performance liquid chromatography apparatus equipped with a chromatographic column, ESA-MD 150 × 3.2 mm with 3-μm particle size (ESA, Chelmsford, MA), and a coulometric detector (5200a Coulochem II; ESA, Inc.) to quantify DA. Potentials for the oxidation and reduction electrodes of the analytical cell (5014B; ESA, Inc.) were set at +125 mV and -125 mV, respectively. The mobile phase, containing 100 mM NaH2PO4, 0.1 mM Na2EDTA, 0.5 mM n-octyl sulfate, and 18% (v/v) methanol (pH adjusted to 5.5 with Na2HPO4), was pumped by an ESA 582 (ESA, Inc.) solvent delivery module at 0.50 ml/min. Assay sensitivity for DA was 2 fmol/sample.
Histology. At the end of the experiment, mice were euthanized by pentobarbital overdose, and their brains were removed and left to fix in 4% formaldehyde in saline solution. Brains were then cut on a Vibratome Plus 1000 (The Vibratome Company, St. Louis, MO) in serial coronal slices oriented according to Paxinos and Franklin (2001) to identify the location of the probes. The location of the probes was verified in all of the experiments. Figure 1 schematically shows the boundaries (superimposed rectangles) of probe tracks that were considered correct probe placements. Data only from those animals for which probe tracks were within those boundaries were used for results described in the article. Although the probes were implanted randomly in the right or left side of the brain, all probe placements have been shown schematically on one side of the brain in Fig. 1. The brain sections are based on drawings of Paxinos and Franklin (2001), and the anterior coordinates (measured from bregma) have been indicated.
Fig. 1.
Drawings of forebrain sections, based on those of Paxinos and Franklin (2001), to show the boundaries (superimposed rectangles) within which microdialysis probe tracks were considered correct probe placements. Data only from those animals for which probe tracks, from the dialyzing portion of the microdialysis probe, were within the box boundaries were used for results described in the article. The anterior coordinate (measured from bregma) is indicated on each section. Sh = NAC shell.
Drugs. The drugs tested were: (-)-cocaine HCl (Sigma-Aldrich, St. Louis, MO), WIN 35,428 [National Institute on Drug Abuse (NIDA) Drug Supply Program], and JHW 007, synthesized in the Medicinal Chemistry Section, NIDA Intramural Research Program, according to methods described previously in Agoston et al. (1997). Drugs were dissolved in saline (0.9% NaCl) and were injected in a volume of 10 ml/kg i.p. Injections of saline (10 ml/kg i.p.) served as vehicle controls.
Data Analysis. Data are shown as percentage increase above basal DA values. Basal DA values were calculated as the mean of three consecutive samples (differing no more than 10%) immediately preceding the first drug or vehicle injection. All results are presented as group means (±S.E.M.). Statistical analysis was carried out by use of a two-way ANOVA (drug dose and time as factors) for repeated measures over time, with results from treatments showing overall changes subjected to post hoc Tukey's test. Changes were considered to be significant when p < 0.05.
The effects of each of the drugs during the 30-min period of maximal stimulation of DA levels (cocaine, 0–30 min; WIN 35,428, 0–30 min; JHW 007, 240–270 min) were analyzed by ANOVA and regression techniques. Nonlinear regression was used to determine the maximal effects of each drug. Linear regression was then used to determine the doses effective in producing an increase in extracellular DA levels to half of the maximal elevation, ED50 values, and their 95% confidence limits (Snedecor and Cochran, 1967). The dose-effect data were analyzed further by standard parallel-line bioassay techniques (Finney, 1964) to determine their potencies relative to cocaine. Isobolographic analysis of the drug combinations was conducted according to the comprehensive descriptions that can be found in previous articles, with related calculations (Tallarida, 2000, 2007; Grabovsky and Tallarida, 2004).
Results
Saline administration did not significantly modify extracellular DA levels in dialysates from the NAC shell. The maximal changes (±S.E.M.) produced by saline over the course of 270 min were approximately +13% (±4.9%) and -13% (±9%) of baseline (Fig. 2C, open circles). Cocaine administration (5–55 mg/kg) produced dose-dependent and significant increases in DA levels in the NAC shell (Fig. 2A; two-way ANOVA resulted in a main-effect dose, F4,36 = 28.002, p < 0.0001; main-effect time, F18,648 = 124.518, p < 0.0001; time × dose interaction, F72,648 = 12.903, p < 0.0001). The effect of cocaine on DA levels had a rapid onset and a relatively rapid offset at all doses tested. DA values reached maximum at approximately 20 min after cocaine administration, with the maximal increase reaching approximately 700% of basal values at the highest dose. DA levels approached basal values approximately 120 min after administration of the highest doses of cocaine (Fig. 2A).
Fig. 2.
Time course of effects of cocaine (A), WIN 35,428 (B), and JHW 007 (C) on extracellular levels of DA in dialysates from the NAC shell. Ordinates, change in extracellular DA concentration as a percentage of basal values. Abscissae, time since injection. Results are means, with vertical bars representing S.E.M., of the amount of DA, expressed as percentage change from basal values, in 10-min dialysate samples, uncorrected for probe recovery. The number of subjects for each experimental group has been reported in the symbol legend. Basal levels of extracellular DA (expressed as femtomoles per 10-μl sample ± S.E.M.) were 26.01 ± 1.56. No significant differences in basal levels of DA were found among the different groups of subjects.
Administration of WIN 35,428 (0.3–5.6 mg/kg) also significantly and dose-dependently increased DA levels in dialysates from the NAC shell (Fig. 2B; two-way ANOVA resulted in a main-effect dose, F3,19 = 9.823, p < 0.0001; main-effect time, F18,342 = 60.120, p < 0.0001; time × dose interaction, F54,142 = 12.236, p < 0.0001). Administration of the higher doses of WIN 35,428 produced a substantial and prolonged increase in DA levels. DA values reached maximum at approximately 30 min after injection, with maximal increases of approximately 750% of basal values. DA levels approached basal values at approximately 180 min after administration of the highest doses of WIN 35,428.
JHW 007 (1.0–17.0 mg/kg) also dose-dependently and significantly increased NAC shell dialysate DA levels (Fig. 2C; two-way ANOVA resulted in a main-effect dose, F3,12 = 19.587, p < 0.0001; main-effect time, F30,360 = 16.656, p < 0.0001; time × dose interaction, F90,360 = 2.796, p < 0.0001). However, in contrast to the other compounds, the increases in DA levels had a slower onset, with accumulation of DA reaching maximum as much as 4 to 5 h after injection, depending on dose. At the highest dose of JHW 007 (17.0 mg/kg) the maximal increase in DA levels reached approximately 400% of basal values from approximately 180 to 360 min after injection. Within the time period examined, DA levels did not return to basal values or show any evidence of a decrease from the maximal elevation obtained. Informal observations of the subjects during the microdialysis sampling indicated that the behavioral effects of JHW 007 were in all important respects similar to those described previously (Desai et al., 2005).
The dose-dependent effects of all of the drugs at the time at which they were most effective (Fig. 3) show that the maximal effect of cocaine and WIN 35,428 were about the same, whereas that for JHW 007 was substantially less than those for the other drugs. Cocaine reached its half-maximal effect with an ED50 value of 14.3 mg/kg (Table 1). WIN 35,428 was approximately 10-fold more potent than cocaine on a milligram per kilogram basis. JHW 007, at 4 h after injection, had effects at doses lower than the active doses of cocaine with a substantially reduced slope of the dose-effect curve.
Fig. 3.
Dose-dependent maximal effects of cocaine, WIN 35,428, and JHW 007 on extracellular levels of DA. Ordinates, change in extracellular DA levels as a percentage of basal values during the 30-min period after drug administration in which maximal stimulation of DA transmission was observed. Abscissae, dose of drug in milligrams per kilogram, log scale. Each point represents the average effect determined in five to nine mice.
TABLE 1.
Dose effects of different dopamine uptake inhibitors alone or in combination with cocaine on extracellular dopamine concentrations in the nucleus accumbens shell in mice For drug combinations the ED50 value is that for the cocaine dose when cocaine was administered with the specified dose of the combined drug.
| Treatment | ED50 Value | Potency Relative to Cocaine |
|---|---|---|
| mg/kg | ||
| Cocaine | 14.3 (11.6–17.1) | |
| WIN 35,428 | 1.50 (1.08–2.06) | 10.3 (7.46–14.2) |
| WIN 35,428 and cocaine (0.3 mg/kg) | 11.3 (8.15–3.9) | 0.644a (0.472–0.856) |
| WIN 35,428 and cocaine (1.0 mg/kg) | 6.78 (2.79–9.81) | 0.386a (0.264–0.532) |
| JHW 007 at 240 min | 2.81 (1.08–4.74) | 1.87 (1.23–2.72) |
| JHW 007 and cocaine (240 min) (3.0 mg/kg) | 7.64 (0.201–13.0) | 1.00 (0.708–1.42) |
| JHW 007 and cocaine (240 min) (10.0 mg/kg) | 9.57 (1.63–15.0) | 0.597a (0.386–0.859) |
| JHW 007 at 10 min | 3.55 (1.48–6.52) | 0.720 (0.397–1.14) |
| JHW 007 and cocaine (10 min) (3.0 mg/kg) | 10.1 (6.73–13.1) | 1.52 (1.17–1.98) |
| JHW 007 and cocaine (10 min) (10.0 mg/kg) | 12.4 (7.23–16.6) | 1.01a (0.745–1.34) |
The value is an estimate because there was a significant difference in the mean response (weighted by N) for the two treatments
Treatment with WIN 35,428 dose-dependently shifted the cocaine dose-effect curve to the left (Fig. 4A). WIN 35,428 at doses of 0.3 and 1.0 mg/kg decreased the cocaine ED50 value from 14.3 to 11.3 and 6.78 mg/kg, respectively. The relative potency values indicated that these shifts were significant (Table 1). The comparison of the obtained (experimental) effects of cocaine and WIN 35,428 in combination with those predicted on the basis of a dose-additive effect are shown in Fig. 4B (dashed line). Also shown is the linear regression of the effects of cocaine alone (dotted line). Combinations of WIN 35,428 with cocaine produced effects that were most often greater than the effects of dose additivity, because the experimental points were above the dashed line. Because the dose-effect curves for these drugs were parallel, the construction of a linear isobole of additivity was allowed as shown in Fig. 4C. For a specified effect level of a 327.5% increase above basal levels, the combination of WIN 35,428 (0.3 mg/kg) yielded a cocaine quantity of 14.59 (±0.51), whereas the greater dose (1.0 mg/kg) resulted in a cocaine quantity of 8.23 (±2.37). These experimental points, shown in the isobologram, are seen to be below the line of additivity (suggestive of synergism); however, the values did not lie significantly off of the line.
Fig. 4.
Effects of combinations of WIN 35,428 and cocaine on extracellular levels of DA. A, observed effects of the combinations. Ordinates, change in extracellular DA levels as a percentage of basal values during the 30-min period after cocaine administration in which maximal stimulation of DA transmission was observed. Abscissae, dose of cocaine in milligrams per kilogram, log scale. B, observed effects compared with the predicted effects of the combinations. Ordinates and abscissae are as in A. The calculated (predicted) additive dose-effect curve for cocaine in the presence of 0.3 (top) or 1.0 (bottom) mg/kg WIN 35,428 is shown by the dashed straight line and the effects of cocaine alone by the dotted line. The experimental (obtained) values are shown by the connected circles. C, the analysis of combination data with use isobolographic methods. The line connects the additive effects of an increase of 327.5%. The approximately constant potency ratio produced by these agents allowed the construction of the linear isobole of additivity. The experimental points are those producing the specified increase as determined by linear regression. The points are below the line of additivity (suggestive of synergism).
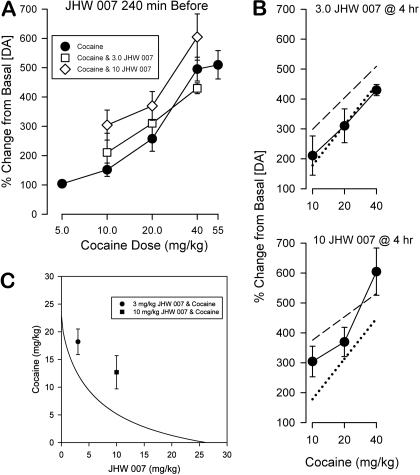
The cocaine dose-effect curve was also shifted leftward by coadministration of 10.0 mg/kg, but not 3.0 mg/kg, JHW 007 240 min before (Fig. 5A). The cocaine ED50 value was decreased by both doses of JHW 007, but the relative potency analysis showed that only at the higher dose of JHW 007 was the effect significant (Table 1). The obtained effects of cocaine and 3.0 mg/kg JHW 007 in combination were consistently less than those predicted from dose additivity (Fig. 5B). This was also seen at the higher dose of JHW 007, with the exception to this occurring at the highest dose of cocaine (Fig. 5B). Because JHW 007 had a lower efficacy than cocaine, and thus a variable potency ratio with cocaine, the calculation of the isobole by use of the nonlinear curves of each results in an additive isobole that is not linear (Grabovsky and Tallarida, 2004). Nonetheless, the criteria for comparing the additive combination and the experimental combination are as in the linear isobole. As seen in Fig. 5C, the experimental points for the two different doses of JHW 007 (3.0 and 10.0 mg/kg) lie well above the additive isobole, suggesting a less than additive effect. However, the large variance precluded a statistically significant effect.
Fig. 5.
Effects of combinations of JHW 007 and cocaine on extracellular levels of DA. A, observed effects of the combinations. Ordinates, change in extracellular DA levels as a percentage of basal values during the 30-min period after cocaine administration in which maximal stimulation of DA transmission was observed. Abscissae, dose of cocaine in milligrams per kilogram, log scale. B, observed effects compared with the predicted effects of the combinations. Ordinates and abscissae are as in A. The calculated (predicted) additive dose-effect curve for cocaine in the presence of 3.0 (top) or 10.0 (bottom) mg/kg JHW 007 is shown by the dashed straight line and the effects of cocaine alone are shown by the dotted line. The experimental (obtained) values are shown by the connected circles. C, the analysis of combination data using isobolographic methods. Because cocaine and JHW 007 displayed a varying dose ratio (evident from their different maximal effects; see Fig. 2) the isobole of additivity is curved (Grabovsky and Tallarida, 2004). The curved isobole is given by the equation: y = 22.95 - (22.95/(0.689 + 8.16/x)0.626). The line connects the additive effects of an increase of 327.5%. The experimental points are those producing the specified increase as determined by linear regression. The points are above the line of additivity (suggestive of subadditivity).
The dose effects for JHW 007 at 10 min after injection were less than those obtained at 240 min after injection (Fig. 6A). The ED50, relative potency values, and isobole were not calculated because the effects were not at steady state (Fig. 2), and there was no semblance of a maximum among the doses tested (Fig. 6A). The lower dose of JHW 007, which alone produced an effect that approached a doubling of DA concentration, did not alter the effects of the lower doses of cocaine, but decreased the effects of the higher doses. The combination of the highest dose of JHW 007 with cocaine at this time point was not appreciably different from the effects of cocaine alone, despite a more than doubling of DA concentration by that dose of JHW 007 alone. The comparison of the obtained effects of cocaine and JHW 007 in combination with those predicted on the basis of a dose-additive effect (Fig. 6C, dashed line) indicated that the effects of the combinations of 3.0 mg/kg JHW 007 and cocaine were less than the effects predicted from dose additivity, and that was also true of the combinations of the higher dose of JHW 007 with the lower doses of cocaine.
Fig. 6.
Effects of combinations of cocaine and JHW 007 at 10 min after injection on extracellular levels of DA. A, dose-dependent maximal effects of JHW 007 alone at 4 h and at 10 min after injection compared with those of cocaine. Ordinates, change in extracellular DA levels as a percentage of basal values from 10 to 40 min after JHW 007 injection (squares), from 4 to 4.5 h after JHW 007 injection (triangles), or during the 30-min period after cocaine injection (circles). Abscissae, dose of drug in milligrams per kilogram, log scale. Each point represents the average effect determined in 5 to 12 mice. B, observed effects of the combinations. Ordinates, change in extracellular DA levels as a percentage of basal values during the 30-min period after cocaine administration. Abscissae, dose of cocaine in milligrams per kilogram, log scale. C, observed effects compared with the predicted effects of the combinations. Ordinates and abscissae are as in B. The calculated (predicted) additive dose-effect curve for cocaine in the presence of 3.0 (top) or 10.0 (bottom) mg/kg JHW 007 is shown by the dashed straight line and the effects of cocaine alone are shown by the dotted line. The experimental (obtained) values are shown by the connected circles.
Discussion
The action of cocaine thought to be involved in its abuse liability is the blockade of DA uptake, and the NAC shell is a critical site for that effect (Kuhar et al., 1991; Di Chiara et al., 1999). In addition, several drugs proposed for development as medications for cocaine abuse target the DAT (Carroll et al., 1999). Consequently, it is important to understand the potential interactions of cocaine with other drugs acting at the DAT. Each of the present compounds binds the DAT and inhibits DA uptake (Reith et al., 1986; Kuhar et al., 1991; Katz et al., 2004). Despite those actions, JHW 007 produces behavioral effects that are reduced compared with cocaine and other cocaine-like DA uptake inhibitors (Katz et al., 2004). Moreover, JHW 007 can attenuate the effects of cocaine (Desai et al., 2005, Hiranita et al., 2009). Thus, the aim of the present study was to compare the in vivo effects of combinations of DA uptake inhibitors with cocaine on stimulation of DA levels.
Each compound produced significant dose- and time-dependent increases in DA concentrations in the NAC shell. The time courses for the effects of cocaine and WIN 35,428 were relatively similar, as were their maxima. JHW 007 increased DA levels at doses lower than effective doses of cocaine, in agreement with its higher DAT affinity (Agoston et al., 1997). However, JHW 007 differed from the other compounds regarding its relatively slow onset and longer duration of effects, and the decreased slope of its dose-effect curve compared with the other drugs. The slow onset and reduced effectiveness are consistent with other effects of the drug (Katz et al., 2004; Desai et al., 2005). The different slope of the dose-effect curve suggests that the mechanism by which JHW 007 increases DA concentrations differs from that for the other drugs.
Several off-target effects of JHW 007 have been explored as possibly interfering with cocaine-like effects and contributing to the present subadditive effects of its combination with cocaine. For example, although in vitro binding studies have shown selectivity of JHW 007 for the DAT over other monoamine transporters, the selectivity may not be fully reflected in vivo. Several previous studies showed DA stimulation in the NAC shell with combinations of DA and norepinephrine or 5-hydroxytryptamine transport inhibitors greater than that obtained with DAT blockade alone (Bubar et al., 2003; Carboni et al., 2006), suggesting that inhibition of norepinephrine or 5-hydroxytryptamine transport by JHW 007 is probably not responsible for the present subadditive effects. A previous study examined the binding of JHW 007 at more than 30 other targets (Katz et al., 2004), and the potential of many of these other actions as modulating influences on the cocaine-like effects of JHW 007 has been investigated (Newman and Katz, 2009). The parent compound, BZT, has well known muscarinic M1 and histaminic H1 antagonist effects, and JHW 007 had affinity for these sites. The possible role of muscarinic M1 receptors in attenuating the effects of BZT analogs was investigated with regard to effects on DA concentrations in the NAC (Tanda et al., 2007). The M1 receptor antagonists, telenzepine and trihexyphenidyl, enhanced rather than attenuated the effects of cocaine on DA levels in the NAC shell, suggesting that the M1 antagonist effects of JHW 007 probably do not contribute to the presently obtained subadditivity. Likewise, selective H1 receptor antagonist effects produced by triprolidine do not alter DA levels in either the NAC shell or core (Tanda et al., 2008). Finally, JHW 007 has affinity for σ-receptors, and actions at this site did not modify the in vivo levels of dopamine produced by cocaine (Tanda et al., 2009). Clearly, JHW 007 is not perfectly DAT-selective, and off-target sites present hypotheses for potential explanation of the present findings. However, available evidence on those sites that have been investigated to date suggests that they are unlikely candidates for these actions.
It is also possible that a depletion of intracellular DA limited increases in DA and contributed to the subadditive effects of cocaine-JHW 007 combinations. There are several reasons, however, why this interpretation cannot fully account for the present findings. First, the effects were generally assessed at doses that alone had effects well below the maximum. For example, among the doses of cocaine and JHW 007 that produced subadditive effects were 20 mg/kg cocaine with 3.0 mg/kg JHW 007. These doses alone increased DA levels to values that were, respectively, 51 and 32% of the maximal effect of cocaine. The predicted dose-additive effect of this combination was 79%, and the obtained combined effect was only 61% of the cocaine maximum. Thus, despite ample room below “ceiling,” the expected additive effect was not obtained. In addition, JHW 007 produced a subadditive effect most prominently at the low dose, and at the earlier time after injection when its own effect on DA concentration was minimal. Finally, the effects alone of JHW 007 were less than those produced by WIN 35,428, which suggests this latter drug as a more likely candidate to produce DA depletion. Yet WIN 35,428 in combination with cocaine was dose-additive or greater than additive. Thus, it seems unlikely that DA depletion contributed to the subadditive effects of JHW 007.
Previous studies have indicated that DA uptake inhibitors have different affinities when the DAT is in an outward as opposed to inward facing conformation, and that some ligands may promote an equilibrium shift toward one conformation. For example, Reith et al. (2001) examined the reaction of a cell-impermeant methanethiosulfonate derivative, methanethiosulfonate ethyltrimethylammonium (MTSET), to various cysteine residues within the DAT. Cocaine, WIN 35,428, mazindol, and DA enhanced the reaction of Cys-90 with MTSET, whereas BZT had no effect on this reaction. The results suggested that different DAT inhibitors can produce different conformational changes in the transporter, with cocaine leaving, in this case, the outward-facing Cys-90 residue more exposed. More recently, Loland et al. (2008) determined how different inhibitors affected accessibility of MTSET to a cysteine inserted at position 159 (I159C) in a DAT background in which the two external endogenous cysteines were mutated to alanines (C90A, C306A). Cys-159 is thought to be accessible when the transporter's extracellular gate is open but inaccessible when the gate is closed. Preincubation of COS-7 cells expressing this mutant with MTSET decreased DA uptake, and the addition of cocaine enhanced the effect. In contrast, adding JHW 007 or other BZT analogs to the preincubation decreased the effects of MTSET, suggesting that cocaine binds an open conformation, whereas BZT analogs bind a closed conformation protecting the Cys-159 residue. Most recently, Beuming et al. (2008) modeled the DAT when complexed with DA and several DAT inhibitors. In contrast to the binding of cocaine, the binding of BZT analogs preserved the hydrogen bond between Asp79 and Tyr156 that was proposed to function as a gate, predicting a closed (inward facing) binding conformation. The DAT model with JHW 007 suggested that this BZT binds more like substrate than the cocaine-like inhibitors, allowing a closed hydrogen-bonded gate. Because BZT analogs bind with much higher affinity than substrates, they are not translocated, consistent with a slow dissociation and the presently observed long duration of action.
One interesting feature of the effects of cocaine-JHW 007 combinations was that the subadditive effects were more pronounced early after JHW 007 injection compared with the later time point. In vivo binding studies (Desai et al., 2005) indicated a relatively shallow displacement curve, with no plateau hours after injection. Nonetheless, there was up to 20% displacement of label within minutes after JHW 007 injection. In vitro binding studies of [3H]JHW 007 have confirmed two phases to the association of JHW 007 (Kopajtic et al., 2006), with a rapid phase that may correspond to this early in vivo displacement and a slower phase that may correspond to the cocaine-like increases in DA that more slowly appeared. It is potentially this fast-onset component of the binding of JHW 007 that is responsible for the subadditivity. Finally, these two components of activity of JHW 007 acting together may contribute to the decreased maximum and the lower slope of its dose-effect curve when acting alone. However, the greater subadditivity at earlier times after administration, and the more pronounced subadditivity at the lower dose of JHW 007, remain a challenge for mechanistic hypotheses.
The results of the present study suggest that DA uptake inhibitors that preferentially bind disparate DAT conformations will act in combination differently from those that preferentially bind similar conformations. Those that prefer the same conformation, e.g., cocaine and WIN 35,428, will combine in an additive or more than additive manner. Those that prefer different conformations, e.g., cocaine and JHW 007, will produce combined effects that are subadditive. If JHW 007 actually induces a shift in equilibrium toward an inward-facing conformation, fewer DAT molecules would be available for cocaine-like actions. That combined with a slow dissociation constant for JHW 007 may be fundamental to subadditive effects of combinations of cocaine and JHW 007.
Acknowledgments
We thank Jianjing Cao (the Medicinal Chemistry Section, NIDA Intramural Research Program, National Institutes of Health) for the synthesis of JHW 007.
This work was supported by the Intramural Research Program of the National Institutes of Health National Institute on Drug Abuse.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.154302.
ABBREVIATIONS: DA, dopamine; DAT, dopamine transporter; BZT, benztropine; NAC, nucleus accumbens; JHW 007, 3-(bis(4-fluorophenyl-)methoxy)-8-butyl-8-azabicyclo[3.2.1]octane; WIN 35,428, 2β-carbomethoxy-3β-(4-fluorophenyl)tropane; GBR 12909, 1-[2-[bis(4-fluorophenyl-)methoxy]ethyl]-4-(3-phenylpropyl)piperazine; ANOVA, analysis of variance; MTSET, methanethiosulfonate ethyltrimethylammonium; NIDA, National Institute on Drug Abuse.
References
- Agoston GE, Wu JH, Izenwasser S, George C, Katz J, Kline RH, and Newman AH (1997) Novel N-substituted 3α-[bis(4′-fluorophenyl)methoxy]tropane analogues: selective ligands for the dopamine transporter. J Med Chem 40 4329-4339. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Char GU, De Costa BR, Rice KC, and Rothman RB (1994) GBR12909 attenuates cocaine-induced activation of mesolimbic dopamine neurons in the rat. J Pharmacol Exp Ther 271 1216-1222. [PubMed] [Google Scholar]
- Berger P, Elsworth JD, Reith ME, Tanen D, and Roth RH (1990) Complex interaction of cocaine with the dopamine uptake carrier. Eur J Pharmacol 176 251-252. [DOI] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, and Spealman RD (1989) Effects of cocaine and related drugs in nonhuman primates III self-administration by squirrel monkeys. J Pharmacol Exp Ther 251 150-155. [PubMed] [Google Scholar]
- Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, Newman AH, Javitch JA, Weinstein H, Gether U, et al. (2008) The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci 11 780-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, McMahon LR, De Deurwaerdère P, Spampinato U, and Cunningham KA (2003) Selective serotonin reuptake inhibitors enhance cocaine-induced locomotor activity and dopamine release in the nucleus accumbens. Neuropharmacology 44 342-353. [DOI] [PubMed] [Google Scholar]
- Carboni E, Silvagni A, Vacca C, and Di Chiara G (2006) Cumulative effect of norepinephrine and dopamine carrier blockade on extracellular dopamine increase in the nucleus accumbens shell, bed nucleus of stria terminalis and prefrontal cortex. J Neurochem 96 473-481. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Howell LL, and Kuhar MJ (1999) Pharmacotherapies for treatment of cocaine abuse: preclinical aspects. J Med Chem 42 2721-2736. [DOI] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, Koffarnus M, Newman AH, and Katz JL (2005) Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J Neurosci 25 1889-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, and Carboni E (1999) Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci 877 461-485. [DOI] [PubMed] [Google Scholar]
- Finney DJ (1964) Statistical Method in Biological Assay, 2nd ed, Hafner, New York.
- Gifford AN, Bergmann JS, and Johnson KM (1993) GBR 12909 fails to antagonize cocaine-induced elevation of dopamine in striatal slices. Drug Alcohol Depend 32 65-71. [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Kimmel HL, Carroll FI, Goodman MM, and Howell LL (2005) Interaction of cocaine and dopamine transporter inhibitors on behavior and neurochemistry in monkeys. Pharmacol Biochem Behav 80 481-491. [DOI] [PubMed] [Google Scholar]
- Grabovsky Y and Tallarida RJ (2004) Isobolographic analysis for combinations of a full and partial agonist: curved isoboles. J Pharmacol Exp Ther 310 981-986. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Newman AH, and Katz JL (2009) Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther 329 677-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman SG (2001) Differential interaction of GBR 12909 a dopamine uptake inhibitor with cocaine and methamphetamine in rats discriminating cocaine. Psychopharmacology 155 180-186. [DOI] [PubMed] [Google Scholar]
- Howell LL and Wilcox KM (2001) The dopamine transporter and cocaine medication development: drug self-administration in nonhuman primates. J Pharmacol Exp Ther 298 1-6. [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC.
- Katz JL, Kopajtic TA, Agoston GE, and Newman AH (2004) Effects of N-substituted analogues of benztropine: diminished cocaine-like effects in dopamine transporter ligands. J Pharmacol Exp Ther 309 650-660. [DOI] [PubMed] [Google Scholar]
- Kopajtic TA, Newman AH, and Katz JL (2006) Comparisons of the in vitro binding of [3H]WIN 354428 with [3H]JHW 007 a dopamine transporter ligand that blocks the effects of cocaine In: LS Harris, editor. Proceedings of the 67th Annual Scientific Meeting of the College on Problems of Drug Dependence; 2005 June 18–23; Orlando, FL. College of Drug Dependence, Philadelphia, PA.
- Kuhar MJ, Ritz MC, and Boja JW (1991) The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci 14 299-302. [DOI] [PubMed] [Google Scholar]
- Li SM, Campbell BL, and Katz JL (2006) Interactions of cocaine with dopamine uptake inhibitors or dopamine releasers in rats discriminating cocaine. J Pharmacol Exp Ther 317 1088-1096. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, and Gether U (2008) Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol Pharmacol 73 813-823. [DOI] [PubMed] [Google Scholar]
- Newman AH and Katz JL (2009) Atypical dopamine uptake inhibitors that provide clues about cocaine's mechanism at the dopamine transporter, in Topics in Medicinal Chemistry, Volume 4: Transporters as Targets for Drugs (Napier S and Bingham M, eds), Springer, Berlin.
- Paxinos G and Franklin KBJ (2001) The Mouse Brain in Stereotaxic Coordinates, 2nd ed, Academic Press, New York.
- Pontieri FE, Tanda G, and Di Chiara G (1995) Intravenous cocaine morphine and amphetamine preferentially increase extracellular dopamine in the “hell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A 92 12304-12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith ME, Berfield JL, Wang LC, Ferrer JV, and Javitch JA (2001) The uptake inhibitors cocaine and benztropine differentially alter the conformation of the human dopamine transporter. J Biol Chem 276 29012-29018. [DOI] [PubMed] [Google Scholar]
- Reith ME, Sershen H, and Lajtha A (1986) Binding sites for [3H]cocaine in mouse striatum and cerebral cortex have different dissociation kinetics. J Neurochem 46 309-312. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, and Kuhar MJ (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237 1219-1223. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Mele A, Reid AA, Akunne HC, Greig N, Thurkauf A, de Costa BR, Rice KC, and Pert A (1991) GBR12909 antagonizes the ability of cocaine to elevate extracellular levels of dopamine. Pharmacol Biochem Behav 40 387-397. [DOI] [PubMed] [Google Scholar]
- Snedecor GW and Cochran WG (1967) Statistical Methods, 6th ed, Iowa State University Press, Ames, IA.
- Tallarida RJ (2000) Drug Synergism and Dose-Effect Data Analysis. CRC/Chapman-Hall, Boca Raton, FL.
- Tallarida RJ (2007) Interactions between drugs and occupied receptors. Pharmacol Ther 113 197-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Ebbs AL, Kopajtic TA, Elias LM, Campbell BL, Newman AH, and Katz JL (2007) Effects of muscarinic M1 receptor blockade on cocaine-induced elevations of brain dopamine levels and locomotor behavior in rats. J Pharmacol Exp Ther 321 334-344. [DOI] [PubMed] [Google Scholar]
- Tanda G, Ebbs A, Newman AH, and Katz JL (2005) Effects of 4′-chloro-3α-(diphenylmethoxy)-tropane on mesostriatal mesocortical and mesolimbic dopamine transmission: comparison with effects of cocaine. J Pharmacol Exp Ther 313 613-620. [DOI] [PubMed] [Google Scholar]
- Tanda G and Katz JL (2007) Muscarinic preferential M1 receptor antagonists minimally alter the discriminative-stimulus effects of cocaine in rats. Pharmacol Biochem Behav 87 400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Ebbs AL, Kopajtic TA, Elias LM, Campbell BL, Newman AH, and Katz JL (2007) Effects of muscarinic M1 receptor blockade on cocaine-induced elevations of brain dopamine levels and locomotor behavior in rats. J Pharmacol Exp Ther 321 334-344. [DOI] [PubMed] [Google Scholar]
- Tanda G, Garcés-Ramírez L, Green JL, Hiranita T and Katz JL (2009) Effects of acute administration of sigma receptor ligands on mesolimbic dopamine neurotransmission in rats. FASEB J 23 745.4. [Google Scholar]
- Tolliver BK, Newman AH, Katz JL, Ho LB, Fox LM, Hsu K Jr, and Berger SP (1999) Behavioral and neurochemical effects of the dopamine transporter ligand 4-chlorobenztropine alone and in combination with cocaine in vivo. J Pharmacol Exp Ther 289 110-122. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, et al. (1997) Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature 386 827-830. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Lindsey KP, Votaw JR, Goodman MM, Martarello L, Carroll FI, and Howell LL (2002) Self-administration of cocaine and the cocaine analog RTI-113: Relationship to dopamine transporter occupancy determined by PET neuroimaging in rhesus monkeys. Synapse 43 78-85. [DOI] [PubMed] [Google Scholar]