Abstract
Studies have shown that long-term (5α,6α)-7,8-didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol (morphine) treatment increases the sensitivity to painful heat stimuli (thermal hyperalgesia). The cellular adaptations contributing to sustained morphine-mediated pain sensitization are not fully understood. It was shown previously (J Neurosci 22:6747–6755, 2002) that sustained morphine exposure augments pain neurotransmitter [such as calcitonin gene-related peptide (CGRP)] release in the dorsal horn of the spinal cord in response to the heat-sensing transient receptor potential vanilloid 1 receptor agonist 8-methyl-N-vanillyl-6-nonenamide (capsaicin). In the present study, we demonstrate that sustained morphine-mediated augmentation of CGRP release from isolated primary sensory dorsal root ganglion neurons is dependent on protein kinase A and Raf-1 kinase. Our data indicate that, in addition to neural system adaptations, sustained opioid agonist treatment also produces intracellular compensatory adaptations in primary sensory neurons, leading to augmentation of evoked pain neurotransmitter release from these cells.
Opioid analgesics such as (5α,6α)-7,8-didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol (morphine) are widely used in the treatment of various pain conditions. It is interesting, however, that long-term treatment with opioid analgesics paradoxically increases the sensitivity of patients (Koppert, 2004) and experimental animals to mildly painful and normally innocuous thermal stimuli (thermal hyperalgesia and allodynia). It was suggested that such paradoxical pain sensitization may contribute to the development of antinociceptive tolerance (Mao et al., 1995; Vanderah et al., 2000; Gardell et al., 2002; Ossipov et al., 2005). The molecular mechanisms leading to sustained morphine-mediated thermal hyperalgesia are not fully identified.
Painful heat stimuli regulate cation influx into small-diameter primary sensory neurons (nociceptors) by activation of nonselective (mostly calcium-permeable) transient receptor potential ion channels (such as the TRPV1-type vanilloid receptors). In addition to noxious heat, TRPV1 receptors also respond to numerous endogenous (such as protons and inflammatory substances released after tissue injury) and exogenous substances [such as 8-methyl-N-vanillyl-6-nonenamide (capsaicin) and 4-hydroxy-3-methoxy-octahydro-6a-hydroxy-8,10-dimethyl-11a-(1-methylethenyl)-7-oxo-2-(phenylmethyl)-7H-2,9-b-epoxyazuleno[5,4-e]-1,3-[benzodioxol-5-yl]benzeneacetate (resiniferatoxin)] (Caterina et al., 1997; Numazaki and Tominaga, 2004). Pain produced by activation of TRPV1 receptors in the primary peptidergic nociceptors is due to calcium influx through the TRPV1 ion channels, depolarization, and an augmented release of excitatory pain neurotransmitters (such as GGRP and substance P) (Caterina et al., 1997; Szallasi and Blumberg, 1999).
Regulation of temperature sensitivity of the transient receptor potential family of ion channels has an important role in the development of inflammatory and neuropathic thermal hyperalgesia (Zhang et al., 2005; Christoph et al., 2006). It was suggested that activation of cAMP-dependent protein kinase (PKA) plays an important role in the sensitization of the primary sensory neurons toward pain mediators (such as capsaicin and heat) by phosphorylation of the TRPV1 channel (Bhave et al., 2002; Rathee et al., 2002; Schnizler et al., 2008). Indeed, under inflammatory conditions, PKA inhibitors were found to attenuate capsaicin-evoked calcium influx in isolated DRG neurons (Lopshire and Nicol, 1998; Hu et al., 2002; Schnizler et al., 2008), to reduce capsaicin-evoked pain neurotransmitter release in the dorsal horn of the spinal cord (Lopshire and Nicol, 1998; Hu et al., 2002), and to alleviate thermal hyperalgesia in inflammatory (Taiwo and Levine, 1991) and neuropathic conditions (Stein et al., 2009).
It is interesting that recent data indicate that opioid agonists may also regulate thermal nociception by regulation of intracellular cAMP formation in primary sensory neurons. Thus, it was shown that acute treatment with opioid agonists attenuates PKA-mediated potentiation of TRPV1 responses in recombinant cells (Vetter et al., 2006) and in cultured primary sensory neurons (Oshita et al., 2005). Although it is well known that acute opioid agonist treatment reduces cellular cAMP concentration in a majority of cells (Sharma et al., 1975; Levine and Taiwo, 1989), sustained opioid agonist treatment causes a paradoxical increase in cellular cAMP formation (cAMP overshoot) after opioid drug removal. Thus, sustained incubation of NG108-15 neuroblastoma × glioma hybrid cells (Sharma et al., 1977) or recombinant human μ-opioid/Chinese hamster ovary (CHO) cells (Yue et al., 2006) with morphine or recombinant human δ-opioid/CHO cells with SNC 80 or deltorphin (Rubenzik et al., 2001) was shown to lead to cAMP overshoot after removal of the opioid ligand. In addition, our recent investigations demonstrate that sustained morphine treatment also causes cAMP overshoot in cultured primary sensory DRG neurons (Yue et al., 2008).
Previously, we investigated the molecular mechanism leading to sustained SNC 80, and morphine-mediated cAMP overshoot in recombinant CHO cells stably expressing human δ-or μ-opioid receptors, respectively. We found that sustained opioid agonist (SNC 80 or morphine) treatment leads to phosphorylation and sensitization of cellular adenylyl cyclase(s) (AC superactivation) in these cells in a Raf-1-dependent manner (Varga et al., 2003b; Yue et al., 2006). Recently, we showed that sustained morphine treatment also leads to AC superactivation in cultured neonatal rat primary sensory neurons in a Raf-1-dependent manner (Yue et al., 2008).
Based on these observations, we hypothesized that by regulation of cellular PKA activity, Raf-1-mediated AC superactivation may have an important role in sensitization of TRPV1 channels in the primary sensory neurons toward capsaicin and noxious heat. In the present study, we investigated the physiological role of Raf-1-mediated AC superactivation, in sustained morphine treatment-mediated augmentation of capsaicin-evoked CGRP release from cultured neonatal rat primary sensory (DRG) neurons in vivo.
Materials and Methods
Primary Culture of Neonatal Rat DRG Neurons. Neonatal (1–3-day-old) Sprague-Dawley rats were decapitated, and DRGs were aseptically dissected from all spinal levels. The protocol for the use of experimental animals was in compliance with the guidelines of the National Institutes of Health and has been approved by the Institutional Animal Care and Use Committee of the University of Arizona.
The isolated tissue was digested with 0.1% collagenase (Sigma-Aldrich, St. Louis, MO) (3–5 min) and 0.25% trypsin (Invitrogen, Carlsbad, CA) (10 min) in Neurobasal A medium (Invitrogen) containing 0.5 mM l-glutamine (LG), penicillin-streptomycin (PS) (1: 100; Sigma-Aldrich) (Neurobasal A/LG/PS medium) in the presence of 0.1 mg/ml DNase I (Sigma-Aldrich) and 5 mM MgSO4 and dissociated by trituration through a siliconized fire-polished Pasteur pipette. After centrifugation, the cells were resuspended in Neurobasal A/LG/PS medium containing 2% B27 (Invitrogen) (Neurobasal A/LG/PS/B27 medium) and 250 ng/ml nerve growth factor (NGF) (Sigma-Aldrich). The cells were seeded onto 24-well plates to a cell density of ∼1.6 × 104 cells/well and incubated in a humidified 5% CO2 incubator at 37°C. After 4-h incubation, antimitotic drugs [uridine (150 μM) and 5-fluo-deoxyuridine (50 μM); Sigma-Aldrich] were added to the medium to prevent the proliferation of non-neuronal cell types. The cells were allowed to differentiate for 7 to 9 days. The medium was changed every other day. On the day before the experiments, the cells were washed with NGF- and mitotic inhibitor-free Neurobasal A/LG/PS/B27 medium, and then the cells were incubated in the absence of NGF/mitotic inhibitors for 24 h.
Membrane Preparation for Radioligand Binding Experiments. DRGs were collected from ∼20 neonatal rats, washed with Ca2+, Mg2+-deficient phosphate-buffered saline (PBS) buffer, and harvested in PBS containing 0.02% EDTA. After centrifugation at 600g for 10 min, the cells were homogenized in ice-cold 10 mM Tris-HCl buffer, pH 7.4, containing 1 mM EDTA. The cells were again homogenized and then centrifuged at 20,000g and 4°C for 20 min. The membrane pellet was resuspended in the assay buffer (50 mM Tris, pH 7.4, containing 50 μg/ml bacitracin, 30 μM bestatin, 10 μM captopril, 100 μM phenylmethylsulfonyl fluoride, and 1 mg/ml bovine serum albumin). Protein concentrations were determined by using the Bradford assay (Bradford, 1976).
Opioid Receptor Binding. To determine the effect of DRG neuron differentiation on the number of opioid binding sites, isolated DRG neurons were kept in culture for increasing times (1–18 days), and cell membranes were isolated as described in the previous section. Membrane preparations (∼20 mg protein) were incubated with the nonselective opioid ligand [3H](5α,7α)-17-(cyclopropylmethyl)-4,5-epoxy-18,19-dihydro-3-hydroxy-6-methoxy-α,α-dimethyl-6,14-ethenomorphinan-7-methanol (diprenorphine) (0.1–10 nM) ([3H]DPN; PerkinElmer Life and Analytical Sciences, Boston, MA) in the assay buffer at 30°C for 90 min. The reaction was terminated by rapid filtration through GF/B glass fiber filters (Whatman, Maidstone, UK), using a cell harvester (Brandel Inc., Gaithersburg, MD). The filters were rinsed five times with 4 ml of ice-cold saline containing 0.01% bovine serum albumin. Filter-bound radioactivity was measured in Ecolite scintillation cocktail (MP Biomedicals, Irvine, CA) using an LS 6000SC liquid scintillation counter (Beckman Coulter, Fullerton, CA). Nonspecific binding was determined in the presence of 10 μM 17-(cyclopropylmethyl)-4,5α-epoxy-3,14-dihydroxymorphinan-6-one (naltrexone) (NTX; Tocris Biosciences, Ellisville, MO). Specific binding was calculated by subtracting nonspecific binding (dpm) from the total radioligand binding (dpm). The data were analyzed to calculate the concentration of specific opioid binding sites per milligram of membrane protein (femtomoles per milligram of protein) using the Prism 7.0 software (GraphPad Software Inc., San Diego, CA) and were plotted as a function of time in culture (days).
TRPV1 Receptor Binding. To assess the number of TRPV1 binding sites, we performed saturation binding assays for the selective TRPV1 agonist [3H]resiniferatoxin ([3H]RTX; PerkinElmer Life and Analytical Sciences) in a concentration range of 0.1 to 5 nM, using a protocol modified from (Endres-Becker et al., 2007). Nonspecific binding was measured in the presence of 10 μM unlabeled RTX (Sigma-Aldrich). Because adenosine, released in the process of sample preparation, was shown to interfere with the assay (Puntambekar et al., 2004), before ligand incubation the DRG cell membranes were incubated with adenosine deaminase (1 U/ml; Sigma-Aldrich) at 37°C for 15 min.
Drugs. Capsaicin and the selective Raf-1 inhibitor GW5074 were purchased from Sigma-Aldrich. H-89 and the cell-permeable PKA inhibitor PKI were purchased from Millipore (Billerica, MA) and Tocris Bioscience, respectively. Morphine sulfate was obtained from the National Institute on Drug Abuse (Bethesda, MD), and naltrexone was purchased from Tocris Cookson. GW5074 (10 mM) and H-89 (1 mM) were dissolved in dimethyl sulfoxide; PKI (1 mM), morphine sulfate (1 mM), and naltrexone (10 mM) were dissolved in double-distilled water. The stock solutions were further diluted in Neurobasal A/LG/PS/B27 medium in each case.
Drug Treatments. Twenty-four hours before the experiment, the DRG neurons were gently washed twice to remove NGF and antimitotic drugs from the culture medium. On the day of the experiment, the DRG neurons were preincubated (at 37°C for 1 h) in the absence (control) or presence of either a PKA inhibitor [H-89 (1 μM) or PKI (50 nM)] or a selective Raf-1 inhibitor [GW5074 (10 μM)]. Optimal inhibitor concentrations were selected based on inhibitor dose-response curves (Varga et al., 2003a; S. Tumati, unpublished observations). After inhibitor pretreatment, the cells were incubated with a saturating concentration of morphine (1 μM) for 24 h, in the continued presence (Mor + GW group, Mor + H-89 group, and Mor + PKI group) or absence (Mor group) of the appropriate kinase inhibitor. The concentration of morphine was adopted from our previous studies (Rubenzik et al., 2001; Varga et al., 2003a; Yue et al., 2006). To test the involvement of opioid receptors, DRG cells were also treated with the nonselective opioid receptor antagonist naltrexone (10 μM) in the presence (Mor + NTX group) or absence (NTX group) of morphine for 24 h. The concentrations of morphine and naltrexone were selected according to our previous study (Yue et al., 2008). Control cells were incubated in Neurobasal A/LG/PS/B27 medium in the absence or presence (GW or H-89 or PKI groups) of the kinase inhibitors.
Measurement of cAMP Formation. Intracellular cAMP formation was measured as described by Rubenzik et al. (2001). Briefly, after sustained morphine (1 μM; 24 h) treatment, DRG neurons were washed three times with Neurobasal A medium and incubated for 20 min with a phosphodiesterase inhibitor, 3-isobutyl-1-methylxanthine (4 mM; Sigma-Aldrich). Cells were subsequently lysed, centrifuged, and 50-μl aliquots of the supernatants were incubated with 4 nM [3H]cAMP (PerkinElmer Life and Analytical Sciences) and protein kinase A (30 μg/ml; Sigma-Aldrich) at 4°C for 2 h. cAMP standards were run in parallel with each assay. After incubation, activated charcoal (26 mg/ml; Norit Nederland BV, Amersfoort, The Netherlands) was added to adsorb free [3H]cAMP. After centrifugation, bound radioactivity was counted in Ecolite scintillation fluid (MP Biomedicals) using an LS 6000SC scintillation counter (Beckman Coulter).
Measurement of Capsaicin-Evoked CGRP Release. CGRP release was measured by modifications to the method described by Yue et al. (2008). Capsaicin (100 mM) stock solution was prepared in ethanol and diluted to the final required concentrations in Neurobasal A/LG/PG medium. CGRP release was assayed in duplicate, using an enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI). Briefly, after drug pretreatment, the cells were gently washed twice with phenol red-free Neurobasal A/LG/PS medium followed by 10-min incubation in 200 μl of fresh phenol red-free Neurobasal A medium containing different concentrations (10 nM–3 μM) of capsaicin at 37°C in a CO2 incubator. After 10 min, the medium was collected, and released CGRP was assessed according to the manufacturer's instructions, by measuring the absorbance of the final calorimetric reaction product at λ= 412 nm. Standard CGRP dose-absorbance curves (range, 0.1–500 pg/ml) were measured in parallel with each assay.
Data Analysis. One-sample t tests were used to compare normalized mean values from each treatment group to the control group (100%). One-way ANOVAs, followed by Newman-Keuls multiple comparison tests, were subsequently performed to evaluate statistical differences between treatment groups (*, p < 0.05; **, p < 0.01; and ***, p < 0.001). Radioligand binding data were analyzed by nonlinear regression. Data were represented as mean ± S.E.M., unless otherwise indicated. Statistical evaluations were performed using Prism 4.0 software (GraphPad Software Inc.).
Results
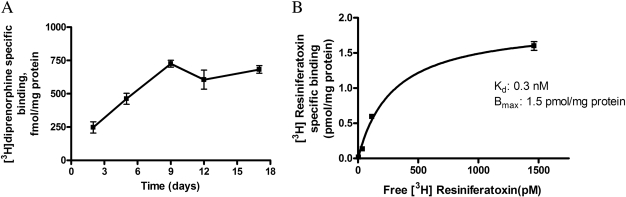
Characterization of Neonatal Rat DRG Neurons in Culture. Expression of the opioid receptor (Fig. 1A) during the course of DRG cell differentiation was monitored by radioligand binding using a nonselective opioid ligand, [3H]DPN. The number of [3H]DPN-specific binding sites steadily increased during differentiation for the first 7 to 9 days in culture and subsequently remained at maximal levels (0.7 ± 0.2 pmol/mg protein) for at least 18 days (Fig. 1A). Consequently, in all subsequent studies, we used neonatal rat DRG neurons differentiated in culture for 7 to 9 days. Expression of vanilloid TRPV1 receptors was also assessed in our model system by performing saturation binding studies with the TRPV1 agonist [3H]RTX. As shown in Fig. 1B, neonatal rat DRG neurons exhibited a high concentration of [3H]resiniferatoxin-specific binding sites (Bmax = 1.5 pmol/mg of protein) after 7 days in culture. Characterization of these 7- to 9-day-old cultures using immunocytochemical analysis showed that most of the cells in the culture were neurons, only 25 to 30% of the total cells were non-neuronal, indicating that the contribution of glia if any, to the overall effect measured in the study would be very minimal or negligible. In addition, the CGRP-expressing neurons in our cultures were found to be over a range of 55 to 65% of the total neuronal population (Tumati et al., 2008).
Fig. 1.
A, [3H]DPN-specific binding in primary sensory neurons reaches a maximal value of 725 fmol/mg protein by 9 days in culture. Values are expressed as mean ± S.E. (n = 3). Nonspecific binding was determined in the presence of 10 μM naltrexone. B, saturation binding isotherm for specific [3H]RTX in cultured (7-day) neonatal rat DRG neuron cell membranes (n = 3). Specific binding was obtained by subtracting total [3H]RTX binding (0.1–5 nM) from [3H]RTX binding in presence of 10 μM unlabeled resiniferatoxin (nonspecific binding).
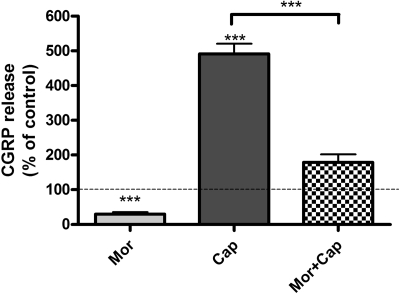
Acute Morphine Treatment Inhibits Basal and Capsaicin-Evoked CGRP Release from Cultured Neonatal Rat DRG Neurons. Acute (30-min) treatment of cultured neonatal rat DRG neurons with morphine (100 μM) significantly inhibited capsaicin-evoked CGRP release from these cells. Basal CGRP release from control neonatal rat DRG neurons after 7 days in culture was 549 ± 11 pg/ml. Incubation with a saturating concentration of morphine resulted in a 70% reduction of basal CGRP release from these cells (**, p < 0.01, one-sample t test; n = 4) (Fig. 2). Treatment of cultured neonatal rat DRG neurons with 1 μM capsaicin (concentration selected based on the dose-response curve shown in Fig. 4) for 10 min increased CGRP release to 492 ± 29% basal (***, p < 0.001, one-sample t test; n = 4). Acute treatment of cultured DRG neurons with morphine (100 μM; 30 min) attenuated capsaicin (1 μM; 10 min)-evoked CGRP release to 179 ± 23% basal, which is a 64% reduction relative to capsaicin alone (492 ± 29% basal) (***, p < 0.001, one-way ANOVA; n = 4).
Fig. 2.
Acute morphine treatment inhibits capsaicin-evoked CGRP release from cultured neonatal rat DRG neurons. Basal CGRP release from unstimulated neonatal rat DRG neurons after 7 days in culture was 549 ± 11 pg/ml. Mor, incubation with morphine (100 μM) for 30 min resulted in a 70 ± 5% reduction of basal CGRP release (**, p < 0.01, one-sample t test; n = 4). Cap, treatment of cultured neonatal rat DRG neurons with 1 μM capsaicin for 10 min increased CGRP release to 492 ± 29% basal (***, p < 0.001, one-sample t test; n = 4). Mor + Cap, acute treatment (30 min) of cultured DRG neurons with morphine (100 μM) attenuated capsaicin (1 μM; 10 min)-evoked CGRP to 179 ± 23% basal, which is a 64% reduction relative to capsaicin alone (***, p < 0.001, one way ANOVA; n = 4). The results (mean ± S.E.M.) are expressed as the percentage of basal CGRP release from unstimulated DRG neurons.
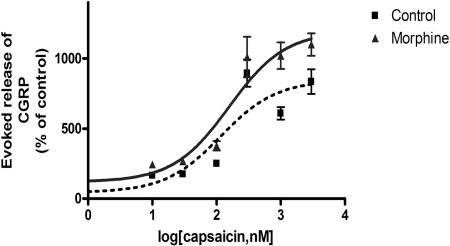
Fig. 4.
Dose-response relationship for capsaicin-evoked CGRP release before and after sustained morphine treatment. DRG neurons (7 days in culture) were incubated without (▪) or with (▴) morphine (1 μM) for 24 h followed by stimulation (10 min) with various doses of capsaicin ranging from 10 nM to 3 μM. CGRP release (mean ± S.E.M.) is expressed as the percentage of basal CGRP release (610 ± 22 pg/ml) from unstimulated DRG neurons. The dose-response curves show the mean ± S.E.M. of three individual experiments, performed in quadruplicates.
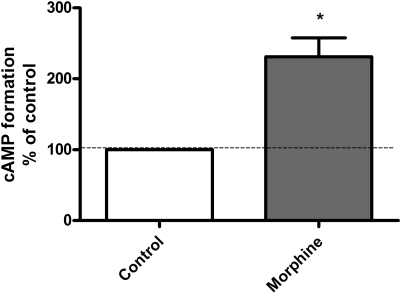
Sustained Morphine Treatment Augments Basal cAMP Formation in DRG Cells. Basal cAMP formation was 0.3 ± 1.7 pmol/50 μl (n = 4) in cultured neonatal rat DRG neurons. Sustained morphine (1 μM; 24 h) treatment augmented basal cAMP formation to 231 ± 27% of control (*, p < 0.05, one-sample t test; n = 4) (Fig. 3), indicating that sustained morphine treatment causes intracellular compensatory adaptations in the primary sensory neurons leading to augmented intracellular cAMP levels, thereby activating cAMP-dependent cellular pathways.
Fig. 3.
Sustained morphine treatment augments basal cAMP formation in DRG cells. Mor, treatment of cultured neonatal rat DRG neurons with 1 μM morphine for 24 h augmented basal cAMP formation to 231 ± 27% of control (*, p < 0.05, one-sample t test; n = 4), which is an increase of 131% over the control. The results (mean ± S.E.M.) are expressed as the percentage of cAMP formation in control unstimulated DRG neurons.
Sustained Morphine Treatment Augments Capsaicin-Evoked CGRP Release from Cultured Neonatal Rat DRG Neurons. To determine whether sustained morphine treatment affects the potency or the efficacy of capsaicin to evoke CGRP release from neonatal rat sensory neurons, we measured capsaicin dose-CGRP release curves for medium and morphine (1 μM; 24 h)-pretreated DRG cell cultures (Fig. 4). Our data indicate that although sustained morphine treatment had no effect on the EC50 value of capsaicin (116 ± 87 versus 157 ± 99 nM in control compared with morphine-pretreated cells, respectively), it significantly augmented the Emax of capsaicin-evoked CGRP release (631 ± 32% basal versus 1014 ± 51% basal; ***, p < 0.001) in control and morphine-pretreated cells, respectively (Fig. 4).
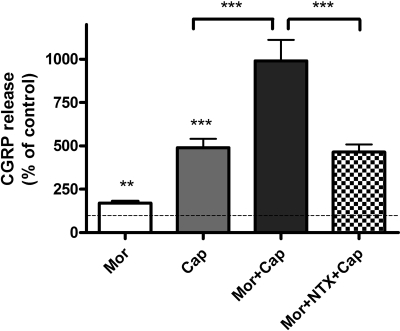
Basal CGRP release in the supernatant of unstimulated DRG cells was 610 ± 22 pg/ml (100%; n = 5). Sustained morphine (1 μM; 24 h) treatment of cultured DRG neurons augmented basal CGRP release from these cells to reach 171 ± 11% basal (**, p < 0.01, one-way ANOVA; n = 5). Capsaicin (1 μM; 10 min) treatment evoked a significant increase in CGRP release from neonatal rat DRG neurons (489 ± 52% basal; ***, p < 0.001, one-sample t test; n = 5) (Fig. 5). Sustained morphine treatment (1 μM; 24 h) significantly augmented capsaicin-evoked CGRP release to 991 ± 120% basal (corresponding to a 104% increase over capsaicin alone; ***, p < 0.001, one-way ANOVA; n = 5) (Fig. 5). Coincubation with opioid receptor antagonist naltrexone prevented sustained morphine-mediated sensitization of capsaicin-evoked CGRP release to 464 ± 44% basal, which is a 113% reduction of CGRP release compared with morphine- + capsaicin-treated cells (***, p < 0.001, compared with Mor + Cap group, one-way ANOVA; n = 5), indicating that the sensitization of CGRP release is opioid receptor-mediated.
Fig. 5.
Sustained morphine-mediated augmentation of capsaicin-evoked CGRP release is opioid receptor-mediated in cultured neonatal rat DRG neurons. Mor, 24-h incubation of cultured DRG neurons with 1 μM morphine augmented CGRP release to 171 ± 11% basal (**, p < 0.01, one-sample t test; n = 5). Cap, treatment of cultured neonatal rat DRG neurons with 1 μM capsaicin for 10 min evoked CGRP release to 489 ± 52% basal (***, p < 0.001, one-sample t test; n = 5). Mor + Cap, 24-h incubation of cultured DRG neurons with 1 μM morphine followed by removal of morphine and stimulation with 1 μM capsaicin for 10 min augmented CGRP release to 991 ± 120% basal, that is, 104% increase relative to capsaicin alone (***, p < 0.001, one-way ANOVA; n = 5). Mor + NTX + Cap, sustained morphine-mediated sensitization of capsaicin-evoked CGRP release was prevented by coincubation with the opioid antagonist naltrexone (464 ± 44% basal; ***, p < 0.001 compared with Mor + Cap group; p > 0.05 compared with Cap group; one-way ANOVA; n = 5). The results (mean ± S.E.M.) are expressed as the percentage of basal CGRP release in unstimulated DRG neurons.
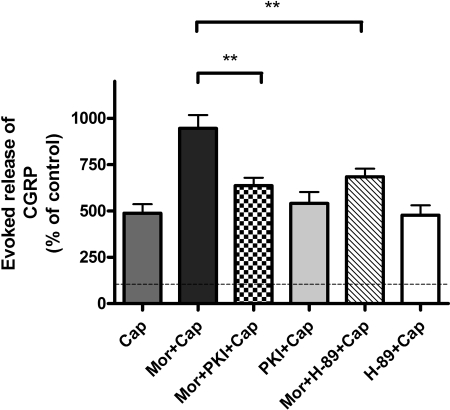
Selective PKA Inhibitors Attenuate Sustained Morphine-Mediated Sensitization of Capsaicin-Evoked CGRP Release from Neonatal Rat DRG Neurons. Treatment of neonatal rat DRG neurons with capsaicin (1 μM; 10 min) evokes CGRP release (487 ± 48% basal; ***, p < 0.001, one-sample t test; n = 5). Sustained morphine (1 μM; 24 h) treatment augmented capsaicin-evoked CGRP release to 946 ± 71% of the basal, which is a 94% increase relative to capsaicin-treated cells (***, p < 0.001 relative to Cap, one-way ANOVA; n = 5).
To determine whether PKA has a role in sustained morphine-mediated augmentation of capsaicin-evoked CGRP release, we pretreated (1 h) the cells with a PKA inhibitor, H-89 (1 μM). H-89 pretreatment attenuated sustained morphine (1 μM; 24 h)-mediated augmentation of capsaicin (1 μM; 10 min)-evoked CGRP release to 684 ± 44% basal, which is a 57% reduction relative to morphine + capsaicin group (946 ± 71% basal) (**, p < 0.01, one-way ANOVA; n = 5) (Fig. 6). Treatment of the DRG neurons with 1 μM H-89 alone had no significant effect on capsaicin-evoked CGRP release [478 ± 53% basal CGRP release; p > 0.05 relative to capsaicin alone (487 ± 48% basal), one-way ANOVA; n = 5], indicating that H-89 inhibitor exhibited no measurable cytotoxicity.
Fig. 6.
Selective PKA inhibitors attenuate sustained morphine-mediated sensitization of capsaicin evoked CGRP release from neonatal rat DRG neurons. Cap, capsaicin (1 μM) treatment (10 min) of neonatal rat DRG neurons evokes CGRP release to 487 ± 48% basal (n = 5). Mor + Cap, sustained morphine (1 μM) treatment augmented capsaicin-evoked CGRP release to 946 ± 71% basal, which is a 94% increase relative to Cap alone (***, p < 0.001 relative to Cap, one-way ANOVA; n = 5). Mor + PKI + Cap, pretreatment (1 h) of the cells with 50 nM PKI followed by 24-h coincubation with morphine (1 μM) attenuated the effect of sustained morphine treatment on capsaicin (1 μM; 10 min)-evoked CGRP release by 67% relative to Mor + Cap group (**, p < 0.01 relative to Mor + Cap, one-way ANOVA; n = 5). PKI + Cap, treatment of the DRG neurons with 50 nM PKI followed by stimulation with 1 μM capsaicin had no effect on capsaicin-evoked CGRP release [542 ± 61% basal, p > 0.05 relative to Cap (487 ± 48% basal), one-way ANOVA; n = 5]. Mor + H-89 + Cap, pretreatment (1 h) of the cells with H-89 (1 μM), followed by 24-h coincubation with the inhibitor in the presence of morphine (1 μM) attenuated sustained morphine-mediated augmentation of capsaicin (1 μM; 10 min)-evoked CGRP release by 57% relative to Mor + Cap group (**, p < 0.01, one-way ANOVA; n = 5). H-89 + Cap, treatment of the DRG neurons with 1 μM H-89 alone caused no difference in basal CGRP release relative to capsaicin alone [478 ± 53% basal, p > 0.05 relative to Cap (487 ± 48% basal), one-way ANOVA; n = 5]. The results (mean ± S.E.M.) are expressed as the percentage of basal CGRP release from unstimulated DRG neurons.
To confirm the role of PKA in sustained morphine-mediated augmentation of capsaicin-evoked CGRP release from neonatal rat DRG neurons, we also tested the effect of a more selective PKA inhibitor, PKI. Pretreatment of the cells with PKI (50 nM; 1 h), followed by 24-h coincubation with morphine (1 μM), also significantly attenuated the effect of sustained morphine treatment on capsaicin (1 μM; 10 min)-evoked CGRP release to 637 ± 43% basal, which is a 67% reduction relative to morphine + capsaicin group (946 ± 71%) (**, p < 0.01, one-way ANOVA; n = 5) (Fig. 6). Treatment of the DRG neurons with 50 nM PKI alone had no significant effect on capsaicin-evoked CGRP release [542 ± 61% basal; p > 0.05 relative to capsaicin alone (487 ± 48% basal), one-way ANOVA; n = 5], indicating that PKI treatment was not toxic for the cultured neonatal rat DRG neurons.
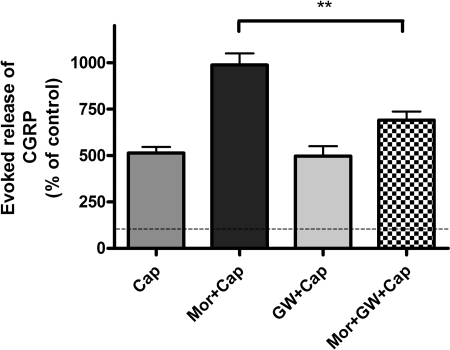
The Selective Raf-1 Inhibitor GW5074 Attenuates Sustained Morphine-Mediated Augmentation of Capsaicin-Evoked CGRP Release from Neonatal Rat DRG Neurons. To test whether Raf-1-mediated AC superactivation contributes to sustained morphine-mediated PKA activation and the resulting augmentation of capsaicin-evoked CGRP release, we pretreated (1 h) the cells with a selective Raf-1 inhibitor, GW5074 (10 μM). Preincubation with the Raf-1 inhibitor, followed by 24-h coincubation in the presence of 1 μM morphine, attenuated capsaicin (1 μM; 10 min)-evoked CGRP release to 691 ± 47% basal [a 67% reduction relative to the Mor + Cap group (989 ± 62% basal); **, p < 0.01, one-way ANOVA; n = 5] (Fig. 7). Treatment of the DRG neurons with 10 μM GW5074 alone had no significant effect on capsaicin-evoked CGRP release from cultured DRG neurons [498 ± 53% basal; p > 0.05 relative to capsaicin alone (472 ± 38% basal), one-way ANOVA; n = 5], indicating that GW5074 exhibited no cytotoxic effects (Fig. 7).
Fig. 7.
The selective Raf-1 inhibitor GW5074 attenuates sustained morphine-mediated augmentation of capsaicin-evoked CGRP release from neonatal rat DRG neurons. Cap, capsaicin (1 μM) treatment (10 min) of neonatal rat DRG neurons evokes CGRP release to 514 ± 33% basal (n = 5). Mor + Cap, sustained morphine (1 μM) treatment augmented capsaicin-evoked CGRP release by 92% (989 ± 62% basal, ***, p < 0.001 relative to Cap, one-way ANOVA; n = 5). Mor + GW + Cap, pretreatment (1 h) of the cells with GW5074 (10 μM), followed by 24-h coincubation in the presence of morphine (1 μM), attenuated capsaicin (1 μM; 10 min)-evoked CGRP release to 691 ± 47% basal, which is a 67% reduction of CGRP release to that of Mor + Cap group (**, p < 0.01, one-way ANOVA; n = 5). GW + Cap, treatment of the DRG neurons with GW5074 (10 μM) alone had no effect on capsaicin-evoked CGRP release [498 ± 53% basal, p > 0.05 to Cap (514 ± 33% basal), one-way ANOVA; n = 5]. The results (mean ± S.E.M.) are expressed as the percentage of basal CGRP release from unstimulated DRG neurons.
Discussion
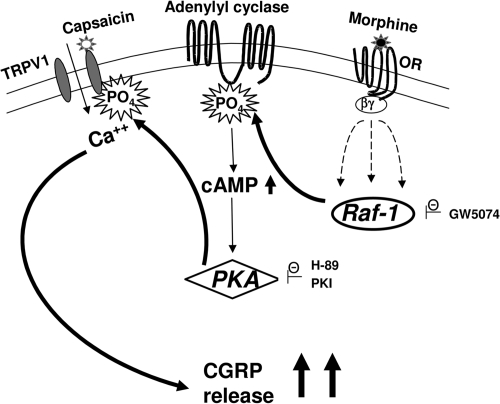
Our present investigation demonstrates the following: 1) sustained morphine treatment augments basal cAMP formation in cultured neonatal rat primary sensory neurons (Fig. 3); 2) sustained morphine treatment augments capsaicin-evoked CGRP release from cultured neonatal rat primary sensory neurons (Figs. 4 and 5); 3) sustained morphine-mediated augmentation of intracellular cAMP formation (cAMP/PKA signaling) has an important role in regulation of capsaicin-evoked CGRP release in primary sensory neurons (Fig. 6); and 4) Raf-1 inhibitors attenuate sustained morphine-mediated regulation of capsaicin-evoked CGRP release (Fig. 7). Accordingly, we hypothesize that, similar to our previous findings in recombinant cell lines (Rubenzik et al., 2001; Varga et al., 2003a,b; Yue et al., 2006, 2008), sustained morphine-mediated activation of Raf-1 may lead to phosphorylation and sensitization of adenylyl cyclase isoenzyme(s) in neonatal rat DRG neurons (AC superactivation). The resulting increase in basal and/or stimulated cellular cAMP formation (cAMP overshoot) increases cAMP-dependent protein kinase (PKA) activity that in turn leads to phosphorylation and sensitization of the heat-sensing TRPV1 vanilloid receptors (Fig. 8). Because we have used isolated neonatal rat DRG neurons in our experiments, our data also demonstrate that in addition to neural system adaptations (such as descending facilitation and activation of spinal glia), sustained morphine treatment also leads to intracellular compensatory adaptations in the primary sensory neurons themselves. We suggest that such intracellular compensatory adaptations may have a crucial trigger role in the further intercellular adaptations.
Fig. 8.
Putative molecular mechanism underlying sustained morphine-mediated sensitization of capsaicin evoked CGRP release in primary sensory neurons. Stimulation of the opioid receptor (OR) by an opioid agonist (morphine) in the primary sensory DRG neurons liberates G protein βγ subunits. The free G protein βγ subunits interact with multiple effectors, leading to the activation of Raf-1 through multiple parallel pathways (Varga et al., 2003a). Activated Raf-1 phosphorylates and sensitizes adenylyl cyclase VI (AC VI), leading to up-regulation of cAMP (cAMP overshoot). Increased cAMP activity leads to increased activation of cAMP-dependent protein kinase (PKA). PKA phosphorylates TRPV1 channels, increasing the efficacy of capsaicin to stimulate calcium influx into the sensory neurons, leading to augmented pain neurotransmitter (CGRP) release. The putative molecular targets of the inhibitors used in the present work are indicated in the figure. GW5074 is a selective Raf-1 inhibitor, whereas H-89 and PKI are nonselective and selective PKA inhibitors, respectively.
Opioid receptors are expressed in neuronal cells throughout the pain-modulatory pathway, including the peripheral and central termini of the primary afferent neurons. Several studies have shown that on the cellular level, acute opioid treatment inhibits intracellular cAMP formation and regulates the activity of multiple ion channels (Sharma et al., 1975; Nestler and Aghajanian, 1997; Mayer et al., 1999), thus reducing neuronal excitability and inhibiting presynaptic excitatory neurotransmitter release in the pain transmission pathway (Mao et al., 1995; Ossipov et al., 2005; Trang et al., 2005). Accordingly, our study demonstrates that acute morphine (opioid agonist) treatment inhibits capsaicin-evoked CGRP release (Fig. 2) from the cultured primary sensory neurons. It is interesting that recent data indicate acute opioid exposure-mediated changes in intracellular cAMP formation may contribute to the regulation of capsaicin (and/or noxious heat)-mediated pain neurotransmitter release from sensory neurons (Endres-Becker et al., 2007). In contrast, it was shown previously that sustained morphine exposure paradoxically augments pain neurotransmitter (such as CGRP) release in the dorsal horn of the spinal cord in response to the heat-sensing TRPV1 receptor agonist capsaicin (Gardell et al., 2002). In the present study, we show for the first time that sustained morphine treatment sensitizes capsaicin-evoked CGRP release from isolated primary sensory neurons in vitro, highlighting that in addition to the suggested mechanisms, such as descending facilitation or glial excitatory neurotransmitter release, intracellular changes in the sensory neurons themselves also contribute to sustained morphine-mediated effects. The molecular mechanism of sustained morphine-mediated augmentation of CGRP release from the sensory neurons is not known. However, cellular cAMP formation was shown to have a role in the regulation of the sensitivity of primary sensory neurons toward noxious heat stimuli. Thus, it was demonstrated that increased cellular cAMP formation and the resulting activation of PKA are involved in the development of inflammatory hyperalgesia and neurogenic inflammation (Taiwo and Levine, 1991). Such increased inflammatory and neuropathic heat sensitivity may, at least in part, be due to PKA-mediated phosphorylation of the vanilloid type 1 (TPRV1) ion channels in primary sensory neurons (Bhave et al., 2002; Rathee et al., 2002; Schnizler et al., 2008).
It is interesting that although acute treatment with opioids inhibit cAMP formation in the majority of mammalian cells, sustained opioid agonist treatment leads to a compensatory increase in the catalytic activity of adenylyl cyclase(s) (AC superactivation) that becomes manifest as a sudden increase in cellular cAMP formation upon withdrawal of the opioid (cAMP overshoot) (Sharma et al., 1977; Rubenzik et al., 2001; Yue et al., 2006). Our recent investigations demonstrate that sustained morphine treatment also augments forskolin-stimulated cAMP formation in a physiological environment, in cultured DRG neurons (Yue et al., 2008). Furthermore, in the present study (Fig. 3), we show that sustained morphine treatment also augments basal cAMP formation in cultured DRG neurons. Accordingly, we hypothesized that cAMP overshoot and the resulting activation of cAMP-dependent protein kinase—upon sustained opioid agonist treatment include similar molecular mechanisms identified in the development of inflammatory hyperalgesia and neurogenic inflammation (Bhave et al., 2002; Rathee et al., 2002; Schnizler et al., 2008)—may lead to PKA-mediated phosphorylation and sensitization of TRPV1 channels in the peripheral termini of morphine-treated peptidergic small-diameter sensory neurons. Immunohistochemical staining studies indicate that TRPV1 channels, opioid receptors, and CGRP are coexpressed in isolectin B4-binding, glycoprotein-positive, small-diameter DRG neurons (Aoki et al., 2005).
Sustained opioids may cause intracellular compensatory adaptations due to multiple molecular mechanisms in the primary sensory neurons. Thus, sustained morphine treatment was found to increase TRPV1 receptor expression (Chen et al., 2008) and to augment CGRP and substance P concentrations in isolated DRG neuron cultures (Ma et al., 2000). Our data (Figs. 4 and 5) indicate that sustained morphine treatment causes no change in the potency (EC50) of capsaicin but increases its intrinsic activity, evoked CGRP release (Emax), in cultured neonatal rat DRG neurons. Because we and others have demonstrated previously that significant increase in receptor density causes a leftward shift in the EC50 values of agonists, we propose that the effect of sustained morphine treatment for 24 h may rather be due to a regulation of the opening kinetics of the TRPV1 ion channel.
In the present study, we investigated the hypothesis that sustained morphine-mediated cAMP overshoot and the resulting activation of PKA play a role in sustained morphine-mediated regulation of TRPV1 channels, by measuring the effect of PKA inhibitors on capsaicin-evoked CGRP release in vitro in neonatal rat DRG neurons. Our data indicate that selective PKA inhibitors, such as H-89 and PKI, significantly inhibit sustained morphine-mediated augmentation of capsaicin-evoked CGRP release from cultured primary sensory neurons (Fig. 6). These data suggest that 1) PKA regulates capsaicin-evoked CGRP release; and 2) augmentation of basal cAMP formation may be sufficient to achieve PKA activation and TRPV1 channel phosphorylation in cultured DRG neurons, even in the absence of extracellular excitatory modulators.
Our previous data indicated that sustained opioid agonist treatment-mediated AC superactivation and the resulting cAMP overshoot are Raf-1-dependent (Varga et al., 2003b; Yue et al., 2006). Recently, we also demonstrated the physiological importance of this molecular mechanism and have shown that sustained morphine treatment also causes a Raf-1-dependent AC superactivation in cultured neonatal rat DRG neurons (Yue et al., 2008). Therefore, in the present study, we investigated the role of Raf-1-mediated cAMP overshoot upon sustained opioid agonist treatment on capsaicin-evoked CGRP release in vitro in neonatal rat DRG neurons. Our data indicate that selective Raf-1 inhibitor GW 5074 significantly attenuates sustained morphine-mediated augmentation of capsaicin-evoked CGRP release from cultured primary sensory neurons (Fig. 7), suggesting the role of Raf-1 kinase in regulation of thermal hypersensitivity.
Raf-1 is activated by multiple parallel intracellular signaling cascades. Raf-1 activation initiates the mitogen-activated protein kinase (MAPK) cascade by activating extracellular signal-regulated kinase that, in turn, phosphorylates p42/44 MAPK and other serine-threonine kinases (Morrison and Cutler, 1997). Raf-1-mediated activation of the MAPK pathway plays an important role in the regulation of neurotransmitter gene transcription and synthesis rate. However, interestingly, that current data show that activation of the MAPK pathway is not the only—and possibly not even the most important—function of Raf-1 (Galabova-Kovacs et al., 2006). Indeed, data from our laboratory (Varga et al., 2003a) and from others (Tan et al., 2001) indicated that Raf-1 may have an important role in regulating cellular cAMP pathways by phosphorylation and sensitization of adenylyl cyclase isoenzymes. Our present data demonstrate that both Raf-1 and the cAMP pathway play an important role in sustained morphine-mediated regulation of CGRP release from primary sensory neurons. However, the relative role of Raf-1-mediated MAPK activation versus Raf-1-mediated AC phosphorylation in regulation of CGRP release is presently not clear, and further investigations are necessary to distinguish between these pathways.
In conclusion, our present study indicates the physiological relevance of Raf-1-mediated AC superactivation. Our results show that Raf-1 and PKA play a significant role in regulation of capsaicin-evoked pain neurotransmitter (CGRP) release upon sustained morphine treatment from primary sensory neurons. We propose that sustained morphine-mediated intracellular compensatory adaptations in the primary sensory neurons play a crucial trigger role in the further neuronal system adaptations.
Acknowledgments
We thank Dr. Paul A. St. John (Department of Cell Biology and Anatomy, University of Arizona) for providing facilities for dissection of DRG neurons.
This work was supported in part by the National Institutes of Health National Institute on Drug Abuse [Grant DA06284]; and the National Institutes of Health National Institute of General Medical Sciences [Grant GM065465].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.151704.
ABBREVIATIONS: TRPV, transient receptor potential vanilloid; CGRP, calcitonin gene-related peptide; PKA, protein kinase A; DRG, dorsal root ganglion; CHO, Chinese hamster ovary; SNC 80, (+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide; AC, adenylyl cyclase; LG, l-glutamine; PS, penicillin-streptomycin; NGF, nerve growth factor; PBS, phosphate-buffered saline; DPN, diprenorphine; NTX, naltrexone; GW5074 (GW), 3-(3,5-dibromo-4-hydroxybenzylidene-5-iodo-1,3-dihydro-indol-2-one; H-89, N-[2-((p-bromocinnamyl)amino)ethyl]-5-isoquinoline sulfonamide, 2HCl; PKI, myristoylated protein kinase A inhibitor 14-22 amide; Mor, morphine; ANOVA, analysis of variance; Cap, capsaicin; MAPK, mitogen-activated protein kinase; B27, 0.1 μg/ml biotin, 2.0 μg/ml l-carnitine, 15 μg/ml d-(+)-galactose, 1 μg/ml ethanolamine, 16.1 μg/ml putrescine, 0.016 μg/ml selenium, 0.02 μg/ml corticosterone, 1 μg/ml linoleic acid, 0.0063 μg/ml progesterone, 0.1 μg/ml retinyl acetate, 1 μg/ml dl-α-tocopherol, 1 μg/ml dl-α-tocopherol acetate, 2.5 μg/ml catalase, 4 μg/ml insulin, 2.5 μg/ml superoxide dismutase, 5 μg/ml human transferrin, 2.5 mg/ml albumin, 1 μg/ml reduced glutathione, 0.002 μg/ml triiodo-l-thyrosine.
References
- Aoki Y, Ohtori S, Takahashi K, Ino H, Douya H, Ozawa T, Saito T, and Moriya H (2005) Expression and co-expression of VR1, CGRP, and IB4-binding glycoprotein in dorsal root ganglion neurons in rats: differences between the disc afferents and the cutaneous afferents. Spine 30 1496-1500. [DOI] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, and Gereau RW 4th (2002) cAMP-Dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 35 721-731. [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248-254. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, and Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389 816-824. [DOI] [PubMed] [Google Scholar]
- Chen Y, Geis C, and Sommer C (2008) Activation of TRPV1 Contributes to morphine tolerance: involvement of the mitogen-activated protein kinase signaling pathway. J Neurosci 28 5836-5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph T, Grünweller A, Mika J, Schäfer MK, Wade EJ, Weihe E, Erdmann VA, Frank R, Gillen C, and Kurreck J (2006) Silencing of vanilloid receptor TRPV1 by RNAi reduces neuropathic and visceral pain in vivo. Biochem Biophys Res Commun 350 238-243. [DOI] [PubMed] [Google Scholar]
- Endres-Becker J, Heppenstall PA, Mousa SA, Labuz D, Oksche A, Schäfer M, Stein C, and Zöllner C (2007) μ-Opioid receptor activation modulates transient receptor potential vanilloid 1 (TRPV1) currents in sensory neurons in a model of inflammatory pain. Mol Pharmacol 71 12-18. [DOI] [PubMed] [Google Scholar]
- Galabova-Kovacs G, Kolbus A, Matzen D, Meissl K, Piazzolla D, Rubiolo C, Steinitz K, and Baccarini M (2006) ERK and beyond: insights from B-Raf and Raf-1 conditional knockouts. Cell Cycle 5 1514-1518. [DOI] [PubMed] [Google Scholar]
- Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP Jr, Lai J, and Porreca F (2002) Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J Neurosci 22 6747-6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingtgen CM, Waite KJ, and Vasko MR (1995) Prostaglandins facilitate peptide release from rat sensory neurons by activating the adenosine 3′,5′-cyclic monophosphate transduction cascade. J Neurosci 15 5411-5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Bhave G, and Gereau RW 4th (2002) Prostaglandin and PKA-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. J Neurosci 22 7444-7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppert W (2004) Opioid-induced hyperalgesia. Pathophysiology and clinical relevance. Anaesthesist 53 455-466. [DOI] [PubMed] [Google Scholar]
- Levine JD and Taiwo YO (1989) Involvement of the mu-opiate receptor in peripheral analgesia. Neuroscience 32 571-575. [DOI] [PubMed] [Google Scholar]
- Lopshire JC and Nicol GD (1998) The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole-cell and single-channel studies. J Neurosci 18 6081-6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Zheng WH, Kar S, and Quirion R (2000) Morphine treatment induced calcitonin gene-related peptide and substance P increases in cultured dorsal root ganglion neurons. Neuroscience 99 529-539. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, and Mayer DJ (1995) Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain 62 259-274. [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Mao J, Holt J, and Price DD (1999) Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci U S A 96 7731-7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison DK and Cutler RE (1997) The complexity of Raf-1 regulation. Curr Opin Cell Biol 9 174-179. [DOI] [PubMed] [Google Scholar]
- Nestler EJ and Aghajanian GK (1997) Molecular and cellular basis of addiction. Science 278 58-63. [DOI] [PubMed] [Google Scholar]
- Numazaki M and Tominaga M (2004) Nociception and TRP channels. Curr Drug Targets CNS Neurol Disord 3 479-485. [DOI] [PubMed] [Google Scholar]
- Oshita K, Inoue A, Tang HB, Nakata Y, Kawamoto M, and Yuge O (2005) CB(1) cannabinoid receptor stimulation modulates transient receptor potential vanilloid receptor 1 activities in calcium influx and substance P Release in cultured rat dorsal root ganglion cells. J Pharmacol Sci 97 377-385. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, King T, Vanderah TW, and Porreca F (2005) Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers 80 319-324. [DOI] [PubMed] [Google Scholar]
- Puntambekar P, Van Buren J, Raisinghani M, Premkumar LS, and Ramkumar V (2004) Direct interaction of adenosine with the TRPV1 channel protein. J Neurosci 24 3663-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, and Kress M (2002) PKA/AKAP/VR-1 module: a common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci 22 4740-4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenzik M, Varga E, Stropova D, Roeske WR, and Yamamura HI (2001) Expression of α-transducin in Chinese hamster ovary cells stably transfected with the human δ-opioid receptor attenuates chronic opioid agonist-induced adenylyl cyclase superactivation. Mol Pharmacol 60 1076-1082. [DOI] [PubMed] [Google Scholar]
- Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, Strack S, Hell JW, and Usachev YM (2008) Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci 28 4904-4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Klee WA, and Nirenberg M (1975) Dual regulation of adenylyl cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci U S A 72 3092-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Klee WA, and Nirenberg M (1977) Opiate-dependent modulation of adenylate cyclase. Proc Natl Acad Sci U S A 74 3365-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C, Clark JD, Oh U, Vasko MR, Wilcox GL, Overland AC, Vanderah TW, and Spencer RH (2009) Peripheral mechanisms of pain and analgesia. Brain Res Rev 60 90-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A and Blumberg PM (1999) Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev 51 159-212. [PubMed] [Google Scholar]
- Taiwo YO and Levine JD (1991) Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience 44 131-135. [DOI] [PubMed] [Google Scholar]
- Tan CM, Kelvin DJ, Litchfield DW, Ferguson SS, and Feldman RD (2001) Tyrosine kinase-mediated serine phosphorylation of adenylyl cyclase. Biochemistry 40 1702-1709. [DOI] [PubMed] [Google Scholar]
- Trang T, Quirion R, and Jhamandas K (2005) The spinal basis of opioid tolerance and physical dependence: involvement of calcitonin gene-related peptide, Substance P and arachidonic acid-derived metabolites. Peptides 26 1346-1355. [DOI] [PubMed] [Google Scholar]
- Tumati S, Yamamura HI, Vanderah TW, Roeske WR, and Varga EV (2008). Sustained morphine treatment augments PGE2-evoked CGRP release from cultured neonatal rat DRG neurons, in Proceedings of the Annual Meeting of the Society of Neuroscience; 2008 November 15–19; Washington, DC. Abstract 630.2/D26, Society of Neuroscience, Washington, DC.
- Vanderah TW, Gardell LR, Burgess SE, Ibrahim M, Dogrul A, Zhong CM, Zhang ET, Malan TP Jr, Ossipov MH, Lai J, et al. (2000) Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J Neurosci 20 7074-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga EV, Rubenzik MK, Stropova D, Sugiyama M, Grife V, Hruby VJ, Rice KC, Roeske WR, and Yamamura HI (2003a) Converging protein kinase pathways mediate adenylyl cyclase superactivation upon chronic δ-opioid agonist treatment. J Pharmacol Exp Ther 306 109-115. [DOI] [PubMed] [Google Scholar]
- Varga EV, Yamamura HI, Rubenzik MK, Stropova D, Navratilova E, and Roeske WR (2003b) Molecular mechanisms of excitatory signaling upon chronic opioid agonist treatment. Life Sci 74 299-311. [DOI] [PubMed] [Google Scholar]
- Vetter I, Wyse BD, Monteith GR, Roberts-Thomson SJ, and Cabot PJ (2006) The μ opioid agonist morphine modulates potentiation of capsaicin-evoked TRPV1 responses through a cyclic AMP-dependent protein kinase A pathway. Mol Pain 2 22-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Tumati S, Navratilova E, Strop D, St John PA, Vanderah TW, Roeske WR, Yamamura HI, and Varga EV (2008) Sustained morphine treatment augments basal CGRP release from cultured primary sensory neurons in a Raf-1 dependent manner. Eur J Pharmacol 584 272-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Varga EV, Stropova D, Vanderah TW, Yamamura HI, and Roeske WR (2006) Chronic morphine-mediated adenylyl cyclase superactivation is attenuated by the Raf-1 inhibitor, GW5074. Eur J Pharmacol 540 57-59. [DOI] [PubMed] [Google Scholar]
- Zhang N, Inan S, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, Caterina M, and Oppenheim JJ (2005) A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci U S A 102 4536-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]