Abstract
In mouse models of cardiac disease, the type 5 (PDE5)-selective cyclic nucleotide phosphodiesterase inhibitor sildenafil has antihypertrophic and cardioprotective effects attributable to the inhibition of cGMP hydrolysis. To investigate the relevance of these findings to humans, we quantified cGMP-hydrolytic activity and its inhibition by sildenafil in cytosolic and microsomal preparations from the left ventricular myocardium of normal and failing human hearts. The vast majority of cGMP-hydrolytic activity was attributable to PDE1 and PDE3. Sildenafil had no measurable effect on cGMP hydrolysis at 10 nM, at which it is selective for PDE5, but it had a marked effect on cGMP and cAMP hydrolysis at 1 μM, at which it inhibits PDE1. In contrast, in preparations from the left ventricles of normal mice and mice with heart failure resulting from coronary artery ligation, the effects of sildenafil on cGMP hydrolysis were attributable to inhibition of both PDE5 and PDE1; PDE5 comprised ∼22 and ∼43% of the cytosolic cGMP-hydrolytic activity in preparations from normal and failing mouse hearts, respectively. These differences in PDE5 activities in human and mouse hearts call into question the extent to which the effects of sildenafil in mouse models are likely to be applicable in humans and raise the possibility of PDE1 as an alternative therapeutic target.
The PDE5-selective cyclic nucleotide phosphodiesterase inhibitor sildenafil is used in the treatment of erectile dysfunction and pulmonary hypertension. Its actions in these diseases result from its inhibition of cGMP-hydrolytic activity in vascular smooth muscle myocytes and the consequent increases in intracellular cGMP content and the potentiation of protein kinase G-mediated vasodilatory responses (Francis and Corbin, 2005; Kass et al., 2007; Movsesian et al., 2008).
Studies in mouse models of cardiac disease suggest that sildenafil may have additional beneficial effects in cardiac disease, including antihypertrophic actions after transverse aortic constriction and cardioprotective effects after ischemic injury or exposure to doxorubicin, that result from inhibition of myocardial cGMP-hydrolytic activity (Salloum et al., 2003; Das et al., 2004, 2005, 2008; Fisher et al., 2005; Hassan and Ketat, 2005; Takimoto et al., 2005; Salloum et al., 2008). “Seminal studies in animal models of pressure overload” are included in the rationale underlying a current clinical trial of sildenafil in patients with diastolic heart failure sponsored by the National Institutes of Health (https://www.hfnetwork.org/hf-trials/relax-trial). In view of interspecies differences in the phosphodiesterases that are expressed in individual tissues, however, the relevance of these effects in mouse models to the treatment of heart disease in humans is unknown. In previous studies in normal human left ventricular myocardium, we found that the vast majority of cGMP-hydrolytic activity was attributable to enzymes in the PDE1 (Ca2+/calmodulin-stimulated) and PDE3 families (Hambleton et al., 2005; Vandeput et al., 2007). However, PDE5 expression has been reported to be low in normal human right-ventricular myocardium but increased in hypertrophic human right-ventricular myocardium, and a recent study has shown an increase in PDE5 activity in the explanted left ventricles of heart transplant recipients with dilated cardiomyopathy (Nagendran et al., 2007; Pokreisz et al., 2009). The issue is further complicated because, at higher concentrations, the selectivity of sildenafil for PDE5 is reduced (Bischoff, 2004), and the relatively high concentrations of sildenafil used in some of the experiments in animal models raise the possibility that some of its myocardial effects may have been attributable to inhibition of PDE1 (Das et al., 2005; Fisher et al., 2005; Nagendran et al., 2007; Pokreisz et al., 2009).
To gain insight into the contributions of different phosphodiesterases to cGMP-hydrolytic activity in failing human left ventricular myocardium and to the potential actions of sildenafil in this tissue, we characterized cGMP-hydrolytic activity and its inhibition by sildenafil in subcellular preparations from nonfailing (referred to herein as “normal”) and failing human left ventricular myocardium and in comparable preparations from the left ventricles of normal mice and of mice that had undergone coronary artery ligation 7 days earlier.
Materials and Methods
Tissue Sources. Human myocardium was obtained from the left ventricular free walls of the hearts of organ donors for whom no suitable recipients were identified at the time of organ procurement (normal hearts) and of the explanted hearts of patients with idiopathic dilated cardiomyopathy undergoing cardiac transplantation (failing hearts) as described previously (Movsesian et al., 1989). Tissue was immediately placed on ice for dissection and quick-frozen in liquid nitrogen immediately thereafter.
The left ventricles of untreated (normal) mouse hearts were obtained from 50 8-week-old male ICR mice. Twenty-five other male ICR mice were anesthetized by injection of pentobarbital (70 mg/kg i.p.), intubated orotracheally, and ventilated on positive pressure at a tidal volume of 0.2 ml and a ventilatory rate of 133 cycles/min. A left thoracotomy was performed at the fourth intercostal space, and the heart was exposed by stripping the pericardium. The left descending coronary artery was occluded by a 7.0 silk ligature. After coronary artery ligation, air was expelled from the chest. Animals were extubated and received doses of analgesia buprenorphine hydrochloride (Buprenex; Bedford Laboratories, Bedford, OH; 0.02 mg/kg i.m. every 12 h for 3 days) and antibiotic (gentamicin; 0.7 mg/kg i.m. for 3 days). Seven days later, after mice were anesthetized with pentobarbital (100 mg/kg i.p.) and echocardiography was performed, the hearts were excised, placed on ice for dissection, and quick-frozen in liquid nitrogen immediately thereafter. All animal studies were performed according to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, the American Physiological Society, and Virginia Commonwealth University.
Functional Characterization of Mouse Hearts. Doppler echocardiography was performed under light anesthesia with pentobarbital (30 mg/kg i.p.) by use of the Vevo770 imaging system (VisualSonics Inc., Toronto, Canada) before euthanizing the animal. A 30-MHz probe was used to obtain two-dimensional, M-mode, and Doppler images from parasternal short-axis views at the level of the papillary muscles. M-mode images were used to measure systolic and diastolic wall thickness (anterior and posterior) and left ventricular end-systolic and end-diastolic diameters. Left ventricular fractional shortening was calculated as (left ventricular end-diastolic diameter minus left ventricular end-systolic diameter)/(left ventricular end-diastolic diameter).
Preparation of Cytosolic and Microsomal Fractions of Left Ventricular Myocardium. With use of samples from three to four hearts for each preparation from human tissue and from 25 to 50 hearts for preparations from mouse tissue, cytosolic and microsomal fractions were prepared by homogenization and differential sedimentation by a protocol adapted from previously published methods (Movsesian et al., 1989). Tissue was homogenized for two 10-s cycles in a Kinematica homogenizer (setting 11; Kinematica GmbH, Littau-Lucerne, Switzerland) in 5 volumes of 0.29 M sucrose, 3 mM NaN3, and 10 mM 3-[N-morpholino]propanesulfonic acid, pH 7.0, at 4°C, to which were added 1 mM dithiothreitol, 1 mM benzamidine, 2 mM EGTA, 0.8 mM phenylmethylsulfonyl fluoride, and 1 μg/ml each of pepstatin A, leupeptin, and antipain (sucrose buffer). After a 20-min sedimentation at 7,740g to remove debris, the supernatant was sedimented for 60 min at 40,445g. The resulting supernatant was used as the “cytosolic” fraction. The pellet was resuspended by hand-homogenization (glass-glass) in 2 volumes (relative to starting material) of 0.6 M KCl, 3 mM NaN3, and 10 mM 3-[N-Morpholino]propanesulfonic acid, pH 7.0, at 4°C, with 1 mM dithiothreitol, 1 mM benzamidine, and 1 μg/ml each of pepstatin A, leupeptin, and antipain. After resedimentation for 40 min at 153,376g, the pellet was resuspended in sucrose buffer (without EGTA and phenylmethylsulfonyl fluoride) by use of a Teflon-glass hand homogenizer, quick-frozen, and stored at -80°C until use as the “microsomal” fraction. Protein was quantified by Bradford's method with bovine serum albumin fraction V as the standard.
Preparation of Recombinant Proteins. Full-length recombinant human PDE1C1 and recombinant human PDE3A1 were expressed in Sf9 cells and prepared as described previously (Hambleton et al., 2005; Vandeput et al., 2007). Recombinant human PDE5 was expressed in Sf9 cells with the baculovirus system and purified to homogeneity as described previously (Zoraghi et al., 2007).
Immunodetection of PDE5 Protein. Subcellular preparations from human and mouse left ventricular myocardium were subjected to SDS-polyacrylamide gel electrophoresis and electrophoretic transfer to nitrocellulose membranes (Vandeput et al., 2007). The latter were incubated with anti-PDE5 antibody (number 2395, 1:1000; Cell Signaling Technology Inc., Danvers, MA), and PDE5 protein was visualized by use of horseradish peroxidase-conjugated anti-rabbit antibody (1:10.000; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and enhanced chemiluminescence reagent (GE Healthcare, Little Chalfont, Buckinghamshire, UK). The density of PDE5 bands was quantified by analyzing autoradiograms made over a range of exposure times with use of the Image J Program (http://rsbweb.nih.gov/ij/).
Cyclic Nucleotide-Hydrolytic Activity. Cyclic nucleotide-hydrolytic activity was quantified at 30°C by the two-step snake-venom method with [3H]cAMP or [3H]cGMP as substrate (Kincaid and Manganiello, 1988). The amount of protein used per assay and the incubation times were adjusted to ensure that no more than 20% of the total cyclic nucleotide was hydrolyzed during the assay. EGTA, CaCl2, and calmodulin (provided by Donald K. Blumenthal, University of Utah) were included as indicated. Activity was measured at 0.1 μM cGMP or cAMP in the presence of 200 μMCa2+ and 50 nM calmodulin or in the presence of 100 μM EGTA to determine Ca2+/calmodulin-stimulated PDE1 activity; Ca2+/calmodulin-stimulated PDE1 activity was quantified as the difference between cyclic nucleotide-hydrolytic activity measured under these conditions. PDE3 and PDE5 activities were measured in the presence of 100 μM EGTA with the PDE3-selective inhibitor cilostazol (Otsuka, Tokyo, Japan) and the PDE5-selective inhibitor sildenafil (Francis et al., 2007; Kambayashi et al., 2007; Vandeput et al., 2007). To minimize possible inhibition of other phosphodiesterase families, measurements were made at concentrations of drug that inhibited the cyclic nucleotide-hydrolytic activity of the corresponding recombinant protein by 30 to 50%. PDE3 activity, for example, was calculated by dividing the amount of activity inhibited by cilostazol in subcellular fractions by the fractional inhibition of recombinant PDE3A1 (rtPDE3A1) activity at the same cilostazol concentration; thus, if the concentration of cilostazol used in the experiment inhibited rtPDE3A1 activity by 50%, the activity inhibited by the drug in subcellular fractions was divided by 0.50 to determine the amount of PDE3 in these fractions (Hambleton et al., 2005; Vandeput et al., 2007).
Statistical Analysis. For comparison of two groups of samples normally distributed, Student's two-tailed t test was used. IC50 values were calculated with use of Prism software version 2.01 (GraphPad Software Inc., San Diego, CA).
Results
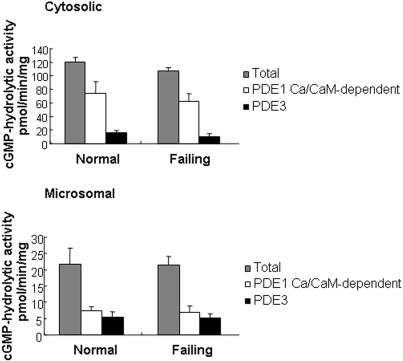
PDE1 and PDE3 Activity in Normal and Failing Hearts. Previous studies demonstrated that PDE1 and PDE3 constitute the majority of cGMP-hydrolytic activity in subcellular fractions prepared from normal human left ventricular myocardium (Hambleton et al., 2005; Vandeput et al., 2007). To determine whether this pattern were changed in patients with heart failure, we quantified Ca2+/calmodulin-stimulated PDE1 and PDE3 activity in cytosolic and microsomal fractions of normal and failing human left ventricular myocardium (Fig. 1). In preparations from both normal and failing hearts, Ca2+/calmodulin-stimulated PDE1 activity accounted for 56 to 60% and for 32 to 34% of the cGMP-hydrolytic activity in cytosolic and microsomal fractions, respectively, whereas PDE3 activity accounted for 10 to 13% and 23 to 25% of the cGMP-hydrolytic activity in cytosolic and microsomal fractions, respectively. These findings demonstrate that the relative levels of PDE1 and PDE3 activity are comparable in normal and failing human left ventricular myocardium.
Fig. 1.
Cyclic GMP-hydrolytic activities in subcellular fractions of human myocardium. Total phosphodiesterase activity was determined at 0.1 μM cGMP in the presence of 200 μMCa2+ and 50 nM calmodulin (CaM). Activity was also measured in the presence of 100 μM EGTA. The difference between total and Ca2+-independent activity was taken as Ca2+/calmodulin-stimulated PDE1 activity. PDE3 activity was calculated based on phosphodiesterase assays performed in the presence of EGTA using the PDE3-selective inhibitor cilostazol (0.3 μM). Each value represents the average ± S.D. of at least three determinations. Differences between normal and failing hearts with respect to Ca2+/calmodulin-dependent PDE1 activity and PDE3 activity were not statistically significant.
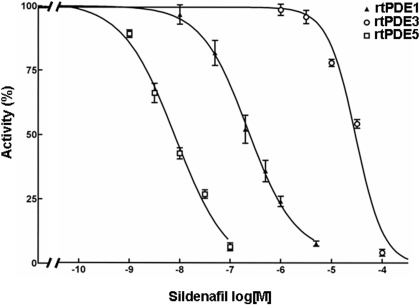
Effects of Sildenafil on cGMP-Hydrolytic Activity in Preparations from Normal and Failing Human Left Ventricular Myocardium. These observations suggested that the relative contribution of PDE5 to cGMP-hydrolytic activity in human left ventricular myocardium is low. To test this directly, we examined the effects of the PDE5-selective inhibitor sildenafil on cGMP-hydrolytic activity in our preparations. We first determined the potency of the inhibition by sildenafil of recombinant forms of PDE1, PDE3, and PDE5 at a cGMP concentration of 0.1 μM (Fig. 2). Under these conditions, sildenafil inhibited purified recombinant PDE5 activity with an IC50 of 7.1 ± 0.8 nM. This inhibition was ∼30-fold more potent than its inhibition of rtPDE1C1 activity (IC50, 228 ± 12 nM) and ∼4000-fold more potent than its inhibition of recombinant PDE3A1 (rtPDE3A1) activity (IC50, 30 ± 3 μM).
Fig. 2.
Inhibition of cGMP-hydrolytic activity of recombinant PDE1, PDE3, and PDE5 by sildenafil. cGMP-hydrolytic activity was measured at 0.1 μM cGMP in the presence of 100 μM EGTA. Each value represents the average ± S.D. of at least three determinations.
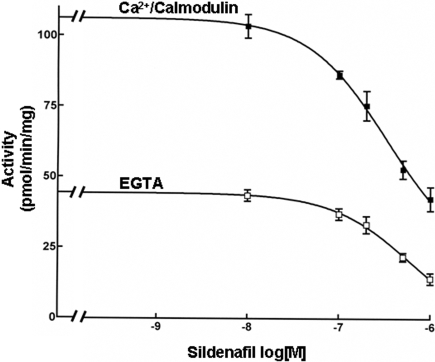
The ∼30-fold difference in the sensitivity of PDE1 and PDE5 to sildenafil made it possible to distinguish the activities of these two cGMP-hydrolytic activities in subcellular preparations based on the concentration range of sildenafil at which inhibition occurred. Results for a cytosolic preparation from human myocardium are shown in Fig. 3. In either the presence or absence of Ca2+/calmodulin, there was no quantifiable inhibition of cGMP-hydrolytic activity at concentrations of sildenafil (≤10 nM) at which it is selective for PDE5. In contrast, there was a marked inhibition of Ca2+/calmodulin-stimulated and Ca2+/calmodulin-independent activity at higher concentrations of sildenafil (>100 nM), at which it inhibits PDE1 and PDE5.
Fig. 3.
Inhibition of the cGMP-hydrolytic activity in cytosolic fractions of failing human left ventricular myocardium by sildenafil. cGMP-hydrolytic activity was measured at 0.1 μM cGMP in the presence of 200 μM Ca2+ and 50 nM calmodulin or in the presence of 100 μM EGTA. Each value represents the average ± S.D. of at least three determinations.
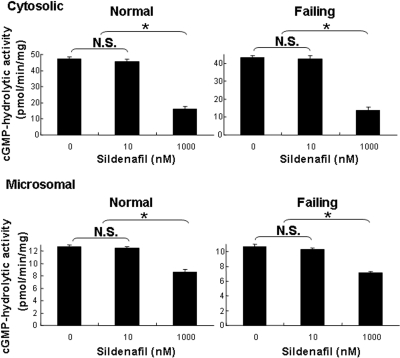
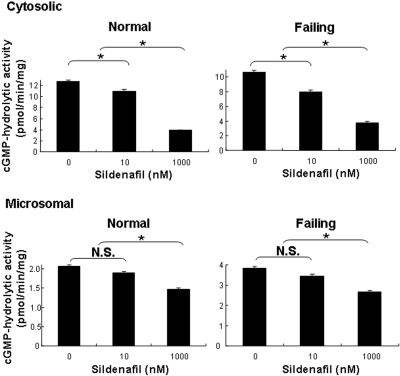
We extended these studies to a comparison of the cGMP-hydrolytic activities of cytosolic and microsomal fractions from normal and failing hearts (Fig. 4). Measurements were performed in the presence of 2.0 mM EGTA to minimize Ca2+/calmodulin-stimulated PDE1 activity and thereby increase our ability to detect PDE5 activity. Results in preparations from failing hearts were indistinguishable from those in normal hearts. There was no quantifiable inhibition of cGMP hydrolysis at 10 nM sildenafil, a concentration at which it inhibits ∼60% of PDE5 activity with minimal inhibition of PDE1. To ensure that there were no substances in the cytosolic fraction that interfered with PDE5 activity or its inhibition, we repeated the experiment in the presence of added rtPDE5. The rtPDE5 activity was inhibited to the same degree in the absence or presence of the cytosolic fraction (data not shown). At 1 μM sildenafil, a concentration at which it inhibits ∼75% of PDE1 activity without inhibiting PDE3 activity, cGMP-hydrolytic activity was reduced by 65 and 68% in the cytosolic fractions and by 32 and 34% in the microsomal fractions of normal and failing hearts, respectively. These results are consistent with the interpretation that PDE1 and PDE3 constitute the majority of cGMP-hydrolytic activity in normal and failing human left ventricular myocardium, that the amount of PDE5 in these preparations was, at best, very low, and that the principal effect of sildenafil on cGMP hydrolysis in these preparations was through inhibition of PDE1.
Fig. 4.
Inhibition of cGMP-hydrolytic activity by sildenafil in subcellular fractions of human myocardium. cGMP-hydrolytic activity was measured at 0.1 μM cGMP in the presence of 100 μM EGTA and in the absence or presence of 10 nM or 1000 nM (1 μM) sildenafil. Each value represents the average ± S.D. of at least three determinations. N.S., not significant. *, P < 0.05.
Effects of Sildenafil on cGMP-Hydrolytic Activity in Subcellular Preparations of Normal and Failing Mouse Left Ventricular Myocardium. Because our results indicated that PDE5 constitutes at most a very small fraction of the cGMP-hydrolytic activity in normal or failing human left ventricular myocardium, we investigated its contribution to cGMP-hydrolytic activity in the left ventricular myocardium of mice, where the beneficial antihypertrophic and cardioprotective effects of sildenafil have been demonstrated (Salloum et al., 2003; Das et al., 2004, 2005, 2008; Fisher et al., 2005; Hassan and Ketat, 2005; Takimoto et al., 2005; Salloum et al., 2008). We quantified the effects of sildenafil on cGMP hydrolysis in cytosolic and microsomal preparations from the left ventricular myocardium of normal mice and of mice in which the left anterior descending artery had been ligated 7 days earlier. In the latter group, hearts had developed left ventricular dilation with impaired contractility and thinning of the left ventricular anterior wall (Fig. 5). In contrast to our findings in humans, Ca2+/calmodulin-independent cGMP-hydrolytic activity in cytosolic fractions from normal mouse hearts was inhibited by ∼15% at 10 nM sildenafil (Fig. 6). In preparations from mice that had undergone coronary ligation 7 days earlier, cGMP-hydrolytic activity was inhibited ∼25% by 10 nM sildenafil. At 1 μM sildenafil, cGMP-hydrolytic activity in cytosolic fractions was reduced by ∼65% in both normal and failing hearts, consistent with the conclusion that PDE1 constitutes the major portion of the cGMP-hydrolytic activity in mouse and human hearts. Because, at a concentration of 10 nM, sildenafil inhibits ∼60% of recombinant PDE5 activity (Fig. 2), we calculated, based on the data represented in Fig. 6, that PDE5 constitutes ∼22% of the Ca2+/calmodulin-independent cGMP-hydrolytic activity in cytosolic preparations from normal mouse left ventricles and ∼43% of this activity in cytosolic preparations from failing mouse left ventricles.
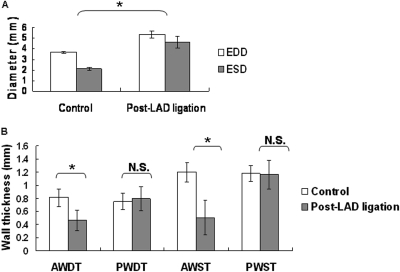
Fig. 5.
Functional characterization of mouse hearts. A, left ventricular end-diastolic diameter (EDD), left ventricular end-systolic diameter (ESD), and fractional shortening in mice 7 days after ligation of the left anterior descending artery compared with control mice. The ejection fraction was 0.42 in control hearts and 0.13 in hearts after infraction. B, anterior wall diastolic thickness (AWDT), posterior wall diastolic thickness (PWDT), anterior wall systolic thickness (AWST), and posterior wall systolic thickness (PSWT) in mice 7 days after ligation of the left anterior descending artery compared with control mice. In the former group, thickness was decreased in the infarcted anterior walls but not in the posterior walls. N.S., not significant; LAD, left anterior descending artery. *, P < 0.05.
Fig. 6.
Contribution of PDE5 activity to total cGMP-hydrolytic activity in subcellular fractions of mouse myocardium. cGMP-hydrolytic activity was measured at 0.1 μM cGMP in the presence of 100 μM EGTA (Ca2+/CaM-independent activity) and in the absence or presence of 10 or 1000 nM (1 μM) sildenafil. Each value represents the average ± S.D. of at least three determinations. N.S., not significant. *, P < 0.05.
We prepared comparable experiments in microsomal preparations from normal and failing mouse left ventricular myocardium (Fig. 6). Although there seemed to be more inhibition of cGMP hydrolysis at 10 nM sildenafil than we observed in humans (and therefore more PDE5 activity), we were not confident that this difference could be quantified reliably.
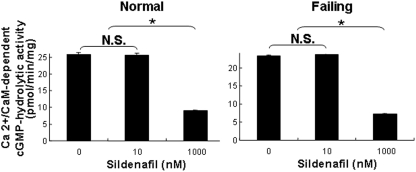
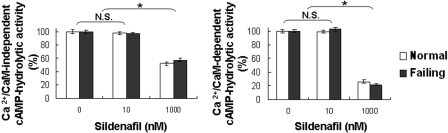
To confirm that inhibition of cGMP-hydrolytic activity at 10 nM sildenafil was due to inhibition of PDE5 and not PDE1, we measured activity in the presence and absence of Ca2+/calmodulin (Fig. 7). Consistent with this interpretation, Ca2+/calmodulin-stimulated cGMP-hydrolytic activity (i.e., PDE1 activity) in normal and failing mouse hearts was inhibited at 1 μM sildenafil but not at 10 nM sildenafil. Because PDE1 is a dual-specificity phosphodiesterase, we also examined the effect of sildenafil on Ca2+/calmodulin-independent and Ca2+/calmodulin-stimulated cAMP-hydrolytic activity in cytosolic fractions of mouse hearts (Fig. 8). cAMP-hydrolytic activity was inhibited at 1 μM sildenafil but not at 10 nM sildenafil, consistent with the interpretation that its effect was mediated through inhibition of PDE1. The Km of Ca2+/calmodulin-stimulated cAMP-hydrolytic activity for cAMP in cytosolic fractions of mouse hearts was 0.75 ± 0.08 μM, consistent with PDE1C (rather than PDE1A or PDE1B, whose affinity for cAMP is significantly lower) being the principal PDE1 isoform in these preparations (Bender, 2007).
Fig. 7.
Effect of sildenafil on Ca2+/calmodulin-stimulated cGMP-hydrolytic activity in cytosolic preparations from mouse myocardium. cGMP-hydrolytic activity was measured at 0.1 μM cGMP in the presence of 200 μMCa2+ and 50 nM calmodulin or in the presence of 100 μM EGTA, and in the absence or presence of 10 or 1000 nM (1 μM) sildenafil. The difference was taken as Ca2+/calmodulin-stimulated activity. Each value represents the mean ± S.D. of at least three determinations. N.S., not significant. *, P < 0.05.
Fig. 8.
Effect of sildenafil on cAMP-hydrolytic activity in cytosolic fractions of mouse myocardium. cAMP-hydrolytic activity was measured at 0.1 μM cAMP in the presence of 200 μMCa2+ and 50 nM calmodulin or in the presence of 100 μM EGTA, and 0, 10, or 1000 nM (1 μM) sildenafil. The difference was taken as Ca2+/calmodulin-stimulated activity. Each value represents the average ± S.D. of at least three determinations. N.S., not significant. *, P < 0.05.
PDE5 Protein Content in Normal and Failing Left Ventricular Myocardium. Our inability to detect an increase in PDE5 activity relative to other cGMP-hydrolytic activities in failing human left ventricular myocardium seemed inconsistent with a report of an increase in PDE5 protein in this tissue (Pokreisz et al., 2009). However, the fraction of total cGMP-hydrolytic activity attributable to PDE5 in human myocardium was sufficiently low that a change in its expression might have been difficult to detect by our approach. For this reason, we examined PDE5 protein content in cytosolic fractions prepared from mouse and human left ventricular myocardium by Western blotting with anti-PDE5 antibodies (Fig. 9). We noted a 6.1 ± 1.0-fold increase in the density of PDE5 bands (migrating at an apparent molecular weight of ∼100,000) in failing mouse left ventricle relative to normal tissue, but only a 2.3 ± 0.3-fold increase in the density of PDE5 bands in failing human left ventricle. These findings leave open the possibility that PDE5 protein content is increased in preparations from failing human hearts, although this increase would seem to have a smaller magnitude than that seen in the mouse model.
Fig. 9.
Immunodetection of PDE5 protein in preparations of mouse and human left ventricular myocardium. Cytosolic fractions of mouse tissue (one preparation each from 25 to 50 normal and 25 to 50 failing hearts) and human tissue (two preparations each from three to four normal and three to four failing hearts) were analyzed by Western blotting with anti-PDE5 antibody as described under Materials and Methods. Fifty micrograms of protein were loaded into each lane before SDS-polyacrylamide gel electrophoresis, electrophoretic transfer, and overlay with anti-PDE5.
Discussion
In a number of studies in mouse models of cardiac disease, the PDE5-selective phosphodiesterase inhibitor sildenafil has been shown to have impressive antihypertrophic and cardioprotective actions (Salloum et al., 2003; Das et al., 2004, 2005, 2008; Fisher et al., 2005; Hassan and Ketat, 2005; Takimoto et al., 2005; Salloum et al., 2008). The implications of these reports with respect to the treatment of cardiac disease in humans depend on the accuracy with which the alterations in the mouse models mimic the pathophysiology that occurs in human disease and on PDE5 being of comparable importance in the mouse and human myocardium. With respect to the latter, our prior studies showing that the great majority of cGMP-hydrolytic activity in normal human left ventricular myocardium is attributable to the activities of PDE1 and PDE3 were a cause for concern (Hambleton et al., 2005; Vandeput et al., 2007). Other investigators, however, have reported an increase in the expression of PDE5 in hypertrophic human right ventricular myocardium, and, more recently, in failing human left ventricular myocardium (Nagendran et al., 2007; Pokreisz et al., 2009). In view of these reports, it was important to characterize cGMP-hydrolytic activity and its inhibition by sildenafil in failing and normal human left ventricular myocardium, and to compare the results with those made in comparable preparations of normal and failing mouse hearts. Our findings demonstrate that the levels of PDE5 activity relative to those of other cGMP-hydrolytic activities are much lower in normal and failing human left ventricular myocardium than in normal and failing mouse left ventricular myocardium.
The large disparity in the relative level of PDE5, the primary target of sildenafil, in mouse and human myocardium raises important questions as to whether PDE5 inhibition is as likely to be beneficial in humans with cardiomyopathies as in the mouse models that have been examined. Before reaching a conclusion, several points need to be considered. First, the relative amount of a particular cyclic nucleotide phosphodiesterase may not be the ultimate indicator of its importance in intracellular signaling. Levels of cyclic nucleotides in different spatial compartments of cardiac myocytes can be regulated with considerable selectivity, owing to a large degree to differences in the intracellular localization of cyclic nucleotide phosphodiesterases (Fischmeister et al., 2006; Zaccolo, 2006). Inhibition of a phosphodiesterase whose abundance is low relative to other phosphodiesterases may still have significant effects if the phosphodiesterase has an important role in a particular intracellular compartment (Mongillo et al., 2006). It is important to note as well that our findings in preparations from normal human hearts and the hearts of transplant recipients with end-stage dilated cardiomyopathy may not apply to all forms of cardiac disease in humans. We cannot exclude the possibility that PDE5 levels may be elevated in other forms of cardiac disease, especially those associated with acute myocardial infarctions and postinfarction remodeling and hypertrophic cardiomyopathy; decreases in the levels of nitric oxide synthase that have been described in failing human myocardium may also affect responses to PDE5 inhibitors (Drexler et al., 1998; Heymes et al., 1999). With these caveats in mind, we nevertheless believe our findings are evidence of the need for great caution in extrapolating from the results of studies of phosphodiesterase inhibition in mouse models of cardiac pathophysiology to predictions of beneficial therapeutic actions for phosphodiesterase inhibitors in humans with cardiac disease.
Our results raise several additional possibilities regarding the mechanism of action of sildenafil in cardiac pathophysiology. It is possible that the effects of sildenafil may be due to inhibition of PDE1 and of PDE5 activity. The high abundance of PDE1 in left ventricular myocardium and the fact that the inhibitory potency of sildenafil is only ∼30-fold higher for PDE5 than for PDE1 make this plausible. Although the intracellular action of an inhibitor cannot be predicted reliably based on its extracellular concentration, in several studies the effects of sildenafil on myocardial cGMP-hydrolytic activity were quantified at a sildenafil concentration of 1 to 10 μM (Das et al., 2005; Fisher et al., 2005; Nagendran et al., 2007; Pokreisz et al., 2009). Our results indicate that sildenafil at these concentrations inhibits PDE1 activity and PDE5 activity. As a corollary, because PDE1 isoenzymes are dual-specificity phosphodiesterases, it is possible that some of the effects of sildenafil in mouse myocardium may be due to the inhibition of cAMP hydrolysis and cGMP hydrolysis. Other studies have shown that the potentiation of cAMP-mediated signaling may be part of the action of sildenafil in cardiac muscle in hypertrophic rat right ventricular myocardium, but the mechanism involved competitive inhibition of cAMP-hydrolytic activity by a sildenafil-induced increase in intracellular cGMP content (Nagendran et al., 2007). Our results suggest that a direct potentiation of cAMP-mediated signaling through inhibition of the cAMP-hydrolytic activity of PDE1 might also occur.
Finally, our results raise the possibility that PDE1 may be a useful target in the treatment of dilated cardiomyopathy in humans. Drugs that elevate intracellular cAMP content in cardiac myocytes by inhibiting the cAMP-hydrolytic activity of PDE3 are frequently used as inotropic agents in the treatment of heart failure, but with chronic administration at higher doses, they have been found to increase mortality (Movsesian et al., 2008). Proapoptotic actions may contribute to these adverse responses (Ding et al., 2005a,b; Yan et al., 2007). An agent that inhibits PDE1 may have a combination of inotropic actions attributable to an increase in intracellular cAMP content and cardioprotective actions attributable to an increase in intracellular cGMP content. The compartmentation of cyclic nucleotide-mediated signaling in cardiac myocytes is again an important consideration. With respect to cAMP-mediated signaling, different cellular responses can be elicited depending on the G-protein-coupled receptors through which adenylyl cyclase activity is stimulated and intracellular cAMP content is increased (Hayes et al., 1980; Xiao et al., 1994; Kuschel et al., 1999; Xiao et al., 1999; Leroy et al., 2008). There are also several examples of differences in the potentiation of individual cAMP-mediated responses depending on the particular phosphodiesterase that is inhibited (Jurevicius et al., 2003; Mongillo et al., 2006; Nikolaev et al., 2006; Rochais et al., 2006). cGMP-mediated signaling in cardiac myocytes has been less thoroughly studied in this regard, but different cellular responses are elicited depending on whether intracellular cGMP levels are increased by stimulating soluble or membrane-associated forms of guanylyl cyclase and depending on which cGMP phosphodiesterases are inhibited (Castro et al., 2006; Piggott et al., 2006). For this reason, one cannot assume that raising intracellular cAMP and/or cGMP levels by inhibiting PDE1 activity will have the same effect as raising their levels by inhibiting PDE3 and/or PDE5 activity. For these reasons, future experiments through which the consequences of PDE1 inhibition can be delineated and compared with those of PDE3 and PDE5 inhibition will constitute an important step in evaluating the potential of PDE1 as a therapeutic target.
Acknowledgments
We thank Donald K. Blumenthal (University of Utah) for providing calmodulin.
This work was supported in part by the National Institutes of Health [Grants HL51045, HL59469, HL79424, HL093685] (to R.C.K.); the National Institutes of Health [Grant DK40029] (to J.D.C.); the United States Department of Veterans Affairs Medical Research Funds; the American Heart Association; and the Leducq Foundation [Grant O6 CVD 02] (to M.A.M.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.154468.
ABBREVIATIONS: PDE, phosphodiesterase; rtPDE, recombinant PDE; CaM, calmodulin.
References
- Bender AT (2007) Calmodulin-stimulated cyclic nucleotide phosphodiesterases, in Cyclic Nucleotide Phosphodiesterases in Health and Disease (Beavo JA, Francis SH, and Houslay MD eds) pp 35-54, CRC Press, Boca Raton, FL.
- Bischoff E (2004) Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int J Impot Res 16 (Suppl 1): S11-S14. [DOI] [PubMed] [Google Scholar]
- Castro LR, Verde I, Cooper DM, and Fischmeister R (2006) Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation 113 2221-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Ockaili R, Salloum F, and Kukreja RC (2004) Protein kinase C plays an essential role in sildenafil-induced cardioprotection in rabbits. Am J Physiol Heart Circ Physiol 286 H1455-H1460. [DOI] [PubMed] [Google Scholar]
- Das A, Xi L, and Kukreja RC (2005) Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem 280 12944-12955. [DOI] [PubMed] [Google Scholar]
- Das A, Xi L, and Kukreja RC (2008) Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem 283 29572-29585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Abe J, Wei H, Huang Q, Walsh RA, Molina CA, Zhao A, Sadoshima J, Blaxall BC, Berk BC, et al. (2005a) Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: implication in heart failure. Circulation 111 2469-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Abe J, Wei H, Xu H, Che W, Aizawa T, Liu W, Molina CA, Sadoshima J, Blaxall BC, et al. (2005b) A positive feedback loop of phosphodiesterase 3 (PDE3) and inducible cAMP early repressor (ICER) leads to cardiomyocyte apoptosis. Proc Natl Acad Sci U S A 102 14771-14776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler H, Kästner S, Strobel A, Studer R, Brodde OE, and Hasenfuss G (1998) Expression, activity and functional significance of inducible nitric oxide synthase in the failing human heart. J Am Coll Cardiol 32 955-963. [DOI] [PubMed] [Google Scholar]
- Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, and Vandecasteele G (2006) Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res 99 816-828. [DOI] [PubMed] [Google Scholar]
- Fisher PW, Salloum F, Das A, Hyder H, and Kukreja RC (2005) Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation 111 1601-1610. [DOI] [PubMed] [Google Scholar]
- Francis SH and Corbin JD (2005) Sildenafil: efficacy, safety, tolerability and mechanism of action in treating erectile dysfunction. Expert Opin Drug Metab Toxicol 1 283-293. [DOI] [PubMed] [Google Scholar]
- Francis SH, Zoraghi R, Kotera J, Ke H, Bessay EP, Bount MA, and Corbin JD (2007) Phosphodiesterase 5: molecular characteristics relating to structure, function and regulation, in Cyclic Nucleotide Phosphodiesterases in Health and Disease (Beavo JA, Francis SH, and Houslay MD eds) pp 131-164, CRC Press, Boca Raton.
- Hambleton R, Krall J, Tikishvili E, Honeggar M, Ahmad F, Manganiello VC, and Movsesian MA (2005) Isoforms of cyclic nucleotide phosphodiesterase PDE3 and their contribution to cAMP hydrolytic activity in subcellular fractions of human myocardium. J Biol Chem 280 39168-39174. [DOI] [PubMed] [Google Scholar]
- Hassan MA and Ketat AF (2005) Sildenafil citrate increases myocardial cGMP content in rat heart, decreases its hypertrophic response to isoproterenol and decreases myocardial leak of creatine kinase and troponin T. BMC Pharmacol 5 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JS, Brunton LL, and Mayer SE (1980) Selective activation of particulate cAMP-dependent protein kinase by isoproterenol and prostaglandin E1. J Biol Chem 255 5113-5119. [PubMed] [Google Scholar]
- Heymes C, Vanderheyden M, Bronzwaer JG, Shah AM, and Paulus WJ (1999) Endomyocardial nitric oxide synthase and left ventricular preload reserve in dilated cardiomyopathy. Circulation 99 3009-3016. [DOI] [PubMed] [Google Scholar]
- Jurevicius J, Skeberdis VA, and Fischmeister R (2003) Role of cyclic nucleotide phosphodiesterase isoforms in cAMP compartmentation following beta2-adrenergic stimulation of ICa, L in frog ventricular myocytes. J Physiol 551 239-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambayashi J, Shakur Y, and Liu Y (2007) Bench to bedside: multiple actions of the PDE3 inhibitor cilostazol, in Cyclic Nucleotide Phosphodiesterases in Health and Disease (Beavo JA, Francis SH, and Houslay MD eds) pp 627-648, CRC Press, Boca Raton, FL.
- Kass DA, Champion HC, and Beavo JA (2007) Phosphodiesterase type 5: expanding roles in cardiovascular regulation. Circ Res 101 1084-1095. [DOI] [PubMed] [Google Scholar]
- Kincaid RL and Manganiello VC (1988) Assay of cyclic nucleotide phosphodiesterase using radiolabeled and fluorescent substrates. Methods Enzymol 159 457-470. [DOI] [PubMed] [Google Scholar]
- Kuschel M, Zhou YY, Cheng H, Zhang SJ, Chen Y, Lakatta EG, and Xiao RP (1999) G(i) protein-mediated functional compartmentalization of cardiac beta(2)-adrenergic signaling. J Biol Chem 274 22048-22052. [DOI] [PubMed] [Google Scholar]
- Leroy J, Abi-Gerges A, Nikolaev VO, Richter W, Lechene P, Mazet JL, Conti M, Fischmeister R, and Vandecasteele G (2008) Spatiotemporal dynamics of {beta}-adrenergic cAMP signals and L-type Ca2+ channel regulation in adult rat ventricular myocytes. Role of phosphodiesterases. Circ Res 102: 1091-1100. [DOI] [PubMed] [Google Scholar]
- Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD, et al. (2006) Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res 98 226-234. [DOI] [PubMed] [Google Scholar]
- Movsesian M, Stehlik J, Vandeput F, and Bristow MR (2008) Phosphodiesterase inhibition in heart failure. Heart Fail Rev, doi: . [DOI] [PubMed]
- Movsesian MA, Bristow MR, and Krall J (1989) Ca2+ uptake by cardiac sarcoplasmic reticulum from patients with idiopathic dilated cardiomyopathy. Circ Res 65 1141-1144. [DOI] [PubMed] [Google Scholar]
- Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St Aubin C, Webster L, Rebeyka IM, Ross DB, et al. (2007) Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation 116 238-248. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bünemann M, Schmitteckert E, Lohse MJ, and Engelhardt S (2006) Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circ Res 99 1084-1091. [DOI] [PubMed] [Google Scholar]
- Piggott LA, Hassell KA, Berkova Z, Morris AP, Silberbach M, and Rich TC (2006) Natriuretic peptides and nitric oxide stimulate cGMP synthesis in different cellular compartments. J Gen Physiol 128 3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokreisz P, Vandenwijngaert S, Bito V, Van den Bergh A, Lenaerts I, Busch C, Marsboom G, Gheysens O, Vermeersch P, Biesmans L, et al. (2009) Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation 119 408-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochais F, Abi-Gerges A, Horner K, Lefebvre F, Cooper DM, Conti M, Fischmeister R, and Vandecasteele G (2006) A specific pattern of phosphodiesterases controls the cAMP signals generated by different Gs-coupled receptors in adult rat ventricular myocytes. Circ Res 98 1081-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum F, Yin C, Xi L, and Kukreja RC (2003) Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res 92 595-597. [DOI] [PubMed] [Google Scholar]
- Salloum FN, Abbate A, Das A, Houser JE, Mudrick CA, Qureshi IZ, Hoke NN, Roy SK, Brown WR, Prabhakar S, et al. (2008) Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol 294 H1398-H1406. [DOI] [PubMed] [Google Scholar]
- Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, and Kass DA (2005) Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med 11 214-222. [DOI] [PubMed] [Google Scholar]
- Vandeput F, Wolda SL, Krall J, Hambleton R, Uher L, McCaw KN, Radwanski PB, Florio V, and Movsesian MA (2007) Cyclic nucleotide phosphodiesterase PDE1C1 in human cardiac myocytes. J Biol Chem 282 32749-32757. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Avdonin P, Zhou YY, Cheng H, Akhter SA, Eschenhagen T, Lefkowitz RJ, Koch WJ, and Lakatta EG (1999) Coupling of beta2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ Res 84 43-52. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Hohl C, Altschuld R, Jones L, Livingston B, Ziman B, Tantini B, and Lakatta EG (1994) Beta 2-adrenergic receptor-stimulated increase in cAMP in rat heart cells is not coupled to changes in Ca2+ dynamics, contractility, or phospholamban phosphorylation. J Biol Chem 269 19151-19156. [PubMed] [Google Scholar]
- Yan C, Miller CL, and Abe J (2007) Regulation of phosphodiesterase 3 and inducible cAMP early repressor in the heart. Circ Res 100 489-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccolo M (2006) Phosphodiesterases and compartmentalized cAMP signalling in the heart. Eur J Cell Biol 85 693-697. [DOI] [PubMed] [Google Scholar]
- Zoraghi R, Francis SH, and Corbin JD (2007) Critical amino acids in phosphodiesterase-5 catalytic site that provide for high-affinity interaction with cyclic guanosine monophosphate and inhibitors. Biochemistry 46 13554-13563. [DOI] [PubMed] [Google Scholar]