Abstract
The non-steroidal anti-inflammatory drug (NSAID) tolfenamic acid (TA), previously thought to be dimorphic, is demonstrated to have at least five polymorphs. The new forms were uncovered through the emerging screening technique of polymer-induced heteronucleation (PIHn). The presence of conformational changes among forms, whole molecule disorder, space group diversity, and varying number of molecules in the asymmetric unit occurring within a very narrow free energy window (∼0.3 kcal/mol) make the solid-state chemistry of this molecule uniquely complex among pharmaceuticals. These aspects make it a particularly suitable benchmark compound for crystal structure prediction methods.
Molecules exhibiting multiple crystalline forms differing solely in the arrangement or conformation of the building units, polymorphic compounds, have long represented an active area of research in solid-state chemistry. In addition to important implications for properties including drug bioavailability and pigment color, such systems offer a challenging test for crystal structure prediction and, in cases where thermodynamic relationships have been derived, an invaluable opportunity to refine computational approaches.
Definitive evidence for polymorphism is offered by single crystal X-ray diffraction. The number of compounds in the Cambridge Structural Database (CSD) with two structurally characterized polymorphs figures in the tens of thousands, whereas structures with three or four structurally characterized forms are considerably more rare. There is only one pharmaceutical in the CSD reaching the level of five structurally characterized polymorphs: sulfathiazole.1-3 Crystals of this highly polymorphic compound were obtained over a period of nearly 30 years using solvent-based methods, remarkably all forms are in the same space group.2,3 Currently, a pharmaceutical precursor, 5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile (ROY), has the largest number of structurally characterized polymorphs in the CSD and three different space groups are represented among its crystal phases.4,5 Six of the seven structurally characterized forms were obtained by solvent-based screening methods between 1995 and 2000, whereas, the last form was first accessed by melt crystallization and solid-state transformation in 2005.4,5 These examples serve to illustrate that there is a need for alternative approaches for polymorph discovery, especially in the pharmaceutical industry. Screening approaches that accelerate the pace of solid form discovery, thus preventing the unexpected appearance of undesirable forms at late stages of evaluation or during manufacturing, would be of considerable value.
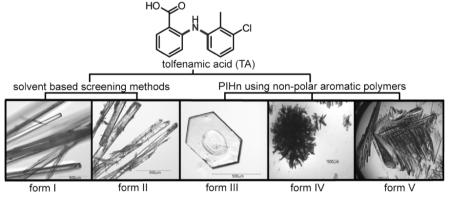
Here, we report structural studies on the conformational polymorphism of a non-steroidal anti-inflammatory drug (NSAID) tolfenamic acid (TA) and demonstrate unambiguously that this pharmaceutical possesses at least five polymorphs. TA (Figure 1) is a member of a large class of NSAIDs that contain a monocarboxylated diphenyl amine nucleus; several of these analogs display polymorphism. As such, TA offers a good test case for the notion that certain molecular motifs, when incorporated into a structure, render the compound polymorphic: the polymorphophore.6 As a well-studied pharmaceutical, it also offers a challenge for polymorph discovery techniques, such as polymer-induced heteronucleation (PIHn).7,8 This is a screening technique in which a single solvent/temperature condition leads to novel form discovery enabled by a diverse array of insoluble polymers acting as heterogeneous growth sites.7,8
Figure 1.
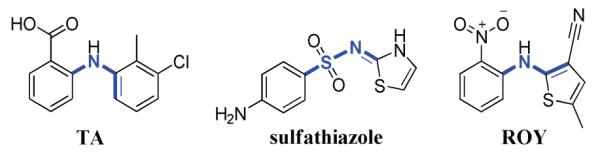
Chemical structures of TA, sulfathiazole, and ROY illustrating the torsion angle (τ, bonds in blue) differing among the conformers.
Three novel phases of TA were grown from an ethanol solution using non-polar aromatic polymers as heteronuclei (Supporting Information).7,8 Optical examination revealed noticeable differences in the morphology of the crystals. The previously reported colorless and yellow needles (form I and II)9 were observed as well as prisms (form III) and colorless plates (forms IV and V). When the samples were examined by Raman spectroscopy, four main spectral regions (3375-3300, 1645-1565, 1180-1040, and 815-760 cm-1) aided the identification of the five polymorphs (Supporting Information).
Powder X-ray diffraction confirms that unique crystalline phases (Supporting Information) were obtained for TA. Structure determination revealed that, as in the case of sulfathiazole and ROY, each form contains different conformers (Figure 1). In contrast to ROY, several structures of TA vary in the number of inequivalent molecules in the asymmetric unit. TA is known to crystallize in two monoclinic forms,9 both containing one molecule in the asymmetric unit. The now accessible form III crystallizes in the space group P21/c with Z’ = 2, whereas, forms IV and V crystallize in the space group 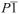 with Z’ = 3 and Z’ = 1, respectively.
with Z’ = 3 and Z’ = 1, respectively.
TA displays conformational polymorphism. Form II posses the smallest torsion angle9 τ[C-N-C-C] of 42.2° whereas form I has the largest torsion angles measuring 107.7° (Figure 1). The small dihedral angle in form II may cause extended conjugation not present in the other TA forms, thus giving this form its characteristic yellow color. Modifications I, II, IV, and V form hydrogen bonded anti dimers that connect the carboxylic acids through an inversion center. Form III displays a similar motif but between crystallographically inequivalent molecules. Intramolecular hydrogen bonding occurs between the amino group and the carbonyl oxygen in all modifications.
In TA form I, each dimer interacts with eight adjacent molecules through close CH···π contacts between the chlorinated aromatic ring and the carboxylated aromatic ring. The contacts alternate between four donors and four acceptors to form a 2D sheet in the ac-plane. Close contacts between the chlorine and the methyl group along the b-axis join these sheets into a 3D structure.9 In form II, the hydrogen bonded dimers pack in columns along the a-axis; additional donation from a neighboring methyl group to the carbonyl above and below it connects the columnar packing along the same direction. Adjacent pairs of dimers pack through Cl···π (3.41 Å) interactions.9
The newly accessed crystal phases pack in columnar arrangements (Figure 2). In form III, heterodimers pack tilted along the columnar axis at an angle of 63°, where a supramolecular ring is formed by two short contacts between the carbon atom of the carboxylic acid and the π system located above and below each heterodimer. Other aromatic CH···π (2.86 Å and 2.85 Å) and methyl CH···π (2.89 Å) short contacts reinforce the columns along the same direction. Adjacent columns of heterodimers are connected by Cl···Cl (3.38 Å) interactions as well as Cl···π (3.44 Å) donor-acceptor interactions between the inequivalent molecules. In form IV, the shortest contacts observed are methyl CH···π (2.65 Å) donation from the chlorinated aromatic ring of the homodimer to the chlorinated aromatic ring of the heterodimer as well as aromatic CH···π interactions (2.75 and 2.79 Å) from the chlorinated aromatic ring of the heterodimer to the carboxylated aromatic ring of the homodimer. These interactions expand the packing of the tilted dimers within the bc-plane. Other important contacts present include Cl···Cl (3.41 Å) interactions, which are only observed between heterodimers, and aromatic CH···O (2.68 Å) contacts between the carboxylated aromatic ring and the carboxylic acid in adjacent heterodimers. In polymorph V, dimers pack through Cl···Cl interactions aligned with the c-axis in one of the disordered models and Cl···H interactions along the b-axis in the other disordered model; however, additional assignments regarding molecular packing are hampered by the complexity of the whole molecule disorder in the crystal structure.
Figure 2.
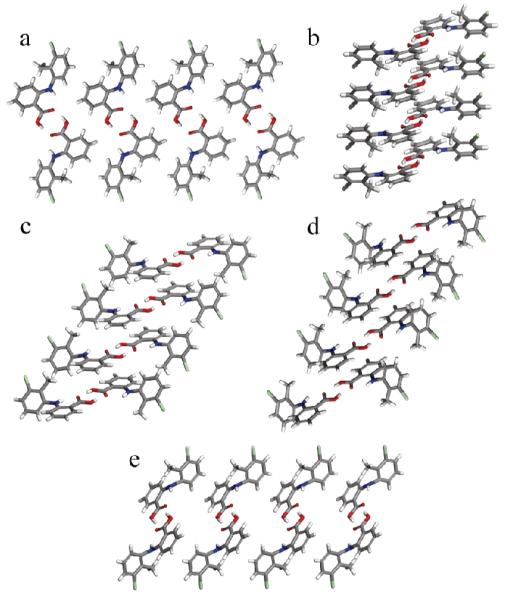
Molecular packing and hydrogen bonding motif of TA polymorphs (a) sheets of TA in form I, columns of TA in forms (b) II, (c) III, (d) IV, and (e) V. Form V represents 50% occupancy of the whole molecule disorder structure.
Hirshfeld surface (HSs)10 analysis was performed to determine the relative contribution of the important intermolecular contacts present in each of the molecules in the asymmetric unit of the ordered TA polymorphs (Supporting Information). The C···H contacts compose 42.6% of the HSs in conformer c of TA form IV, and only 10.0% in form II (Figure 3). In addition, examination of the HSs demonstrates the great contribution (11.9%) of C···C contacts to the planar conformation present in form II as well as the low contribution of the C···H contacts resulting from extensive π···π interactions in this form. A higher contribution (1.8-1.3% vs. 0.5-0.2%) of the Cl···Cl contacts is also observed in all of the newly accessed polymorphs of TA. Remarkably, although the crystal phases differ significantly in packing arrangements and conformations, the strong hydrogen bonding contacts (O···H and N···H) remain fairly constant among all of the five forms of TA. To assess the effect of these structural differences on the energies of TA polymorphs, relative free energies were established experimentally and enthalpies were determined by experiment and theory: all five polymorphs exist within a ∼0.3 kcal/mol window (Supporting Information).
Figure 3.
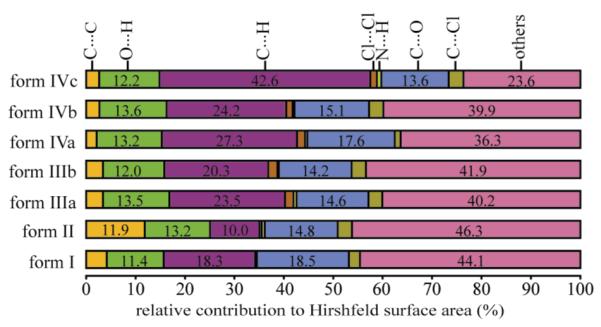
Percent relative contribution to the HSs of the important intermolecular interactions present in each of the molecule in the asymmetric unit of the ordered TA polymorphs. The letters a, b, and c represent different conformers in the structures with Z’ > 1.
PIHn has allowed access to three new metastable phases of TA, making this compound one of the few highly polymorphic pharmaceutical compounds structurally characterized. The range of different conformations, space groups, packing motifs, varying number of inequivalent molecules, and very narrow range of free energy differences make TA a particularly challenging model compound for polymorph prediction methods, in addition, to a valuable test case to assess the robustness of other solid form discovery methods.
Supplementary Material
Acknowledgment
This work was supported by the National Institute of Health Grant Number GM072737.
Footnotes
Supporting Information Available: Experimental preparation, Raman spectra, powder X-ray diffraction patterns, CIF files of forms III, IV, and V, Hirshfeld surface analysis, and relative stability studies. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.If the definition of polymorph is expanded to include tautomers, sulfapyridine, a pharmaceutical compound which can assume two tautomeric forms can also be considered as a pentamorphic. Four of the structures determined adopt the imide form (II-V), whereas the fifth form (VI) presents a combination of the imide and amide forms; Gelbrich T, Threlfall T, Bingham AL, Hursthouse MB. Acta Crystallogr. Sect. C: Cryst. Struct. Comm. 2007;63:323. doi: 10.1107/S010876810701395X.
- 2.Blagden N, Davey RJ, Lieberman HF, Williams L, Payne R, Roberts R, Rowe R, Docherty R. J. Chem. Soc., Faraday Trans. 1998;94:1035. [Google Scholar]
- 3.Hughes DS, Hursthouse MB, Threlfall T, Tavener S. Acta Crystallogr. Sect. C: Cryst. Struct. Comm. 1999;55:1831. [Google Scholar]
- 4.Stephenson GA, Borchardt TB, Byrn SR, Bowyer J, Bunnell CA, Snorek SV, Yu L. J. Pharm. Sci. 1995;84:1385. doi: 10.1002/jps.2600841122. [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Guzei IA, Yu L. J. Am. Chem. Soc. 2005;127:9881. doi: 10.1021/ja052098t. [DOI] [PubMed] [Google Scholar]
- 6.Lutker KM, Tolstyka ZP, Matzger AJ. Cryst. Growth Des. 2008;8:136. doi: 10.1021/cg700921w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang MD, Grzesiak AL, Matzger AJ. J. Am. Chem. Soc. 2002;124:14834. doi: 10.1021/ja0286526. [DOI] [PubMed] [Google Scholar]
- 8.Price CP, Grzesiak AL, Matzger AJ. J. Am. Chem. Soc. 2005;127:5512. doi: 10.1021/ja042561m. [DOI] [PubMed] [Google Scholar]
- 9.Andersen KV, Larsen S, Alhede B, Gelting N, Buchardt O. J. Chem. Soc., Perkin Trans. 2. 1989:1443. [Google Scholar]
- 10.McKinnon JJ, Fabbiani FPA, Spackman MA. Cryst. Growth Des. 2007;7:755. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.