Abstract
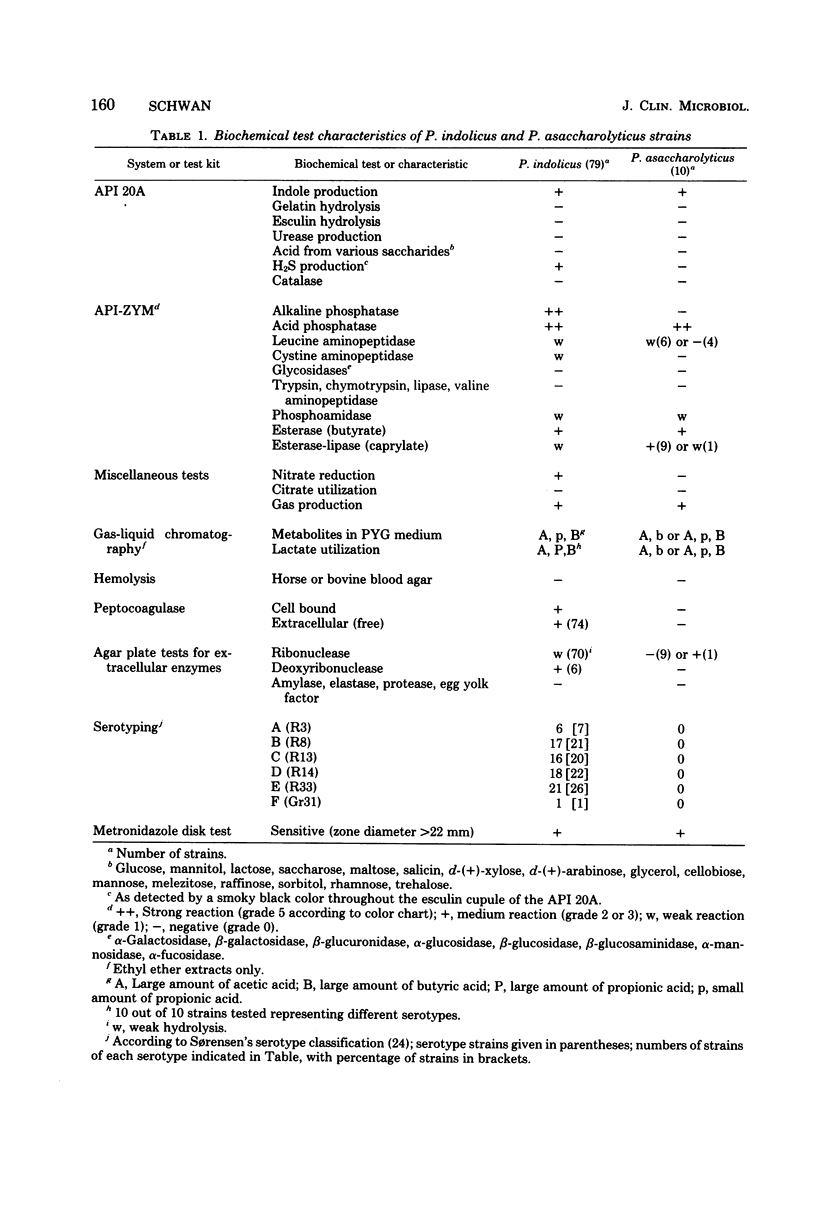
Peptococcus indolicus (formerly Micrococcus indolicus) is an asaccharolytic anaerobic coccus that is frequently isolated from udder secretions from cases of heifer and dry-cow mastitis (summer mastitis). To facilitate better identification and its differentiation from Peptococcus asaccharolyticus, a variety of biochemical, enzymatic, and serological properties were studied. Seventy-nine strains of P. indolicus of bovine origin and 10 strains of P. asaccharolyticus of human origin were examined using the API 20A and API-ZYM test kit systems. In addition, production of extracellular enzymes by using sensitive substrate-containing agar plate tests, production of peptocoagulase (a plasma-clotting factor), hemolytic properties, metabolic end products by gas chromatography, and serological characteristics with a set of P. indolicus typing antisera were investigated. P. indolicus and P. asaccharolyticus were not satisfactorily differentiated solely by the API 20A system. P. indolicus differed from P. asaccharolyticus in producing H2S, reducing nitrate to nitrite, producing peptocoagulase, possessing alkaline phosphatase, and producing large amounts of propionate from lactate. Moreover, none of the strains of P. asaccharolyticus was typable with the P. indolicus typing antisera. The majority (88%) of P. indolicus strains also gave weak hydrolysis of ribonucleic acid, and 6 out of 79 produced deoxyribonuclease. All strains in this study were sensitive to metronidazole (5 μg) by disk diffusion tests.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. R., Foster J. H. A simple diagnostic milk medium for Pseudomonas aeruginosa. J Clin Pathol. 1970 Mar;23(2):172–177. doi: 10.1136/jcp.23.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISALVO J. W. Desoxyribonuclease and coagulase activity of micrococci. Med Techn Bull. 1958 Sep-Oct;9(5):191–196. [PubMed] [Google Scholar]
- Hoeffler U. Enzymatic and hemolytic properties of Propionibacterium acnes and related bacteria. J Clin Microbiol. 1977 Dec;6(6):555–558. doi: 10.1128/jcm.6.6.555-558.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbeck H., Tirunarayanan M. O. Investigation on the enzymes and toxins of staphylococci. Study of the "egg yolk reaction" using an agar plate assay method. Acta Pathol Microbiol Scand. 1966;68(1):123–134. doi: 10.1111/apm.1966.68.1.123. [DOI] [PubMed] [Google Scholar]
- Miller A., 3rd, Sandine W. E., Elliker P. R. Extracellular nuclease in the genus Lactobacillus. J Bacteriol. 1971 Oct;108(1):604–606. doi: 10.1128/jb.108.1.604-606.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H. B., Sutter V. L., Finegold S. M. Comparison of three procedures for biochemical testing of anaerobic bacteria. J Clin Microbiol. 1975 Jan;1(1):15–24. doi: 10.1128/jcm.1.1.15-24.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord C-E, Dahlbäck A., Wadström T. Evaluation of a test kit for identification of anaerobic bacteria. Med Microbiol Immunol. 1975 Sep 19;161(4):239–242. doi: 10.1007/BF02122711. [DOI] [PubMed] [Google Scholar]
- Porschen R. K., Spaulding E. H. Phosphatase activity of anaerobic organisms. Appl Microbiol. 1974 Apr;27(4):744–747. doi: 10.1128/am.27.4.744-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulverer G., Ko H. L., Wegrzynowicz Z., Jeljaszewicz J. Clotting and fibrinolytic activities of Bacteroides melaninogenicus. Zentralbl Bakteriol Orig A. 1977 Dec;239(4):510–513. [PubMed] [Google Scholar]
- SBARRA A. J., GILFILLAN R. F., BARDAWIL W. A. A plate assay for elastase. Nature. 1960 Oct 22;188:322–323. doi: 10.1038/188322b0. [DOI] [PubMed] [Google Scholar]
- STUART P., BUNTAIN D., LANGRIDGE R. G. Bacteriological examination of secretions from cases of "summer mastitis" and experimental infection of non-lactating bovine udders. Vet Rec. 1951 Jul 7;63(27):451–453. doi: 10.1136/vr.63.27.451. [DOI] [PubMed] [Google Scholar]
- Sorensen G. H. Micrococcus indolicus. Some biochemical properties, and the demonstration of sex antigenically different types. Acta Vet Scand. 1973;14(2):301–326. doi: 10.1186/BF03547448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen G. H. Peptococcus (S. Micrococcus) indolicus. The demonstration of two varieties of hemolysin forming strains. Acta Vet Scand. 1975;16(2):218–225. doi: 10.1186/BF03546676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen G. H. Studies on the occurrence of Peptococcus indolicus and Corynebacterium pyogenes in apparently healthy cattle. Acta Vet Scand. 1976;17(1):15–24. doi: 10.1186/BF03547939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr S. E., Thompson F. S., Dowell V. R., Jr, Balows A. Micromethod system for identification of anaerobic bacteria. Appl Microbiol. 1973 May;25(5):713–717. doi: 10.1128/am.25.5.713-717.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Schwam O., Smyth C. J., Wadström T. Peptocoagulase: clotting factor produced by bovine strains of Peptococcus indolicus. J Clin Microbiol. 1978 Apr;7(4):361–367. doi: 10.1128/jcm.7.4.361-367.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharagonnet D., Sisson P. R., Roxby C. M., Ingham H. R., Selkon J. B. The API ZYM system in the identification of Gram-negative anaerobes. J Clin Pathol. 1977 Jun;30(6):505–509. doi: 10.1136/jcp.30.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt B., Jack E. P. What are anaerobic cocci? J Med Microbiol. 1977 Nov;10(4):461–468. doi: 10.1099/00222615-10-4-461. [DOI] [PubMed] [Google Scholar]
- Weeke B. A manual of quantitative immunoelectrophoresis. Methods and applications. 1. General remarks on principles, equipment, reagents and procedures. Scand J Immunol Suppl. 1973;1:15–35. doi: 10.1111/j.1365-3083.1973.tb03776.x. [DOI] [PubMed] [Google Scholar]
- Wegrzynowicz Z., Ko H. J., Pulverer G., Jeljaszewicz J. The nature of clotting and fibrinolytic activities of Bacteroids melaninogenicus. Zentralbl Bakteriol Orig A. 1978 Jan;240(1):106–111. [PubMed] [Google Scholar]


