Abstract
Obstructive sleep apnoea (OSA) is a disease of ever-increasing importance due to its association with multiple impairments and rising prevalence in an increasingly susceptible demographic. The syndrome is linked with loud snoring, disrupted sleep and observed apnoeas. Serious co-morbidities associated with OSA appear to be reversed by continuous positive airway pressure (CPAP) treatment; however, CPAP is variably tolerated leaving many patients untreated and emphasising the need for alternative treatments. Virtually all OSA patients have airways that are anatomically vulnerable to collapse, but numerous pathophysiological factors underlie when and how OSA is manifested. This review describes how the complexity of OSA requires multiple treatment approaches that are individually targeted. This approach may take the form of more specific diagnoses in terms of the mechanisms underlying OSA as well as rational pharmacological treatment directed toward such disparate ends as arousal threshold and ventilatory control/chemosensitivity, and mechanical treatment in the form of surgery and augmentation of lung volumes.
Keywords: motoneurones, motor units, obstructive sleep apnoea, respiratory, sleep, therapeutic
1. Definitions and epidemiology of obstructive sleep apnoea
Obstructive sleep apnoea syndrome (OSA) is recognised as a common disease with prevalence figures of at least 4% of the population depending on the criteria used [1]. The prevalence of OSA is likely to have risen subsequent to these estimates, due to the aging of the population, the obesity pandemic, improved techniques to identify breathing abnormalities, and the recognition that even asymptomatic patients can have important apnoea. Currently, the standard treatment for OSA is continuous positive airway pressure treatment (CPAP) [2]. However, due to the variable adherence associated with CPAP, alternative approaches for treating OSA are sometimes used such as mandibular advancements (in the form of oral appliances) [3,4] and upper airway surgery (attempting to reduce anatomical susceptibility to collapse). Experimental treatments include electrical stimulation of the hypoglossal nerves [5-7], training the upper airway muscles (e.g., through didgeridoo playing) [8] and various pharmacological approaches. However, an undisputed goal of many investigators is to provide a better understanding of the underlying physiology thereby opening targeted pharmacological approaches as a treatment strategy.
1.1 Negative sequelae
OSA is defined by symptoms resulting from episodes of reduced or absent airflow through a narrow or collapsed pharyngeal airway during sleep [9]. These include loud snoring, disrupted sleep and apnoeas despite ongoing respiratory efforts [10] OSA is a major public health concern due to its association with excessive sleepiness, strokes/cardiovascular disorders, depression, attention deficit hyperactivity disorder in children, memory and learning impairments and motor vehicle accidents [1,11-16]. Alarmingly evidence suggests that moderate to severe sleep apnoea is an independent risk factor for mortality after controlling for other factors that are already known to cause premature death [17].
1.2 Abnormality of obstructive sleep apnoea
There is a spectrum of sleep-associated upper airway dysfunction. Abnormalities include increased upper airway resistance, vibration of pharyngeal mucosal tissues (snoring), flow limitation and partial airway closure (hypopnoea), and finally, complete obstruction (apnoea). Episodes of hypopnoea and apnoea can last from 10 to more than 60 sec [1]. Sleep-disordered breathing also encompasses non-obstructive hypoventilation in its various forms [18]. Nocturnal hypoventilation is attributed to either decreased ventilatory drive (‘won’t breathe’) or impaired respiratory system mechanics (‘can’t breathe’) [18]. Coexistence of obstructive and non-obstructive events is not uncommon [e.g., 19,20]. OSA is characterised by repetitive episodes of ‘valve-like’ pharyngeal closure during sleep [21] with a resulting decrease in oxygen saturation and hypercapnia. Contributors to collapse of the upper airway include a combination of constricted space due to upper airway anatomy [22-25], impaired neuromuscular drive [26-28], intraluminal negative (sub-atmospheric) pressure, and external influences on the pharyngeal space (such as extraluminal tissue pressure) [29,30]. With compromised stabilisation of the airway walls and increasing negative pressure behind the soft palate, pharyngeal obstruction is common [31-37]. Because pharyngeal obstruction often occurs at end-exhalation, factors other than intraluminal negative pressure are also likely to be important for example end-expiratory lung volume and expiratory (tonic) upper airway dilator muscle activity. These obstructive apnoeic events often terminate with an arousal yielding restoration of pharyngeal patency but not before exposing the patient to periods of hypoxemia, hypercapnia and consequent autonomic reflexes [26]. However, some OSA patients may occasionally restore their airway patency without awakening, presumably via recruitment of upper airway muscles through sufficient increases in respiratory drive [38,39].
2. Structural anatomical basis
The upper airway has to serve multiple roles in speech, swallowing and breathing and the human airway is assisted with specialised features such as the free-floating hyoid morphology, unlike in most mammals [40,41]. Although the structure of the upper airway is anatomically fairly well understood [42,43], the biomechanical interaction of human upper airway muscles has received little attention. Only a few researchers have considered how these muscles perform and interact in vivo [44]. This lack of attention is largely because human airways are difficult to study and, until the recent advances in dynamic imaging techniques, interpretations were based solely on still-frame images of the airway muscles. The airway itself has received more attention, with changes in the cross-sectional area based on cine-computed tomography, optical coherence tomography and MRI [32,33,45,46]. These images have led investigators to appreciate where and under what conditions various sites in the upper airway are susceptible to collapse. Nonetheless, descriptions of this complex, three-dimensional structure in health and disease remain incomplete, therefore models have by necessity been greatly simplified and extrapolated from available data. There are well-characterised differences in basic anatomical features that distinguish airways of normal individuals from those of patients who suffer from OSA. The human upper airway is constrained anatomically by the mandibular skeletal framework in the anterior and lateral aspects while the posterior aspect is constrained by the morphology of the anterior aspect of the spine. The maxilla provides support and defines the rostral portion of the retroglossal airway but this bony vault is relatively unconstrained in its caudal aspect. In some OSA patients, these bony compartments are abnormally small, thereby any deformation or further change in the local anatomy may cause narrowing or even obstruction of the airway. Within the constraints of the skeletal framework, the tongue can markedly alter the upper airway dimensions; hence much research has focused on this specific area of the airway. The tongue includes four pairs of extrinsic muscles (which have their point of origin outside the tongue and insert into the musculature of the tongue body [42]: genioglossus, styloglossus, hyoglossus and palatoglossus) and four pairs of intrinsic muscles (with both their origin and insertion within the tongue body; superior longitudinal, inferior longitudinal, vertical and transverse muscles). The tongue, is referred to as a ‘muscular hydrostat’, with the movement of the tongue reliant upon the co-ordinated actions of individual muscles fibres from each muscle [44,47,48]. The fibres of the intrinsic muscles intermesh together [49] and are assembled by a structural collagen lattice [50] that interact closely with the contractile elements of the skeletal muscles to transmit force. Therefore, this dynamic ‘muscular hydrostat’ environment allows movements of the tongue concurrently in multiple dimensions and is not constricted to a protrusion and retraction plane of movement. The tongue muscles are supported by the muscles of the floor of the mouth. This group includes the geniohyoid that is separated from the genioglossus muscle by fibrous aponeurosis, in turn supported by thin muscle layer that acts like a hammock to elevate the tongue called the mylohyoid muscle, with the two digastric jaw muscles situated most proximal to the surface of the skin. These muscles act together with the infra-hyoid muscle group to dilate the airway, but mostly during ballistic swallowing movements.
Histological biopsy studies have confirmed differences in the muscle properties between OSA patients and control subjects. Muscle fibre types are presumably remodelled with a shift towards a higher representation of type II muscle fibres and decreased type I in OSA patients [51-55]. This may be driven by repetitive exposure to hypoxia which has been demonstrated in animal models to produce myogenic changes similar to those seen in humans [56-59]. However, in humans suffering from OSA, there are also differences in the distribution and angulation of the muscle fibres [60]. Taken together, these descriptions of modifications in the muscle fibres possibly make the tongues of OSA patients slower to recover from exercise and cause them to fatigue more easily than those of controls [28,51], although no compelling evidence exists that fatigue is important in apnoea pathogenesis. To date no complete cadaver study has been performed in detail to map out the level of these changes throughout the whole tongue in OSA patients. Furthermore, no studies in vivo have examined the level of fatigue on the tongue in OSA patients separating the influence of motivation, central and peripheral influences [61].
Obesity and anatomical abnormalities are common in OSA [29,62,63]. Clinically, measures of local adiposity (such as neck circumference), as well as witnessed apnoeas and snoring are the strongest predictors of the OSA syndrome [10]. Although obesity is not essential for the development of this disorder, this risk factor has been causally linked to OSA and its importance is highlighted by the observation that weight loss in obese patients with OSA can eliminate this condition. Increased body mass index is suggestive of visceral obesity but the accumulation of upper body fat is more important than the total amount of body fat for the risk of sleep apnoea [29]. Any increase in adipose tissue may load the longer and more vulnerable airways of men to a greater extent than those of women [64]. Interestingly, the ratio of adipose tissue to muscle fibres in the tongue is higher than that in peripheral limb muscles, and expected to be even higher in OSA patients [65,66]. Selected anatomical factors such as the size of the lateral pharyngeal walls, tongue, and total soft tissue volume have been demonstrated to also have genetic links to OSA [36,46]. There are also racial differences in sleep-disordered breathing with African-Americans more likely to develop OSA at an earlier age than Caucasians [67]. The reasons for racial predisposition for OSA are not obvious, but differences in the soft tissue and bony structure of upper airway are likely explanations [10] as are different demographics for other predisposing risk factors [68,69].
3. Ventilatory stability
Ventilation is regulated with optimal feedback control to maintain stable levels of oxygen and carbon dioxide. However, the cohesive framework involved allows a number of potential mechanisms to influence the stability of the ventilatory system [70]. Several investigators have indicated that ventilatory instability can lead to periodic breathing and impaired airway patency throughout the respiratory cycle [71,72]. Indeed, instability in respiratory control has been linked to OSA in some patients [72]. Evidence from mathematical models suggests that measurements of ventilatory instability may be predictive of obstructive events [71]. Respiratory instability is quantified by ‘loop gain’, the ventilatory response to disturbance ratio [70,72,73]. A system with high loop gain will react in a robust manner to a perturbation, while a low-gain system will respond in a blunted manner [74]. The two key variables that influence the loop gain are the controller gain and plant gain, which can both modify the stability of ventilation. Controller gain is essentially chemoresponsiveness (i.e., chemosensitivity plus response to stimulation), which is determined by the brainstem pathways that sense and respond to changes in oxygen and CO2 levels (e.g. the change in acidosis within the blood supply). Plant gain comprises the efficiency of CO2 excretion, dependent on the size of the stores of oxygen and CO2. Mathematically loop gain can be expressed as the response to a disturbance divided by the perturbation itself. A loop gain less than 1 will lead to a small controlled response, so that ventilation will return to a stable pattern [74]. If loop gain is greater than 1, a respiratory disturbance will lead to an overcompensation that leads ventilation to wax and wane in a periodic manner. Therefore, an elevated loop gain is destabilising to ventilation [74], particularly in the context of sleep apnoea. The combined influence of structural abnormalities and the reduced activation of respiratory motoneurones at sleep onset leaves the stability of respiration especially dependent upon loop gain [75]. Thus in some individual OSA patients carefully titrated oxygen, which decreases loop gain, may decrease fluctuations in central output which could otherwise cause apnoeas (when central output to the diaphragm and upper airway muscles is at its nadir) [73].
4. End expiratory lung volume
There are both external and internal influences that affect the collapsing and dilating forces that determine patency of the airway during wakefulness. Changes in lung volume can affect pharyngeal size and stiffness, probably through caudal traction forces. Such forces can improve upper airway collapsibility [76], independent of the neural drive to the airway. Throughout wakefulness, passive increases in lung volume positively influence the pharyngeal airway size and its collapsibility [77,78]. Furthermore, the upper airway dimensions of patients with OSA may have greater changes than controls with alterations in lung volume, described as a greater ‘lung volume dependence’. Sleep-induced decreases in the functional residual capacity of the lung contribute to inspiratory flow limitation [76] and with the dependence on lung volume, the upper airways of OSA patients are vulnerable to collapse in sleep. Therefore, increased end expiratory lung volume in OSA patients reduces the respiratory disturbance index [79,80] and stabilises the upper airway during sleep. This effect of stabilisation has been suggested to be larger in OSA patients compared with normal controls [81].
5. Arousal threshold
Awakening is associated with a surge in ventilation [e.g., 82,83] that, when combined with the abrupt decrease in CO2 set point (which is higher during sleeping than when awake) and the presence of hypercapnia consequent to the apnoea, can produce ventilatory instability, particularly in individuals with high loop gain. Because ventilatory instability in turn increases susceptibility to apnoeas and hypopnoeas [74] a vicious cycle can exist. It is commonly assumed that awakening is necessary for restoration of airway patency and that it is therefore a critical defence mechanism against asphyxia. However, this may not be the case for all patients. Activation of respiratory muscles, sometimes called ‘brainstem arousal’ can occur in the absence of cortical electroencephalogram desynchonisation [38]. In these patients delay of awakening long enough to allow effective recruitment of pharyngeal dilator muscles may allow restoration of airway patency without sleep fragmentation [38]. This strategy would be particularly helpful in those patients with low arousal thresholds who are able to reopen their airways given sufficient chemostimulation. By contrast, raising arousal threshold could be theoretically deleterious for patients that are arousal-dependent and if this characteristic varies at different times in the same patient as does arousal threshold [84], determining a safe treatment plan could be tricky. Nevertheless, a better understanding of the role of arousal threshold in OSA pathogenesis is clearly warranted.
6. Upper airway muscle control
Normal breathing (eupnoea) requires no conscious input and can be clearly observed when environmental stimuli and behavioural influences are minimised. The automatic control of breathing originates from the brainstem with the output shaped not only by chemo- and mechanosensory feedback but also the influence of volitional inputs from supra-pontine structures such as the cortex [85]. Under normal homeostatic conditions the respiratory output is directed through the motoneuron pools (e.g. hypoglossal and phrenic) to the muscles so that adequate ventilation is maintained. The most pronounced state-related change to affect respiratory neural drive is the transition through sleep and wake states; these transitions produce abrupt changes in the activity of the respiratory system especially with the elimination of volitional influences at sleep onset [e.g., 86,87]. These sleep-wake transition changes are responsible for some sleep-related disorders, the most common being OSA, where intrathoracic pressure swings and the chest wall moves without airflow at the nose or mouth.
Due to its importance in OSA, ease of accessibility and shear size, the genioglossus is the most widely studied muscle of the upper airway [e.g., 26,42]. Neural drive to the genioglossus has been quantified using multiunit electromyographic techniques, as it gives an estimate of the overall neural drive. The results from these techniques are limited and have described respiratory activity patterns as simply ‘tonic’ (i.e., constant activity) or ‘phasic’ (i.e. bursts of activity with inspiration) [e.g., 88 - 91]. While these studies are unable to provide information about the discharge frequency or pattern of firing of single motor units (a motor unit is a group of muscle fibres innervated by a single motoneuron) they have shed light on the activity in the genioglossus, which is greater during wakefulness and the neural drive is diminished at sleep onset which may lead to repetitive upper airway collapse [26]. A seminal observation from early multiunit electromyographic recordings has been that the signal is higher in OSA patients compared with that in controls during wakefulness [27]. It was presumed that the higher electromyographic signal was due to increased descending neural drive that resulted in high firing rates in genioglossus motor units. The universal theory suggested that during wakefulness the increased neural drive acts as a compensatory mechanism to upper airway muscles in OSA patients, and is lost at the onset of sleep yielding airway collapse.
Recently, selective single motor unit recordings have been employed to better understand the mechanisms behind genioglossus activity patterns. These studies indicate that control of the genioglossus muscle is more complex than multiunit recordings would suggest. Six different classes of single motor units have been identified based on respiratory (inspiratory phasic and tonic; expiratory phasic and tonic) and non-respiratory related activity (tonic and tonic other) [92] and unlike the activity within the respiratory chest wall muscles [93,94] the genioglossus motor units display high firing frequencies during periods of quiet breathing [94-96]. These methods have also been exploited to provide some understanding of the mechanisms that underlie the increased activity of the genioglossus in OSA subjects. Single motor unit recordings from the genioglossus in OSA patients during wakefulness have revealed the same proportions of the six classes of motor unit activity compared with control subjects with subtle changes in the central neural drive in both the timing and discharge firing frequencies between the groups [96]. Furthermore, this study was the first to reveal increased duration of single motor unit action potentials in the genioglossus of OSA subjects. This is a clear neurophysiological marker for peripheral neurogenic changes in the motor units [96]. This finding is supported by the sensory changes, abundance of additional nerve endings from biopsy specimens in the mucosa and the degeneration of myelinated nerve fibres and axons seen in sleep apnoea patients [97-101]. Furthermore, the histological findings can correspond to a neurogenic alteration. These observations have led to the suggestion that there may be a progression from mild snoring to heavy habitual snoring to OSA. However, the clinical studies are equivocal as to whether sleep apnoea progresses above and beyond that attributable to weight gain over time.
The increased multiunit electromyogram (EMG) signal in the genioglossus muscle of OSA patients may be due in part to the neurogenic involvement; however, there also is most probably additional neural drive reaching these muscles due to afferent feedback from mechanoreceptor activation. Currently, there is no evidence or techniques available to determine whether the increased or compensatory neural drive in the genioglossus is independent of these two interactive factors.
Respiratory motoneurons inhibited at sleep onset may lead to apnoeic events, however, it is not known if all stages of sleep actively inhibit all classes of respiratory motoneurons or if there is a selective subgroup that is not affected by the wakefulness stimulus for example expiratory motoneurons [87,102]. During normal quiet breathing the effects of oscillating motor units in the upper airway prevent the collapse, however, with the state-related change there is a loss of input to the motoneurones. This may allow the patency of the airway to be affected by the anatomical constraints to the systems such as the additional mass of the neck.
7. Phenotype - targeting the right population
As described in the previous sections, OSA is a surprisingly complex and highly individualized disease with different factors making varying contributions to the disease process in each patient. The primary physiological factors are anatomy, muscle responsiveness, arousal threshold and loop gain. In addition behavioural factors, such as poor sleep hygiene, and drugs, including alcohol, and excessive body weight may exacerbate the condition and should be addressed first to the extent possible. Finally, people have individual psychosocial conditions affecting their willingness to use CPAP, ingest pharmaceuticals, or submit to surgical procedures. Clearly implementing a strategy that is tailored to the individual is an essential ingredient for the best outcome (Table 1 outlines factors affecting airway patency and approaches that may be adopted to treat these conditions in OSA).
Table 1. Factors affecting patency and possible treatment options.
| Factors affecting patency | Treatment options | Pathophysiological effect |
|---|---|---|
| Anatomical | ||
| Enlarged uvula and soft palate | Uvulopalatopharyngoplasty | Enlarge the velopharynx |
| Enlarged tonsils | Tonsillectomy | Removal of tonsils |
| Recessed mandible | Oral appliances | Temporary anterior displacement of mandible |
| Mandibular advancement | Permanent anterior displacement of mandible | |
| Impaired retrolingual airway | Genioglossal advancement | Enlarged retrolingual airway |
| Hyoid myotomy suspension | Enlarged retrolingual airway | |
| Collapsed airway | Tracheostomy | Bypass upper airway |
| Small upper airway | CPAP | Splints the airway |
| Increased airflow resistance | Corticosteroids | Reduces resistance |
| High surface tension forces | Topical lubricants | Reduce the tension |
| Lung volume | ||
| Decreased functional residual capacity | CPAP | Inflates the lungs and enlarges the airways |
| Upper airway muscle activity | ||
| Loss of neural muscle activity at sleep onset | Pharmacological (see Table2) | Re-established increased activity |
| Electrical stimulation | Provide patency to airways | |
Usually multiple approaches are adopted within the patients interest.
8. Therapies
Currently, CPAP remains the gold standard therapy for symptomatic OSA. However, long-term compliance is an important problem even in patients with severe OSA [103]. Among patients who are adherent with therapy, CPAP maintains pharyngeal patency by maintaining a positive transmural pressure in the upper airway. The CPAP strategy is beneficial among those who can tolerate it. Randomised trials have shown benefits from standpoint of daytime sleepiness [104], blood pressure [105,106], quality of life, and vascular function [107]. However, whether CPAP prevents hard cardiovascular complications is currently unclear. In addition, there are many patients who are either intolerant of CPAP or avoid the OSA diagnosis due to reluctance to try CPAP [108]. Thus, new therapeutic approaches are clearly required.
For patients who have difficulty tolerating CPAP treatments, alternative options are currently limited [109]. One alternative approach is oral appliance therapy, including mandibular retention devices, which has potential advantages over CPAP. Oral appliances may be less obtrusive than CPAP, do not make noise, and are more portable, especially advantageous for travel [110]. Although oral appliance therapy may be effective across all grades of obstructive sleep apnoea severity [111] (especially mild to moderate disease), these devices are limited in their effectiveness, they are expensive and response to therapy is somewhat unpredictable [111-113] (see: Phenotype - targeting the right population above). Oral appliances have been shown to improve adherence as compared with CPAP in some patients [112]. Oral appliances can lead to some improvements in daytime sleepiness, blood pressure [114] and endothelial function [115]. However, oral appliances can be uncomfortable and expensive, and not particularly effective for more severe disease.
Each of these treatments options discussed herein should be utilised in conjunction with altering the other possible predisposing factors which may affect OSA including: excessive weight, poor sleep hygiene, nasal obstructions, and drugs, including alcohol. The efficacy of treatment with any intervention will benefit from altering these factors.
8.1 Surgery
Patients who seek improvements in health and quality of life outcomes occasionally turn to a range of permanent surgical procedures specifically designed to improve their upper airway mechanics via alteration of the local anatomy. An attractive advantage of utilising surgical options for treatment is that they are a ‘permanent fix’ and the ‘compliance’ factor becomes irrelevant. The evidence for the effectiveness of surgery is not compelling and complications can become problematic. A severe problem with these therapies is that the effects on related co-morbidities are not always addressed thus the positive outcomes can be hard to measure and their effectiveness remains controversial.
Many of the anatomical and physiological abnormalities corrected with surgery aim to increase the area of the pharyngeal airway space through the partial removal of soft tissues, suspension of existing tissues or the general remodelling of the anatomical space [116]. The organisation and structure of the musculo-skeletal system has a profound influence on the conversion of muscle forces into providing airway patency of each of these surgical procedures (listed below). Uvulopalatopharyngoplasty is the most common surgical operation performed for OSA with removal of the distal edge of the uvula and soft palate [117]. The incidence of complications and mortality are low after this type of surgery, thereby prompting its prevalence in the literature and in clinical practice. More far-reaching measures of permanent correction include mandibular advancement, genioglossal advancement, and hyoid myotomy suspension [118]. Hyoid myotomy suspension is achieved by placing a suture over the hyoid bone and pulling it anteriorly towards the mandible. Similarly, a genioglossal advancement leads to advancement of the suprahyoid and tongue muscles fixed to the mandible [119]. Mandibular advancement has high success rates in reducing the apnoea hypopnoea index in some studies, as it enlarges the retrolingual and retropalatal airways but this somewhat morbid option is often left as a second line of treatment [4]. Thus, methods to predict the responsiveness to surgery (e.g., with computational modelling [120,121]) will be required to improve the benefits to patients. Predictors of failure to respond to particular procedures would also be useful to help redirect attention towards potentially effective approaches.
Indirectly treating OSA through weight loss including laparoscopic adjustable gastric banding surgery has positive outcomes. This procedure involves the placement of an adjustable band below the level of the gastro-oesophageal junction [109]. The restriction on the bands can be tailored such that it allows significant weight loss with major benefits for severely obese subjects with OSA, including sleep architecture in addition to a reduction in overall sleep disturbances [109].
8.2 Manipulating loop gain
The efforts devoted to understanding ventilation have assisted in determining possible causes of breathing instability. These mathematical modelling studies allow investigators the ability to recognise gaps in our knowledge that have enabled new alternatives to treatment options to be considered. However, not all instances of periodic breathing are easily explained as control system instability by current models. For example, we are currently unable to predict the neural response to arousal using the loop gain models. The ability to use these models to provide ongoing feedback to ventilators etc. will obviously provide additional mechanisms by which compliance to treatments may be assessed. Emerging data suggest that a subgroup of sleep apnea patients may have particularly high loop gain [122]. Based on the prior literature suggesting that a subgroup of sleep apnea patients may respond to oxygen therapy (i.e. to lower loop gain), we would predict that in pre-selected patients, methods to lower ventilatory loop gain may be an effective approach [73]. However, randomised controlled trials will be required to assess clinical outcome benefits to patients. In addition, further theoretical work is still needed to break down the processes of feedback control and respiratory instability into more physiologically probable models that can help to guide future experimental studies. However, it remains unlikely that such modelling efforts will accomplish the level of detail that is present and included in the respiratory brainstem circuitry.
8.3 Manipulation of lung volume
The decrease in functional residual capacity that occurs at sleep onset, may also promote upper airway collapsibility, particularly in supine, obese subjects [79,123]. Not only does thoracic inflation increase upper airway pharyngeal size and stiffness through caudal traction of the trachea, this lung volume effect is independent of activity in the upper airway muscles [76]. Therefore, as lung volume has an independent influence on upper airway collapsibility it has been hypothesised that CPAP prevents upper airway obstruction in patients with sleep apnoea through an increased functional residual capacity [79,80,123]. The responses of patients to increased functional residual capacity may vary depending on the movement of the trachea and the weight of the individual. However, further work is required to determine whether manipulation of lung volume by itself represents a viable therapeutic target.
8.4 Controlling airway muscle activity and arousal threshold
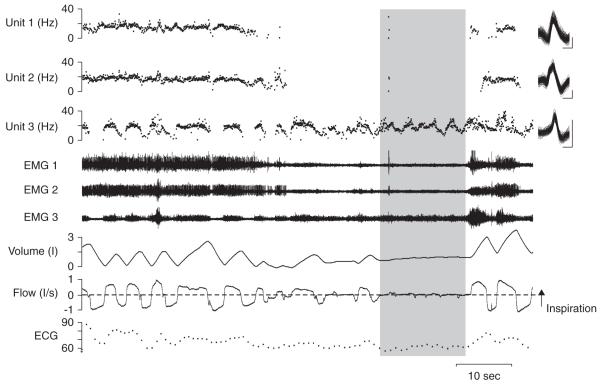
The motor system controlling ventilation is complex as it is responsible for the conversion of afferent inputs and multiple descending pacemaker signals to shape and control respiration. The control of respiration is affected greatly by behavioural state changes, with respiratory brainstem neuron activity reduced at sleep onset [75] and the reduction is reflected in the multiunit muscle signal [124]. This change is reflected in detailed activity of single motor units which reduce the period of a breath for which they are active and often ceasing completely [87]. It is not yet clear which motor units in the genioglossus affect the patency of the airway. Figure 1 shows the pattern activation of three motor units in the genioglossus muscle with a non-uniform derecruitment of two units that may have a function that relates to the ensuing 15 second apnoea. These two units may receive similar premotor neurone influences while control of the third unit is clearly distinct as it displays ongoing activity through the apnoea. This finding provides direct evidence that the responses of neurones to state-related changes are non-uniform and therefore that there is differential organisation of drive at the pre-motoneuronal level. Future neural recordings will be required in various brain and muscle regions to determine how the feedback control and state changes are distributed through the respiratory motor system. These changes coincide with a rise in upper airway resistance (see Lung volume) and reductions in multiple neural reflex pathways. This combined change in the physiology at sleep onset leaves the airway vulnerable to collapse and provides a potential mechanism for pharmacological intervention or external electrical stimulation.
Figure 1. Simultaneous recordings from genioglossus muscle in one subject with 15 sec apnoea.
From bottom to top: electrocardiogram, airflow, volume, genioglossus raw electromyogram (EMG) and instantaneous discharge frequency with overlaid motor unit potentials from the unit (at right) are shown. The three decomposed signals (Units 1, 2 and 3) are from the corresponding three EMG signals (EMG 1, 2 and 3) shown. Inspiration is upwards in the airflow and volume trace. Vertical calibrations for superimposed motor unit potentials are: 200 mV and horizontal calibration is 2 ms. Shaded area shows the complete apnoea. Units 1 and 2 are both suppressed during the apnoea while unit 3 remains active.
Where and how respiratory signals and state-related changes are integrated at the single-cell level remains poorly understood. Clearly there remains substantive integration of inputs to the motoneuron pools themselves, however, complex premotor influences remain incompletely understood. One of the primary difficulties in controlling the airway with pharmacological treatments remains our lack of knowledge of the respiratory pathways themselves. However, there have been pharmacological trials using a variety of drugs that are proposed to act on ventilatory drive, which may increase or decrease respiratory responses globally to respiration or specifically to a single motoneuron pool. Many of these trials have not been fruitful, with some studies limited in design with small sample sizes or lack of long-term trials, often with short periods of intervention. Despite these limitations some of the trials have specifically targeted the hypoglossal or phrenic motoneurone pools with differing results (Table 2).
Table 2. Categories of pharmacological treatment options that have been used for OSA.
| Drug | Mechanism of action | Outcome |
|---|---|---|
| Benzodiazepine | ||
| Diazepam | Binds to the GABAA receptor | Increased XII output [125] |
| Trazodone | Not entirely clear, but, has antagonistic effects on 5 HT2 receptors | Increased respiratory-arousal threshold in OSA [128] |
| Lorazepam | Systemic action not a direct XII effect | Increases GG activity systemically Decreases XII output directly |
| Zolpidem | Systemic action not a direct XII effect | Increases GG activity systemically Decreases XII output direct |
| Non benzodiazepine hypnotics | ||
| Eszopiclone | Interaction with GABAA receptor | Promote sleep, increased respiratory-arousal threshold in OSA [147] |
| Zolpidem | Interaction with GABAA receptor | Promote sleep, increased respiratory-arousal threshold in OSA [147] |
| Antidepressants | ||
| Protriptyline | Tricyclic antidepressant | Increased XII output, reduced rapid eye movement sleep, reduced AHI [148] |
| Fluoxetine | Selective serotonin reuptake inhibitor | Reduced rapid eye movement sleep, Reduced AHI |
| Paroxetine | Selective serotonin reuptake inhibitor | Increased peak genioglossus activity NREM sleep. No change in tonic activity or OSA index [149] |
| Ventilatory stimulants | ||
| Thyroxine | Thyroid substitution treatment | Reduced OSA severity |
| Acetazolamide | Decreases blood pH | Increased incidents of apnoeas Increased ventilation |
| Medroxyprogesterone | Increased ventilation (mixed responses, used in hypercapnic patients) | |
| Theophylline | Inhibits adenosine | Reduced central apnoeas Not useful in OSA |
Under each category some examples of each type are given along with the proposed mechanism of action and outcomes.
AHI: Apnea/hyponea index; GG: Genioglossus; NREM: Non rapid eye movement; XII: Hypoglossal (group XII) motoneurons.
The hypoglossal motor nucleus has been targeted with tricyclic antidepressant protriptyline which increases its overall output activity without altering the phrenic nerve discharge in cats [125]. In humans, protriptyline was associated with subjective improvements in daytime hypersomnolence, possibly by suppression of rapid eye movement sleep [126]. By contrast, the benzodiazepine derivative, diazepam, reduced hypoglossal but did not alter phrenic nerve discharge [125]. However, sedative effects of benzodiazepines have been assessed and suggested to assist in prolonging the time to arousal particularly in sleep apnoea patients following airway occlusion thereby resulting in an increased arousal threshold [127]. Another sedative, trazodone has been shown to increase the respiratory-related arousal threshold in OSA patients thereby allowing higher levels of CO2 to be tolerated [128]. Therefore, in selected patients with a low arousal threshold these non-myorelaxant sedatives may contribute to reduced apnoea severity [128].
Some have considered the manipulation of the arousal threshold to be somewhat of a double-edged sword. That is, among patients with high arousal threshold, profound hypoxemia and hypercapnia may develop prior to arousal and therefore a sedative/hypnotic may well be deleterious. On the other hand, among patients with a low arousal threshold, inadequate time may be present for the accumulation of respiratory stimuli (i.e., negative intrapharyngeal pressure and CO2) to allow the activation of pharyngeal dilator muscles. A recent report suggested that the phasic pharyngeal dilator muscles (as represented by the genioglossal EMG) were necessary and sufficient for the stabilization of breathing among sleep apnoea patients [129,130]. These data suggest that among OSA patients with a low arousal threshold that an intervention to raise arousal threshold (e.g., trazodone) may be effective at allowing sufficient dilator muscle activation to yield stabilisation of ventilation. However, again, outcome data are currently lacking for this approach.
Mixed responses have been reported to the effectiveness of medroxyprogesterone, a ventilatory stimulant; it may be useful to treat sleep apnoea of chronically hypercapnic patients [131]. Recent work has lessened enthusiasm for a role of serotonin as a modulator of the hypoglossal contribution to upper airway patency [132]. Recent developments in the use of ampakines [133] have generated enthusiasm for the viability of this approach; however, clinical trial results will be required before any definitive conclusions can be drawn.
The favoured drugs for OSA treatment are currently used to supplement rather than replace CPAP treatment. Topical lubricants such as phosphocholinamin benefit some OSA patients by reducing surface tension forces in the upper airway to facilitate recovery from obstructions [134]. The use of corticosteroids such as fluticasone has benefits in some patients with OSA, potentially by reducing tonsillar tissue [135].
Electrical stimulation of upper airway muscles and nerves can produce contractions of the tongue and can be utilised for OSA treatment as either a training device for the muscles or for night time relief of symptoms [5-7,136-139]. These types of stimulation can induce transformations of the fibre types in the upper airway muscles that may improve the fatigability of the muscle [6]. It is debatable whether direct muscle stimulation or nerve stimulation is preferable. Either may theoretically be helpful as genioglossus stimulation alone or concomitant stimulation of retracting and protruding muscles stabilizes the airway [e.g., 136,140 - 143]. Reported techniques include implanted wire electrodes, surface stimulating electrodes and hypoglossal nerve stimulation. Unfortunately, inconsistent results to date do not currently justify the risks associated with the invasive nature of implanting devices into the upper airway. Nonetheless stimulation may be a viable option for some patients pending safety and efficacy.
8.5 Genetics/stem cell therapy
It is still debated as to the potential means by which stem cell and genetics research will influence the future of OSA therapies, but there is a great deal of research focused directly or indirectly into these areas. There are investigators using genetic screening to identify those most at risk of suffering from sleep disorders resulting from vulnerabilities in inherited traits. As genetic factors related to cranio-facial shapes and features also play a role in pathogenesis of OSA.
Stem cell research into obesity is attempting to identify means to decrease patients’ adipose tissue deposition and thereby preventing a myriad of diseases including OSA [144]. The underlying hypothesis is that stem cells, having more than one potential outcome, can theoretically be induced to differentiate ex vivo into any of the 200 or so cell types in the human body or be able to restore or replace organs that have been damaged or lost through injury or chronic degenerative disease [144]. Therefore in obese individuals control over the tissue mass of these individuals may facilitate their treatment.
9. Expert opinion
The areas of physiology, surgery and regenerative medicine are involved in the ongoing research and in future treatments of OSA patients. Areas which are likely to have new alternative breakthroughs in providing treatment approaches for OSA may include stem cell technology, cellular or genetic therapies, and drug delivery systems.
A direct determinant of upper airway mechanics is the activity present in the upper airway muscles, which is influenced by both respiratory [92,94,96], and non-respiratory behaviors [145,146]. Taking advantage of neural pathways that independently control upper airway and respiratory pump muscles is likely to help prevent apnoeas [93]. Approaches which attempt to modify the behaviour of the motoneurones offer a potentially interesting means of controlling the airway. However, the human afferent and motor pathways that regulate eupnoea are still poorly understood. The premotoneurones and the sensory pathways which control of the upper airway during sleep may offer viable therapeutic targets in the future, once they have been adequately characterized pharmacologically and neurophysiologically.
The research that has been completed to date with mandibular splints, pharmacological treatment interventions (low arousal threshold), electrical stimulation and feedback control over existing CPAP machines offer viable options for OSA treatments. The future of other means of treatments should remain open with the ability to screen patients for genetic markers or phenotypic traits that may yield positive outcomes in terms of compliance. Ultimately the vision for the treatment of sleep apnoea will be a multidisciplinary targeted approach based on underlying neurophysiological mechanism. However, further research is clearly required to realise this goal.
9.1 Conclusion
OSA is widely accepted as a major public health problem, as it is strongly associated with cardiovascular disorders, depression, attention deficit hyperactivity disorder in children, memory and learning impairments and motor vehicle accidents [11-14]. There are a variety of different treatments available either using direct surgical interventions, continuous positive airway pressure, or indirectly including weight loss.
Clinicians are now presented with a variety of options to treat their patients with OSA, however, the best options for individual patients are not yet predictable. Clearly an understanding of the distinct pathophysiology in each patient and research into the most probably successful approach for each pathophysiological type will inform treatment decisions in the future. At this time it is still uncertain whether or not single pharmacological measures will ever be possible or if genetics and stem cell research will offer possible screening and possible alternative approaches to treat OSA.
Acknowledgments
Declaration of interest
This work was supported by grants from the National Institutes of Health (NIH) P50 HL60292, R01 HL073146, R01 HL085188, K23 AG 024837 and the American Heart Association.
Bibliography
Papers of special note have been highlighted as either of interest (·) or of considerable interest (··) to readers.
- 1.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 3.Cistulli PA. Rapid maxillary expansion in obstructive sleep apnea-hope on the horizon? Sleep. 2004;27:606–7. [PubMed] [Google Scholar]
- 4.Dekeister C, Lacassagne L, Tiberge M, et al. Mandibular advancement surgery in patients with severe obstructive sleep apnea uncontrolled by continuous positive airway pressure. A retrospective review of 25 patients between 1998 and 2004. Rev Mal Respir. 2006;23:430–7. doi: 10.1016/s0761-8425(06)71813-7. [DOI] [PubMed] [Google Scholar]
- 5.Eisele DW, Schwartz AR, Smith PL. Tongue neuromuscular and direct hypoglossal nerve stimulation for obstructive sleep apnea. Otolaryngol Clin North Am. 2003;36:501–10. doi: 10.1016/s0030-6665(02)00178-0. [DOI] [PubMed] [Google Scholar]
- 6.Pae EK, Hyatt JP, Wu J, Chien P. Short-term electrical stimulation alters tongue muscle fibre type composition. Arch Oral Biol. 2007;52:544–51. doi: 10.1016/j.archoralbio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Tran W, Loeb G, Richmond FJR, et al. First subject evaluated with simulated BION treatment in genioglossus to prevent obstructive sleep apnea. Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2004;6:4287–9. doi: 10.1109/IEMBS.2004.1404194. [DOI] [PubMed] [Google Scholar]
- 8.Puhan MA, Suarez A, Lo Cascio C, et al. Didgeridoo playing as alternative treatment for obstructive sleep apnoea syndrome: randomised controlled trial. Br Med J (Clin Res Ed) 2006;332:266–70. doi: 10.1136/bmj.38705.470590.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 10.Malhotra A, White D, editors. Pathogenesis of obstructive sleep apnea. Saunders; Philadelphia: 2002. [Google Scholar]
- 11.Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166:1709–15. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- 12.Wei JL, Mayo MS, Smith HJ, et al. Improved behavior and sleep after adenotonsillectomy in children with sleep-disordered breathing. Arch Otolaryngol Head Neck Surg. 2007;133:974–9. doi: 10.1001/archotol.133.10.974. [DOI] [PubMed] [Google Scholar]
- 13.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 14.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J, Cooperative Group Burgos-Santander The association between sleep apnea and the risk of traffic accidents. N Engl J Med. 1999;340:847–51. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 15.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, In K, You S, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170:1108–13. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 17.Marshall NS, Wong KK, Liu PY, et al. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–85. [PMC free article] [PubMed] [Google Scholar]
- 18.Casey KR, Cantillo KO, Brown LK. Sleep-related hypoventilation/hypoxemic syndromes. Chest. 2007;131:1936–48. doi: 10.1378/chest.06-2334. [DOI] [PubMed] [Google Scholar]
- 19.Xie A, Rutherford R, Rankin F, et al. Hypocapnia and increased ventilatory responsiveness in patients with idiopathic central sleep apnea. Am J Respir Crit Care Med. 1995;152:1950–5. doi: 10.1164/ajrccm.152.6.8520761. [DOI] [PubMed] [Google Scholar]
- 20.White DP, Gleeson K, Pickett CK, et al. Altitude acclimatization: influence on periodic breathing and chemoresponsiveness during sleep. J Appl Physiol. 1987;63:401–12. doi: 10.1152/jappl.1987.63.1.401. [DOI] [PubMed] [Google Scholar]
- 21.Safar P, Escarraga LA, Chang F. Upper airway obstruction in the unconscious patient. J Appl Physiol. 1959;14:760–4. doi: 10.1152/jappl.1959.14.5.760. [DOI] [PubMed] [Google Scholar]
- 22.Robinson S, Lewis R, Norton A, Mcpeake S. Ultrasound-guided radiofrequency submucosal tongue-base excision for sleep apnoea: a preliminary report. Clin Otolaryngol Allied Sci. 2003;28:341–5. doi: 10.1046/j.1365-2273.2003.00719.x. [DOI] [PubMed] [Google Scholar]
- 23.Morikawa S, Safar P, Decarlo J. Influence of the headjaw position upon upper airway patency. Anesthesiology. 1961;22:265–70. doi: 10.1097/00000542-196103000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Ozbek MM, Miyamoto K, Lowe AA, Fleetham JA. Natural head posture, upper airway morphology and obstructive sleep apnoea severity in adults. Eur J Orthod. 1998;20:133–43. doi: 10.1093/ejo/20.2.133. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert RJ, Napadow VJ. Three-dimensional muscular architecture of the human tongue determined in vivo with diffusion tensor magnetic resonance imaging. Dysphagia. 2005 Winter;20:1–7. doi: 10.1007/s00455-003-0505-9. [DOI] [PubMed] [Google Scholar]
- 26.Remmers JE, Degroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–8. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 27.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J ClinInvest. 1992;89:1571–9. doi: 10.1172/JCI115751.Quantified the increased multiunit electromyographic signal in OSA patients versus controls.
- 28.Blumen MB, de La Sota AP, Quera-Salva MA, et al. Tongue mechanical characteristics and genioglossus muscle EMG in obstructive sleep apnoea patients. Respir Physiol Neurobiol. 2004;140:155–64. doi: 10.1016/j.resp.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Resta O, Foschino-Barbaro MP, Legari G, et al. Sleep-related breathing disorders, loud snoring and excessive daytime sleepiness in obese subjects. Int J Obes RelatMetab Disord. 2001;25:669–75. doi: 10.1038/sj.ijo.0801603.Differentiated that neck circumference in men and BMI in women were the strongest predictors of OSA severity.
- 30.Berry RB, White DP, Roper J, et al. Awake negative pressure reflex response of the genioglossus in OSA patients and normal subjects. J Appl Physiol. 2003;94:1875–82. doi: 10.1152/japplphysiol.00324.2002. [DOI] [PubMed] [Google Scholar]
- 31.Eastwood PR, Szollosi I, Platt PR, Hillman DR. Collapsibility of the upper airway during anesthesia with isoflurane. Anesthesiology. 2002;97:786–93. doi: 10.1097/00000542-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong JJ, Leigh MS, Sampson DD, et al. Quantitative upper airway imaging with anatomic optical coherence tomography. Am J Respir Crit Care Med. 2006;173:226–33. doi: 10.1164/rccm.200507-1148OC. [DOI] [PubMed] [Google Scholar]
- 33.Horner RL, Shea SA, Mcivor J, Guz A. Pharyngeal size and shape during wakefulness and sleep in patients with obstructive sleep apnoea. Q J Med. 1989;72:719–35. [PubMed] [Google Scholar]
- 34.Ryan CF, Love LL. Mechanical properties of the velopharynx in obese patients with obstructive sleep apnea. A Journal of Respiratory and Critical Care Medicine. 1996;154:806–12. doi: 10.1164/ajrccm.154.3.8810623. [DOI] [PubMed] [Google Scholar]
- 35.Isono S, Remmers JE, Tanaka A, et al. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–26. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 36.Ciscar MA, Juan G, Martinez V, et al. Magnetic resonance imaging of the pharynx in OSA patients and healthy subjects. Eur Respir J. 2001;17:79–86. doi: 10.1183/09031936.01.17100790. [DOI] [PubMed] [Google Scholar]
- 37.Suratt PM, Dee P, Atkinson RL, et al. Fluoroscopic and computed tomographic features of the pharyngeal airway in obstructive sleep apnea. Am Rev Respir Dis. 1983;127(4):487–92. doi: 10.1164/arrd.1983.127.4.487. [DOI] [PubMed] [Google Scholar]
- 38.Younes M, Ostrowski M, Atkar R, et al. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol. 2007;103:1929–41. doi: 10.1152/japplphysiol.00561.2007. [DOI] [PubMed] [Google Scholar]
- 39.Younes M, Park E, Horner RL. Pentobarbital sedation increases genioglossus respiratory activity in sleeping rats. Sleep. 2007;30:478–88. doi: 10.1093/sleep/30.4.478. [DOI] [PubMed] [Google Scholar]
- 40.Martinez I, Arsuaga JL, Quam R, et al. Human hyoid bones from the middle Pleistocene site of the Sima de los Huesos (Sierra de Atapuerca, Spain) J Hum Evol. 2008;54:118–24. doi: 10.1016/j.jhevol.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Arensburg B, Tillier AM, Vandermeersch B, et al. A Middle Palaeolithic human hyoid bone. Nature. 1989;338:758–60. doi: 10.1038/338758a0. [DOI] [PubMed] [Google Scholar]
- 42.Anderson RJ. The morphology of the muscles of the tongue and pharynx. J Anat Physiol. 1881;15:382–91.Detailed anatomy of the upper airway muscles.
- 43.Lowe AA. The neural regulation of tongue movements. Prog Neurobiol. 1980;15:295–344. doi: 10.1016/0301-0082(80)90008-8. [DOI] [PubMed] [Google Scholar]
- 44.Cheng S, Butler JE, Gandevia SC, Bilston LE. Movement of the tongue during normal breathing in awake healthy humans. J Physiol. 2008;586:4283–94. doi: 10.1113/jphysiol.2008.156430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwab RJ, Gefter WB, Pack AI, Hoffman EA. Dynamic imaging of the upper airway during respiration in normal subjects. J Appl Physiol. 1993;74:1504–14. doi: 10.1152/jappl.1993.74.4.1504. [DOI] [PubMed] [Google Scholar]
- 46.Schwab RJ, Pasirstein M, Kaplan L, et al. Family aggregation of upper airway soft tissue structures in normal subjects and patients with sleep apnea. Am J Respir Crit Care Med. 2006;173:453–63. doi: 10.1164/rccm.200412-1736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keir WM, Smith KK. Tongues, tentacles, and trunks: the biomechanics of movement in muscular hydrostats. Zoolog J Linnean Soc. 1985;83:307–24. [Google Scholar]
- 48.Smith KK, Kier WM. Trunks, tongues, and tentacles: Moving with skeletons of muscle. Am Sci. 1989;77:28–35. [Google Scholar]
- 49.Slaughter K, Li H, Sokoloff AJ. Neuromuscular organization of the superior longitudinalis muscle in the human tongue. Cells Tissues Organs. 2005;181:51–64. doi: 10.1159/000089968. [DOI] [PubMed] [Google Scholar]
- 50.Miller AJ. Oral and pharyngeal reflexes in the mammalian nervous system: their diverse range in complexity and the pivotal role of the tongue. Crit Rev Oral Biol Med. 2002;13:409–25. doi: 10.1177/154411130201300505. [DOI] [PubMed] [Google Scholar]
- 51.Carrera M, Barbe F, Sauleda J, et al. Patients with obstructive sleep apnea exhibit genioglossus dysfunction that is normalized after treatment with continuous positive airway pressure. Am J Respir Crit Care Med. 1999;159:1960–6. doi: 10.1164/ajrccm.159.6.9809052.Anatomy of the genioglossus suggesting differences between OSA patients versus controls in the fibre type.
- 52.Series F, Cote C, Simoneau JA, et al. Physiologic, metabolic, and muscle fiber type characteristics of musculus uvulae in sleep apnea hypopnea syndrome and in snorers. J Clin Invest. 1995;95:20–5. doi: 10.1172/JCI117640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferini-Strambi LJ, Smirne S, Moz U, et al. Muscle fibre type and obstructive sleep apnea. Sleep Res Online. 1998;1:24–7. [PubMed] [Google Scholar]
- 54.Lindman R, Stal PS. Abnormal palatopharyngeal muscle morphology in sleep-disordered breathing. J Neurol Sci. 2002;195:11–23. doi: 10.1016/s0022-510x(01)00676-1. [DOI] [PubMed] [Google Scholar]
- 55.Smirne S, Iannaccone S, Ferini-Strambi L, et al. Muscle fibre type and habitual snoring. Lancet. 1991;337:597–9. doi: 10.1016/0140-6736(91)91651-a. [DOI] [PubMed] [Google Scholar]
- 56.Mcguire M, Macdermott M, Bradford A. The effects of chronic episodic hypercapnic hypoxia on rat upper airway muscle contractile properties and fiber-type distribution. Chest. 2002;122:1400–6. doi: 10.1378/chest.122.4.1400. [DOI] [PubMed] [Google Scholar]
- 57.Mcguire M, Macdermott M, Bradford A. Effects of chronic episodic hypoxia on rat upper airway muscle contractile properties and fiber-type distribution. Chest. 2002;122:1012–7. doi: 10.1378/chest.122.3.1012. [DOI] [PubMed] [Google Scholar]
- 58.Pae EK, Wu J, Nguyen D, et al. Geniohyoid muscle properties and myosin heavy chain composition are altered after short-term intermittent hypoxic exposure. J Appl Physiol. 2005;98:889–94. doi: 10.1152/japplphysiol.00978.2004. [DOI] [PubMed] [Google Scholar]
- 59.Sauleda J, Garcia-Palmer FJ, Tarraga S, et al. Skeletal muscle changes in patients with obstructive sleep apnoea syndrome. Respir Med. 2003;97:804–10. doi: 10.1016/s0954-6111(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 60.Edstrom L, Larsson H, Larsson L. Neurogenic effects on the palatopharyngeal muscle in patients with obstructive sleep apnoea: a muscle biopsy study. J Neurol Neurosurg Psychiatry. 1992;55:916–20. doi: 10.1136/jnnp.55.10.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81(4):1725–89. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 62.Arzt M, Young T, Finn L, et al. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carrera M, Barbe F, Sauleda J, et al. Effects of obesity upon genioglossus structure and function in obstructive sleep apnoea. Eur Respir J. 2004;23:425–9. doi: 10.1183/09031936.04.00099404. [DOI] [PubMed] [Google Scholar]
- 64.Whittle AT, Marshall I, Mortimore IL, et al. Neck soft tissue and fat distribution: comparison between normal men and women by magnetic resonance imaging. Thorax. 1999;54:323–8. doi: 10.1136/thx.54.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller JL, Watkin KL, Chen MF. Muscle, adipose, and connective tissue variations in intrinsic musculature of the adult human tongue. J Speech Lang Hear Res. 2002;45:51–65. doi: 10.1044/1092-4388(2002/004). [DOI] [PubMed] [Google Scholar]
- 66.Chmielewski L, Kim E, Pack A, et al. The role of tongue fat in patients with OSA compared to BMI-matched normals. Am J Respir Crit Care Med. 2007;175:A755. [Google Scholar]
- 67.Redline S, Tishler PV, Hans MG, et al. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–92. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 68.Wetter DW, Young TB, Bidwell TR, et al. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med. 1994;154:2219–24. [PubMed] [Google Scholar]
- 69.Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax. 1991;46:85–90. doi: 10.1136/thx.46.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cherniack NS, Longobardo GS. Mathematical models of periodic breathing and their usefulness in understanding cardiovascular and respiratory disorders. Exp Physiol. 2006;91:295–305. doi: 10.1113/expphysiol.2005.032268. [DOI] [PubMed] [Google Scholar]
- 71.Longobardo GS, Evangelisti CJ, Cherniack NS. Analysis of the interplay between neurochemical control of respiration and upper airway mechanics producing upper airway obstruction during sleep in humans. Exp Physiol. 2008;93:271–87. doi: 10.1113/expphysiol.2007.039917. [DOI] [PubMed] [Google Scholar]
- 72.Khoo MC. Determinants of ventilatory instability and variability. Respir Physiol. 2000;122:167–82. doi: 10.1016/s0034-5687(00)00157-2. [DOI] [PubMed] [Google Scholar]
- 73.Wellman A, Malhotra A, Jordan AS, et al. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol. 2008;162:144–51. doi: 10.1016/j.resp.2008.05.019.Translation of loop gain theories into stabilizing the ventilatory control system and reducing the risk of upper airway collapse in patients with OSA.
- 74.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–70. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 75.Orem J, Osorio I, Brooks E, Dick T. Activity of respiratory neurons during NREM sleep. J Neurophysiol. 1985;54:1144–56. doi: 10.1152/jn.1985.54.5.1144.Quantified the activity of medullary respiratory neurones finding a decreased level of activity in sleep compared to wakefulness.
- 76.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol. 1988;65:2124–31. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- 77.Series F, Marc I. Influence of lung volume dependence of upper airway resistance during continuous negative airway pressure. J Appl Physiol. 1994;77:840–4. doi: 10.1152/jappl.1994.77.2.840. [DOI] [PubMed] [Google Scholar]
- 78.Series F, Cormier Y, Desmeules M. Influence of passive changes of lung volume on upper airways. J Appl Physiol. 1990;68:2159–64. doi: 10.1152/jappl.1990.68.5.2159. [DOI] [PubMed] [Google Scholar]
- 79.Heinzer RC, Stanchina ML, Malhotra A, et al. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:114–7. doi: 10.1164/rccm.200404-552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heinzer RC, Stanchina ML, Malhotra A, et al. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax. 2006;61(5):435–9. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heinzer R, White DP, Malhotra A, et al. Effect of expiratory positive airway pressure on sleep disordered breathing. Sleep. 2008;31:429–32. doi: 10.1093/sleep/31.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trinder J, Ivens C, Kleiman J, et al. The cardiorespiratory activation response at an arousal from sleep is independent of the level of CO2. J Sleep Res. 2006;15:174–82. doi: 10.1111/j.1365-2869.2006.00513.x. [DOI] [PubMed] [Google Scholar]
- 83.Horner RL, Rivera MP, Kozar LF, Phillipson EA. The ventilatory response to arousal from sleep is not fully explained by differences in CO2 levels between sleep and wakefulness. J Physiol (Lond) 2001;534(Pt 3):881–90. doi: 10.1111/j.1469-7793.2001.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol. 2008;105(5):1389–405. doi: 10.1152/japplphysiol.90408.2008. [DOI] [PubMed] [Google Scholar]
- 85.Mckay LC, Evans KC, Frackowiak RS, Corfield DR. Neural correlates of voluntary breathing in humans. J Appl Physiol. 2003;95:1170–8. doi: 10.1152/japplphysiol.00641.2002. [DOI] [PubMed] [Google Scholar]
- 86.Worsnop C, Kay A, Kim Y, et al. Effect of age on sleep onset-related changes in respiratory pump and upper airway muscle function. J Appl Physiol. 2000;88:1831–9. doi: 10.1152/jappl.2000.88.5.1831. [DOI] [PubMed] [Google Scholar]
- 87.Wilkinson V, Malhotra A, Nicholas CL, et al. Discharge patterns of human genioglossus motor units during sleep onset. Sleep. 2008;31(4):525–33. doi: 10.1093/sleep/31.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mcginley BM, Schwartz AR, Schneider H, et al. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol. 2008;105(1):197–205. doi: 10.1152/japplphysiol.01214.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol. 1976;51:160–70. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- 90.Sauerland EK, Mitchell SP. Electromyographic activity of the human genioglossus muscle in response to respiration and to positional changes of the head. Bull Los Angeles Neurol Soc. 1970;35:69–73. [PubMed] [Google Scholar]
- 91.Sauerland EK, Mitchell SP. Electromyographic activity of intrinsic and extrinsic muscles of the human tongue. Tex Rep Biol Med. 1975;33:444–55. [PubMed] [Google Scholar]
- 92.Saboisky JP, Butler JE, Fogel RB, et al. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol. 2006;95:2213–21. doi: 10.1152/jn.00940.2005. [DOI] [PubMed] [Google Scholar]
- 93.Saboisky JP, Gorman RB, De Troyer A, et al. Differential activation among five human inspiratory motoneuron pools during tidal breathing. J Appl Physiol. 2006;102:772–80. doi: 10.1152/japplphysiol.00683.2006. [DOI] [PubMed] [Google Scholar]
- 94.Saboisky JP, Butler JE, Walsh LD, Gandevia SC. New display of the timing and firing frequency of single motor units. J Neurosci Methods. 2007;162:287–92. doi: 10.1016/j.jneumeth.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 95.Saboisky JP, Butler JE, Gorman RB, et al. European Respiratory Society Behaviour of single motor units in genioglossus in patients with obstructive sleep apnoea; Annual Congress; 2006.p. 642s.p. P3744. [Google Scholar]
- 96.Saboisky JP, Butler JE, Mckenzie DK, et al. Neural drive to human genioglossus in obstructive sleep apnoea. J Physiol. 2007;585:135–46. doi: 10.1113/jphysiol.2007.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Woodson BT, Garancis JC, Toohill RJ. Histopathologic changes in snoring and obstructive sleep apnea syndrome. Laryngoscope. 1991;101:1318–22. doi: 10.1002/lary.5541011211. [DOI] [PubMed] [Google Scholar]
- 98.Friberg D, Gazelius B, Hokfelt T, Nordlander B. Abnormal afferent nerve endings in the soft palatal mucosa of sleep apnoics and habitual snorers. RegulPept. 1997;71:29–36. doi: 10.1016/s0167-0115(97)01016-1. [DOI] [PubMed] [Google Scholar]
- 99.Nguyen AT, Jobin V, Payne R, et al. Laryngeal and velopharyngeal sensory impairment in obstructive sleep apnea. Sleep. 2005;28:585–93. doi: 10.1093/sleep/28.5.585.Evidence for the need to phenotype OSA patients as this paper demonstrates that there may exist subgroups with normal laryngeal sensation and others with abnormal sensory values.
- 100.Boyd JH, Petrof BJ, Hamid Q, et al. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:541–6. doi: 10.1164/rccm.200308-1100OC. [DOI] [PubMed] [Google Scholar]
- 101.Kimoff RJ, Sforza E, Champagne V, et al. Upper airway sensation in snoring and obstructive sleep apnea. Am J Respir Crit Care Med. 2001;164:250–5. doi: 10.1164/ajrccm.164.2.2010012. [DOI] [PubMed] [Google Scholar]
- 102.Bailey EF, Fridel KW, Rice AD. Sleep/Wake firing patterns of human genioglossus motor units. J Neurophysiol. 2007;98(6):3284–91. doi: 10.1152/jn.00865.2007. [DOI] [PubMed] [Google Scholar]
- 103.Zozula R, Rosen R. Compliance with continuous positive airway pressure therapy: assessing and improving treatment outcomes. Curr Opin Pulm Med. 2001;7:391–8. doi: 10.1097/00063198-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 104.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353:2100–5. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 105.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–10. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 106.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 107.Massie CA, Mcardle N, Hart RW, et al. Comparison between automatic and fixed positive airway pressure therapy in the home. Am J Respir Crit Care Med. 2003;167:20–3. doi: 10.1164/rccm.200201-022OC. [DOI] [PubMed] [Google Scholar]
- 108.Lin HS, Zuliani G, Amjad EH, et al. Treatment compliance in patients lost to follow-up after polysomnography. Otolaryngol Head Neck Surg. 2007;136:236–40. doi: 10.1016/j.otohns.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 109.Dixon JB, Schachter LM, O’Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes. 2005;29:1048–54. doi: 10.1038/sj.ijo.0802960. [DOI] [PubMed] [Google Scholar]
- 110.Ng AT, Gotsopoulos H, Qian J, Cistulli PA. Effect of oral appliance therapy on upper airway collapsibility in obstructive sleep apnea. Am J Respir Crit care Med. 2003;168:238–41. doi: 10.1164/rccm.200211-1275OC. [DOI] [PubMed] [Google Scholar]
- 111.Mehta A, Qian J, Petocz P, et al. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Criti Care Med. 2001;163:1457–61. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 112.Ferguson KA, Ono T, Lowe AA, et al. A randomized crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest. 1996;109:1269–75. doi: 10.1378/chest.109.5.1269. [DOI] [PubMed] [Google Scholar]
- 113.Engleman HM, Mcdonald JP, Graham D, et al. Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: continuous positive airway pressure and mandibular repositioning splint. Am J Respir Crit Care Med. 2002;166(6):855–9. doi: 10.1164/rccm.2109023. [DOI] [PubMed] [Google Scholar]
- 114.Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial. Sleep. 2004;27:934–41. doi: 10.1093/sleep/27.5.934. [DOI] [PubMed] [Google Scholar]
- 115.Itzhaki S, Dorchin H, Clark G, et al. The effects of 1-year treatment with a herbst mandibular advancement splint on obstructive sleep apnea, oxidative stress, and endothelial function. Chest. 2007;131:740–9. doi: 10.1378/chest.06-0965. [DOI] [PubMed] [Google Scholar]
- 116.Sundaram S, Bridgman SA, Lim J, Lasserson TJ. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev (Online) 2005;4 doi: 10.1002/14651858.CD001004.pub2. CD001004. Published online 19 October 2005, doi: 10.1002/14651858.CD001004.pub2. [DOI] [PubMed] [Google Scholar]
- 117.Kezirian EJ, Weaver EM, Yueh B, et al. Incidence of serious complications after uvulopalatopharyngoplasty. Laryngoscope. 2004;114(3):450–3. doi: 10.1097/00005537-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 118.Puricelli E. A new technique for mandibular osteotomy. Head face Med. 2007;3:15. doi: 10.1186/1746-160X-3-15. Published online 13 March 2007, doi:10.1186/1746-160X-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nagler RM, Laufer D. Genioglossal advancement-a simple surgical procedure for sleep apnea. Case report and literature review. Eur Surg Res. 2002;34:373–7. doi: 10.1159/000063999. [DOI] [PubMed] [Google Scholar]
- 120.Huang Y, Malhotra A, White DP. Computational simulation of human upper airway collapse using a pressure-/state-dependent model of genioglossal muscle contraction under laminar flow conditions. J Appl Physiol. 2005;99:1138–48. doi: 10.1152/japplphysiol.00668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang Y, White DP, Malhotra A. Use of computational modeling to predict responses to upper airway surgery in obstructive sleep apnea. Laryngoscope. 2007;117:648–53. doi: 10.1097/MLG.0b013e318030ca55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–32. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stanchina ML, Malhotra A, Fogel RB, et al. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26(7):851–6. doi: 10.1093/sleep/26.7.851. [DOI] [PubMed] [Google Scholar]
- 124.Worsnop C, Kay A, Pierce R, et al. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol. 1998;85:908–20. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- 125.Bonora M, St John WM, Bledsoe TA. Differential elevation by protriptyline and depression by diazepam of upper airway respiratory motor activity. Am Rev Respir Dis. 1985;131:41–5. doi: 10.1164/arrd.1985.131.1.41. [DOI] [PubMed] [Google Scholar]
- 126.Smith PL, Haponik EF, Allen RP, Bleecker ER. The effects of protriptyline in sleep-disordered breathing. Am Rev Respir Dis. 1983;127:8–13. doi: 10.1164/arrd.1983.127.1.8. [DOI] [PubMed] [Google Scholar]
- 127.Berry RB, Mccasland CR, Light RW. The effect of triazolam on the arousal response to airway occlusion during sleep in normal subjects. Am Rev Respir Dis. 1992;146:1256–60. doi: 10.1164/ajrccm/146.5_Pt_1.1256. [DOI] [PubMed] [Google Scholar]
- 128.Heinzer RC, White DP, Jordan AS, et al. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur Respir J. 2008;31(6):1308–12. doi: 10.1183/09031936.00067607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jordan AS, White DP, Lo YL, et al. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep. 2009;32:361–8. doi: 10.1093/sleep/32.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–33. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 131.Hudgel DW, Thanakitcharu S. Pharmacologic treatment of sleep-disordered breathing. Am J Respir Crit Care Med. 1998;158:691–9. doi: 10.1164/ajrccm.158.3.9802019. [DOI] [PubMed] [Google Scholar]
- 132.Horner RL. Neuromodulation of hypoglossal motoneurons during sleep. Respir Physiol Neurobiol. 2008;164:179–96. doi: 10.1016/j.resp.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 133.Ren J, Poon BY, Tang Y, et al. Ampakines alleviate respiratory depression in rats. Am J Respir Crit Care Med. 2006;174:1384–91. doi: 10.1164/rccm.200606-778OC.Apakines have some promise that they may alleviate respiratory depression and be useful in treating sleep apnoea.
- 134.Jokic R, Klimaszewski A, Mink J, Fitzpatrick MF. Surface tension forces in sleep apnea: the role of a soft tissue lubricant: a randomized double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 1998;157:1522–5. doi: 10.1164/ajrccm.157.5.9708070. [DOI] [PubMed] [Google Scholar]
- 135.Kiely JL, Nolan P, Mcnicholas WT. Intranasal corticosteroid therapy for obstructive sleep apnoea in patients with co-existing rhinitis. Thorax. 2004;59:50–5. [PMC free article] [PubMed] [Google Scholar]
- 136.Oliven A, Schnall RP, Pillar G, et al. Sublingual electrical stimulation of the tongue during wakefulness and sleep. Respir Physiol. 2001;127:217–26. doi: 10.1016/s0034-5687(01)00254-7. [DOI] [PubMed] [Google Scholar]
- 137.Decker MJ, Haaga J, Arnold JL, et al. Functional electrical stimulation and respiration during sleep. J Appl Physiol. 1993;75:1053–61. doi: 10.1152/jappl.1993.75.3.1053. [DOI] [PubMed] [Google Scholar]
- 138.Isono S, Tanaka A, Nishino T. Effects of tongue electrical stimulation on pharyngeal mechanics in anaesthetized patients with obstructive sleep apnoea. Eur Respir J. 1999;14:1258–65. doi: 10.1183/09031936.99.14612589. [DOI] [PubMed] [Google Scholar]
- 139.Ludwig A. Results of electromyostimulation therapy in obstructive sleep apnea. Artif Organs. 2008;32:655–8. doi: 10.1111/j.1525-1594.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 140.Oliven A, O’Hearn DJ, Boudewyns A, et al. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. J Appl Physiol. 2003;95:2023–9. doi: 10.1152/japplphysiol.00203.2003.Chronically implanted hypoglossal nerve electrodes in OSA patients assessed with Pcrit measures.
- 141.Schwartz AR, Bennett ML, Smith PL, et al. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2001;127:1216–23. doi: 10.1001/archotol.127.10.1216. [DOI] [PubMed] [Google Scholar]
- 142.Bailey EF, Huang YH, Fregosi RF. Anatomic consequences of intrinsic tongue muscle activation. J Appl Physiol. 2006;101:1377–85. doi: 10.1152/japplphysiol.00379.2006. [DOI] [PubMed] [Google Scholar]
- 143.Yoo PB, Durand DM. Effects of selective hypoglossal nerve stimulation on canine upper airway mechanics. J Appl Physiol. 2005;99:937–43. doi: 10.1152/japplphysiol.00652.2004. [DOI] [PubMed] [Google Scholar]
- 144.Bernardo AS, Docherty K. Stem cells and metabolic diseases. Biochem Soc Trans. 2008;36:363–5. doi: 10.1042/BST0360363. [DOI] [PubMed] [Google Scholar]
- 145.Pittman LJ, Bailey EF. Genioglossus and intrinsic electromyographic activities in impeded and unimpeded protrusion tasks. J Neurophysiol. 2009;101(1):276–82. doi: 10.1152/jn.91065.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bailey EF, Rice AD, Fuglevand AJ. Firing patterns of human genioglossus motor units during voluntary tongue movement. J Neurophysiol. 2007;97:933–6. doi: 10.1152/jn.00737.2006. [DOI] [PubMed] [Google Scholar]
- 147.Jia F, Goldstein PA, Harrison NL. The modulation of synaptic GABAA receptors in the thalamus by eszopiclone and zolpidem. J Pharmacol Exp Ther. 2009;328:1000–6. doi: 10.1124/jpet.108.146084. [DOI] [PubMed] [Google Scholar]
- 148.Hanzel DA, Proia NG, Hudgel DW. Response of obstructive sleep apnea to fluoxetine and protriptyline. Chest. 1991;100(2):416–21. doi: 10.1378/chest.100.2.416. [DOI] [PubMed] [Google Scholar]
- 149.Berry RB, Yamaura EM, Gill K, Reist C. Acute effects of paroxetine on genioglossus activity in obstructive sleep apnea. Sleep. 1999;22:1087–92. doi: 10.1093/sleep/22.8.1087. [DOI] [PubMed] [Google Scholar]