Abstract
Objective
This study was performed to elucidate the technical and patient-specific risk factors for postoperative ischemia in patients undergoing temporary arterial occlusion (TAO) during the surgical repair of their aneurysms.
Methods
Eighty-nine consecutive patients in whom TAO was performed during surgical repair of an aneurysm were retrospectively analyzed. The demographics of the patients were analyzed with respect to age, Hunt and Hess grade on admission, Fisher grade of hemorrhage, aneurysm characteristics, timing of surgery, duration of temporary occlusion, and number of temporary occlusive episodes. Outcome was analyzed at the 3-month follow-up, along with the occurrence of symptomatic and radiological stroke.
Results
In overall, twenty-seven patients (29.3%) had radiologic ischemia attributable to TAO and fifteen patients (16.3%) had symptomatic ischemia attributable to TAO. Older age and poor clinical grade were associated with poor clinical outcome. There was a significantly higher rate of symptomatic ischemia in patients who underwent early surgery (p = 0.007). The incidence of ischemia was significantly higher in patients with TAO longer than 10 minutes (p = 0.01). In addition, patients who underwent repeated TAO, which allowed reperfusion, had a lower incidence of ischemia than those who underwent single TAO lasting for more than 10 minutes (p = 0.011).
Conclusion
Duration of occlusion is the only variable that needs to be considered when assessing the risk of postoperative ischemic complication in patients who undergo temporary vascular occlusion. Attention must be paid to the patient's age, grade of hemorrhage, and the timing of surgery. In addition, patients undergoing dissection when brief periods of temporary occlusion are performed may benefit more from intermittent reperfusion than continuous clip application. With careful planning, the use of TAO is a safe technique when used for periods of less than 10 minutes.
Keywords: Temporary arterial occlusion, Intracranial aneurysm, Cerebral ischemia
INTRODUCTION
Temporary arterial occlusion (TAO) is an established technique for the repair of intracranial aneurysms (ICAs), and it facilitates the dissection of aneurysms that are densely adherent to adjacent vasculature or the repair of giant aneurysms. Apart from the reduced risk of rupture associated with aneurysm manipulation, TAO allows evacuation of intramural calcification and thrombosis before definitive clipping in large aneurysms. A number of authors have attempted to establish safe time periods for temporary occlusion, defining their end points variously as clinical ischemic deficit or radiological evidence of ischemia2,4,10,14,15,18,20). However, the period of stroke-free temporary occlusion varies considerably depending on clinical factors as well as which vessel is occluded2,3,11,16,21,24,25). When estimating the potential risks of ischemia in patients undergoing temporary vessel occlusion during surgery for ruptured aneurysms, factors other than the time of vessel occlusion need to be taken into account. These factors may include older patient age, poor grade of hemorrhage, location of aneurysm, size of aneurysm, and the risk of intraoperative aneurysm rerupture. This study was performed to assess factors that may increase the risk of ischemic complication resulting from temporary arterial occlusion in patients with ruptured aneurysms.
MATERIALS AND METHODS
Patient population
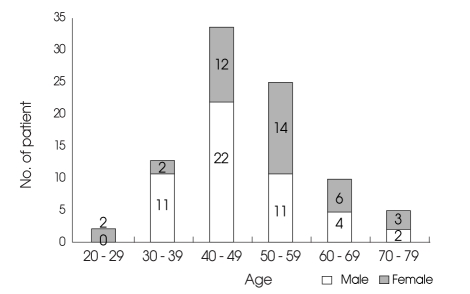
Between March 2001 and February 2006, 139 patients with aneurysmal subarachnoid hemorrhage (SAH) were treated surgically. Temporary arterial occlusion was performed in 95 of these patients to facilitate dissection and permanent aneurysm repair. Inadvertent permanent vessel occlusion was identified in six patients, and these patients were excluded from further analysis. The final study population included 89 patients. The patients were 50 men and 39 women with a mean age of 48.8 years (range 22 - 73 years) (Fig. 1). The medical records, operative anesthesiology and radiology reports for these patients were retrospectively reviewed.
Fig. 1.
Graph showing age and sex distribution of the candidates.
Surgical procedure
All of the patients underwent microsurgery performed by one surgeon. General anesthesia was induced using thiopentone and maintained with isoflurane and fentanyl. Normothermia and normotension were maintained during this time. Mannitol and barbiturate infusion were administered during temporary clipping for brain protection.
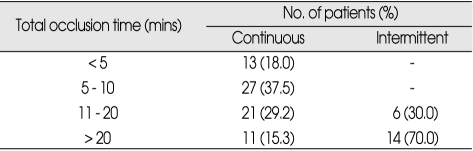
In all patients undergoing intermittent occlusion, a reperfusion period of more than 5 minutes was allowed between occlusive episodes. This amount of time has been shown to be protective after short periods of temporary occlusion (Table 1).
Table 1.
Stratification of patients by type and time of occlusion
Assessment of variables
The patients' medical and radiological records were reviewed with respect to patient age, Hunt and Hess grade on admission, Fisher grade of hemorrhage, aneurysm characteristics, timing of surgery, duration of temporary occlusion, and number of occlusive episodes.
All patients underwent CT scanning during the early postoperative period to screen for the development of new ischemic areas. Assessment of postoperative ischemia can be difficult when there are coincidental confounding factors such as inadvertent permanent vessel occlusion, surgical trauma related to retraction injury and distal hypodensity following hematoma evacuation and vasospasm. However, with careful appraisal of surgical methods, perioperative angiography, and clinical conditions, these factors can be detected and avoided during temporary clipping. In patients who developed vasospasm, the clinical time course and distribution of the infarcts allowed for an assessment of whether they were related to temporary occlusion or vasospasm, and they were recorded accordingly. However, ischemic change from inadvertent vessel occlusion during aneurysm clipping was not sorted out from ischemic change from temporary arterial occlusion. As a result, six patients were excluded from further analysis due to inadvertent vessel occlusion during aneurysm clipping.
In the 89 patients included in the final analysis, ischemic change was assessed on the basis of radiological and clinical findings. If patients had ischemic change on radiologic imaging but not had a neurologic deficit clinically, it was defined as radiologic ischemia. If the patient had a neurologic deficit and ischemic change was seen on radiologic imaging, it was defined as symptomatic ischemia. Clinical outcome was assessed using the Glasgow Outcome Scale (GOS) after 3 months after the surgery. A good outcome was defined as a GOS score of 4 or 5, and a poor outcome was defined as a GOS score of 1, 2, or 3.
Statistical analysis
Univariate and multivariate analyses were conducted. Categorical variables were compared using the chi-square or Fisher tests, and continuous variables were compared using the t-test when appropriate. Differences with a probability value of less than 0.05 were considered statistically significant. The end points considered included the occurrence of radiological documented stroke determined on postoperative CT scan, the development of symptomatic stroke, and clinical outcome. Commercially available software (SPSS) was used for all statistical calculations.
RESULTS
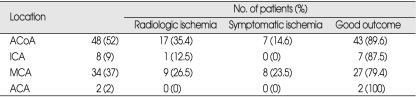
Nineteen (20.6%) of the ruptured aneurysms treated were larger than 10 mm in diameter. The ruptured aneurysms were located in the anterior communicating artery area in 48 (52%) patients, on the middle cerebral artery (MCA) in 34 (37%) patients, the ICA in 8 (9%) patients, and the ACA in 2 (2%) patients (Table 2). The low incidence of posterior circulation aneurysms in this series reflects our policy of performing endovascular therapy as the primary mode of treatment for these aneurysms, especially when they are located on the BA.
Table 2.
Clinical outcome and the incidence of symptomatic ischemia according to the location of aneurysm
ACoA : anterior communicating artery, ICA : internal carotid artery, MCA : middle cerebral artery, ACA : anterior cerebral artery
Overall, twenty-seven patients (29.3%) had radiologic ischemia attributable to TAO and fifteen patients (16.3%) had symptomatic ischemia attributable to TAO. With the exception of 1 patient who died within 2 weeks after the onset of hemorrhage, the mean follow-up time was 11.5 months (range, 3 months-29 months). A good outcome (GOS 4-5) was achieved in 75 (84.3%) patients, and 14 (15.7%) patients had poor outcomes (GOS 1-3). One of the patients who experienced a poor outcome died.
Age as a prognostic factor
There was no distinct increase in the risk of ischemic complication in the patients 50 years of age or older in our series. Age younger than 50 years was associated with a 16% risk of symptomatic ischemia, while age 50 years or older was associated with a 16.7% risk of ischemia. Univariate analysis revealed that age older than 50 years was not significantly associated with symptomatic (p = 0.931) or radiologic ischemic complication (p = 0.881). However, clinical outcome was significantly associated with patient age (p = 0.07). In addition, the incidence of symptomatic ischemia tended to be higher in patients over 60 years of age. Age younger than 60 years was associated with a 14% risk of symptomatic ischemia, while age 60 years or older was associated with a 26.7% risk of symptomatic ischemia (p = 0.235).
Hunt and Hess grade of hemorrhage
The Hunt and Hess grade of hemorrhage was related to the overall outcome but not to the risk of ischemic complication. The term "good grade" has been used throughout the text to describe patients with SAH of Hunt and Hess grade I to III, and "poor grade" was used to describe those with SAH of Hunt and Hess Grades IV or V. A favorable outcome was achieved in 59.1% of the patients with poor-grade hemorrhages. Univariate analysis revealed that the grade of hemorrhage was significantly associated with outcome (p = ≤ 0.001), but not with symptomatic ischemia (p = 0.698) or radiologic ischemia (p = 0.806).
Fisher grade
Fisher grade was related to overall outcome but not the risk of ischemic complication. The term "good grade" was used throughout the text to desc-ribe patients with Fisher grade 1-2, and "poor grade" was used for those with Fisher grade 3-4. All of the patients with good grades had satisfactory outcomes, whereas 77.6% of the patients with poor grades had satisfactory outcomes. Univariate analysis revealed that Fisher grade was signifi-cantly associated with outcome (p = 0.01), but not sympto-matic ischemia (p = 0.495) or radiologic ischemia (p = 0.862).
Aneurysm characteristics
Aneurysm size larger than 10 mm and the location of the aneurysm were not significantly associated with a poor outcome. Similarly, the incidence of symptomatic or radiologic ischemic complication was not significantly related to the size or location of the aneurysm. On univariate analysis, the location of the aneurysm was not significantly associated with outcome (p = 0.429), symptomatic ischemia (p = 0.234), or radiologic ischemia (p = 0.361). However, the incidence of symptomatic ischemia was relatively high in patients with MCA aneurysms (Table 2). The size of the aneurysm was not significantly associated with outcome (p = 0.529), symptomatic ischemia (p = 0.529) or radiologic ischemia (p = 0.373).
Timing of surgery
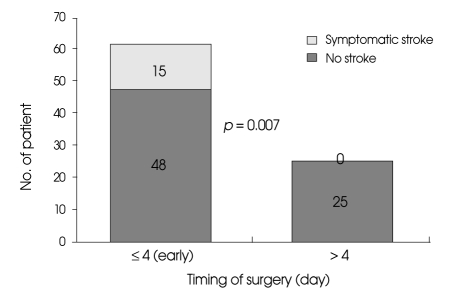
Univariate analysis revealed that patients who underwent delayed surgery (post-day 4 after SAH) had significantly better outcomes than those who underwent early surgery (between days 0 and 4 following SAH) (p = 0.040). Similarly, the results of univariate analysis revealed that there was a significantly higher rate of symptomatic ischemic complication in patients who underwent early surgery (p = 0.007) (Fig. 2).
Fig. 2.
Bar graph showing the incidence of symptomatic ischemic complication resulting from temporary arterial occlusion according to the timing of surgery.
Duration of temporary occlusion
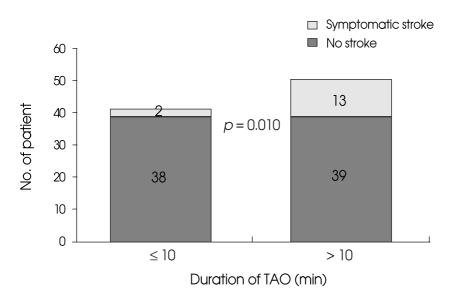
The incidence of symptomatic ischemia was 5% (2 cases) in patients undergoing temporary occlusion for periods of less than 10 minutes and 25% (13 cases) in patients undergoing temporary occlusion for more than 10 minutes. The incidence of radiologic ischemia was 10% (4 cases) in patients undergoing temporary occlusion for less than 10 minutes and 44% (23 cases) in those undergoing temporary occlusion for more than 10 minutes. There was no significant association between the outcome and the duration of temporary occlusion (p = 0.151). Univariate analysis revealed that increased duration of temporary occlusion was associated with a significant increase in symptomatic ischemic complication (p = 0.010) (Fig. 3) and radiologic ischemic complication (p = ≤ 0.001).
Fig. 3.
Bar graph showing an increased incidence of symptomatic ischemic complication in patients undergoing temporary arterial occlusion lasting longer than 10 minutes. TAO : temporary arterial occlusion.
Repeated temporary arterial occlusion and effect of reperfusion
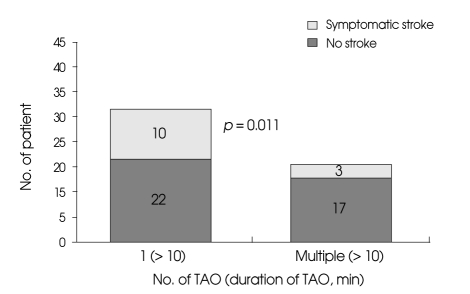
The incidence of symptomatic ischemia was 15% (3 cases) in patients undergoing temporary occlusion with reperfusion and 31% (10 cases) in patients undergoing temporary occlusion without reperfusion. The incidence of radiologic ischemia was 35% (7 cases) in patients undergoing temporary occlusion with reperfusion and 50% (16 cases) in patients undergoing temporary occlusion without reperfusion. Univariate analysis revealed that temporary occlusion with reperfusion was associated with a significant increase in symptomatic ischemic complication (p = 0.011) (Fig. 4) and radiologic ischemic complication (p = 0.001), but the outcome was not significantly associated with reperfusion (p = 0.081).
Fig. 4.
Bar graph showing an increased incidence of symptomatic ischemic complication in patients undergoing single episode of temporary arterial occlusion longer than 10 minutes than those undergoing intermittent occlusion, which allowed reperfusion. TAO : temporary arterial occlusion.
DISCUSSION
As the use of TAO during aneurysm surgery gains wider acceptance in the neurosurgical community1,4-6), it becomes crucial to define the risks of this procedure in patients with ruptured aneurysms and to identify a reasonably safe period of occlusion, with emphasis on the anatomical level of occlusion. With this aim, we evaluated the incidence of ischemic complication, both radiologic demonstrated and symptomatic, in our series of patients who underwent temporary arterial occlusion. Radiologic demonstrated ischemia related to temporary clip application was predominantly observed in the form of cortical ischemia rather than in the territory supplied by the perforating arteries. Deep ischemic areas tended to be small, often measuring approximately 1 cm in diameter and were often seen in the insulonuclear or caudate region. These lesions are frequently seen after intraoperative aneurysm rupture, particularly in association with temporary occlusion of the ICA, and they often resolve spontaneously, rarely causing persistent neurological deficits. Deep ischemia may be more difficult to ascribe to temporary occlusion because the vessel dissection and final clip placement may affect perforating vessels without the surgeon's knowledge.
The anesthesiology regimen has been quite uniform in our surgical series, consisting of induction of normothermia and normotension in all cases. Barbiturate infusion was initiated a few minutes before aneurysm clipping in the majority of cases of deliberate temporary occlusion. Some authors have recommended hypertension or hypothermia, during temporary clipping, while others have advocated the use of different monitoring techniques to warn the surgeon of impending stroke so that it can be prevented1,7,8,17,24). However, these monitoring techniques remain imperfect and can be misleading26). Neurophysiological monitoring was not used in our series.
Patient age
In previous studies, older age has been associated with poor outcomes and a greater risk of stroke13). This result was probably affected by the incidence of associated medical conditions. Medical illnesses, especially hypertension and other vascular diseases, are more common in older patients, and thus they are more susceptible to borderline cerebral perfusion and they have less compensation capacity6). Furthermore, older patients may be more symptomatic after cerebral ischemia due to a diminished functional reserve. In our study, the incidence of symptomatic ischemia tended to be higher in patients over 60 years. The reason for the tendency of increased symptomatic ischemia in patients over 60 years may be the same as that described in the previous study.
Hunt and Hess grade
In our series, Hunt and Hess grade was significantly associated with clinical outcome in the univariate analyses. However, there was no significant association between stroke and Hunt and Hess grade. In another study, there was a significant associated between Hunt and Hess grade and ischemia16). This discrepancy may have resulted from the bias in our study design. The other study was performed using multivariate analysis, but our study was performed with univariate study, and thus the bias led to a different result.
Fisher grade
In many other studies, the Fisher grade was significantly related to ischemia risk. They hypothesized that the existence of subarachnoid blood clots surrounding the cerebral arteries was the most important factor in eliciting vasospasm secondary to a ruptured aneurysm, and the distribution of the blood clot in the site of aneurysm rupture may determine the distribution of the vasospasm23). In addition, patients with poor-grade hemorrhage (Grades IV-V) and greater brain injury may be less able to compensate for ischemia and therefore have a higher rate of symptomatic ischemia. In addition, patients who experience such hemorrhages have more cortical areas in which there is marginal perfusion7). With the additional insult of temporary vascular occlusion, the brain may not be able to sustain cellular integrity, and this may be the reason for the increased rate of cortical distribution ischemia.
However, in our study, there was a significant relationship between Fisher grade and outcome, and this consistent with the findings of a previous study. However, it was less closely associated with ischemia risk. This result was different from the findings of another previous study. This difference may result from the bias in our study design. The other study was performed using multivariate analysis, but our study was performed with univariate analysis, and thus the bias led to a different result.
Aneurysm characteristics
In some studies, patients with large aneurysms have a greater chance of having a poor outcome after SAH than patients with small aneurysms. In addition, patients with large aneurysms tend to have a poor clinical condition on admission. However, in our study, the size of the aneurysm was not a significant factor for outcome. The relationship between outcome and aneurysm size reported in the previous study was that larger aneurysms were associated with greater accumulation of subarachnoid hematoma. However, there was no correlation between aneurysm size and amount of hematoma in our series, and this may have led to the discrepancy in the results.
Location was not a significant factor for ischemia, but the incidence of symptomatic ischemia was relatively high in patients with MCA aneurysms. Some authors have reported that certain vascular territories, especially the BA and MCA, are at particular risk when TAO is performed. There did seem to be less risk when the ICA was occluded, which may be related to the effects of the circle of Willis and other collateral circulation sources through various anastomoses. Occlusion of the MCA involved a higher risk of ischemia than occlusion of the ICA or other locations combined20).
Timing of surgery
Time of surgery was a significant factor for the development of ischemia as well as for the clinical outcome. This can be due to the fact that the brain may be less tolerant of focally reduced perfusion pressure from retraction in the acute stage, and surgery may precipitate cerebral vasospasm and cerebral dysfunction of immediate or delayed onset12,22). Furthermore, in the early days following SAH, patients experience decreased cerebral perfusion, which is present independent of vasospasm, suggesting an increased susceptibility to stroke. The advantage of delayed over early surgery in regard to resistance to ischemia is obviously counterbalanced by the risks of a wait-and-see attitude, especially when the overall management of patients with ruptured aneurysms is considered.
Duration of temporary occlusion and repeated temporary arterial occlusion (effect of reperfusion)
The duration of temporary occlusion remains the most investigated factor in clinical series of patients who undergo temporary vessel occlusion14,17,20). In our study, we observed a trend for an increased incidence of ischemic complication and good outcome in patients in whom temporary vessel occlusion was performed for more than 10 minutes. Other authors have also noted a tendency for increased ischemia rates with temporary occlusion lasting more than 10 minutes. Furthermore, in our study, patients with repeated TAO, which allowed reperfusion, showed a lower incidence of stroke than those with long single TAO over 10 minutes. There have been conflicting reports regarding the mode of temporary occlusion17,20,23). Some authors have claimed that there is a higher risk of ischemia with intermittent occlusion based on the results of experimental studies, whereas others have claimed that the risk is higher when continuous occlusion is performed5,9,14,19). The temporary occlusion times reported in the studies in which the rate of ischemia was lower when continuous occlusion was performed and compared with intermittent occlusion also involved substantially longer occlusion times17,20). In our series, the time of temporary occlusion before a period of reperfusion instituted was less than 10 minutes in nearly all cases. These relatively short occlusion times between periods of reperfusion might be the reason for the significantly longer duration of stroke-free intermittent occlusion when compared with the stroke-free group undergoing continuous occlusion.
CONCLUSION
Duration of occlusion is the only variable that needs to be considered when assessing the risk of postoperative stroke in patients who undergo temporary vascular occlusion. Attention must be paid to the patient's age, grade of hemorrhage, and the timing of surgery. In addition, patients undergoing dissection when brief periods of temporary occlusion are performed may benefit more from intermittent reperfusion than continuous clip application. With careful planning, the use of TAO is a safe technique when used for periods of less than 10 minutes.
References
- 1.Artiola i Fortuny L, Prieto-Valiente L. Long-term prognosis in surgically treated intracranial aneurysms. Part 2 : Morbidity. J Neurosurg. 1981;54:35–43. doi: 10.3171/jns.1981.54.1.0035. [DOI] [PubMed] [Google Scholar]
- 2.Batjer HH, Frankfurt AI, Purdy PD, Smith SS, Samson DS. Use of etomidate, temporary arterial occlusion, and intraoperative angiography in surgical treatment of large and giant cerebral aneurysms. J Neurosurg. 1988;68:234–240. doi: 10.3171/jns.1988.68.2.0234. [DOI] [PubMed] [Google Scholar]
- 3.Buchthal A, Belopavlovic M, Mooij JJ. Evoked potential monitoring and temporary clipping in cerebral aneurysm surgery. Acta Neurochir (Wien) 1988;93:28–36. doi: 10.1007/BF01409899. [DOI] [PubMed] [Google Scholar]
- 4.Charbel FT, Ausman JI, Diaz FG, Malik GM, Dujovny M, Sanders J. Temporary clipping in aneurysm surgery : technique and results. Surg Neurol. 1991;36:83–90. doi: 10.1016/0090-3019(91)90223-v. [DOI] [PubMed] [Google Scholar]
- 5.David CA, Prado R, Dietrich WD. Cerebral protection by intermittent reperfusion during temporary focal ischemia in the rat. J Neurosurg. 1996;85:923–928. doi: 10.3171/jns.1996.85.5.0923. [DOI] [PubMed] [Google Scholar]
- 6.Fortuny LA, Adams CB, Briggs M. Surgical mortality in an aneurysm population : effects of age, blood pressure and preoperative neurological state. J Neurol Neurosurg Psychiatry. 1980;43:879–882. doi: 10.1136/jnnp.43.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman WA, Chadwick GM, Verhoeven FJ, Mahla M, Day AL. Monitoring of somatosensory evoked potentials during surgery for middle cerebral artery aneurysms. Neurosurgery. 1991;29:83–88. doi: 10.1097/00006123-199107000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Friedman WA, Kaplan BL, Day AL, Sypert GW, Curran MT. Evoked potential monitoring during aneurysm operation : observations after fifty cases. Neurosurgery. 1987;20:678–687. doi: 10.1227/00006123-198705000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Goldman MS, Anderson RE, Meyer FB. Effects of intermittent reperfusion during temporal focal ischemia. J Neurosurg. 1992;77:911–916. doi: 10.3171/jns.1992.77.6.0911. [DOI] [PubMed] [Google Scholar]
- 10.Jabre A, Symon L. Temporary vascular occlusion during aneurysm surgery. Surg Neurol. 1987;27:47–63. doi: 10.1016/0090-3019(87)90107-8. [DOI] [PubMed] [Google Scholar]
- 11.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 12.Kassell NF, Drake CG, Peerless SJ. Occlusion of major cerebral arteries : new concepts. Surg Forum. 1978;29:527–529. [PubMed] [Google Scholar]
- 13.Kassell NF, Torner JC, Jane JA, Haley EC, Jr, Adams HP. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 2 : Surgical results. J Neurosurg. 1990;73:37–47. doi: 10.3171/jns.1990.73.1.0037. [DOI] [PubMed] [Google Scholar]
- 14.Lanzino G, Kassell NF, Germanson TP, Kongable GL, Truskowski LL, Torner JC, et al. Age and outcome after aneurysmal subarachnoid hemorrhage : why do older patients fare worse? J Neurosurg. 1996;85:410–418. doi: 10.3171/jns.1996.85.3.0410. [DOI] [PubMed] [Google Scholar]
- 15.Ljunggren B, Säveland H, Brandt L, Kågström E, Rehncrona S, Nilsson PE. Temporary clipping during early operation for ruptured aneurysm : preliminary report. Neurosurgery. 1983;12:525–530. doi: 10.1227/00006123-198305000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Momma F, Wang AD, Symon L. Effects of temporary arterial occlusion on somatosensory evoked responses in aneurysm surgery. Surg Neurol. 1987;27:343–352. doi: 10.1016/0090-3019(87)90009-7. [DOI] [PubMed] [Google Scholar]
- 17.Ogilvy CS, Carter BS, Kaplan S, Rich C, Crowell RM. Temporary vessel occlusion for aneurysm surgery : risk factors for stroke in patients protected by induced hypothermia and hypertension and intravenous mannitol administration. J Neurosurg. 1996;84:785–791. doi: 10.3171/jns.1996.84.5.0785. [DOI] [PubMed] [Google Scholar]
- 18.Pool JL. Aneurysms of the anterior communicating (ACC) artery--indications for surgery. Trans Am Neurol Assoc. 1961;86:232–233. [PubMed] [Google Scholar]
- 19.Raftopoulos C, Mathurin P, Boscherini D, Billa RF, Van Boven M, Hantson P. Prospective analysis of aneurysm treatment in a series of 103 consecutive patients when endovascular embolization is considered the first option. J Neurosurg. 2000;93:175–182. doi: 10.3171/jns.2000.93.2.0175. [DOI] [PubMed] [Google Scholar]
- 20.Samson D, Batjer HH, Bowman G, Mootz L, Krippner WJ, Jr, Meyer YJ, et al. A clinical study of the parameters and effects of temporary arterial occlusion in the management of intracranial aneurysms. Neurosurgery. 1994;34:22–28. discussion 28-29. [PubMed] [Google Scholar]
- 21.Schramm J, Koht A, Schmidt G, Pechstein U, Taniguchi M, Fahlbusch R. Surgical and electrophysiological observations during clipping of 134 aneurysms with evoked potential monitoring. Neurosurgery. 1990;26:61–70. doi: 10.1097/00006123-199001000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Sonesson B, Ljunggren B, Säveland H, Brandt L. Cognition and adjustment after late and early operation for ruptured aneurysm. Neurosurgery. 1987;21:279–287. doi: 10.1227/00006123-198709000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg GK, Panahian N, Sun GH, Maier CM, Kunis D. Cerebral damage caused by interrupted, repeated arterial occlusion versus uninterrupted occlusion in a focal ischemic model. J Neurosurg. 1994;81:554–559. doi: 10.3171/jns.1994.81.4.0554. [DOI] [PubMed] [Google Scholar]
- 24.Symon L. European Association of Neurosurgical Societies Fifth European lecture. Barcelona, February 24, 1984. Thresholds of ischaemia applied to aneurysm surgery. Acta Neurochir (Wien) 1985;77:1–7. doi: 10.1007/BF01402298. [DOI] [PubMed] [Google Scholar]
- 25.Symon L. Management of giant intracranial aneurysms. Clin Neurosurg. 1990;36:21–47. [PubMed] [Google Scholar]
- 26.Taylor CL, Selman WR, Kiefer SP, Ratcheson RA. Temporary vessel occlusion during intracranial aneurysm repair. Neurosurgery. 1996;39:893–905. doi: 10.1097/00006123-199611000-00001. discussion 905-906. [DOI] [PubMed] [Google Scholar]