Abstract
Objective
To evaluate the potential effects of risedronate (RIS) which shows a higher anti-resorptive effect among bisphosphonates, after a posterolateral lumbar intertransverse process spinal fusion using both autograft and allograft in a rat model.
Methods
A totoal of 28 Sprague-Dawley rats were randomized into 2 study groups. A posterolateral lumbar intertransverse process spinal fusion was peformed using both autograft and allograft in a rat model. Group I (control) received 0.1 mL of steril saline (placebo) and Group II (treatment) received risedronate, equivalent to human dose (10 µg/kg/week) for 10-weeks period.
Results
The fusion rates as determined by manual palpation were 69% in the group I and 46% in the group II (p = 0.251). According to radiographic score, the spinal segment was considered to be fused radiographically in 7 (53%) of the 13 controls and 9 (69%) of the 13 rats treated with RIS (p = 0.851). The mean histological scores were 5.69 ± 0.13 and 3.84 ± 0.43 for the control and treatment groups, respectively. There was a significant difference between the both groups (p = 0.001). The mean bone density of the fusion masses was 86.9 ± 2.34 in the control group and 106.0 ± 3.54 in the RIS treatment group. There was a statistical difference in mean bone densities of the fusion masses comparing the two groups (p = 0.001).
Conclusion
In this study, risedronate appears to delay bone fusion in a rat model. This occurs as a result of uncoupling the balanced osteoclastic and osteoblastic activity inherent to bone healing. These findings suggest that a discontinuation of risedronate postoperatively during acute fusion period may be warranted.
Keywords: Rats, Risedronate, Spinal fusion, Spine
INTRODUCTION
Osteoporosis is a systemic disease, characterised by reduced bone mass and structural deterioration of bone tissue. It is considered a public health issue threatening a large portion of the population above fifty years of age. Often presenting as a silent disease, it generally occurs asymptomatically and has a progressive course, with a tendency to entail fractures, and requires medical treatment. Bisphosphonates are being used widely in diseases that present increase in bone resorption, such as senile or postmenapause osteopenia and osteoporosis11,26,43,47). Studies indicate that they stimulate a higher bone density increase than other drugs used with the same purpose, such as raloxifene or calcitonin42). Chemically, bisphosphonates are synthetic analogs of endogenous pyrophosphate2,28).
Among bisphosphonates (risedronate, alendronate, pamidronate, etidronate) risedronate (RIS) shows a higher antiresorptive effect3,7,18,34). It affects bone remodeling and increase bone mass through the inhibition of osteoclasts23,24). Its effect on osteoblasts, and the balance between osteoblastic and osteoclastic activity on bone turnover and healing, is not completely understood. Specifically, the effect of RIS on spinal fusion has yet to be determined, and with the increasing use of bisphosphonates in elderly population, this effect needs to be delineated.
The aim of this study was to evaluate the potential effects of RIS after a posterolateral lumbar intertransverse process spinal fusion using with both autograft and allograft in a rat model.
MATERIALS AND METHODS
After approval by the Institutional Animal Care and Use Committee, 28 Sprague-Dawley rats (3 mo of age with weights of 300 - 350 g) were randomized into 2 study groups. General health and activity were monitored daily, and both-groups were administered equal volumes of dailiy gavage for 10 weeks. Group I (control) received 0.1 mL of sterile saline (placebo) and Group II (treatment) received risedronate, equivalent to human dose (10 µg/kg/week). Risedronate (Actonel, Aventis pharma, Inc.) was dissolved in 0.1 mL of sterile saline solution and administered subcutaneously every week.
In humans, oral dose of risedronate is approximately 0.5 mg/kg/week and its oral bioavailability 0.63%29); this is approximately equivalent to 3.15 µg/kg/week. Because the rate of drug elimination is higher in rats than humans beings, allometric scaling equations were used to calculate the equivalent dose in rats and the result was approximately 9.45 µg/kg/week30). Therefore we selected doses 10 µg/kg/week (human equivalent dose).
After general anesthesia was induced with an intraperitoneal injection of pentobarbital (30 - 50 mg/kg body weight) and the rats were placed prone on the operating table. After removal of hair and sterilization with clorhexidine at the operation site, a longitudinal midline skin incision was made from the L3 to the L6 spinous processes. Under the loope magnification, the L4 and L5 transverse processes were exposed via a paravertebral muscle splitting (Wiltse) approach. A high-speed drill was used to decorticate the transverse processes. After decortication, 2 × 2 × 25 mm DBM fiber sheet (Grafton DBM Flex) and harvested from posterior iliac crest of both sides 0.4 gr autograft was placed in the intertransverse space bilaterally. Bone for grafting was obtained through the same surgical incision. After hemostasis was ensured, the incisions were closed in layers with absorbable sutures. All rats were kept in standard cages with water, rat chow, and activity ad libitum. They were euthanized at the end of 10 weeks by intraperitoneal injection of phenobarbital.
Manual palpation
Lumbar spine was extracted with adjacent paravertebral musculature en bloc. A manual manipulation test was done after the most of the soft tissues were removed from the spine and the spine was manipulated in the sagittal plane (flexion-extension). The specimens with complete absence of motion between L4 and L5 were rated as fused. Two independent observers blinded to the treatment groups of the animals rated the specimens for fusion and graded as fused or not fused. The specimens were scored as 0, when motion was present, 1 if there was absolutely no motion was detected.
Radiographic analysis
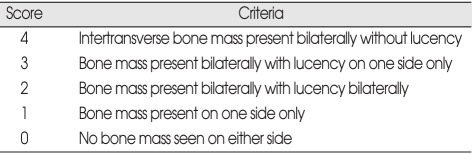
After resection of the fusion segment, each specimen was radiographed anteroposteriorly (Siemens multix 41 kVp, 1.60 mAmp and 3.11 sec). After the collection of data, two independent evaluators reviewed and graded the radiographs according to Table 1.
Table 1.
Definitions for radiographic scores
Density bone fusion mass
Spiral computed tomography was used to evaluate the density of the formed bony fusion mass. The digital CT scans (Siemens somatom emotion duo) acquired for each specimen (section slice thickness of 1 mm). The fusion mass area of each section was determined with 3D (syngo fastView 3 Build VX57G27 Software). The bone density of the fusion masses was recorded for 0.8 cm square bilaterally and averaged for five slices of every specimen. An independent evaluator performed the density measurements.
Histological analysis
Sections of the L4-5 intertransverse process fusion mass were fixed in 10% neutral buffered formalin for 24 hours; then decalcified in 5% nitric acid for 4 days and washed in phosphate-buffered saline solution. After decalcification, specimens were sectioned in the midsagittal plane and dehydrated sequentially in 95% alcohol for 2 days, two changes of 100% alcohol for 2 days, and finally cleared in xylene for 1 day. A sagittal section from the right and left side of L4-5 level was taken for processing and evaluated separately. Each sagittal section included the graft site and adjacent transverse processes were then processed routinely and embedded in paraffin. For histopathologic study, 5-µm-thick tissue sections were deparaffinized, rehydrated, and stained with hematoxylin and eosin.
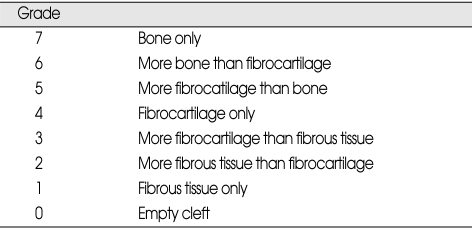
For each histological section, 3 random × 10 fields were evaluated and scored at transverse process interfaces. In total, 312 graft-transverse fusion sites (26 rats×2 sides/rat × 3 × 10 fields/section × 2 fusion sites/section) were graded by two independent blinded pathologists. The quality of the fusion was graded for each section by assigning a histological score, as described by Emery et al.13). The scores for each side were recorded, and the maximum score for each side was reported and used to calculate the average histological fusion rate (Table 2). A fusion mass was considered to have been fused when the maximum Emery scale score was greater than or equal to 6, because this represents the lowest score where bone is the predominant constituent. For purposes of reporting histological fusion rate, total 52 sides in 26 rats were analyzed in Groups I and II.
Table 2.
Hisological scoring of the fusion mass
Statistics
Nonparametric descriptive statistics, including the median and range, were recorded for each group. Kappa values for interobserver and intraobserver repeatability of fusion assessment were calculated. According to J.L. Fleiss's the value of kappa exceeding 0.75 represents excellent agreement, values between 0.4 - 0.75 indicate fair to good agreement, and values less than 0.4 indicate poor agreement16). The differences in fusion rates between Group I and Group II according to manual palpation, radiographic, fusion mass density and histological assessment were analyzed using one way-ANOVA test. Data were presented as mean ± SEM. Values were considered statistically significant at p < 0.05. The statistical analysis was performed using SPSS (SPSS Inc., Statistical Software, Ver. 11.0, Chicago, Illinois, USA).
RESULTS
All rats survived the surgical procedure, but two (one from each group), (7%) of them died in the peri-operative period. There were no medication-related complications and the remaining twenty-six rats had an uneventful postoperative course. No neurological injury, infection or wound complication was seen. They were euthanized at the end of 10 weeks by intraperitoneal injection of phenobarbital.
Results of manual palpation
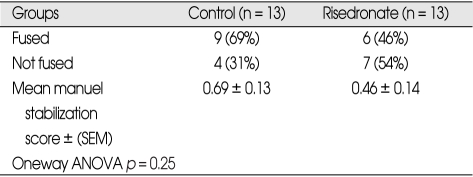
The fusion rates as determined by manual palpation were 69% (9 of 13) in the Group I and 46% (6 of 13) in the Group II. Fusion rates between the two groups were not statistically significant (Table 3) (p = 0.251). Mean Kappa values of 0.78 - 0.86 showed excellent interobserver and intraobserver agreement in fusion assesment.
Table 3.
Results of manuel palpation
Results of radiographic analysis
Radiographic fusion was considered present with a grade of 3 or 4. According to this score, the spinal segment was considered to be fused radiographically in 7 (53%) of the 13 controls and 9 (69%) of the 13 rats treated with RIS (Table 4). Again, this difference was not significant (p = 0.851). The mean radiographic scores were 2.69 ± 0.20 for the control and 2.92 ± 0.21 for the treatment group. The differences were not statistically significant. Radiographic fusion in RIS-treated animals did not correlate with fusion assessed by manual palpation (p = 0.44).
Table 4.
Results of radiographic evaluation
Results of histological analysis
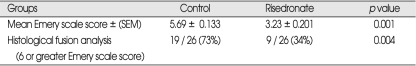
The mean histological scores were 5.69 ± 0.13 and 3.84 ± 0.43 for the control and treatment groups, respectively. There was a significant difference between the both groups (p = 0.001) (Table 5). The histological fusion was considered if Emery scale scores were equal or greater than 6. When evaluating the histological fusion rate for each side individually, the control group had 19 of 26 fusion masses (73%; 11 of 13 left side and 8 of 13 right side) and the treatment group had 9 of 26 fusion masses (34%; 4 left side and 5 right side) (p = .004). The lower median scores and fusion rates were indicated significantly lower content of bone in the fusion masses obtained in the treatment group versus the control group.
Table 5.
Results of histological evaluation
Results of CT bone fusion density measurement
The mean bone density of the fusion masses was 86.9 ± 2.34 Hounsfield units in the control group and 106.0 ± 3.54 in the RIS treatment group (Table 6). There was a statistical difference in mean bone densities of the fusion masses comparing the two groups (p = 0.001).
Table 6.
Results of CT bone density measurement
DISCUSSION
Spinal fusion is a common surgical procedure for the treatment of degenerative spondylosis, symptomatic scoliosis, and instability caused by various conditions. Posterolateral intertransverse process fusion is a commonly performed procedure in the lumbar spine; however, the incidence of failure to achieve fusion has ranged from 5% to 45% in large series because of inadequate new bone mass caused by the limited mass of graft bone or poor surgical technique5,10,41,46). Although autogenous bone graft is the gold Standard for spinal fusion, there is a limit to its mass and complications are associated with bone harvest in as many as 20% of the patients, even in the hands of experienced surgeons8,19,20,37,38). In recent years, a variety of materials have been developed as alternatives to autogenous bone graft to avoid their disadvantages. The use of allogenic bone as a graft material for spinal arthrodesis has expanded. The major disadvantages of allografts include the potential for transmission of infectious disease, immunogenicity and rejection, and decreased biologic activity after sterilization. Furthermore, there are many clinical situations where graft alternatives are desirable. The advances in material engineering have led to renewed interest in osteoconductive materials and yielded materials that may function not only as traditional bone graft expanders, but also as a scaffold for bone ingrowth. To solve these problems, a variety of bone substitutes and bone tissue engineering strategies are already being used9,22,27). In the present study, to increase the fusion rate we applied allograft (DMB Grafton flex) together with autograft in both study groups.
The treatment of osteoporosis is gaining increased attention as our population ages. Many patients are now on therapeutic regimens directed at altering the balance between bone formation and bone resorption with a goal of influencing bone mineral density, and hence, fracture risk. Bisphosphonates and selective estrogen modulators have been investigated extensively12,36,44). Bisphosphonates have been used therapeutically for the last three decades to treat variety of metabolic bone disorders. More recently, they are a class of antiresorptive drugs indicated in patients with osteoporosis, Paget disease of bone, osteogenesis imperfecta, multiple myeloma and malignancies with propensity for skeletal metastasis in which patients have excessive bone resorption, leading to pathologic fractures. Bisphosphonates bid to bone at sites of active bone remodeling to slow down osteolytic activity. The mechanisms include the inhibition of osteoclast formation from its precursors and inhibitive or toxic effects on mature osteoclasts31).
RIS is a potent amino-bisphosphonate that is also known to regulate cell proliferation, differentiation and gene expression in osteoblasts35). The drug has an affinity for bone tissue and approximately 50% of the systemically absorbed drug is deposited in bone, its presumed site of action21). One hypothesis is that RIS prevents bone resorption by altering osteoclasts cytoskeleton's proteins or inhibiting cholesterol synthesis which are necessary for the formation of the ruffled border, interfering directly with the fixation mechanisms of the osteoclasts to bone matrix7). The other hypothesis suggests that it induces the apoptosis of osteoclasts, by means of direct cytotoxic effects1). RIS is not metabolized but can inhibit enzymes of the mevalonate pathway, thereby preventing the biosynthesis of isoprenoid compounds, which are essential for the posttranslational modification of small GTPases disturbing intracellular function38).
Several preclinical studies have reported the efficiacy of RIS for cancellous osteopenia in osteoporosis. It suppresses bone resorption and prevents cancellous bone loss14,33). The influence of RIS on spinal fusion is unknown. The bone graft resorption phase occurs during the healing process25). RIS treatment may modify bone graft healing and remodeling process of spine fusion. Recently, RIS has been shown to have a direct affect by reducing osteoclastic resorption of grafted bone by up-regulating the osteoclast inhibitory factor (OCIF) mRNA expression, while inducing osteoblastic activation by up-regulating osteocalcin and alkaline phosphatase mRNA expression in graft tissue32). One hypothesis for RIS's effect on fusion is that with decreased osteoclast activity, there is decreased osteoblast signaling and thus decreased osteoblast activity26). In a spinal fusion scenario, the delicate balance between bone resorption and bone formation is instrumental in a successful fusion.
Although there are many studies showing the effect of allendronate on spinal fusion rate, there is no any study related with the effects of risedronate. Because of this, we could compare our results only with the studies which had been used aledronate. Many investigators consider manual palpation as the gold standard in fusion assessment in animal models5,44). In the present study, 69% of specimens in control group and 46% of specimens in risedronate group were thought to have a solid fusion by manual palpation and it was not found to be statistically significant (p = .251). Previous works by Lehman et al.26), Huang et al.21) and Sama et al.39) reported their manual fusion rates of 52%, 95% and 43%, in control groups, 59%, 50%, 43% in alendronate groups, with therapeutic doses at the end of six weeks, respectively. Excluded Huang et al., they found no statistical significant effect of alendronate in spine fusion rates assessed by manual palpation similar as in our study. Although there was not a statistical difference in manual palpation, nonsignificant trend toward lower fusion rates in alendronate-treated animals was identified like our risedronate group.
In humans, oral dose of risedronate is approximately 0.5 mg/kg/week and its oral bioavailability 0.63%29), this is approximately equivalent to 3.15 µg/kg/week. Because the rate of drug elimination is higher in rats than humans, allometric scaling equations were used to calculate the equivalent dose in rats30). Oral route reduces bioavailability of alendronate by approximately 85%17) in treated groups because the presence of food in the stomach. Also, some invastigators15) postulated that the daily handling of animals after posterolateral spine fusion reduced fusion rates from 58% to 14%. So, we chose parenteral route and human equivalent dosage in the present study.
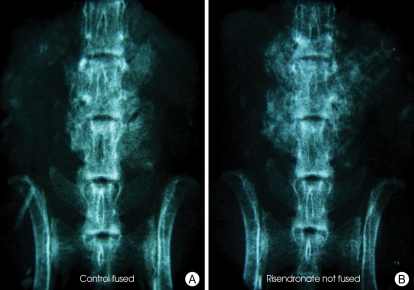
In radiographic evaluation, Boden et al.4), showed that the radiographs were incorrect when compared with fusion status as assessed by manual palpation and confirmed by biomechanical testing. In our study, 54% of specimens in control group and 69% of specimens in risedronate group were thought to have a solid fusion by radiographic evaluation (3 or greater). Radiographic fusion rates in the risedronate group were higher than the control group, but it was not found to be statistically significant (p = 0.85). Besides, in our control and RIS groups, radiographic fusion rates were not consistent with our manual palpation fusion rates like as in other studies1,21,26,39). Radiographically, there was more bone tissue in the fusion beds of RIS-treated animals and this resulted from unincorporated bone graft whose resorption was inhibited by risedronate (Fig. 1). Our radiographic fusion rate in RIS group was also consistent with previous work by Lehman et al.26), Sama et al.39) and Xue et al.43), who reported radiographic fusion rates of 55%, 50% and 55% in the alendronate treated groups, respectively. Numerous studies have shown that plain radiographs are poorly correlated with fusion in animal models6,27,45). Also, fusion mass evaluation through optical density analysis does not faithfully describe the bone callus' morphological course because it does not consider the differences between the trabecular and lamellar bones and also because it adds the fibrous connective tissue to the measurements42). Therefore, we can conclude that radiographic evaluation is not an exact and sufficient method to assess a solid fusion versus a pseudarthrosis in an animal model fusion assessment4).
Fig. 1.
Posteroanterior radiograph of a spinal segment of control group that was interpreted as showing solid fusion (A) and posteroanterior radiograph of a spinal segment of risedronate group that was interpreted as not showing fusion (B).
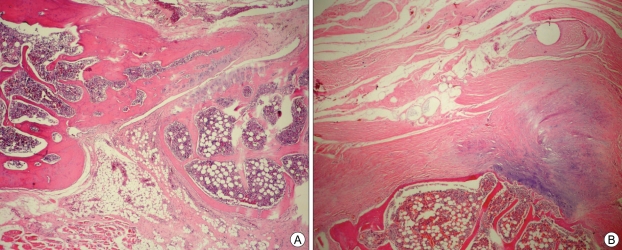
In histological evaluation, we found a significantly higher median Emery scale score for control group (5,69 ± 0,133) versus risedronate group (3,23 ± 0,201) (p = .0001). Also, there was a higher histological fusion rate (6 or greater Emery scale score) in control group (73%) versus risedronate group (34%) (p = .004). The lower median score of the risedronate sodium group is indicative of decreased mature bone present in the fusion mass at harvest. This may reflect an inhibitory effect of risedronate on bone formation or simply a delay in bone maturation. Risedronate shows a higher anti-resorptive effect, and acts as an osteoclast inhibitor18). Whereas, graft resorption and incorporation are essential components of the fusion process, and are mediated by the coupled action of osteoclasts and osteoblasts. A potential explanation for this could be a secondary effect on osteoblasts, thereby decreasing new bone formation. The small quantity of bone and poor remodeling observed in the RIS group was probably due to a response to risedronate treatment, leading to a low bone formation and low resorption (Fig. 2). In the control group, the remodeling and maturation process was almost complete, resulting in trabecular and lamellar bone formation (Fig. 2).
Fig. 2.
Morphologic aspects observed in the control group at 10 weeks of treatment, the remodeling and maturation process was almost complete, resulting in trabecular and lamellar bone formation (H & E×100) (A). More fibrocartilage than fibrous tissue observed in the RIS-treatment group (H & E×100) (B).
In the results of CT bone fusion density, the mean bone densities of the spinal fusion mass were significantly different comparing the two animal groups (p = 0.001) (Table 6). We thought that there was more bone tissue in which not incorporated and resorpted in the fusion beds of risedronate-treated animals. That's why; the density of bone fusion mass in RIS-treated animals was higher than the control group.
In conclusion, we found that RIS inhibited formation or at least maturation of the fusion mass and decreased the spinal fusion rate in the present study. Because spinal fusion requires a delicate balance between bone resorption and bone formation which may even need to be skewed in favor of bone formation, it is possible that the inhibition of osteoclast activity is enough to alter the balance and negatively affect the fusion process in the acute phase of spinal fusion. According to our results of RIS therapy in 10 week-period, we recommend that RIS not to be used in the immediate postoperative period in order to increase the fusion rate. Our data support the need for more research on the long-term effects of RIS before clinical trials and future studies are needed to define the optimum time sequence for reinitiating of RIS treatment and to further evaluate and validate their direct and secondary effects on bone formation and resorption in spinal fusion.
References
- 1.Bae DC, Stein BS. The diagnosis and treatment of osteoporosis in men on androgen deprivation therapy for advanced carcinoma of the prostate. J Urol. 2004;172:2137–2144. doi: 10.1097/01.ju.0000141515.67372.e5. [DOI] [PubMed] [Google Scholar]
- 2.Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, et al. Myeloma Aredia Study Group. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. N Engl J Med. 1996;334:488–493. doi: 10.1056/NEJM199602223340802. [DOI] [PubMed] [Google Scholar]
- 3.Blake GM, Fogelman I. Bone densitometry, steroids and osteoporosis. Curr Opin Nephrol Hypertens. 2002;11:641–647. doi: 10.1097/00041552-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Boden SD, Schimandle JH, Hutton WC. An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine (Phila Pa 1976) 1995;20:412–420. doi: 10.1097/00007632-199502001-00003. [DOI] [PubMed] [Google Scholar]
- 5.Boden SD. The biology of posterolateral lumbar spinal fusion. Orthop Clin North Am. 1998;29:603–619. doi: 10.1016/s0030-5898(05)70034-1. [DOI] [PubMed] [Google Scholar]
- 6.Boden SD. Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine (Phila Pa 1976) 2002;27:S26–S31. doi: 10.1097/00007632-200208151-00007. [DOI] [PubMed] [Google Scholar]
- 7.Boonen S, Haentjens P, Vandenput L, Vanderschueren D. Preventing osteoporotic fractures with antiresorptive therapy : implications of microarchitectural changes. J Intern Med. 2004;255:1–12. doi: 10.1046/j.0954-6820.2003.01258.x. [DOI] [PubMed] [Google Scholar]
- 8.Colterjohn NR, Bednar DA. Procurement of bone graft from the iliac crest. An operative approach with decreased morbidity. J Bone Joint Surg Am. 1997;79:756–759. doi: 10.2106/00004623-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Choi Y, Oldenburg FP, Sage L, Johnstone B, Yoo JU. A bridging demineralized bone implant facilitates posterolateral lumbar fusion in New Zealand white rabbits. Spine (Phila Pa 1976) 2007;32:36–41. doi: 10.1097/01.brs.0000250982.41666.55. [DOI] [PubMed] [Google Scholar]
- 10.Cotler JM, Star AM. Complications of spinal fusion. In: Cotler JM, Cotler HM, editors. Spinal fusion : Science and Technique. New York: Springer-Verlag; 1990. pp. 361–387. [Google Scholar]
- 11.Das H, Wang L, Kamath A, Bukowski JF. Vgamma2Vdelta2 T-cell receptor-mediated recognition of aminobisphonates. Blood. 2001;98:1616–1618. doi: 10.1182/blood.v98.5.1616. [DOI] [PubMed] [Google Scholar]
- 12.Delmas PD. Treatment of postmenopausal osteoporosis. Lancet. 2002;359:2018–2026. doi: 10.1016/S0140-6736(02)08827-X. [DOI] [PubMed] [Google Scholar]
- 13.Emery SE, Brazinski MS, Koka A, Bensusan JS, Stevenson S. The biological and biomechanical effects of irradiation on anterior spinal bone grafts in a canine model. J Bone Joint Surg Am. 1994;76:540–548. doi: 10.2106/00004623-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Erben RG, Mosekilde L, Thomsen JS, Weber K, Stahr K, Leyshon A, et al. Prevention of bone loss in ovariectomized rats by combined treatment with risedronate and 1alpha, 25-dihydroxyvitamin D3. J Bone Miner Res. 2002;17:1498–1511. doi: 10.1359/jbmr.2002.17.8.1498. [DOI] [PubMed] [Google Scholar]
- 15.Feiertag MA, Boden SD, Schimandle JH, Norman JT. A rabbit model for nonunion of lumbar intertransverse process spine arthrodesis. Spine (Phila Pa 1976) 1996;21:27–31. doi: 10.1097/00007632-199601010-00006. [DOI] [PubMed] [Google Scholar]
- 16.Fless JL. Statistical methods for rates and proportion. ed 2. New York: Jon Wiley & Sons; 1981. [Google Scholar]
- 17.Gertz BJ, Holland SD, Kline WF, Matuszewski BK, Porras AG. Clinical pharmacology of alendronate sodium. Osteoporos Int. 1993;3(Suppl 3):S13–S16. doi: 10.1007/BF01623002. [DOI] [PubMed] [Google Scholar]
- 18.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmanopausal osteoporosis : a randomized controlled trial. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 19.Hill NM, Horne JG, Devane PA. Donor site morbidity in the iliac crest bone graft. Aust N Z J Surg. 1999;69:726–728. doi: 10.1046/j.1440-1622.1999.01674.x. [DOI] [PubMed] [Google Scholar]
- 20.Hoard MA, Bill TJ, Campbell RL. Reduction in morbidity after iliac crest bone harvesting : the concept of preemptive analgesia. J Craniofac Surg. 1998;9:448–451. doi: 10.1097/00001665-199809000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Huang RC, Khan SN, Sandhu HS, Metzl JA, Cammisa FP, Jr, Zheng F, et al. Alendronate inhibits spine fusion in a rat model. Spine (Phila Pa 1976) 2005;30:2516–2522. doi: 10.1097/01.brs.0000186470.28070.7b. [DOI] [PubMed] [Google Scholar]
- 22.Itoh H, Ebara S, Kamimura M, Tateiwa Y, Kinoshita T, Yuzawa Y. Experimental spinal fusion with use of recombinant human bone morphogenetic protein 2. Spine. 1999;24:1402–1405. doi: 10.1097/00007632-199907150-00003. [DOI] [PubMed] [Google Scholar]
- 23.Iwamoto J, Takeda T, Sato Y, Shen CL, Yeh JK. Beneficial effect of pretreatment and treatment continuation with risedronate and vitamin K2 on cancellous bone loss after ovariectomy in rats : a bone histomorphometry study. J Nutr Sci Vitaminol (Tokyo) 2006;52:307–315. doi: 10.3177/jnsv.52.307. [DOI] [PubMed] [Google Scholar]
- 24.Iwamoto J, Seki A, Takeda T, Sato Y, Yamada H, Yeh JK. Effect of risedronate on the corticol and cancellous bone mass and mechanical properties ovariectomized rats : a comparison with the effects of alfacalcidol. J Nutr Sci Vitaminol (Tokyo) 2006;52:393–401. doi: 10.3177/jnsv.52.393. [DOI] [PubMed] [Google Scholar]
- 25.Kaynak D, Meffert R, Günhan M, Günhan O, Ozkaya O. A histopathological investigation on the effects of the bisphosphonate alendronate on resorptive phase following mucoperiosteal flap surgery in the mandible of rats. J Periodontol. 2000;71:790–796. doi: 10.1902/jop.2000.71.5.790. [DOI] [PubMed] [Google Scholar]
- 26.Lehman RA, Jr, Kuklo TR, Freedman BA, Cowart JR, Mense MG, Riew KD. The effect of alendronate sodium on spinal fusion : a rabbit model. Spine J. 2004;4:36–43. doi: 10.1016/s1529-9430(03)00427-3. [DOI] [PubMed] [Google Scholar]
- 27.Long J, Lewis S, Kuklo T, Zhu Y, Riew KD. The effect of cyclooxygenase-2 inhibitors on spinal fusion. J Bone Joint Surg Am. 2002;84-A:1763–1768. doi: 10.2106/00004623-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 28.McCloskey EV, MacLennan IC, Drayson MT, Chapman C, Dunn J, Kanis JA. A randomized trial of the effect of clodronate on skeletal morbidity in multiple myeloma. MRC Working Party on Leukaemia in Adults. Br J Haematol. 1998;100:317–325. doi: 10.1046/j.1365-2141.1998.00567.x. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell DY, Barr WH, Eusebio RA, Stevans KA, Duke FP, Russell DA, et al. Risedronate pharmacokinetics and intra- and inter-subject variability upon single-dose intravenous and oral administration. Pharm Res. 2001;18:166–170. doi: 10.1023/a:1011024200280. [DOI] [PubMed] [Google Scholar]
- 30.Morris TH. Dose estimation among species. In: Leary SL, editor. Formulary for Laboratory Animals. ed 2. Ames, IA: Iowa State Press; 1999. pp. 3–14. [Google Scholar]
- 31.Mundy GR, Yoneda T, Hiraga T. Preclinical studies with zolendronic acid and other bisphosphonates: impact on the bone microenvironment. Semin Oncol. 2001;28:35–44. doi: 10.1016/s0093-7754(01)90263-5. [DOI] [PubMed] [Google Scholar]
- 32.Myoung H, Park JY, Choung PH. Effects of 堢isphosphonate on the expression of bonespecific genes after autogenous free bone grafting in rats. J Periodontal Res. 2001;36:244–251. doi: 10.1034/j.1600-0765.2001.036004244.x. [DOI] [PubMed] [Google Scholar]
- 33.Otomo H, Sakai A, Ikeda S, Tanaka S, Ito M, Phipps RJ, et al. Regulation of mineral-to-matrix ratio of lumbar trabecular bone in ovariectomized rats treated with risedronate in combination with or without vitamin K2. J Bone Miner Metab. 2004;22:404–414. doi: 10.1007/s00774-004-0502-6. [DOI] [PubMed] [Google Scholar]
- 34.Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, et al. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int. 2000;11:83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 35.Reinholz GG, Gertz B, Pederson L, Sanders ES, Subramaniam M, Ingle JN, et al. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000;60:6001–6007. [PubMed] [Google Scholar]
- 36.Riggs BL, Hartmann LC. Selective estrogen-receptor modulators--mechanisms of action and application to clinical practice. N Engl J Med. 2003;348:618–629. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- 37.Robertson PA, Wrag AC. Natural history of posterior iliac crest bone graft donation for spinal surgery : a prospective analysis of morbidity. Spine (Phila Pa 1976) 2001;26:1473–1476. doi: 10.1097/00007632-200107010-00018. [DOI] [PubMed] [Google Scholar]
- 38.Rogers MJ, Frith JC, Luckman SP, Coxon FR, Benford HL, Mönkkönen J, et al. Molecular mechanism of action of bisphosphonate. Bone. 1999;24:73S–79S. doi: 10.1016/s8756-3282(99)00070-8. [DOI] [PubMed] [Google Scholar]
- 39.Sama AA, Khan SN, Myers ER, Huang RC, Cammisa FR, Jr, Sandhu HS, et al. High-dose alendronate uncouples osteoclast and osteoblast function : a study in a rat spine pseudarthrosis model. Clin Orthop Relat Res. 2004:135–142. doi: 10.1097/00003086-200408000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Seiler JG, 3rd, Johnson J. Iliac crest autogenous bone grafting: donor site complications. J South Orthop Assoc. 2000;9:91–97. [PubMed] [Google Scholar]
- 41.Steinmann JC, Herkowitz HN. Pseudarthrosis of the spine. Clin Orthop Relat Res. 1992:80–90. [PubMed] [Google Scholar]
- 42.Werkman C, Senra GS, Rocha RF, Brandão AA. Comparative therapeutic use of Risendronate and Calcarea phosphorica--allopathy versus homeopathy--in bone repair in castrated rats. Braz Oral Res. 2006;20:196–201. doi: 10.1590/s1806-83242006000300003. [DOI] [PubMed] [Google Scholar]
- 43.Xue Q, Li H, Zou X, Bünger M, Egund N, Lind M, et al. The influence of alendronate treatment and bone graft volume on posterior lateral spine fusion in a porcine model. Spine (Phila Pa 1976) 2005;30:1116–1121. doi: 10.1097/01.brs.0000162929.19985.d2. [DOI] [PubMed] [Google Scholar]
- 44.Yee AJ, Bae HW, Friess D, Robbin M, Johnstone B, Yoo JU. Accuracy and interobserver agreement for determinations of rabbit posterolateral spinal fusion. Spine (Phila Pa 1976) 2004;29:1308–1313. doi: 10.1097/01.brs.0000127184.43765.61. [DOI] [PubMed] [Google Scholar]
- 45.Yee AJ, Hyun HW, Friess D, Roth SM, Whyne C, Robbin M, et al. The use of simvastatin in rabbit posterolateral lumbar intertransverse process spine fusion. Spine J. 2006;6:391–396. doi: 10.1016/j.spinee.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 46.Zdeblick TA. A prospective, randomized study of lumbar fusion. Preliminary results. Spine (Phila Pa 1976) 1993;18:983–991. doi: 10.1097/00007632-199306150-00006. [DOI] [PubMed] [Google Scholar]
- 47.Zou X, Xue Q, Li H, Bunger M, Lind M, Bünger C. Effect of alendronate on bone ingrowth into porous tantalum and carbon fiber interbody devices : an experimental study on spinal fusion in pigs. Acta Orthop Scand. 2003;74:596–603. doi: 10.1080/00016470310018027. [DOI] [PubMed] [Google Scholar]